Abstract
Dengue is one of the highest and rapidly spreading vector-borne viral diseases with high mortality rates. The infection causes acute febrile illness, a major public health concern in the tropics and subtropics globally. The disease is caused by an RNA virus that belongs to the Flaviviridae family. The virus is transferred to humans by the mosquito vector called Aedvrves aegypti, which is the cause of new prevalent sicknesses worldwide. These vector-borne viral diseases spread very fast and pose public health and economic challenges that deemed various prevention and control techniques. The Flavivirus genus consists of five different types of viruses starting from DENV-1 to DENV-5. Thus, the present review focuses on the origin of the virus, how the Dengue virus can be detected, infection, the morphology of the virus, its classifications as proposed by ICTV, the replication and genome of the dengue virus, translation, receptor binding, and some vaccine trial volunteers. In addition, it highlights the current challenges and limitations of effective dengue treatment.
Keywords: Dengue virus, Genome, RNA, Replication, Morphology, Vaccines
1. Introduction
Viral infections have been of great concern with increase in the rise of epidimics and pandamics leading death of millions of people (Mujtaba et al., 2021: Irfan et al., 2017a, Irfan et al., 2017). Dengue is known as a mosquito-borne viral infection that has very high mortality and morbidity rates. Mosquitos are the primary transmitters of this viral infection. Globally, around 2.5 billion individuals have been affected by the virus (Khursheed et al., 2013: Caraballo and King, 2014). The Pan American Health Organization (PAHO) reported around 20,368 cases of dengue virus infection in the Americas region six weeks into 2021. PAHO reported that Nicaragua has about 4,297 cases, which is the highest. The next highest is Colombia, with 4,118 cases. Paraguay has 3790 cases, while 1,951 and 1,670 cases were reported in Mexico and Ecuador, respectively. In early 2021, DENVI 1–4 variants were reported in the Americas; thus, severe cases keep going up. The Americas has seen up to 55,800 Dengue virus cases from 1st Jan 2021 to now. Out of the 55,800 cases, 210 were severe, while 13 cases resulted in deaths (PAHO, March 2021).
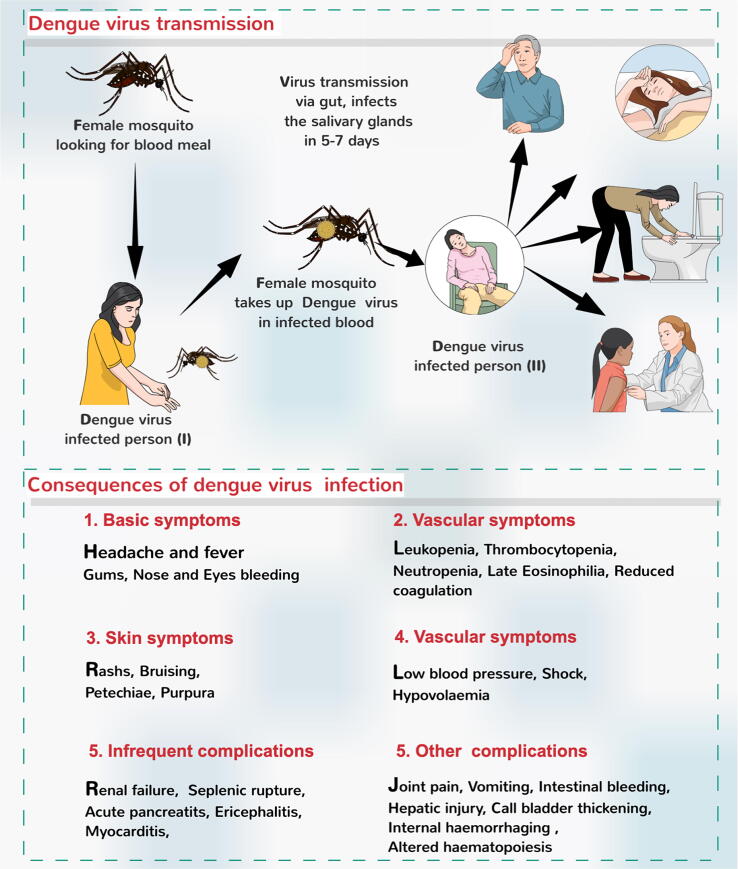
There has been a rise in Dengue virus infection cases in recent years, resulting in around 100–400 million cases every year (WHO). Aedes aegypti transmits the dengue virus through various species of female Aedes mosquito. The infection starts with mild fever; however, after three to fifteen days of infection, the individual can develop a very high fever and other consequences, as shown in Fig. 1 (Anderson and Rico-Hesse, 2006, Tuiskunen-B and Lundkvist, 2012, Bhatt et al., 2013, Basurko et al., 2018).
