Abstract
Obesity has major adverse effects on reproductive performance and fertility potential especially in women with polycystic ovary syndrome. In this study, we aimed to evaluate the consequences of excess weight reduction by bariatric surgery on androgen levels, and ovarian volume by ultrasonography in obese polycystic ovary patients. This one year Cohort study was carried out in Zagazig university hospitals. The study included 36 infertile women with PCOS and obesity, who underwent bariatric surgery(sleeve gastrectomy or gastric bypass). Patients were evaluated for free and total serum testosterone levels, Sex hormone binding globulin (SHBG), free androgen index (FAI) and also ovarian volume by ultrasound pre-operatively, 6 months and 1 year after surgery. The results showed significant reduction in Body Mass Index, free and total serum testosterone levels and rise in SHBG and regulation of menstrual cycle at 6 and 12 months after operation. Free androgen index and ovarian volume by ultrasound also significantly decreased (p < 0.001) .As a conclusion, Bariatric surgery results in durable loss of weight and restores the normal physiological balance of androgenic milieu and ovarian morphology by ultrasound, in infertile women who have Polycystic ovary syndrome.
Keywords: Bariatric surgery, Polycystic ovary, Obesity, Infertility
1. Introduction
Polycystic ovary syndrome (PCOS) has the biggest share in female infertility. “The Rotterdam criteria” is one of the most commonly utilized by clinicians. To build a diagnosis of PCOS using such criteria, the case is supposed to comprise no less than two of; clinical and/or biochemical hyper-androgenic features, menstrual cycle abnormalities, and the characteristic picture of polycystic ovary on ultrasound examination, after ruling other disorders of the endocrine system (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004, Panidis et al., 2014). The national institute of health and disease required the identification of clinical features of hyper-androgenism and/ or elevated serum androgen levels, oligo- or anovulation besides ruling-out other conditions that can result in hyper-androgenism, and excluded ultrasound in diagnostic essentials (Leerasiri et al., 2015, Fulghesu et al., 2017). Even though no clearly identified aetio-pathiology for PCOS exists till now, yet it’s thought to comprise a multifaceted interplay between environmental and gene-related factors. Resistance to insulin and elevated serum insulin are believed to be main mechanisms (Singh et al., 2020).
Recently, two new phenotypes have been proposed besides patients with ovulatory problems and hyper-androgenism. This includes patients with disordered ovulation and polycystic ovary but no evidence of hyper-androgenism, and patients with the ultrasound picture of PCO and hyper-androgenism but with normal menstrual cycles (Belosi et al., 2006, Jamal et al., 2012) Moreover, over 50% of PCOS cases are obese. Obesity is often associated with anovulation, infertility, miscarriage, and perinatal adverse outcomes. Thus it has been advised that treatments targeting fertility should be deferred till woman’s BMI falls below 35 kg/m2 (March et al., 2010, Makaronidis et al., 2017). Actually, spontaneous ovulation can ensue following even<6% of the present body weight (Butterworth et al., 2016).
Bariatric surgery represents a durable option for sustained weight reduction in obese people and also results in alleviation of the metabolic profile disturbances (Fulghesu et al., 2017). After the advances in the laparoscopic techniques, bariatric procedures have advanced to become a favored resort for PCOS obese patients with infertility (Escobar-Morreale et al., 2005, Hector et al., 2017, Mette et al., 2017). Bariatric procedures fall into three main categories; mal-absorptive such as bilio-pancreatic diversion, and restrictive such as; gastric band application and “sleeve gastrectomy”, while “Roux-en-Y gastric bypass” is considered a combined approach (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004, Skubleny et al., 2016). Recent research has proposed that the fast and persistent weight reduction following bariatric procedures can escalate women’s possibility of getting pregnant and also impact both the clinical and laboratory features of PCOS. However, there are not much data regarding pregnancy outcomes post-bariatric (Karmon and Sheiner, 2008). We aimed at this study to evaluate the effect of excess weight loss through bariatric surgery on PCOS patients.
