Abstract
The Indian poultry industry is one of the fast-growing sectors of which duck farming plays an important role. Duck population in India is 33.51 million that is concentrated towards north-east and southern parts of the country who rears mainly for eggs and meat. Duck diseases are of great concern as they badly affect the financial status of the small, landless farmers. Databases such as Google Scholar, PubMed, J gate were used to search articles between 2000 and 2019 that showed the prevalence of viral, bacterial, and parasitic duck diseases. R open source software was used to derive forest plots by statistical analysis. Pooled prevalence estimates of duck diseases worldwide was found to be 20% (95%-CI:15–26). Also, continent-wise analysis of all duck diseases has revealed highest prevalence in North America, followed by Asia, Africa, Europe,Oceania and South America. This prevalence of data would be helpful to the policymakers to develop appropriate intervention strategies to prevent and control diseases in their respective locations.
Keywords: Duck diseases, India, Meta-analysis, Prevalence, Systematic review
1. Introduction
Ducks constitute a major part of the poultry industry worldwide. Very little information is available on the duck population in different countries. As per FAO, 2017 there were 1.15 billion ducks (Anas spp.) worldwide and 1.0 billion (88 percent) were in Asia. The largest duck populations are found in China, Vietnam, Bangladesh, and Indonesia (FAO, 2017). In India, the poultry industry is one of the fastest growing agricultural sectors today. Presently, the production of crops has been rising at a rate of 1.5 to 2% per annum while that of production of eggs and meat has been rising at a rate of 8 to 10% per annum (Indian mirror, 2019). According to the 20th Indian livestock census, the total poultry in India is 851.81 million, registered an increase of 16.8% over the previous census (DAHD, 2019). There are 33.51 million of ducks as per 20th livestock census against 23.53 million in 19th livestock census that shows a change of 42.36% which means that there is an increase in demand of duck and duck farming which further warrants the need for proper surveillance and monitoring of diseases affecting ducks thereby controlling them. Small, marginal farmers and nomadic tribes practice duck farming in India which is sometimes seasonal (Jeyathilakan et al., 2016). Ducks play an important role in rural livelihood as they cater to sustained meat and egg production. One of the important criteria is to keep the ducks healthy to prevent disease outbreaks and in cases where ducks encounter infection, administration of appropriate treatment is practiced to minimize the rate of mortality and morbidity.
The distribution and demographic dynamics of the duck population revealed that they are concentrated in East, North-East, and Southern states of the country. The leading states in the duck population are West Bengal, Assam, Kerala, Manipur, Jharkhand, Tripura, Bihar, Andhra Pradesh, Tamil Nadu, UP, and Orissa (DAHD, 2019). Traditionally, West Bengal and Kerala are the major consumer states for duck egg and meat and one of the reasons is that duck egg and meat highly suits and remain tastier for their fish based culinary preparations (Rajput et al., 2014). In India, farmers practice different systems of duck rearing viz., free range system, confined system, indoor system, integrated duck rearing system, duck keeping combined with paddy cultivation, duck keeping combined with fish ponds (Rajput et al., 2014)
Among the diseases affecting ducks in India, viral diseases have been known to have more serious repercussions to duck production. Farm workers are thus essential in ensuring that strict biosecurity are observed to reduce potential transmission of the disease. Of the most infectious include avian influenza (HPAI/LPAI), duck viral enteritis, West Nile disease, Japanese Encephalitis, Newcastle Disease, duck plague, duck viral hepatitis. Usually, ducklings between the age of 1–28 days are most susceptible to diseases and gradually become immune as they grow older. It would be mandatory to establish and maintain good and viable biosecurity programs that will prevent the invasion of disease in the duck farms.
This study concentrates on estimating the prevalence of the infectious disease of duck in the world including India. The comprehensive information generated from this study would assist the policymakers to formulate prevention and control measures.
2. Methodology
2.1. Literature search
A comprehensive systematic literature search was conducted in electronic databases including PubMed, Google Scholar, Science Direct, Scopus, J gate, BioMed databases from 2000 to 2019 using a combination of keywords “Duck”, “Disease”, “prevalence”, “India”. Meanwhile, for the studies of different countries, the database was searched randomly without any restrictions imposed on year. Bibliographies/cross references of eligible studies were also manually searched to identify additional significant articles. The search was restricted to articles in English. Articles were extracted individually by two authors to avoid bias. All the search and scrutiny was conducted according to the PRISMA protocol (http:// www.prisma-statement.org) (Table S1)
2.2. Study selection criteria
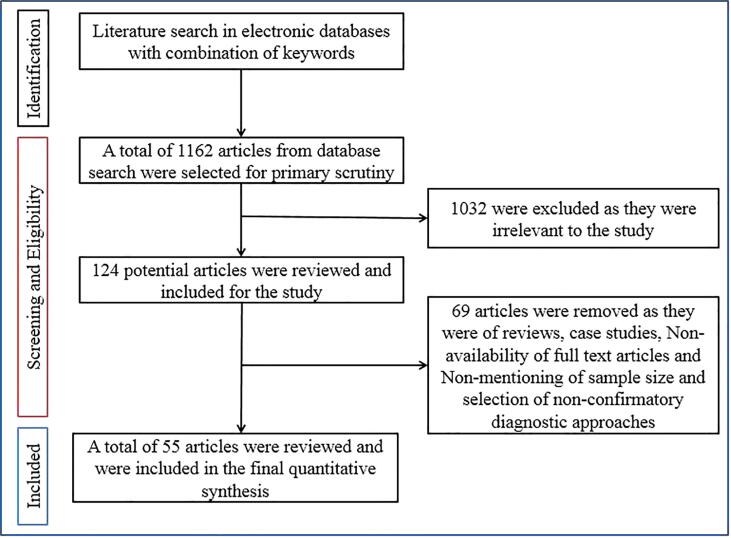
All the articles that described the prevalence rate of various Duck diseases were considered eligible and included in the study. A total of 1,163 articles were identified, of which 1,032 were excluded following the exclusion criteria described above. This comprehensive database searches returned 124 potential articles based on the search for combination of keywords. A total of 55 articles were selected suitable for the study including 80 studies for systematic review and meta-analysis (Fig. 1). Articles were restricted to the English language only. One of the major drawbacks of duck diseases are under-reporting; hence we have tried to pool data as much as possible.
Fig. 1.
Schematic diagram of selection of articles used for the systematic review of this study.
2.3. Data extraction
The data was extracted from qualified studies that included first author, year of publication, total sample size, the location where the study was conducted, detection technique, and the type of infection (viral, bacterial, or parasitic). Articles were stratified according to individual diseases including the studies from India and World. Continent-wise stratification of articles was also performed. Data was extracted independently from each selected article and inconsistency in data was rectified by double-checking the articles until consensus was reached.
2.4. Quality assessment
The quality assessment of different studies was done on a fixed rating scale (Suresh et al., 2019). The scoring was on a scale of 0 to 5, which included evaluation of author and year of study, representativeness of the sample used in the study, ascertainment of the exposure, comparability, and outcome, with each section having the maximum number of two stars. Hence, the overall quality assessment has a maximum score of 5 and a minimum score of 3 (Table 1).
Table 1.
Risk of bias and quality assessment of studies included.