Fig. 1.
Dengue virus infection and associated consequences. (Modified from Rather et al., 2017).
Different preventive strategies are developed to control the spread of dengue virus infection. Nevertheless, different variants of DENV can be found globally, and its transmission is made easier because of human travel globally. Deaths caused by the dengue virus increased from 960 in the year 2000–4032 in 2015. However, at present, the deaths due to DENV infections have drastically reduced.
2. Origin of dengue virus
There is no evidence yet confirming when Dengue Virus was first reported in humans. This is primarily due to the asymptomatic nature of the disease. As a result, it is not easily diagnosed. Dengue fever (DF), although it is not a new illness, was first discovered and documented in Batavia in 1779; after a year, it was deemed a pandemic in Philadelphia, USA (McCallum, 2008). The World Health Organization reported that about 1.2 million people got the DF in 1998 (Dengue: global alert response GAR). Then 50 years later, the number of infections increased by 30 times. Another case was reported in China in 1992 by a medical encyclopedia. Between the 18th and 19th centuries, world shipping expanded, bringing urbanization to port cities, which became ideal for the primary mosquito vector, Aedes aegypti, to transmit the disease.
Similarly, when Southeast Asia expanded very quickly, it resulted in increased transmission of the virus. The first dengue pandemic took the world by storm, happening simultaneously in Africa, Asia, and North America (WHO, 2021). At the moment, the various reports obtainable suggest that there are around 50 million DF cases in hundred nations of the world (Guzman and Istúriz, 2010).
DENV is transmitted between individuals when an infected Aedes mosquito species (Ae. aegypti or Ae. albopictus) bites a human host (Carrington and Simmons, 2014). These mosquitoes can also spread the West Nile virus. Southeast Asia witnessed a dengue hemorrhagic fever (DHF) epidemic due to a change in ecology (Gubler, 2006). Its symptoms include plasma leakage, hypovolemia, an increase in vascular permeability, and shock (World Health Organization (WHO), 1997, Gubler, 2002). In 2019, WHO reported about 4.2 million cases of the dengue virus.
3. ICTV dengue virus taxonomy
Less than fifty species of arthropod-borne viruses are contained in the Flavivirus (Gubler, 2006, Mukhopadhyay et al., 2005). These small-enveloped viruses have RNA genomes that range from 9000 to 13,000 bases (Simmonds et al., 2017).
Usually, birds and mammals are the primary hosts for transmitting this virus by ticks or arthropod vectors and mosquitoes (Simmonds et al., 2017). However, viruses transmitted by mosquitoes are mostly limited to dengue virus, yellow fever virus, West Nile virus, and Japanese encephalitis virus (Simmonds et al., 2017).
4. Virus morphology
The flavivirus has a spherical shape with a diameter of 50 nm. Mature virions consist of virus-encoded, membrane-associated proteins M and E (Fig. 2). Intracellular immature virions have the precursor prM divided into M as they mature (Stadler et al., 1997). X-ray crystallography has been used to obtain the virion structures of the dengue virus (Kuhn et al., 2002, Mukhopadhyay et al., 2003). The envelope protein, E, is a rod-shaped dimeric molecule (Yu et al., 2008). Reconstructing the image of the virion envelope with cryo-electron micrographs has shown that it has icosahedral symmetry, with the E protein dimers arranged in a herringbone-like pattern.
Fig. 2.
Structural components of Dengue virus.
5. Dengue virus genome and replication
There are five different classes of dengue viruses, DENV–1, DENV–2, DENV–3, DENV–4, and DENV–5 (Mukhopadhyay et al., 2005). They have 65–70% similarity with amino acid sequence (Azhar et al., 2015). The structure of the genome has a positive-sense RNA genome of 10.6 to 11.0 kb (Shrivastava et al., 2018), is flanked by 5′ UTR and 3′ UTR (Wadood et al., 2017), and encodes for a single open reading frame (~3400 codons). The genome encodes for three structural proteins, C protein, prM protein, E protein, and non-structural proteins, such as NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5 as shown in Fig. 3.
Fig. 3.