2. Materials and methods
2.1. Study design and settings
This Cohort study took place in General Surgery out-patient clinic and General Gynecology and Infertility outpatient clinic, Zagazig University Hospitals. The study was carried out in the period from January 2019 to January 2020. The study was approved by Zagazig University Institutional Board Review (ZU-IRB#511212–1-2019), and informed consent has been taken from all patients prior to start of the study.
2.2. Patients
The study included 36 females; inclusion criteria included the following: obese women suffering from infertility due to PCOS (after other infertility causes have been excluded), who underwent bariatric surgery or who were planning to do so, age range 22–40 years old, post-surgery BMI ≤ 35 kg/m2. The exclusion criteria were: cases with medical disorders, age above 40 years , post-surgery BMI > 35 kg/m2, other factors of infertility and use of oral contraceptives.
2.3. Methods
Patients were actively involved in the study by recruitment, history taking, and follow up by examination, serum samples’ withdrawal and ultrasound evaluation before , 6 and 12 months’ after surgery. The results are disseminated to participants by direct contact.
2.3.1. History taking
Full history was taken from all cases (personal, present and past medical, and reproductive history, including duration since marriage, date since any previous pregnancies if present and number of live births or abortions if any, use of any oral contraceptives and previous history of any infertility treatments intake. Regarding patients that were recruited before undergoing bariatric surgery, detailed menstrual history is taken. In patients who were recruited to the study after performing the surgery, detailed history is taken, and all patient’s data from the system were reviewed to exclude any other causes of infertility pre-operatively.
2.3.2. Examination
Before bariatric surgery, women’s weight in kilograms is divided by the square of their height in meters to calculate body mass index (BMI), and later compared to the BMI postoperatively at 6 months and 1 year after the operation). General physical and gynecological examination and inspection for signs of clinical hyper-androgenism as acne and hirsutism was performed.
2.3.3. Ultrasonography
Trans-vaginal ultrasound using 2D trans-vaginal ultrasound transducer with 8 MHz frequency was used to assess ovarian volume (either by machine or manually by multiplying the values of ovarian length, depth and width and then multiplying by 0.52, as approved by the international society of Ultrasound in obstetrics and gynecology “ISUOG”. Increased ovarian volume was defined when values spanned over 10 ml, insuring no cysts and the absence of dominant follicle or corpus luteum.
2.3.4. Laboratory investigations
Free and total serum testosterone levels as well as Sex Hormone Binding Globulin (SHBG) levels were either obtained from patients’ files or assessed pre-operatively using enzyme linked immunoassay kit supplied by Elabscience, USA. Levels were re-assessed 6 months and 1 year post-operative. Free androgen index (FAI) was calculated by dividing total serum testosterone value by the value of sex hormone binding globulin (SHBG) and then multiplying by a constant (1 0 0).
2.3.5. Bariatric procedure
All patients who presented pre-operatively underwent standard laparoscopic bariatric procedures (sleeve gastrectomy or gastric bypass), and if patient presented post-operative, type of the procedure and any complications were identified from patients’ files.
2.3.6. Follow up
Follow up of the patients for one year post-operative and if pregnancy occurs, time from bariatric surgery to conception is calculated and pregnancy outcome is observed. In case post-bariatric surgery woman with previous history of PCO & obesity related infertility has undergone bariatric surgery within the past 3 years attends with current pregnancy, time from bariatric surgery to conception is calculated and pregnancy outcome is observed.
2.3.7. Measurable outcomes
The primary outcome was to appraise the influence of bariatric surgery on the alleviation of biochemical hyper-androgenism and ovarian volume in PCOS obese women with infertility. The secondary outcome is to assess conception rate and time lapse between operation and conception and outcome (if occurred).