| Sl. No. | Author and year of publication | Selection |
Comparability | Outcome |
Overall Quality Assessment score | |
|---|---|---|---|---|---|---|
| Representativeness of the sample | Ascertainment of exposure | Assessment of outcome | ||||
| 1 | AbouLaila et al., 2011 | *Truly representative serum samples | *Identification of T. gondii infection confirmed by MAT | Study did not control for other factors | *Independent blind assessment | 4 |
| 2 | Adzitey et al., 2012a | *Truly representative fecal swabs, cloacal swabs, intestinal tissue and other environmental samples | **Identification of Campylobacter spp. by mPCR | Study did not control for other factors | *Independent blind assessment | 3 |
| 3 | Adzitey et al., 2012b | *Truly representative fecal swabs, cloacal swabs, intestinal tissue and other environmental samples | *Identification of Salmonella isolates by Gram staining, LATEX agglutination test and Biochemical tests | Study did not control for other factors | *Independent blind assessment | 4 |
| 4 | Ahamed et al., 2015 | *Truly representative cloacal swabs & visceral organs samples | **Identification of DPV isolates by AGIT, PHA test and PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 5 | Cha et al., 2013a | *Truly representative sample of bursa of Fabricious and other tissue samples | **Identification of duck Circovirus by PCR | Study did not control for other factors | *Independent blind assessment | 3 |
| 6 | Cha et al., 2013b | *Truly representative cloacal swabs and tissue swabs | *Identification of the Salmonella by isolation and testing by Latex test kit and API 20E | Study did not control for other factors | *Independent blind assessment | 4 |
| 7 | Cha et al., 2015 | *Truly representative Pharyngeal and cloacal swabs | **Identification of Riemerella by PCR, API-20NE and API-ZYM tests | Study did not control for other factors | *Independent blind assessment | 4 |
| 8 | Chen et al., 2016 | *Truly representative cloacal swabs and serum samples | **Identification of goose parvovirus-related parvovirus was detected by PCR, ELISA and IFA | Study did not control for other factors | *Independent blind assessment | 3 |
| 9 | Cong et al., 2012 | *Truly representative blood samples | *Identification of T. gondii infection by MAT test | Study did not control for other factors | *Independent blind assessment | 3 |
| 10 | Das et al., 2005 | *Truly representative samples of poultry birds | *Identification of Colibacillosis, Duck Cholera, DEV/DP, DHAV, Coccidiosis and Salmonellosis by post-mortem lesions and microscopic examination | Study did not control for other factors | *Independent blind assessment | 4 |
| 11 | Douglas et al., 2007 | *Truly representative sample of cloacal swabs | **Identification of Avian Influenza virus and Newcastle disease virus by virus isolation and RT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 12 | El-Massry et al., 2000 | *Truly representative serum samples | Identification of T. gondii infection by MAT test | Study did not control for other factors | *Independent blind assessment | 3 |
| 13 | Erfan et al., 2015 | *Truly representative tissue samples | **Identification of DHAV by RT-PCR assays | Study did not control for other factors | *Independent blind assessment | 4 |
| 14 | Ferenczi et al., 2016 | *Truly representative fecal samples | **Identification of Avian Influenza by PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 15 | Germundsson et al., 2010 | *Truly representative cloacal and tracheal swabs | **Identification of Avian Influenza confirmed by RT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 16 | Ghersi et al., 2009 | *Truly representative sample of fecal swabs | **Identification of Avian Influenza virus by virus isolation, antigen capture tests, Haemagglutination and RT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 17 | Gonzalez-Reiche et al., 2012 | *Truly representative sample of cloacal and tracheal swabs | **Identification of Avian Influenza virus by RRT-PCR | Study did not control for other factors | *Independent blind assessment | 3 |
| 18 | Houque et al., 2011 | *Truly representative tissue samples | *Identification of DEV/DP, Duck cholera, Colibacillosis, DHAV by microscopicexamination, biochemical test | Study did not control for other factors | *Independent blind assessment | 4 |
| 19 | Islam et al., 2009 | *Truly representative tissue samples | **Identification of DEV/DP, Duck cholera, Coccidiosis, Colibacillosis and Salmonellosis by histo-pathological examinations and PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 20 | Jamali et al., 2014 | *Truly representative tissue samples | *Identification of Listeriosis, Salmonellosis and Yersiniosis by API 20E, Kirby-Bauer disc diffusion method and USDA method | Study did not control for other factors | *Independent blind assessment | 3 |
| 21 | Jamali et al., 2015 | *Truly representative tissue samples | *Identification of Campylobacteriosis by Kirby Bauerdisc diffusion method | Study did not control for other factors | *Independent blind assessment | 4 |
| 22 | Kalaiyarasu et al., 2016 | *Truly representative sera, oral and cloacal swabs | *Identification of West Nile virus and Japanese encephalitis virus by ELISA and Virus Neutralization test | Study did not control for other factors | *Independent blind assessment | 5 |
| 23 | Kamomae et al., 2017 | *Truly representative tissue samples | *Identification of DHAV Histological, Bacteriological and biochemical tests | Study did not control for other factors | *Independent blind assessment | 4 |
| 24 | Karki et al., 2014 | *Truly representative serum samples | *Identification of Avian Influenza by IDEXX Influenza A Ab test kit | Study did not control for other factors | *Independent blind assessment | 3 |
| 25 | Karlsson et al., 2013 | *Truly representative sample of cloacal and fecal swabs | **Identification of Avian Influenza virus by RRT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 26 | Khatun et al., 2013 | *Truly representative cloacal swabs and serum samples | **Identification of Avian influenza by RT-PCR and ELISA | Study did not control for other factors | *Independent blind assessment | 4 |
| 27 | Li et al., 2017 | *Truly representative tissue samples | **Identification of Goose parvovirus by RT-PCR and ELISA | Study did not control for other factors | *Independent blind assessment | 4 |
| 28 | Liu et al., 2010 | *Truly representative serum and tissue samples | **Identification of Duck Circovirus by ELISA, PCR and Western Blot | Study did not control for other factors | *Independent blind assessment | 4 |
| 29 | Liu et al., 2018 | *Truly representative tissue samples | **Identification of DHAV, Avian influenza, DEV/DP, Duck Parvovirus and Respiratory enteric orphan virus by PCR | Study did not control for other factors | *Independent blind assessment | 3 |
| 30 | Madsen et al., 2013 | *Truly representative Serum, tracheal, and cloacal swabs | **Identification of Newcastle disease, Infectious laryngotracheitis,M. gallisepticum and Salmonella by PCR and ELISA | Study did not control for other factors | *Independent blind assessment | 3 |
| 31 | Mandal et al., 2017 | *Truly representative tissue samples | **Identification of DEV/DP by PCR | Study did not control for other factors | *Independent blind assessment | 5 |
| 32 | Mbuko et al., 2010 | *Truly representative serum and tissue samples | *Identification of Infectious Bursal Disease by Histological, and biochemical tests | Study did not control for other factors | *Independent blind assessment | 4 |
| 33 | Mishra et al., 2012 | *Truly representative tracheal swabs, cloacal swabs, serum and tissue samples | **Identification of West Nile virus by ELISA and RT-PCR | Study did not control for other factors | *Independent blind assessment | 5 |
| 34 | Molia et al., 2017 | *Truly representative blood samples | *Identification of NDV by ELISA | Study did not control for other factors | *Independent blind assessment | 4 |
| 35 | Mondal et al., 2008 | *Truly representative cloacal swabs | *Identification of Salmonella by biochemical test | Study did not control for other factors | *Independent blind assessment | 4 |
| 36 | Montalvo-Corral et al., 2011 | *Truly representative sample of cloacal and oropharyngeal swabs | **Identification of Avian Influenza virus by RRT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 37 | Neher et al., 2019 | *Truly representative blood samples, cloacal andtracheal swabs | **Identification of DEV/DP by PCR | Study did not control for other factors | *Independent blind assessment | 5 |
| 38 | Ninvilai et al., 2019 | *Truly representative tissue samples | **Identification of Duck Tembusu virus by RT-PCR | Study did not control for other factors | *Independent blind assessment | 3 |
| 39 | OIE report., 2015a | *Truly representative cloacal swabs and serum samples | *Identification of Avian Influenza by Antigen detection test. | Study did not control for other factors | *Independent blind assessment | 5 |
| 40 | OIE report., 2015b | *Truly representative cloacal swabs and serum samples | *Identification of Avian Influenza by Antigen detection test. | Study did not control for other factors | *Independent blind assessment | 5 |
| 41 | Rimondi et al., 2011 | *Truly representative sample of cloacal swabs | **Identification of Avian Influenza virus by RRT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 42 | Slemons et al., 2003 | *Truly representative cloacal swabs | *Identification of Avian influenza by virus isolation and typing of HA and NA genes | Study did not control for other factors | *Independent blind assessment | 4 |
| 43 | Soliman et al., 2015 | *Truly representative tissue samples | **Identification of DHAV by RT-PCR assays | Study did not control for other factors | *Independent blind assessment | 3 |
| 44 | Spackman et al., 2006 | *Truly representative sample of cloacal swabs | **Identification of Avian Influenza virus by RRT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 45 | Tarnagda et al., 2011 | *Truly representative tracheal and cloacal swabs | **Identification of Infectious bronchitis and Newcastle disease virus by PCR | Study did not control for other factors | *Independent blind assessment | 3 |
| 46 | Wang et al., 2010 | *Truly representative fecal samples | **Identification of Cryptosporidium by PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 47 | Wei et al., 2016 | *Truly representative tissue samples | **Identification of Campylobacter by bacterial isolation and confirmation by PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 48 | Wille et al., 2016 | *Truly representative fecal samples and cloacal swabs | **Identification of Avian Coronavirus by RT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 49 | Wilson et al., 2013 | *Truly representative serum samples and cloacal swabs | **Identification of Avian Influenza by RT-PCR and ELISA | Study did not control for other factors | *Independent blind assessment | 4 |
| 50 | Wojnarowicz et al., 2007 | *Truly representative tissue samples | *Identification of West Nile virus by Histo-pathological Examination | Study did not control for other factors | *Independent blind assessment | 3 |
| 51 | Yang et al., 2012 | *Truly representative serum samples | *Identification of T. gondii infection by MAT | Study did not control for other factors | *Independent blind assessment | 4 |
| 52 | Yeh et al., 2017 | *Truly representative tissue samples | *Identification of E. coli infection by API 20E system | Study did not control for other factors | *Independent blind assessment | 4 |
| 53 | Yun et al., 2015 | *Truly representative liver samples | *Identification of Novel Duck Reovirus by Western Blot | Study did not control for other factors | *Independent blind assessment | 3 |
| 54 | Zhang et al., 2011 | *Truly representative serum samples | **Identification and confirmation of New castle disease by HA and HI test and RT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
| 55 | Zhao et al., 2013 | *Truly representative cloacal swabs | **Identification and confirmation of Avian Influenza by HI test and RT-PCR | Study did not control for other factors | *Independent blind assessment | 4 |
RT-PCR: Reverse Transcriptase Polymerase Chain Reaction; MAT: Modified agglutination test, IFA: Indirect Immunofluorescence Assay, (*) Stars represent the number of points awarded for the category; * = 1, ** = 2.