Genome organization and polyprotein processing of members of the genus Flavivirus. Boxes below the genome indicate viral proteins generated by proteolytic processing. NCR, non-coding region.
The Dengue Virus lashes to the host's cell surface, entering the cell via a process called endocytosis (Acosta et al., 2014). The virus mixes with the endosomal membrane and enters the cytoplasm. The virus particle releases the viral genome (Pagni and Fernandez-Sesma, 2012). The viral RNA is transformed into Polypeptide, which is divided into 10 Proteins. Afterward, the viral genome is divided into vesicle packets (VPs), which are probably the site of viral RNA replication (Welsch et al., 2009, Junjhon et al., 2014). The surface of the endoplasmic reticulum is where virus assembly occurs. The viral particles that are yet to mature are conveyed via the trans-Golgi network. In the process, they become mature and also infectious. Then the viruses are let loose, ready to damage other cells. To facilitate viral replication, the nonstructural protein 1 (NS1) of the dengue virus has to react with another viral protein known as NS4A-2K-4B (Salazar et al., 2007).
6. Detection of dengue virus
An accurate and efficient diagnosis of dengue is crucial to carry out clinical care (Dengue, 2009). To perform the diagnosis of the virus in the laboratory, either the dengue virus, viral nucleic acid, antibodies or antigens are detected, or a combination of these techniques is used. The virus can be seen in plasma, serum, blood cells in circulation, and other tissues. At the onset of the disease, the detection of either antigen, nucleic acid, or virus isolation can serve as the diagnosis of the infection. When the acute stage of the infection has ended, serology can then be used for diagnosis. The response of antibodies to infection differs depending on the immunity status of the hosts (Vorndam et al., 1997). Various laboratory diagnostic techniques have been put in place to help control the disease and manage patients.
6.1. Virologic diagnosis
For virus isolation, blood is usually taken between five and six days once symptoms are noticed in the acute phase. The collected blood sample can equally be used to detect viral RNA and NS1 antigen by RT-PCR. In addition, the isolated viruses can be distinguished through indirect immunofluorescence utilizing monoclonal antibodies to fight all five serotypes (Henchal et al., 1982).
6.2. Molecular diagnostics by RT-PCR
Specific primers are used in this method for DENV found in gene prM and gene C. A sequence common to all DENV five variants, which allow for the amplification of genomes, flank this segment. A unique primer is then used to identify each variant in a semi-nested PCR during the second amplification process. To view the cDNA, use 1% agarose gel electrophoresis, after which it is then digitalized (Lanciotti et al., 1992). Real-time RT-PCR can process many samples at a time and can be used both quantitatively and qualitatively.
Using the regular RT-PCR to diagnose early for Dengue virus, cases have shown that are very valuable. One of RT-PCR benefits is that its sensitivity in both primary and secondary cases is insignificant (Cordeiro et al., 2007).
6.3. Detection of NS1 antigen
Highly present in all five variants of DENV is the hexameric type of NS1 protein (Young et al., 2000). In the early diagnosis of the disease, NS1 antigen is utilized as a marker. ELISA can detect NS1 very fast and even gives the same precision as RT-PCR. However, ELISA cannot distinguish the different virus variants. The infection can affect the sensitivity of the test. NS1 test produces more accuracy when the disease has become very acute (Hang et al., 2009).
6.4. Serological diagnosis
Excluding urine, saliva, blood on filter paper, and serum can be utilized to detect IgM if within five or more days samples were taken once the fever has begun. Serum specimens may be tested at either single dilution or several dilutions. The majority of the antigens used for this test are obtained from the dengue virus envelope protein (Hunsperger et al., 2009).
7. Infection
During the viraemic and acute stage of the infection, a female mosquito can be infected when it feeds on an individual. The virus infects the mosquitos’ midgut cells and other tissues before it spreads to the salivary glands. Several people can become infected through an infected mosquito, and symptoms begin to manifest between four and seven days, after which an infected individual can also infect a new mosquito. Mosquitoes can become infected with the virus through both asymptomatic and symptomatic people (Gubler, 2014). Throughout the entire lifetime of the mosquito, it will continue to spread the virus to people as it feeds on them (Siler et al., 1926, Gubler, 2014).