3. Results
Basic pre-operative characteristics of the patients are shown in Table 1. The mean age was 27.2 ± 4.2 years and the mean BMI was 43.6 ± 1.76 kg/m2. All patients had irregular cycles at the start of the study. Twenty (55.5%) patients suffered from 1ry infertility, and the remaining sixteen patients (44.5%) had 2ry infertility due to PCOS. Pre-operatively, all women had biochemical hyper-androgenism, with mean free serum testosterone level of 51.5 ± 16.5 pg/ml and mean total testosterone level of 3.67 ± 0.85 ng/dl. The mean SHBG level was 16.3 ± 2.31 nmol/L. Twenty two cases (61%) underwent laparoscopic sleeve gastrectomy, while 14 cases (39%) underwent laparoscopic gastric bypass. No cases encountered complications during or after surgery. All the 36 patients (100%) were followed up 6-month and 1-year post procedure for excess weight loss, the level of androgens and SHBG, and the change in ovarian volume by ultrasound. No cases got pregnant during the 1 year follow up period. Data collected were categorized and tabulated. Appropriate statistical tests were used for statistical analysis (Independent sample t-test, Mann-whitney test, chi-square test and Fisher exact test).
Table 1.
Basic characters of the patients.
| Studied group n = 36 |
|
|---|---|
| Age in years, mean ± SD (range) | 27.2 ± 4.21 (21 – 36) |
| Type of infertility 1ry infertility, n (%) 2ry infertility, n (%) |
20 (55.5%) 16 (44.5%) |
| Surgery done Laparoscopic sleeve gastrectomy, n (%) Laparoscopic gastric bypass, n (%) |
22 (61%) 14 (39%) |
N = number, SD = standard deviation.
3.1. Effect of bariatric surgery on body mass index
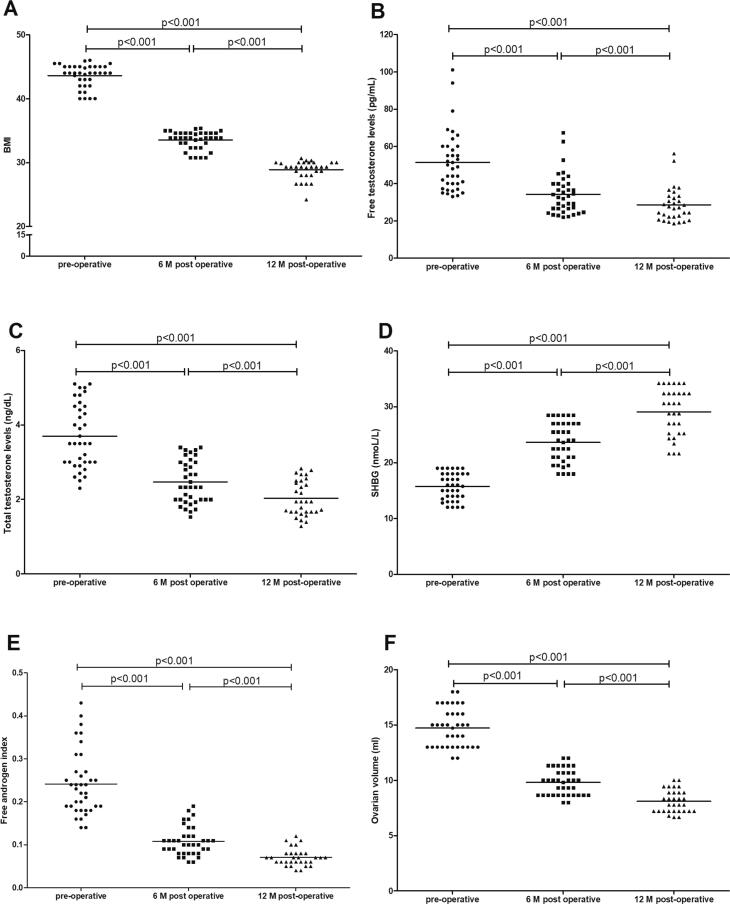
All cases showed significant reduction in BMI with mean pre-operative value of 43.6 ± 1.76 kg/m2. The mean post-operative BMI was 33.5 ± 1.36 kg/m2 (at 6 months follow up) and 29.1 ± 1.17 kg/m2 at completing 1 year follow up (Fig. 2A) Fig. 1.
Fig. 2.
Changes in BMI (A), levels of serum free testosterone (B), total testosterone (C) and sex hormone binding globulin (SHBG) (D), free androgen index (E) and ovarian volume (F) among studied group before operation, 6 and 12 months after bariatric surgery.
Fig. 1.
Basic pre-operative values of the studied group at recruitment.