2.5. Meta-analysis
Meta-analysis was carried out using the R Open source scripting software (version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/). This analysis facilitates generating a weighted average proportion of prevalence of various studies that provides a way forward for proper planning. Metafor, metaprop, and Meta of R packages were used for statistical analysis. Tau square, I2 (Higgin’s I2), and p value were computed to determine the percentage of variation due to heterogeneity among various reports included in this study. Both the random effect and fixed effect model were used to calculate the pooled prevalence of individual diseases since substantial heterogeneity was expected. The funnel plot generated with the y-axis showing the Standard Error (SE) of each study, with larger studies plotted on top of the y-axis indicates indicates publication bias and subsequently, the x-axis showed the effect of each study. The studies with high precision concentrate along the line of average when the publication bias is almost nil, whereas those with low precision distribute evenly on either side of the average line, creating generally a funnel shaped scatter (Egger et al., 1997). The symmetry of the funnel plot was adjusted by the Trim-and-fill method. Graphical representation of the data was depicted in Forest Plot. The restricted maximum-likelihood estimator was used to determine between study variance τ2. The prevalence estimates for duck diseases was expressed as a percentage with a Confidence Interval (CI) at the 95% level. Subgroup analysis was conducted based on species affected, a diagnostic method used, zones of India and continents of the world for determining the heterogeneity in each group and their comparison. In the present study, the data was stratified based on type of diseases and forest plots generated using the R software.
3. Results
3.1. Study details
Articles reporting the prevalence of duck diseases were thoroughly screened and irrelevant ones were excluded. A total of 55 articles were selected suitable for the study including 80 studies for systematic review and meta-analysis. All the articles described the prevalence of various duck diseases of bacterial, viral, and parasitic infections. Systematic Review was conducted to study the reported duck diseases worldwide including those in India. Articles retrieved were from countries belonging to Asia, Europe, Africa, North America, South America, and Oceania regions. All articles used in our study were restricted to the English language only and the study period selected was between 2000 and 2019.
3.2. Meta-analysis of the prevalence of Duck diseases
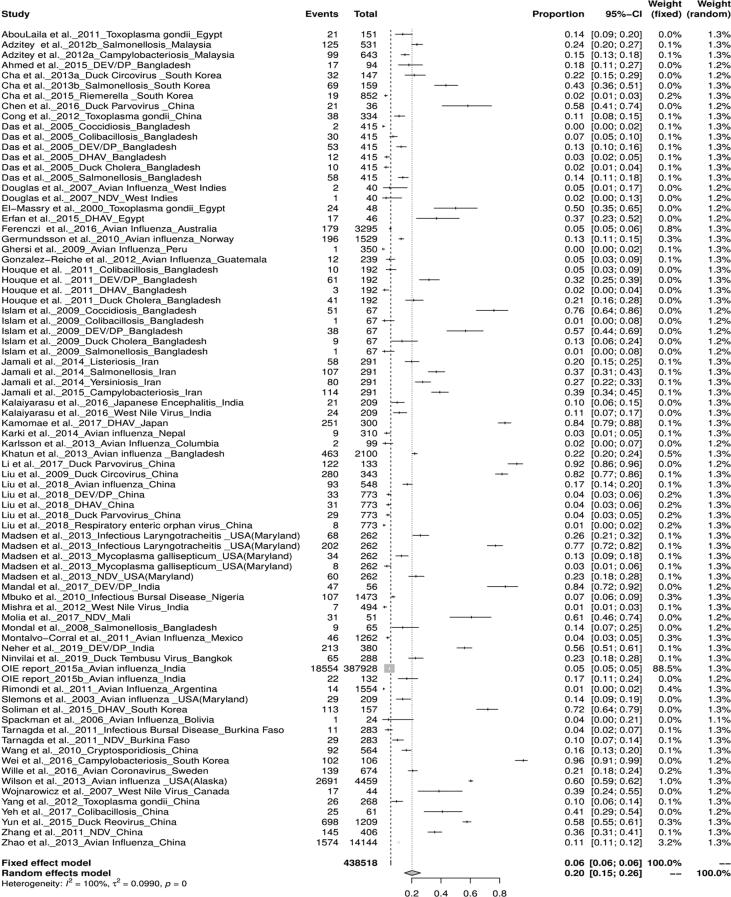
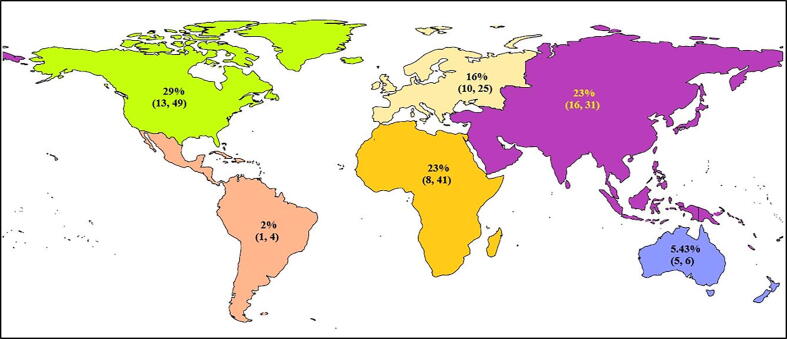
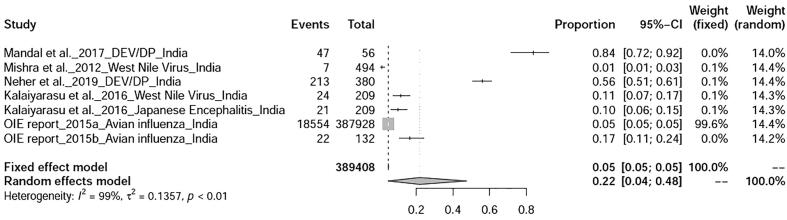
The worldwide percentage prevalence of different duck diseases was estimated statistically using R software to generate forest and funnel plots, of which, the viral diseases were found to be the most prevalent. Articles retrieved were from the countries belonged to Asia (9 countries), Europe (2 countries), Africa (4 countries), North America (3 countries), South America (7 countries), and Oceania (Australia) regions (Table 2). The pooled prevalence of duck diseases worldwide was found to be 20% (95%-CI: 15–26%), I2 = 100%, and τ2 value was 0.0990, p = 0 (Fig. 2). Continent-wise analysis of all duck diseases has revealed highest prevalence in North America 29% (95% CI = 13–49%) , followed by Asia 23% (95% CI = 16–31%) , Africa 23% (95% CI = 8–41%) , Europe 16% (95% CI = 10–25%) , Oceania 5.43% and South America 2% .(Fig. 3) The total number of studies included for meta-analysis was 55 with 438,518 samples for the period 2000–2019. The meta-analysis indicated that the heterogeneity was high between studies, I2 = 100% (τ2 = 0.0990 with P = 0), and hence the random effect model was considered. With the available reports on the prevalence of duck diseases from India, the pooled prevalence of various diseases of duck were also calculated. The prevalence rate of duck diseases reported in India during 2000–2019 was found to be 22% (95%-CI: 4–48%), with I2 = 99% and τ2 value 0.1357, p < 0.01 (Fig. 4).
Table 2.