8. Vaccines
Progress has been continuously made toward developing a functional and safe dengue vaccine that could help reduce the number of dengue cases globally. The first Dengue vaccine was licensed many years later. The vaccine was named Dengvaxia®; it is a dengue/live-attenuated chimeric yellow fever vaccine (Zellweger et al., 2013, Hadinegoro et al., 2015). The vaccine was produced by Sanofi Pasteur and is used in several nations like Thailand, Mexico, Brazil, Costa Rica, and El Salvador (Aguiar et al., 2016). TV003/TV005 and DENVax are two dengue vaccines currently being used in efficacy trials in Latin America and Asia. Besides, other vaccines are now being utilized in early preclinical and clinical studies. Fig. 4 below shows the classification of dengue vaccine development.
Fig. 4.
Classification of dengue vaccine candidates.
8.1. Live-attenuated virus
The only licensed dengue vaccine is Dengvaxia®, and its use has been authorized in a few countries (Guy et al., 2015). Efficacy varied in Phase 3 trials in both the Americas and Asia depending on age, serotype, and dengue serostatus at baseline (ClinicalTrials.gov. in National Library of Medicine US). The method that is now used involves attenuation by serially passing it through cell lines. This method was started by both Mahidol University (Thailand, Bangkok) and the Walter Reed Army Institute of Research (WRAIR), where they target mutagenesis and the construction of chimeric vaccine viruses (Bhamarapravati et al., 1978, Bhamarapravati and Sutee, 2000). The candidates for the Mahidol vaccine have not achieved a balanced immune response to the four components. However, systemic symptoms were observed in the recipients of the tetravalent vaccine (Kanesa-Thasan et al., 2001: Kitchener et al., 2006).
8.2. Chimeric dengue vaccine
The vaccine was designed using two approaches: the chimeric vaccine is produced by combining an attenuated flavivirus with a strain of an attenuated DENV. Sanofi Pasteur is the manufacturer of this vaccine, which is licensed as “Deng- vaxia” (Osorio et al., 2014). The phase I clinical trial of high and low doses of DENVax in healthy recipients shows that the vaccine recipients were immunogenic and safe (Martinez et al., 2015).
8.3. Purified inactivated virus
This inactivated virus is safer. At the moment, only a few quantities of purified formalin-inactivated virus (PIV) are undergoing clinical trials. So far, the results have shown that the vaccine, which WRAIR manufactures, was safe and produced an immune response in Rhesus macaques and mice after measuring the antibody responses by ELISA and carrying out neutralization tests in a few volunteers (Kanesa-Thasan et al., 2001, Kitchener et al., 2006, Guy et al., 2015).
8.4. Recombinant subunit vaccine
Insect and yeast expression systems are utilized to express Recombinant E proteins of dengue and are assessed for vaccine efficacy in monkeys and mice. Twenty percent of the E protein is deleted at the C-terminal. This deletion paves the way for purification and extracellular secretion, allowing it to retain its antigenicity. Also known as r80E, the recombinant 80% E proteins of the four DENV variants are now being manufactured by Hawaii Biotech Inc., HI, USA, and Merck and Co., NJ, USA (Kitchener et al., 2006).
8.5. Dengue DNA vaccine
A plasmid vector in the vaccine contains the genes for encoding an antigen. The plasmid codes the antigen associated with the MHC class I molecules once it enters the cell. It is also displayed on the surface of the cell, resulting in a protective immune response. The NMRC produced the DENV-1 DNA vaccine cloning the E and prM genes of DENV-1 into a plasmid vector (Putnak, et al., 1996). The number of responders and the antibody titers were not adequate even though the vaccine was received well (Putnak et al., 1996, Putnak et al., 2005).
8.6. Replication-defective virus vectored vaccines
This vector transmits antigenic genes that can induce neutralizing antibody reaction as the virus itself. This virus can be transmitted by adenovirus vectors (Osorio et al., 2014). One example of the virus-vectored dengue vaccine is the cAdVax. This vaccine contains prM and E proteins obtained from two dengue variants, DENV-1 and DENV-3, in one construct in another construct (Raviprakash et al., 2008). When the two constructs are mixed, it has proven to offer more protection against all variants of DENV (Raviprakash et al., 2008).
8.7. Virus-like particle (VLP) vaccines
In heterologous hosts, the DENVs E and prM proteins could co-express, and they can also co-assemble into VLPs (Clements et al., 2010). The yeast system could be more appropriate for using VLPs for vaccine production because it could produce higher yields and glycosylated antigens (Danko et al., 2011).