3.2. Effect of weight loss after surgery on serum androgen level
All patients experienced maximum drop in mean serum free testosterone at 6 months after surgery, from a mean of 51.5 ± 16.5 pg/ml pre-operatively to 34.3 ± 11.1 pg/ml (P < 0.001) at 6 months and continued to fall to reach a mean of 28.6 ± 9.2 pg/ml at 1 year- post operatively (P < 0.001) (Fig. 2B). Similarly, total serum testosterone showed highly significant improvement from a mean of 3.67 ± 0.85 ng/dl pre-operatively, to 2.45 ± 0.57 ng/dl at 6 months follow up, and continued to decrease to 2.04 ± 0.47 ng/dl 1 year after surgery (P < 0.001) (Fig. 2C). Levels of SHBG increased with a pre-operative mean of 16.3 ± 2.31 nmol/L that increased to reach 24.4 ± 3.51 nmol/L at 6 months follow up and increased to 29.3 ± 4.21 nmol/L at 1 year post-operatively (P < 0.001) (Fig. 2D). Free androgen index showed high significant decrease, with a mean of 0.23 ± 0.07 pre-operative, that dropped to 0.103 ± 0.03 after 6 months and reached a mean of 0.07 ± 0.02 after one year post- operative (P < 0.001) (Fig. 2E).
3.3. Effect of weight loss after surgery on ovarian volume by ultrasound
There was a gradual reduction in the ovarian morphology and volume, with a mean ovarian volume of 14.7 ± 1.79 ml, that showed progressive and sustained reduction to a mean of 9.78 ± 1.19 ml, 6 months post-operative, and reached a mean of 8.15 ± 0.99 ml, 1 year post-operative, and that is highly significant(P < 0.001) (Fig. 2F).
4. Discussion
In all cases included in this study, bariatric surgery leads to sustained and effective drop in the excess body weight with decrease in the BMI and also improvement of the free and total testosterone levels, SHBG increased, and free androgen index decreased. Also, ovarian volume by ultrasound decreased to normal.
This agrees with the results from (Singh et al., 2020), who recruited 26 women among whom, where 8 women had cycle disturbances and the remaining 18 were diagnosed with PCOS. The mean age of cases was 29.7 ± 5.9 years (18–40 years), 12 out of 18 cases were operated upon by laparoscopic sleeve gastrectomy, 5 cases underwent laparoscopic gastric bypass and one underwent mini gastric bypass. The mean serum testosterone decreased from 0.8 ± 0.4 ng/ml before surgery to 0.5 ± 0.3 ng/ml (P < 0.05) and 0.4 ± 0.3 ng/ml (P < 0.05) after surgery, at 3 and 6 months respectively. At the completion of one year after surgery, values reached 0.4 ± 0.2 ng/ml (P < 0.01). Regarding ultrasonography, follicle numbers and volume of the ovary decreased in all cases apart from one woman who didn’t show any changes in ovarian ultrasound appearance.
Also (Li et al., 2019), in their systemic review and meta-analysis of 9 studies that enrolled 234 obese cases with polycystic ovarian syndrome undertook bariatric surgery and concluded a significant reduction in body mass index “MD = –14.51; 95% CI –17.88 to –11.14; P < 0.00001” and a highly significant drop in the total serum testosterone levels “mean difference equal –25.82; 95% CI –30.06 to –21.58; P < 0.00001”, and also a significant drop in free serum testosterone and this agrees with our results.
Also, (Christ and Falcone, 2018) studied 109 women, among whom Forty-four had PCOS and 65 were controls, the body mass index in all cases decreased after bariatric surgery from 44.2 ± 2.1 pre-operative to a mean of 35.4 ± 1.5 post-operative. The authors proved that PCOS group had substantial declines in androgen hormonal levels (P < 0.05) , with a mean free testosterone level of 29.8 ± 4.5 pg/ml pre-operative, that improved significantly to 29.8 ± 4.5 pg/ml at the studied PCOS women, and total testosterone mean value equal to 2.2 ± 0.2 ng/dl pre-operative that improved post-operative to a mean of 1.2 ± 0.2 in the PCOS group, the ovarian volume declined from 8.4 ± 1.2 pre-operative to a mean of 6.0 ± 1.5 post-operative but didn’t give a statistical significant difference .They concluded that bariatric surgical procedures improve key features of PCOS and reduce ovarian volume, free testosterone. And this is consistent with the results from our study.