Continent-wise stratification of studies.
| Continent/Region with total prevalence | Country | Articles | Disease(s)/Infection |
|---|---|---|---|
| Asia (23%) |
South Korea | Cha et al., 2013a, Cha et al., 2013b, Cha et al., 2015, Soliman et al., 2015, Wei et al., 2016 | Duck Circovirus, Salmonellosis, Riemerella, DHAV, Campylobacteriosis |
| China |
Wang et al., 2010 Zhang et al., 2011 Cong et al., 2012, Yang et al., 2012 Zhao et al., 2013 Yun et al., 2015, Chen et al., 2016 Yeh et al., 2017 Li et al., 2017, Liu et al., 2010, Liu et al., 2018 |
Duck Parvovirus, T. gondii, Duck Parvovirus, Duck Circovirus, DHAV, Avian Influenza, DEV/DP, Respiratory enteric orphan virus, Cryptosporiodiosis, Colibacillosis, Duck Reovirus, Newcastle disease |
|
| Nepal | Karki et al., 2014 | Avian Influenza | |
| Japan | Kamomae et al., 2017 | DHAV | |
| Bangladesh | Mondal et al., 2008, Islam et al., 2009, Hoque et al., 2011, Khatun et al., 2013, Das et al., 2005, Ahamed et al., 2015 | Colibacillosis, Duck Cholera, DEV/DP, DHAV, Coccidiosis, Salmonellosis, avian Influenza | |
| India | Mishra et al., 2012, OIE, 2015a, OIE, 2015b, Kalaiyarasu et al., 2016, Mandal et al., 2017, Neher et al., 2019 | West Nile Virus, DEV/DP, Avian Influenza, West Nile Virus, Japanese encephalitis, DEV/DP | |
| Bangkok | Ninvilai et al., 2019 | Duck Tembusu virus | |
| Iran | Jamali et al., 2014, Jamali et al., 2015 | Listeriosis, Salmonellosis, Yersiniosis | |
| Malaysia | Adzitey et al., 2012a, Adzitey et al., 2012b, Ahamed et al., 2015 | Salmonellosis, Campylobacteriosis, DEV/DP | |
| Europe (16%) |
Norway | Germundsson et al., 2010 | Avian Influenza |
| Sweden | Wille et al., 2016 | Avian Coronavirus | |
| Africa (23%) |
Mali | Molia et al., 2017 | Newcastle disease |
| Egypt | AbouLaila et al., 2011, Erfan et al., 2015, El-Massry et al., 2000 | T. gondii, DHAV | |
| Burkina Faso | Tarnagda et al., 2011 | Newcastle disease | |
| Nigeria | Mbuko et al., 2010 | Infectious Bursal Disease | |
| North America (29%) |
Canada | Wojnarowicz et al., 2007 | West Nile virus |
| Maryland | Madsen et al., 2013, Slemons et al., 2003 | Avian Influenza Newcastle disease, M. gallisepticum, Infectious laryngotracheitis |
|
| Alaska | Wilson et al., 2013 | Avian Influenza | |
| South America (2%) |
Mexico | Montalvo-Corral et al., 2011 | Avian Influenza |
| West Indies | Douglas et al., 2007 | Avian Influenza, Newcastle disease | |
| Peru | Ghersi et al., 2009 | Avian Influenza | |
| Guatemala | Gonzalez-Reiche et al., 2012 | Avian Influenza | |
| Columbia | Karlsson et al., 2013 | Avian Influenza | |
| Argentina | Rimondi et al., 2011 | Avian Influenza | |
| Bolivia | Spackman et al., 2006 | Avian Influenza | |
| Oceania (5.43%) |
Australia | Ferenczi et al., 2016 | Avian Influenza |
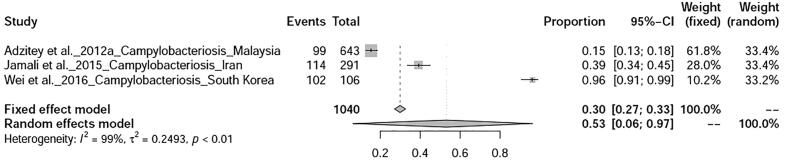
Fig. 2.
Forest plot of prevalence of duck diseases world-wide.
Fig. 3.
Continent-wise pooled prevalence of infectious disease of ducks. Figures in bracket indicate the range at 95% Confidence Interval.
Fig. 4.
Forest plot of pooled prevalence of duck diseases in India.
3.2.1. Viral diseases
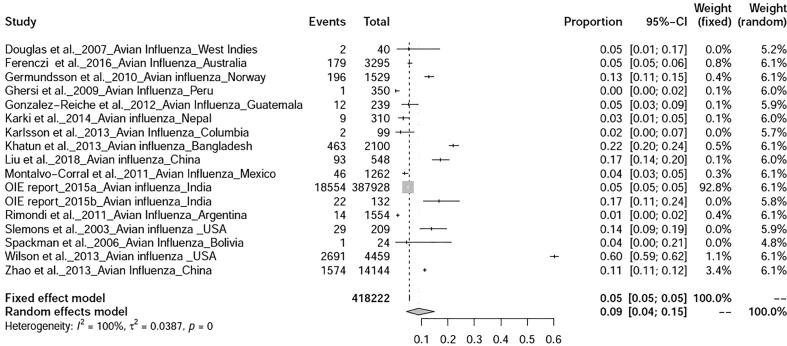
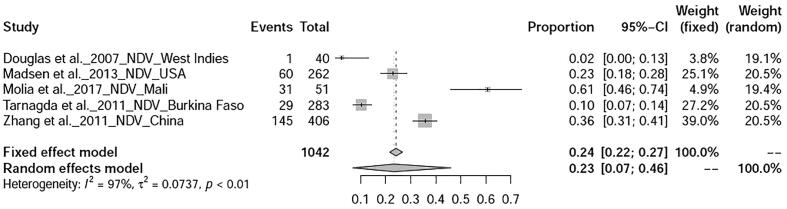
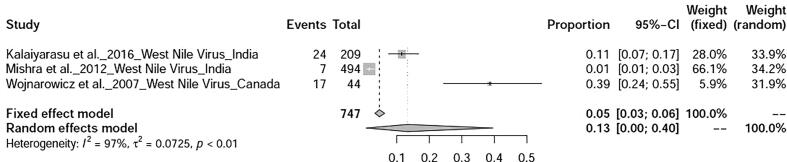
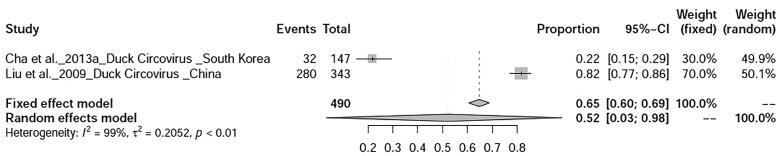
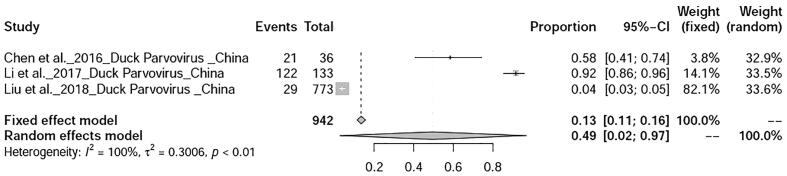
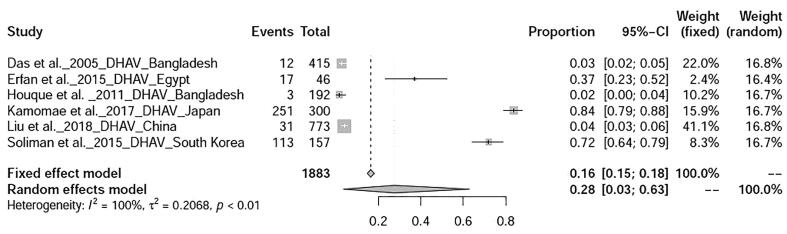
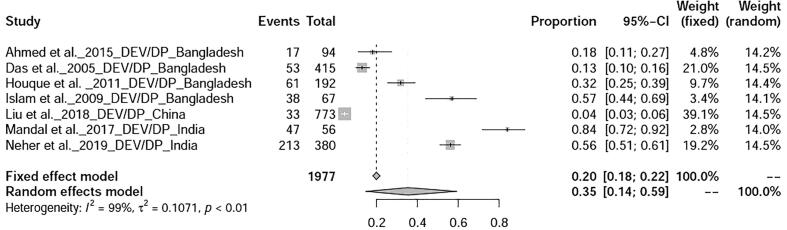
The prevalence avian influenza was 9% (95%-CI: 4–15%), I2 = 100%, τ2 value was 0.0387p = 0 (Fig. 5), Meanwhile, studies on duck tembusu virus infection revealed a prevalence of 23% (95%-CI: 18–28%) in Bangkok. Whereas, prevalence of Newcastle Disease was found to be 23% (95%-CI: 7–46%), I2 = 97%, τ2 value was 0.0737, p < 0.01 (Fig. 6). Prevalence of West Nile virus infection was found to be 13% (95%-CI: 0–40%), I2 = 97%, τ2 value was = 0.0725, p < 0.01 as shown in Fig. 7. Two articles of duck circovirus from South Korea and China showed 52% (95%-CI: 3–98%) prevalence (Fig. 8). Duck parvovirus infection from China (3 articles) revealed a prevalence of 49% (95%-CI: 2–97%) (Fig. 9), whereas the duck hepatitis A virus infection showed a prevalence of 28% (95%-CI: 3–63%) (Fig. 10). In the case of duck plague infection in Asian countries showed a prevalence of 35% (95%-CI: 14–59%) (Fig. 11). A single article on Japanese encephalitis from India showed a 10% prevalence (95%-CI: 6–15%). Infectious bursal disease and infectious laryngotracheitis showed a prevalence estimate of 6% (95%-CI: 3–9%) and 52% (95%-CI: 7–94%) respectively. Duck respiratory enteric orphan virus infection and duck reovirus infection, both articles from China showed a prevalence of 1% and 58% respectively, while avian coronavirus infection showed 21% prevalence in ducks from Sweden.