9. Conclusion
According to several findings, DENV has killed many people worldwide, and most of the increasing cases of Dengue fever have been linked to urbanization. There are no know origins of the virus, which can be transmitted between people. Several methods have also been proposed for detecting the virus. In addition, different vaccines are being licensed and produced to tackle the virus. However, the most pragmatic advice is to maintain and strengthen existing dengue control practices, particularly during the current pandemic of Covid-19 and associated lockdowns. Of note, public health organizations and community-based associations in tropical and subtropical countries should address COVID-19 and dengue.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Centre of Excellence in Bionanoscience Research, King Abdulaziz University (KAU) for proving us research lab facility. All the figures were drawn using a paid software program “mindthegrapgh.com”.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mernan Jamal Sabir, Email: mernan.j.s@hotmail.com.
Sabah Mohmoud Hassan, Email: smmhassan1@kau.edu.sa.
References
- Acosta E.G., Kumar A., Bartenschlager R. Revisiting dengue virus-host cell interaction: new insights into molecular and cellular virology. Adv. Virus. Res. 2014;88:1–109. doi: 10.1016/B978-0-12-800098-4.00001-5. [DOI] [PubMed] [Google Scholar]
- Aguiar M., Stollenwerk N., Halstead S.B. The impact of the newly licensed dengue vaccine in endemic countries. PLoS Negl. Trop Dis. 2016;10:12. doi: 10.1371/journal.pntd.0005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.R., Rico-Hesse R. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am. J. Trop. Med. Hyg. 2006;75:886–892. [PMC free article] [PubMed] [Google Scholar]
- Azhar E.I., Hashem A.M., El-Kafrawy S.A., Abol-Ela S., Abd-Alla A.M., Sohrab S.S., Farraj S.A., Othman N.A., Ben-Helaby H.G., Ashshi A., Madani T.A., Jamjoom G. First complete genome sequence of circulating dengue virus serotype 3 in Jeddah, Saudi Arabia. New Microbes New Infections. 2015;21:9–11. doi: 10.1016/j.nmni.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basurko C., Matheus S., Hildéral H., Everhard S., Marion Restrepo M., Cuadro-Alvarez E., Lambert V., Boukhari R., Duvernois J., Favre A., Nacher M., Carles G. Estimating the risk of vertical transmission of dengue: A prospective study. Am. J. Trop. Med. Hyg. 2018;98:1826–1832. doi: 10.4269/ajtmh.16-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhamarapravati N., Sutee Y. Live attenuated tetravalent dengue vaccine. Vaccine. 2000;18:44–47. doi: 10.1016/s0264-410x(00)00040-2. [DOI] [PubMed] [Google Scholar]
- Bhamarapravati N., Yoksan S., Chayaniyayothin T., Angsubphakorn S., Bunyaratvej A. Immunization with a live attenuated dengue-2-virus candidate vaccine (16681-PDK 53): clinical, immunological and biological responses in adult volunteers. Bull. World Health Organ. 1978;65:189–195. [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Dark J.M., Brownstein J.S., Hoen A.G., Sankoh O.S., Myers M.F., George D.B., Jaenisch T., Wint G.R.W.T., Simmons C.P., Scott T.W., Farrar J.J., Hay S.I. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo H., King K. Emergency Department management of mosquito-borne illness: malaria, Dengue and west Nile virus. Emergency Med. Practice. 2014;16:5. [PubMed] [Google Scholar]
- Carrington L.B., Simmons C.P. Human to mosquito transmission of dengue viruses. Front. Immunol. 2014;5:1–8. doi: 10.3389/fimmu.2014.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements D.E., Coller B.-A.G., Lieberman M.M., Ogata S., Wang G., Harada K.E., Putnak J.R., Ivy J.M., McDonell M., Bignami G.S., Peter I.R.S., Leung J., Weeks-Levy C., Nakano E.T., Humphreys T. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28:2705–2715. doi: 10.1016/j.vaccine.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro M.T., Silva A.M., Brito C.A., Nascimento E.J., Magalhães M.C., Guimarães G.F. Characterization of a dengue patient cohort in Recife, Brazil. Am. J. Trop. Med. Hyg. 2007;77:3328–3334. [PubMed] [Google Scholar]
- Danko J.R., Beckett C.G., Porter K.R. Development of dengue DNA vaccines. Vaccine. 2011;29:7261–7266. doi: 10.1016/j.vaccine.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Dengue, 2009. Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization. 2009. 4, LABORATORY DIAGNOSIS AND DIAGNOSTIC TESTS [PubMed]
- Gubler D.J. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp. 2006;277:3–16. doi: 10.1002/0470058005.ch2. [DOI] [PubMed] [Google Scholar]
- Gubler, D.J., 2014. Dengue and Dengue Hemorrhagic Fever. In: Gubler, D.J., Ooi, E.E., Vasudevan, S., Farrar, J. (Eds.), 2nd edn. CAB International, 1–29.