The results from our study also agree with (Hector et al., 2005) who also noticed the decline in total testosterone levels with pre-operative value of 69 ± 32 ng/dl, that dropped to 42 ± 19 ng/dl; P < 0.02, and free serum testosterone levels declined from 1.6 ± 0.7 to 0.6 ± 0.3 ng/dl; P < 0.005, after bariatric surgery. Also the results from (Lee et al., 2020) come in agreement with our results as the authors proved that the effective short term weight loss achieved by bariatric surgery results in restoration of the regular menstrual pattern and ameliorates hyperandrogenism and its related manifestations and restores fertility in polycystic ovary women. Also (Christinajoice et al., 2020) who studied 45 women with polycystic ovary syndrome who underwent bariatric procedures and showed marked improvement in menstrual abnormalities associated with PCOS and also fertility improved along with better maternal outcomes.
In their systematic review and meta-analysis, (Hector et al., 2017) also found that after bariatric surgery, resolution of PCOS was found in 96% of affected women and sex hormone-binding globulin concentrations increased after bariatric surgery. On the contrary, sex-specific changes were observed in serum androgen concentrations: for example, total testosterone concentration decreased in women and was accompanied by resolution of hirsutism in 53% (95CI 29–76), and of menstrual dysfunction in 96% of cases who suffered from this before bariatric surgery.
In addition, (Mette et al., 2017) evaluated the effect of RYGB on polycystic ovary women and observed that SHBG increased progressively and was doubled after 12 months. On the contrary, both total and free androgens and decreased by about 50% after bariatric surgery and 85% of women restored normal menstrual pattern one year post bariatric surgery.
Moreover, (Różańska-Walędziak et al., 2020) followed up 515 premenopausal women with PCOS for 1 year after bariatric surgery, and observed a significant difference in menstrual pattern after bariatric surgery but in contrast to our study, the authors actually did not observe any improvement in hirsutism or any other clinical symptoms of hyperandrogenism in the studied group of patients after bariatric surgery.
5. Conclusions
Efficient and durable weight reduction can be obtained by bariatric surgery in obese PCOS patients. Bariatric surgery alleviates biochemical abnormalities such as serum androgen levels as free and total testosterone, increases SHBG level and also decreases free androgen index. The androgenic and reproductive milieu and also ovarian volume improve significantly after bariatric surgery in obese PCOS patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Belosi, C., Selvaggi, L., Apa, R., et al., 2006. Is the PCOS diagnosis solved by ESHRE/ASRM 2003 consensus or could it include ultrasound examination of the ovarian stroma? Hum Reprod. 21(12), 3108-15. doi: 10.1093/humrep/del306. Epub 2006 Oct 19. PMID: 17053004. [DOI] [PubMed]
- Butterworth J., Deguara J., Borg C.-M. Bariatric Surgery, Polycystic Ovary Syndrome, and Infertility. J Obes. 2016;2016:1–6. doi: 10.1155/2016/1871594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ J.P., Falcone T. Bariatric Surgery Improves Hyperandrogenism, Menstrual Irregularities, and Metabolic Dysfunction Among Women with Polycystic Ovary Syndrome (PCOS) Obes. Surg. 2018;28(8):2171–2177. doi: 10.1007/s11695-018-3155-6. [DOI] [PubMed] [Google Scholar]
- Christinajoice S., Misra S., Bhattacharya S., Kumar S.S., Nandhini B.D., Palanivelu C., Raj P.P. Impact of Bariatric Surgery on Female Reproductive Health and Maternal Outcomes. Obes. Surg. 2020;30(2):383–390. doi: 10.1007/s11695-019-04245-0. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale H.F., Botella-Carretero J.I., Álvarez-Blasco F., Sancho J., San Millán J.L. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90(12):6364–6369. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- Fulghesu A.M., Canu E., Porru C. Ultrasound Diagnosis of Polycystic Ovarian Syndrome: Current Guidelines, Criticism and Possible Update. Austin J Obstet Gynecol. 2017;4(2):1074. [Google Scholar]