Fig. 5.
Forest plot of prevalence of avian influenza.
Fig. 6.
Forest plot of prevalence of Newcastle disease.
Fig. 7.
Forest plot of prevalence of West Nile fever infection.
Fig. 8.
Forest plot of prevalence of duck circovirus infection.
Fig. 9.
Forest plot of prevalence of duck parvovirus infection.
Fig. 10.
Forest plot of prevalence of duck hepatitis A virus infection.
Fig. 11.
Forest plot of prevalence of duck plague.
3.2.2. Bacterial diseases
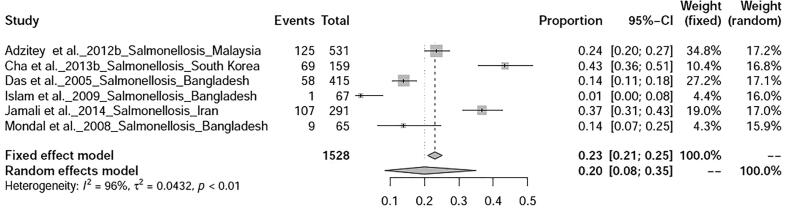
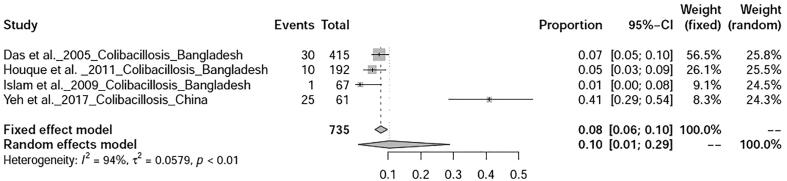
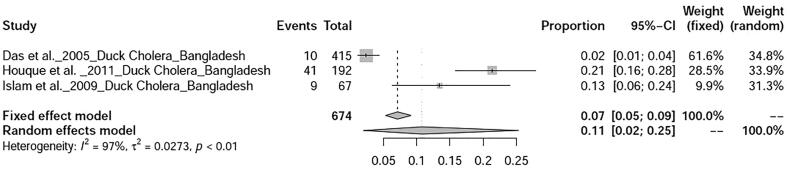
Six bacterial diseases of ducks were analysed in this study. Prevalence of salmonellosis in ducks was found to be 20% (95%-CI: 8–35%) with heterogeneity I2 = 96%, τ2 value was 0.0432, p < 0.01 (Fig. 12), whereas duck campylobacteriosis showed prevalence of 53% (95%-CI: 6–97%) with heterogeneity I2 = 99%, τ2 value was 0.2493, p < 0.01 (Fig. 13). Duck colibacillosis revealed the prevalence of 10% (95%-CI: 1–29%) with heterogeneity I2 = 94%, τ2 value was 0.0579, p < 0.01 (Fig. 14). A total of three articles on duck cholera reported from Bangladesh showed a prevalence of 11% (95%-CI: 2–25%) with heterogeneity I2 = 97%, τ2 value was 0.0273, p < 0.01 (Fig. 15). Prevalence of duck Mycoplasma gallisepticum infection was found to be 7% (95%-CI: 1–20%). Prevalence of a single study on three bacterial diseases viz., Riemerella infection, listeriosis, and yersiniosis was found to be 2%, 20%, and 27% respectively.
Fig. 12.
Forest plot of prevalence of salmonellosis.
Fig. 13.
Forest plot of prevalence of campylobacteriosis.
Fig. 14.
Forest plot of prevalence of Colibacillosis.
Fig. 15.
Forest plot of prevalence of duck cholera.
3.2.3. Parasitic diseases
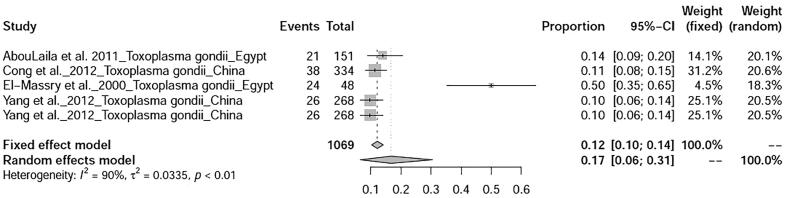
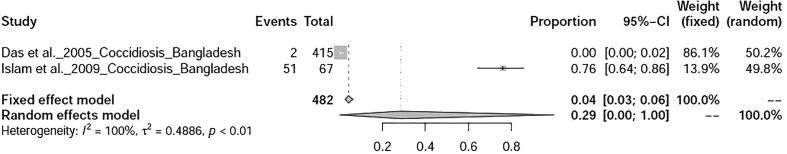
Two studies on parasitic diseases of ducks were selected in this study viz., toxoplasmosis, and coccidiosis whose prevalence was found to be 17% (95%-CI: 6–31%) and 29% (95%-CI: 0–1%) respectively (Fig. 16, Fig. 17).
Fig. 16.
Forest plot of prevalence of T. gondii.
Fig. 17.
Forest plot of coccidiosis.
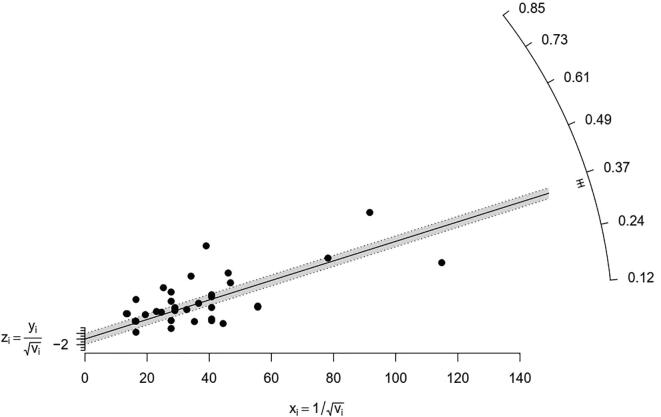
To assess the heterogeneity between-study reports, a Galbraith plot was generated (Fig. 18). The standardized effect estimates against inverse standard error were shown as scattered points in the plot. The points representing the study reports outside confidence bounds may be contributing to the heterogeneity. In the absence of heterogeneity, all points (reports) are expected to lie within the confidence limits centring around the line.
Fig. 18.
Galbraith Plot showing heterogeneity between-study reports.
4. Discussion
Information on the world duck population is very scanty in general and reports on disease prevalence is very less in particular. As per FAO, 2017 there were 1.15 billion ducks (Anas spp.) worldwide and 1.0 billion (88 percent) were in Asia. The largest duck populations are found in China, Vietnam, Bangladesh, and Indonesia FAO, 2017). India has 33.5 million of ducks and the majority of them are domesticated in the northeast including the West Bengal state of India. Duck farming is becoming a popular one and is usually practiced by economically disadvantaged people of the society in some countries. Duck meat contributes to food security in low and middle-income countries. Vast majority of the ducks are raised in households or subsistence-based production system (backyard or small flocks) There are no systematic reports of the occurrence of infectious diseases in ducks in India and elsewhere. Hence the efforts were made to gather information on prevalence of duck disease available in public domains. The information on duck diseases was reviewed and analysed using different statistical tools/methods including meta-analysis. A meta-analysis combines the results from two or more studies conducted by different individuals to provide a single value with high statistical power. In the present study, a systematic review of scientific publications on the prevalence of duck diseases was conducted for 19 years (2000–2019). After the screening of articles, data was extracted from 55 cross-sectional studies published in peer-reviewed journals that reported the prevalence of various duck diseases, reviewed systematically, and conducted a meta-analysis. Meta-analysis showed high heterogeneity, I = 100%, τ2 = 0.0990 indicating a true heterogeneity among the studies. Further, asymmetry in the funnel plot showed heterogeneity of studies since very few studies on the prevalence of different duck diseases were available in a limited number of countries within the continents.