- Gubler D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Guy B., Briand O., Lang J., Saville M., Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine. 2015;33:7100–7111. doi: 10.1016/j.vaccine.2015.09.108. [DOI] [PubMed] [Google Scholar]
- Guzman A., Istúriz R.E. Update on the global spread ofdengue. Int. J. Antimicrob. Agents. 2010;36:S40–S42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Hadinegoro S.R., Arredondo-García J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- Hang V.T., Nguyet N.M., Trung D.T., Tricou V., Yoksan S., Dung N.M. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PloS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchal E.A., Gentry M.K., McCown J.M., Brandt W.E. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 1982;31:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- Hunsperger E.A. Evaluation of commercially available anti–dengue virus immunoglobulin M tests. Emerg. Infect. Dis. 2009;15:436–440. doi: 10.3201/eid1503.080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfan, A.R., Jameel, B.L., Vivek, K.B., Yong-Ha, Park, 2017a. Irfan A Rather, Jameel B Lone, Vivek K Bajpai, Yong-Ha Park. Zika virus infection during pregnancy and congenital abnormalities. Front. Microbiol. 8; 581. [DOI] [PMC free article] [PubMed]
- Irfan A.R., Sanjay K., Vivek K.B., Jeongheui L., Yong-Ha P. Prevention and control strategies to counter ZIKA epidemic. Front. Microbiol. 2017;8:305. doi: 10.3389/fmicb.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junjhon J., Pennington J.G., Edwards T.J., Perera R., Lanman J., Kuhn R.J. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J. Virol. 2014;88:4687–4697. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanesa-Thasan, N., Sun, W., Kim-Ahn, G., Van- Albert, S., Putnak, J., King, A., Raengsakulsrach, B., Christ-Schmidt, H., Gilson, K., Zahradink, J.M., Vaughn, D.W., Innis, B., Saluzzo, J.F., Hoker-Jr, Ch., 2001. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine 19, 3179–3188. [DOI] [PubMed]
- Khursheed, M., Khan, U.R., Ejaz, K., Fayyaz, J., Qamar, I., Razzak, J.A., 2013. A comparison of WHO guidelines issues in 1997 and 2009 for dengue fever- single senter experience. J. Park Med. Assoc. 36, 670–674. [PubMed]
- Kitchener S., Nissen M., Nasveld P., Forrat R., Yoksan S., Lang J., Fransois-Saluzzo J. Immunogenicity and safety of two live-attenuated tetravalent dengue vaccine formulations in healthy Australian adults. Vaccine. 2006;24:1238–1241. doi: 10.1016/j.vaccine.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., Jones C.T., Mukhopadhyay S., Chipman P.R., Strauss E.G., Baker T.S., Strauss J.H. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R.S., Calisher C.H., Gubler D.J., Chang G.J., Vorndam A.V. Rapid detection and typing of dengue viruses from clinical samples by using Reverse Transcriptase Polymerase Chain Reaction. J. Clin. Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L.J., Lin L., Blaylock J.M., Lyons A.G., Bauer K.M., De La Barrera R. Safety and Immunogenicity of a Dengue Virus Serotype-1 Purified -Inactivated Vaccine: Results of a Phase 1 Clinical Trial. Am. J. Trop Med. Hyg. 2015;93:454–460. doi: 10.4269/ajtmh.14-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum, J.E., 2008. Military medicine from ancient times to the 21st century: Dengue fever ABC-CLIO, Inc., California, USA.