- Escobar-Morreale H.F., Santacruz E., Luque-Ramírez M., Botella Carretero J.I. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Human Reproduction Update. 2017;23(4):390–408. doi: 10.1093/humupd/dmx012. [DOI] [PubMed] [Google Scholar]
- Jamal M., Gunay Y., Capper A., Eid A., Heitshusen D., Samuel I. Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: a 9-year analysis. Surgery for Obesity and Related Diseases. 2012;8(4):440–444. doi: 10.1016/j.soard.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Karmon A., Sheiner E. Pregnancy after bariatric surgery: a comprehensive review. Arch Gynecol Obstet. 2008;277(5):381–388. doi: 10.1007/s00404-008-0608-5. [DOI] [PubMed] [Google Scholar]
- Lee R, Joy Mathew C, Jose MT, et al., 2020.A Review of the Impact of Bariatric Surgery in Women With Polycystic Ovary Syndrome. Cureus. 12(10), e10811. [DOI] [PMC free article] [PubMed]
- Leerasiri P., Wongwananuruk T., Rattanachaiyanont M., Indhavivadhana S., Techatraisak K., Angsuwathana S. Ratio of ovarian stroma and total ovarian area by ultrasound in prediction of hyperandrogenemia in reproductive-aged Thai women with polycystic ovary syndrome: a diagnostic test. J Obstet Gynaecol Res. 2015;41(2):248–253. doi: 10.1111/jog.12514. [DOI] [PubMed] [Google Scholar]
- Li Y.J., Han Y., He B. Effects of bariatric surgery on obese polycystic ovary syndrome: a systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(6):942–950. doi: 10.1016/j.soard.2019.03.032. Epub 2019 Apr 3 PMID: 31113751. [DOI] [PubMed] [Google Scholar]
- Makaronidis J., Pucci A., Manning S., Cheung W.H., Kingett H., Buckley G., Kirk A., Carr-Rose K., Tshiala A., Jenkinson A., Elkalaawy M., Hashemi M., Adamo M., O'Brien P., Richens Y., Batterham R. Pregnancy after bariatric surgery: a single-centre retrospective cohort study. Endocrine Ab-stracts. 2017 doi: 10.1530/endoabs.48.OC7. [DOI] [Google Scholar]
- March W.A., Moore V.M., Willson K.J., Phillips D.I.W., Norman R.J., Davies M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- Kjær M.M., Madsbad S., Hougaard D.M., Cohen A.S., Nilas L. The impact of gastric bypass surgery on sex hormones and menstrual cycles in premenopausal women. Gynecological Endocrinology. 2017;33(2):160–163. doi: 10.1080/09513590.2016.1236243. [DOI] [PubMed] [Google Scholar]
- Panidis D., Tziomalos K., Papadakis E., Chatzis P., Kandaraki E.A., Tsourdi E.A., Katsikis I. The role of orlistat combined with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin. Endocrinol. 2014;80(3):432–438. doi: 10.1111/cen.12305. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility ;81,19–25. [DOI] [PubMed]
- Różańska-Walędziak A., Bartnik P., Kacperczyk-Bartnik J., Czajkowski K., Walędziak M. The Impact of Bariatric Surgery on Menstrual Abnormalities—a Cross-Sectional Study. Obes. Surg. 2020;30(11):4505–4509. doi: 10.1007/s11695-020-04840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Arumalla K., Aggarwal S., Singla V., Ganie A., Malhotra N. Impact of Bariatric Surgery on Clinical, Biochemical, and Hormonal Parameters in Women with Polycystic Ovary Syndrome (PCOS) Obes Surg. 2020;30(6):2294–2300. doi: 10.1007/s11695-020-04487-3. [DOI] [PubMed] [Google Scholar]
- Skubleny D., Switzer N.J., Gill R.S., Dykstra M., Shi X., Sagle M.A., de Gara C., Birch D.W., Karmali S. The Impact of Bariatric Surgery on Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis. Obes Surg. 2016;26(1):169–176. doi: 10.1007/s11695-015-1902-5. [DOI] [PubMed] [Google Scholar]