In the present study, articles on the prevalence of infectious diseases of duck in different countries between 2000 and 2019 were analysed. The reports were scanty. The continent-wise analysis revealed a diversified prevalence of duck diseases. In the Asian continent (23% prevalence), China reported the majority of duck diseases that may be due to the highest population of ducks in that country, followed by India, Bangladesh, South Korea, Malaysia, Bangkok. India and Bangladesh have reported a maximum of duck diseases. West Bengal and Assam states of India shares border with Bangladesh which is porous in nature. There is no restriction of movement of men and materials hence there are possibilities of transboundary movement of ducks without proper health records in these borders. Meanwhile, only one report per country was retrieved from Nepal, Japan, and Iran. The articles from Norway and Sweden reporting on the prevalence of Avian influenza in ducks were from Europe (16% prevalence). In Africa (23% prevalence), reports on the prevalence of duck diseases were from Mali, Egypt, Burkina Faso, and Nigeria. North America reported a 29% prevalence of duck diseases from Canada, Maryland, and Alaska, whereas South America including Latin America reported a 2% prevalence of duck diseases from Peru, Columbia, Argentina, Bolivia, Mexico, Guatemala, and West Indies. There was only a report on prevalence (5.43%) of avian Influenza from Australia (Oceania continent).
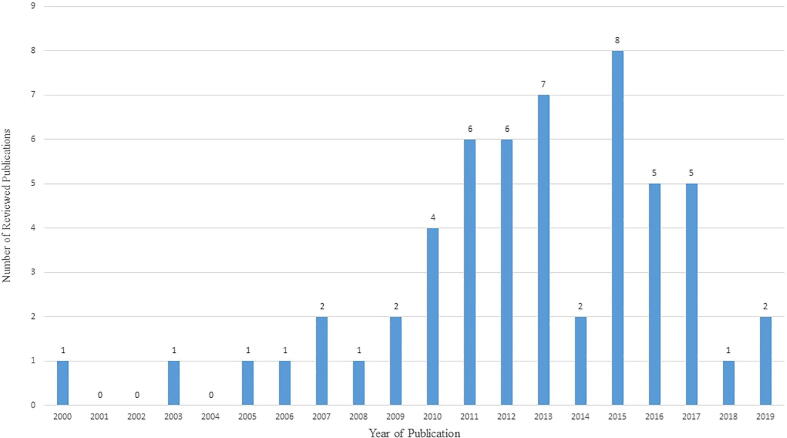
During 2000–2007, duck diseases were under reported and gradually a number of reports on disease prevalence showed an increasing trend from 2008 to 2015 that may be due to adoption of more precise tools in disease diagnosis (Fig. 19), thereafter a declining trend was observed 2016 onwards that may be due to better health care management.
Fig. 19.
Details of year-wise number of publications reviewed in the study.
From the analysis, it is evident that the viral disease remains predominant when compared to bacterial, and parasitic infections. It was found that the viral disease incidence is highly concentrated towards the eastern countries such as China, Korea, Japan and Bangladesh. This may be due to the robust disease reporting system available. However, under reporting of the disease is one of the major drawbacks. During our study, we observed that the reports of duck disease are very scanty which causes the poor availability of previous references. This causes hindrance in evaluating out a strategic plan to control the diseases.
Despite being an important factor in the poultry industry, duck diseases often tend to bring great economic loss to the farmers. Hence, it is important to take precautionary measures by vaccination, better health management practices and also other farm related biosecurity procedures to avoid infections.
Further to meta-analysis, barring selection bias, systematic reviews helps the revision of all the scientific evidence on a given topic. Based on the output, the summarized information can be used to propose hypotheses that explain the behaviour of the data and to identify areas of gaps where further research is needed (Afanador-Villamizar et al., 2017, Moher et al., 2010). However, it is a controversial tool because several conditions are critical and even small violations of these can lead to misleading conclusions. While designing and performing a meta-analysis, several decisions concerning personal judgment and expertise need to be made that may eventually create bias or expectations that influence the result (Greco et al., 2013).
5. Conclusion
This meta-analysis indicated that pooled prevalence of various duck diseases worldwide during the period 2000–2019 was found to be 20% (95% CI = 15–26%) and the pooled prevalence estimate for India was found to be 22% (95% CI: 4–48%) which might be due to increased reporting of duck disease during recent years using precise tools for disease diagnosis. Concerning viral diseases, it was observed that the disease occurrence was concentrated towards the Asian subcontinent especially countries like India, China, and Korea as they have a high number of ducks. Among the viral diseases reported, Avian Influenza was found to be the most predominant followed by Duck Plague and Duck Hepatitis Viral Infections. In the case of bacterial infections in ducks, Salmonellosis was the most prevalent in Bangladesh, North Korea, China, and Malaysia. Among Parasitic diseases, Toxoplasma gondii infection was found to be most prevalent in China. Very little information is available concerning parasitic infection of ducks. Although there is an increase in the total duck population, India still faces a high threat of economic loss due to infectious diseases. Furthermore, awareness amongst farmers about disease reporting to their nearest veterinary doctors, following prevention, control measures, and biosecurity practices can drastically help to reduce duck mortality.
Ethical approval
Not applicable as the study utilised the published data available in the web.
7. Data availability statement
Not applicable since the data used in this study was from peer-reviewed published articles and are available in the public domain for which DOI is mentioned against each reference.
Funding
Not applicable since the entire study utilised the published data available in the internet.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors acknowledge the guidance provided by the Department of Biotechnology, New Delhi, Government of India. Authors thank Dr. Mahesh, Director and Dr. Abhijit of CPDO & TI, Hessarghatta, Bengaluru for providing duck population data. Thanks are due to Dr. Siju, Scientist, ICAR-NIVEDI for providing world map shape files. Authors are grateful to the Director ICAR-NIVEDI for his constant help and support during the work. Authors acknowledge the Head of the Institute, JSS Academy of Higher Education, Mysuru and the Director, Amrita School of Arts and Sciences, Amrita Vishwa Vidyapeetham, Mysuru Campus, Mysuru for the support.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.05.034.
Contributor Information
Sharanagouda S. Patil, Email: sharanspin13@gmail.com.
Asad Syed, Email: assyed@ksu.edu.sa.
Ali H. Bahkali, Email: abahkali@ksu.edu.sa.
Chandan Shivamallu, Email: chandans@jssuni.edu.in.