- Mujtaba A.B., Safikur R., Irfan A.R., Iqbal B., Shafiya S., Hamira K., Mohammad A.K., Rinki M., Arif T.J. Coronavirus Disease-2019 (COVID-19) in 2020: A perspective study of a global pandemic. Curr. Pharm. Des. 2021 doi: 10.2174/1381612826666201118112912. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S., Kim B.S., Chipman P.R., Rossmann M.G., Kuhn R.J. Structure of West Nile virus. Science. 2003;302:5643248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S., Kuhn R.J., Rossmann M.G. A structural perspective of the Flavivirus life cycle. Nat. Rev. Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- Osorio, J.E., Velez, I.D., Omson, C., Thomson, C., a Lopez, L., Jimenez, A., Haller, A.A., Silengo, S., Scott, J., Boroughs, K.L., 2014. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in avivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. e Lancet Infectious Diseases 14, 830–838. [DOI] [PMC free article] [PubMed]
- Pagni S., Fernandez-Sesma A. Evasion of the human innate immune system by dengue virus. Immunol. Res. 2012;54:152–159. doi: 10.1007/s12026-012-8334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnak J.R., Coller B.A., Voss G., Vaughn D.W., Clements D., Peters I., Bignami G., Houng H.U., Chen R.C., Barvir D.A., Seriwatana J., Cayphas S., Garçon N., Gheysen D., Kanesa-Thasan N., McDonell M., Humphreys T., Eckels K.H., Prieels J.P., Innis B.L. An evaluation of dengue type-2 inactivated, recombinant subunit, andlive-attenuated vaccine candidates in the rhesus macaque model. Vaccine. 2005;23:4442–4452. doi: 10.1016/j.vaccine.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Putnak, P., Barvir, D.A.J., Burrous, M., Dubois, D.R., D'Andrea, V.M., Hoke, C.H., Sadoff, J.C., Eckels, K.H., 1996. Development of a puri ed, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. e J. Infect. Dis. 174, 1176–1184. [DOI] [PubMed]
- Rather I.A., Parray H.A., Lone J.B., Paek W.K., Lim J., Bajpai V.K., Park Y.H. Prevention and control strategies to counter dengue virus infection. Front. Cell Infect. Microbiol. 2017;7:336. doi: 10.3389/fcimb.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviprakash K., Wang D., Ewing D., Holman D.H., Block K., Woraratanadharm J., Chen L., Hayes C., Dong J.Y., Porter K. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J. Virol. 2008;82:6927–6934. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M.I., Richardson J.H., Sánchez-Vargas I., Olson K.E., Beaty B.J. How the dengue virus replicates in infected cells. BMC Microbiol. 2007;7:1–12. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava, S., Tiraki, D., Diwan, A., Lalwani, S.K., Modak, M., Mishra, A.C. and Arankalle, V.A., 2018. Co-circulation of all the four dengue virus serotypes and detection of a novel clade of DENV-4 (genotype I) virus in Pune, India during 2016 season PLoS One. 13, 1-19. [DOI] [PMC free article] [PubMed]
- Siler J.F., Hall M.W., Hitchens A.P. Dengue: its history, epidemiology, mechanism of transmission, etiology, clinical manifestations, inmunity and prevention. Philippine J. Sci. 1926;29:1–304. [Google Scholar]
- Simmonds P., Becher P., Bukh J. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017;98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler K., Allison S.L., Schalich J., Heinz F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuiskunen-B A.N., Lundkvist A. Dengue viruses an overview. Infect. Ecol. Epidemiol. 2012;3:19839. doi: 10.3402/iee.v3i0.19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorndam V., Kuno G., Gubler D.J., Kuno G. CAB International. Laboratory diagnosis of dengue virus infections; New York: 1997. Dengue and dengue hemorrhagic fever; pp. 313–333. [Google Scholar]
- Wadood A., Mehmood A., Khan H., Ilyas M., Ahmad A., Alarjah M., Abu-Izneid T. Epitopes based drug design for dengue virus envelope protein: A computational approach. Comput. Biol. Chem. 2017;71:152–160. doi: 10.1016/j.compbiolchem.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C.K., Walther P., Fuller S.D., Antony C., Krijnse-Locker J., Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO), 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed.Geneva WHO.
- world Health Organization (WHO), 2021. History and Origin of Dengue Virus. [online].
- Young P.R., Hilditch P.A., Bletchly C., Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000;38:1053–1057. doi: 10.1128/jcm.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.M., Zhang W., Holdaway H.A., Li L., Kostyuchenko V.A., Chipman P.R., Kuhn R.J., Rossmann M.G., Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- Zellweger R., Miller R., Eddy W., White L., Johnston R., Shresta S. Role of Humoral versus Cellular Responses Induced by a Protective Dengue Vaccine Candidate. PLoS Pathogens. 2013;9 doi: 10.1371/journal.ppat.1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]