Kollur Shiva Prasad, Email: shivachemist@gmail.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- AbouLaila M., El-Bahy N., Hilali M., Yokoyama N., Igarashi I. Serodiagnosis of Toxoplasma gondii in ducks from Behera Governorate, Egypt. J. Protozool. Res. 2011;21:45–49. http://id.nii.ac.jp/1588/00001440 [Google Scholar]
- Adzitey F., Rusul G., Huda N., Cogan T., Corry J. Prevalence, antibiotic resistance and RAPD typing of Campylobacter species isolated from ducks, their rearing and processing environments in Penang, Malaysia. Int. J. Food Microbiol. 2012;154:197–205. doi: 10.1016/j.ijfoodmicro.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Adzitey F., Rusul G., Huda N. Prevalence and antibiotic resistance of Salmonella serovars in ducks, duck rearing and processing environments in Penang, Malaysia. Food Res. Int. 2012;45:947–952. doi: 10.1016/j.foodres.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Afanador-Villamizar A., Gomez-Romero C., Diaz A., Ruiz-Saenz J. Avian influenza in Latin America: A systematic review of serological and molecular studies from 2000–2015. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0179573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed M.M., Hossain M.T., Rahman M., Nazir N.H., Rahman Khan M.F., Parvej M.S., Rahman M.B. Molecular characterization of Duck Plague virus isolated from Bangladesh. J. Adv. Veterinary Animal Res. 2015;2:296–303. http://bdvets.org/JAVAR [Google Scholar]
- Cha S.-Y., Kang M., Cho J.-G., Jang H.-K. Genetic analysis of duck circovirus in Pekin ducks from South Korea. Poultry Sci. 2013;92:2886–2891. doi: 10.3382/ps.2013-03331. [DOI] [PubMed] [Google Scholar]
- Cha S.-Y., Kang M., Yoon R.-H., Park C.-K., Moon O.-K., Jang H.-K. Prevalence and antimicrobial susceptibility of Salmonella isolates in Pekin ducks from South Korea. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:473–479. doi: 10.1016/j.cimid.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Cha S.-Y., Seo H.-S., Wei B., Kang M., Roh J.-H., Yoon R.-H., Kim J.-H., Jang H.-K. Surveillance and characterization of Riemerella anatipestifer from wild birds in South Korea. J. Wildl. Dis. 2015;51:341–347. doi: 10.7589/2014-05-128. [DOI] [PubMed] [Google Scholar]
- Chen H., Tang Y., Dou Y., Zheng X., Diao Y. Evidence for vertical transmission of novel duck-origin goose parvovirus-related parvovirus. Transboundary Emerg. Dis. 2016;63:243–247. doi: 10.1111/tbed.12487. [DOI] [PubMed] [Google Scholar]
- Cong W., Huang S.-Y., Zhou D.-H., Xu M.-J., Wu S.-M., Yan C., Zhu X.-Q. First report of Toxoplasma gondii infection in market-sold adult chickens, ducks and pigeons in northwest China. Parasites Vect. 2012;5:110. doi: 10.1186/1756-3305-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHD, 2019. 20th Livestock census, Department of Animal Husbandry and Dairying, Govt of India. Retrieved from http://dadf.gov.in/sites/default/filess/Livestock%20%205_0.pdf
- Das P.M., Rajib D.M.M., Noor M., Islam M.R. A retrospective analysis on the proportional incidence on poultry diseases in greater Mymensingh district of Bangladesh. Proc. Seminar, World’s Poultry Sci. Associat., Bangladesh. 2005:33–37. [Google Scholar]
- Douglas K.O., Lavoie M.C., Kim L.M., Afonso C.L., Suarez D.L. Isolation and Genetic Characterization of Avian Influenza Viruses and a Newcastle Disease Virus from Wild Birds in Barbados: 2003–2004. Avian Dis. 2007;51:781–787. doi: 10.1637/0005-2086(2007)51[781:IAGCOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Massry A., Mahdy O.A., El-Ghaysh A., Dubey J.P. Prevalence of Toxoplasma gondii antibodies in sera of turkeys, chickens, and ducks from Egypt. J. Parasitol. 2000;86:627–628. doi: 10.1645/0022-3395(2000)086[0627:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Erfan A.M., Selim A.A., Moursi M.K., Nasef S.A., Abdelwhab E.M. Epidemiology and molecular characterization of duck hepatitis A virus from different duck breeds in Egypt. Veterinary Microbiology. 2015;177:347–352. doi: 10.1016/j.vetmic.2015.03.020. [DOI] [PubMed] [Google Scholar]
- FAO (2017). Gateway to poultry production and products - Duck species, Rome. Retrieved from http://www.fao.org/poultry-production-products/production/poultry-species/ducks/en/
- Ferenczi M., Beckmann C., Warner S., Loyn R., O’Riley K., Wang X., Klaassen M. Avian influenza infection dynamics under variable climatic conditions, viral prevalence is rainfall driven in waterfowl from temperate, south-east Australia. Vet. Res. 2016;47:1–12. doi: 10.1186/s13567-016-0308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germundsson A., Madslien K.I., Hjortaas M.J., Handeland K., Jonassen C.M. Prevalence and subtypes of Influenza A Viruses in wild waterfowl in Norway 2006–2007. Acta Vet. Scand. 2010;52:1–5. doi: 10.1186/1751-0147-52-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersi B.M., Blazes D.L., Icochea E., Gonzalez R.I., Kochel T., Tinoco Y., Montgomery J.M. Avian Influenza in wild birds, central coast of Peru. Emerg. Infect. Dis. 2009;15:935–938. doi: 10.3201/eid1506.080981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Reiche A.S., Morales-Betoulle M.E., Alvarez D., Betoulle Jean-Luc, Muller M.L., Sosa S.M., Perez D.R. Influenza A viruses from wild birds in Guatemala belong to the North American lineage. PLoS ONE. 2012;7:1–11. doi: 10.1371/journal.pone.0032873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T., Zangrillo A., Biondi-Zoccai G., Landoni G. Meta-analysis: pitfalls and hints. Heart, Lung Vessels. 2013;5:219–225. [PMC free article] [PubMed] [Google Scholar]
- Hoque M.A., Skerratt L.F., Cook A.J.C., Khan S.A., Grace D., Alam M.R., Vidal-Diez A., Debnath N.C. Factors limiting the health of semi-scavenging ducks in Bangladesh. Trop. Anim. Health Prod. 2011;43:441–450. doi: 10.1007/s11250-010-9712-1. [DOI] [PubMed] [Google Scholar]
- Indian Mirror, 2019. Data on production of eggs and meat in India. Retrieved from https://www.indianmirror.com/indian-industries/poultry.html
- Islam A., Trisha A.A., Das M., Amin M.R. Retrospective study of some poultry diseases at Gaibandha district in Bangladesh. Bangladesh Journal of Veterinary Medicine. 2009;7:239–247. doi: 10.3329/bjvm.v7i1.5067. [DOI] [Google Scholar]
- Jamali H., Radmehr B., Ismail S. Prevalence and antimicrobial resistance of Listeria, Salmonella, and Yersinia species isolates in ducks and geese. Poult. Sci. 2014;93:1023–1030. doi: 10.3382/ps.2013-03699. [DOI] [PubMed] [Google Scholar]
- Jamali H., Ghaderpour A., Radmehr B., Wei K.S.C., Chai L.C., Ismail S. Prevalence and antimicrobial resistance of Campylobacter species isolates in ducks and geese. Food Control. 2015;50:328–330. doi: 10.1016/j.foodcont.2014.09.016. [DOI] [Google Scholar]
- Jeyathilakan N., Basheer-Ahamad D., Selvaraj J. First report of Anatoecus dentatus in domestic duck (Anas platyrhynchos domesticus, Linnaeus, 1978) from Southern India. Parasite Epidemiology and Control. 2016;1:131–135. doi: 10.1016/j.parepi.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaiyarasu S., Mishra N., Khetan R.K., Singh V.P. Serological evidence of widespread West Nile virus and Japanese encephalitis virus infection in native domestic ducks (Anas platyrhynchos var domesticus) in Kuttanad region, Kerala, India. Comp. Immunol. Microbiol. Infect. Dis. 2016;48:61–68. doi: 10.1016/j.cimid.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Kamomae M., Kameyama M., Ishii J., Nabe J., Ogura Y., Iseki H., Yamamoto Y., Mase M. An outbreak of duck hepatitis A virus type 1 infection in Japan. J. Vet. Med. Sci. 2017;79:917–920. doi: 10.1292/jvms.16-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S., Lupiani B., Budke C.M., Manandhar, Ivanek S.R. Cross-sectional sero-survey of avian influenza antibodies presence in domestic ducks of Kathmandu Nepal. Zoonoses Public Health. 2014;61:442–448. doi: 10.1111/zph.12097. [DOI] [PubMed] [Google Scholar]
- Karlsson E.K., Ciuoderis K., Freiden P.J., Seufzer B., Jones J.C., Johnson J., Schultz-Cherry S. Prevalence and characterization of influenza viruses in diverse species in Los Llanos Colombia. Emerg. Microbes Infect. 2013;2 doi: 10.1038/emi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun A., Giasuddin M., Islam K.M., Khanom S., Samad M.A., Islam M.R., Rahman M.M. Surveillance of avian influenza virus type A in semi-scavenging ducks in Bangladesh. Veterinary Res. 2013;9:196. doi: 10.1186/1746-6148-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Zhang R., Chen J., Sun D., Lan J., Lin S., Jiang S. Development of a duplex semi-nested PCR assay for detection of classical goose parvovirus and novel goose parvovirus-related virus in sick or dead ducks with short beak and dwarfism syndrome. J. Virol. Methods. 2017;249:165–169. doi: 10.1016/j.jviromet.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Liu R., Chen C., Huang Y., Cheng L., Lu R., Fu G., Lin J. Microbiological identification and analysis of waterfowl livers collected from backyard farms in southern China. J. Vet. Med. Sci. 2018;80:667–671. doi: 10.1292/jvms.17-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.-N., Zhang X.-X., Zou Z.-F., Xie Z.-J., Zhu Y.-L., Zhao Q., Zhou E.-M., Jiang S.-J. Development of an indirect ELISA for the detection of duck circovirus infection in duck flocks. Vet. Microbiol. 2010;145:41–46. doi: 10.1016/j.vetmic.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Madsen J.M., Zimmermann N.G., Timmons J., Tablante N.L. Prevalence and differentiation of diseases in Maryland backyard flocks. Avian Dis. 2013;57:587–594. doi: 10.1637/10423-101612-Reg.1. [DOI] [PubMed] [Google Scholar]
- Mandal P.S., Mukhopadhayay S.K., Pradhan S., Mondal S., Jana C., Patra N.C., Hansda R.N. Development of nested polymerase chain reaction-based diagnosis of duck enteritis virus and detection of DNA polymerase gene from non-descriptive duck breeds of West Bengal India. Veterinary World. 2017;10:336–341. doi: 10.14202/vetworld.2017.336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbuko I.J., Musa W.I., Ibrahim S., Saidu L., Abdu P.A., Oladele S.B., Kazeem H.M. A retrospective analysis of infectious bursal disease diagnosed at poultry unit of Ahmadu Bello University, Nigeria. Int. J. Poultry. 2010;9:784–790. doi: 10.3923/ijps.2010.784.790. [DOI] [Google Scholar]
- Mishra N., Kalaiyarasu S., Nagarajan S., Rao M.V., George A., Sridevi R., Newman S.H. Serological evidence of West Nile virus infection in wild migratory and resident water birds in Eastern and Northern India. Comparative Immunol., Microbiol. Infect. Dis. 2012;35:591–598. doi: 10.1016/j.cimid.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. PMID: 20171303. [DOI] [PubMed] [Google Scholar]
- Molia M., Grosbois V., Kamissoko B., Sidibe M.S., Sissoko K.D., Traore I., Diakite A., Pfeiffer D.U. Longitudinal study of Avian Influenza and Newcastle Disease in village poultry, Mali, 2009–2011. Avian Dis. 2017;61:165–177. doi: 10.1637/11502-092616-Reg.1. [DOI] [PubMed] [Google Scholar]
- Mondal T., Khan M.S.R., Alam M., Purakayastha M., Das M., Siddique M.P. Isolation, identification and characterization of salmonella from duck Bangladesh. J. Veterinary Med. 2008;6:7–12. [Google Scholar]
- Montalvo-Corral M., Lopez-Robles G., Hernandez J. Avian Influenza survey in migrating waterfowl in Sonora, Mexico. Transboundary Emerg. Dis. 2011;58:63–68. doi: 10.1111/j.1865-1682.2010.01182.x. [DOI] [PubMed] [Google Scholar]
- Neher S., Barman N.N., Bora D.P., Deka D., Tamuly S., Deka P., Das S.K. Detection and isolation of Duck Plague virus from field outbreaks in Assam, India. Indian J. Animal Res. 2019;53:790–798. doi: 10.18805/ijar.B-3588. [DOI] [Google Scholar]
- Ninvilai P., Tunterak W., Oraveerakul K., Amonsin A., Thontiravong A. Genetic characterization of duck Tembusu virus in Thailand, 2015–2017: Identification of a novel cluster. Transboundary Emerg. Dis. 2019;66:1982–1992. doi: 10.1111/tbed.13230. [DOI] [PubMed] [Google Scholar]
- Rajput D.S., Singh S.P., Sudipta G., Nema R.P. Duck farming, fascinating option in India. J. Veterinary Sci. Technol. 2014;5(3) [Google Scholar]
- OIE. (2015a). Outbreak of H5N1 in Chandigarh, India. Retrieved from https://www.oie.int/wahis_2/temp/reports/en_fup_0000017621_20150519_124103.pdf.
- OIE. (2015b). Outbreak of H5N1 of Avian Influenza in Kerala, India. Retrieved from https://www.oie.int/wahis_2/temp/reports/en_fup_0000018081_20150710_122733.pdf.
- Rimondi A., Xu K., Craig M.I., Shao H., Ferreyra H., Rago M.V., Pereda A. Phylogenetic analysis of H6 influenza viruses isolated from rosy-billed Pochards (Netta peposaca) in Argentina reveals the presence of different HA gene clusters. J. Virol. 2011;85:13354–13362. doi: 10.1128/JVI.05946-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemons R.D., Hansen W.R., Converse K.A., Senne D.A. Type A Influenza Virus surveillance in free-flying, nonmigratory ducks residing on the eastern shore of Maryland. Avian Dis. 2003;47:11107–11110. doi: 10.1637/0005-2086-47.s3.1107. [DOI] [PubMed] [Google Scholar]
- Soliman M., Alfajaro M.M., Lee M.-H., Jeong Y.-J., Kim D.-S., Son K.-Y., Kang M.-I. The prevalence of duck hepatitis A virus types 1 and 3 on Korean duck farms. Arch. Virol. 2015;160:493–498. doi: 10.1007/s00705-014-2264-3. [DOI] [PubMed] [Google Scholar]
- Spackman E., McCracken K.G., Winker K., Swayne D.E. H7N3 Avian Influenza virus found in a South American wild duck is related to the Chilean 2002 poultry outbreak, contains genes from equine and North American wild bird lineages, and is adapted to domestic turkeys. J. Virol. 2006;80:7760–7765. doi: 10.1128/JVI.00445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K.P., Patil S.S., Hamsapriya S., Shinduja R., Roy P., Amachawadi R.G. Prevalence of extended-spectrum beta-lactamase producing bacteria from animal origin: A systematic review and meta-analysis report from India. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnagda Z., Yougbaré I., Kam A., Tahita M.C., Ouedraogo J.B. Prevalence of infectious bronchitis and Newcastle disease virus among domestic and wild birds in H5N1 outbreaks areas. J. Infect. Develop. Countries. 2011;5:565–570. doi: 10.3855/jidc.1441. [DOI] [PubMed] [Google Scholar]
- Wang R., Jian F., Sun Y., Hu Q., Zhu J., Wang F., Ning C., Zhang L., Xiao L. Large-scale survey of Cryptosporidium spp. in chickens and Pekin ducks (Anas platyrhynchos) in Henan, China: Prevalence and molecular characterization. Avian Pathol. 2010;39:447–451. doi: 10.1080/03079457.2010.518314. [DOI] [PubMed] [Google Scholar]
- Wei B., Cha S.Y., Yoon R.H., Kang M., Roh J.H., Seo H.S., Jang H.K. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from retail chicken and duck meat in South Korea. Food Control. 2016;62:63–68. doi: 10.1016/j.foodcont.2015.10.013. [DOI] [Google Scholar]
- Wille M., Muradrasoli S., Nilsson A., Järhult J.D. High prevalence and putative lineage maintenance of avian coronaviruses in Scandinavian waterfowl. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.M., Hall J.S., Flint P.L., Franson J.C., Ely C.R., Schmutz J.A., Samuel M.D. High seroprevalence of antibodies to avian influenza viruses among wild waterfowl in Alaska: implications for surveillance. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0058308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnarowicz C., Olkowski A., Schwean-Lardner K. First Canadian outbreak of West Nile Virus disease in farmed domestic ducks in Saskatchewan. Can. Vet. J. 2007;48:1270–1271. [PMC free article] [PubMed] [Google Scholar]
- Yang N., Mu M.Y., Li H.K., Long M., He J.B. Seroprevalence of Toxoplasma gondii infection in slaughtered chickens, ducks, and geese in Shenyang, northeastern China. Parasites Vectors. 2012;5:237. doi: 10.1186/1756-3305-5-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun T., Chen H., Yu B., Zhang C., Chen L., Ni Z., Ye W. Development and application of an indirect ELISA for the detection of antibodies to novel duck reovirus. J. Virol. Methods. 2015;220:55–59. doi: 10.1016/j.jviromet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Karlsson EA, Ciuoderis K, Freiden PJ, Seufzer B, Jones JC, Johnson J, Parra R, Gongora A, Cardenas D, Barajas D, Osorio JE, Schultz-Cherry S. Prevalence and characterization of influenza viruses in diverse species in Los Llanos, Colombia. Emerg Microbes Infect. 2013 Apr; 2(4):e20. doi: 10.1038/emi.2013.20. Epub 2013 Apr 24. PMID: 26038461; PMCID: PMC3636595. [DOI] [PMC free article] [PubMed]
- Yeh JC, Lo DY, Chang SK, Chou CC, Kuo HC. Prevalence of plasmid-mediated quinolone resistance in Escherichia coli isolated from diseased animals in Taiwan. J Vet Med Sci. 2017 Apr 8;79(4):730–735. doi: 10.1292/jvms.16-0463. Epub 2017 Mar 2. PMID: 28250288; PMCID: PMC5402195. [DOI] [PMC free article] [PubMed]
- Zhang S., Zhao L., Wang X., Zhang D., Zhao J., Zhang G. Serologic and virologic survey for evidence of infection with velogenic Newcastle disease virus in Chinese duck farms. Avian Dis. 2011 Sep;55(3):476–479. doi: 10.1637/9649-010611-ResNote.1. PMID: 22017050. [DOI] [PubMed] [Google Scholar]
- Zhao G., Pan J., Gu X., Zhao K., Chen H., Chen S., Wang X., Peng D., Liu X. Prevalence of low pathogenic avian influenza virus in one live bird market in Eastern China from 2002–2011. Avian Dis. 2013 Mar;57(1):155–158. doi: 10.1637/10293-062812-Case.1. PMID: 23678747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable since the data used in this study was from peer-reviewed published articles and are available in the public domain for which DOI is mentioned against each reference.