Abstract
Water deficit stress negatively affects wheat growth, physiology, and yield. In lab and hydroponic experiments, osmotic stress levels (control, −2, −4, −6 and −8 Bars) created by PEG-6000, caused a significant decline in germination, mean germination time, root, shoot, and coleoptile length in both wheat genotypes examined. Germination was inhibited more in Wafaq-2001 than in Chakwal-50. Wafaq-2001 showed a higher susceptibility index based on root and shoot dry weight than did Chakwal-50. Wheat plants exhibited osmotic adjustment through the accumulation of proline, soluble sugars, soluble proteins, and free amino acids, and increased antioxidation activities of superoxide dismutase, peroxidase, catalase, and malondialdehyde. Increasing water deficit stress caused a linear decline in chlorophyll contents, leaf membrane stability, and relative water content in all wheat plants, with Wafaq-2001 showing a more severe negative impact on these parameters with increasing stress levels. The results suggest the possibility of utilizing some of these parameters as quantitative indicators of water stress tolerance in plants. Gas exchange measurements (photosynthesis, transpiration, stomatal conductance), leaf osmotic potential, water potential, and yield attributes decreased more abruptly with increasing water deficit, whereas leaf cuticular wax content increased in both genotypes, with more severe impacts on Wagaq-2001. More reduction in biochemical, physiological, and yield attributes was observed in Wafaq-2001 than was observed in Chakwal-50. Based on these results, we can conclude that Chakwal-50 is a more drought-tolerant genotype, and has excellent potential for future use in breeding programs to improve wheat drought tolerance.
Keywords: Mean germination time, Proline, Chlorophyll contents, Antioxidant enzymes, Photosynthesis, Wheat
1. Introduction
Wheat (Triticum aestivum L.) is a staple food contributing 20% of calories to the world’s population, with a total harvest area of 2.1 million km−2, and global production of 700 million tones (Shiferaw et al., 2013). Because it is typically cultivated in rainfed conditions, it commonly faces high temperatures combined with an irregular water supply (inducing drought stress) during both vegetative and reproductive stages, a condition that results in yield reduction (Asseng et al., 2015).
Within an agricultural context, drought is a prolonged period of unusually low precipitation, and this has negative impacts on crop growth or yield. A warming climate is expected to intensify the frequency and severity of drought in the near future (Yu et al., 2017). As such, identifying key physiological limitations to productivity under drought and mechanisms of crop tolerance to water deficit stress will be important for improving yield stability in a changing climate. Moreover, limited genetic diversity within important crop species coupled with ecological constraints to productivity needs to be overcome to adapt crops to episodic drought events in the future (Han et al., 2015). The ability of plants to maintain physiological functions at low plant water status, and recover quickly once the stress is removed, will be important for ensuring sustainable crop production under intermittent drought events (Izanloo et al., 2008). The effects of drought stress have been well-documented in many crop species. However, reports addressing physiological responses to progressive drought and recovery upon re-watering are relatively limited. Reduced plant growth and productivity under drought are caused by altered plant water relations, decreased CO2 assimilation, cellular oxidative stress, membrane damage of affected tissues, and in some instances, inhibition of enzyme activity. Plants respond to drought stress by exploiting the following mechanisms: (1) drought escape by completing the life cycle before the onset of severe water limitation (Izanloo et al., 2008); (2) drought avoidance through enhanced water-conserving mechanism via stomatal closure and reduction of leaf area or canopy cover (Yu et al., 2017); (3) drought tolerance through osmotic adjustment and increased cell wall elasticity (Yi et al., 2016), and (4) drought resistance through altered metabolic changes such as an increased antioxidant metabolism (Reddy et al., 2004). Plants can employ one or a combination of all the above-mentioned mechanisms in response to drought stress.
Plants can respond and adapt to water stress by perceiving the stimulus, generating, and transmitting the signals, and initiating various defense mechanisms (Ming-Yi and Jian-Hua, 2004). The antioxidant system, including antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POX), catalase (CAT), and small-molecule non-enzymatic antioxidants such as glutathione, ascorbate, flavonoids, and α-tocopherol, play a key role in controlling reactive oxygen species (ROS) levels (Burke and Mahan, 1991). The level of damage may be limited by enzymatic and non-enzymatic scavengers of free radicals (Aroca et al., 2003). The degree to which the activities of antioxidant enzymes, as well as the amounts of antioxidants, are elevated under drought stress is extremely variable among plant species (Zhang and Kirkham, 1995), and even between two genotypes of the same species (Bartoli et al., 1999). The biochemical and physiological responses to stress within a plant are closely associated with the overall agronomic response in a field cropping situation. Characterization of the agronomic response of wheat crops to variable water deficit stress could help to stabilize production at present levels and to identify appropriate stress tolerance mechanisms for use in future breeding efforts to increase yield. During water deficit stress, many plants show significantly increased accumulation of superoxide and hydrogen peroxide, resulting from the increased rate of O2 photoreduction in chloroplasts (Robinson and Bunce, 2000). It has been reported that much of the injury to plants caused by exposure to various stresses is associated with oxidative damage at the cellular level (Allen, 1995). Mechanisms of ROS detoxification exist in all plants (Mundree et al., 2002), and changes in the amount of antioxidants can be a direct reflection of the impact of environmental stresses on a plant’s metabolism (Herbinger et al., 2002). The level of response depends on the species, the development, and the metabolic status of the plant, as well as the duration and intensity of the stress.
Water deficit stress is caused by a reduction in the relative amount (or concentration) of water within plant tissues. In this sense then, one of the most reliable and widely used indicators for defining both the sensitivity and the tolerance to water deficit stress in plants is leaf relative water content (RWC) (Soltys-Kalina et al., 2016). Soltys-Kalina et al. (2016) reported that RWC was one of the best indicators for separating tolerant and sensitive genotypes. Increasing amounts of free proline accumulation and soluble sugars typically occurs in the leaves of crop plants when exposed to moderate to severe water deficit stress (Qayyum et al., 2011). It has been suggested that proline and sugar accumulating potentials could serve as primary indices of drought tolerance. However, the physiological significance of this metabolic response to water is contentious. Others emphasize traits like cell membrane stability (ElBasyoni et al., 2017), a technique often used for screening drought tolerance in various crops such as sorghum (Ali et al., 2009), wheat and wild relatives of wheat (Sallam et al., 2019).
Although attempts have been made to investigate the recovery of photosynthesis from drought stress in different crop species including wheat (Souza et al., 2004), studies addressing membrane stability, oxidative stress, antioxidative process, and osmolyte dynamics during drought recovery are limited. Moreover, studies quantifying the impact of plant metabolic changes during drought and recovery periods during vegetative development on final productivity in the reproductive stage in wheat are, to our knowledge, non-existent because physiological changes during reproductive stages are understandably related to grain yields and have received far more attention (Çakir, 2004). Nonetheless, stress events during vegetative growth periods can significantly influence grain yield of wheat and should be investigated further. After drought stress is removed, the availability of even a small amount of rainfall can have a significant effect on plant physiological functions, ranging from whole-plant responses to biochemical responses. Therefore, it is of particular importance to investigate the underlying mechanisms contributing to drought tolerance (Izanloo et al., 2008). We hypothesized that 1) the ability to osmotically adjust and protect cellular components from oxidative stress will be critical factors influencing tolerance to episodic drought during the seedling phase; 2) final productivity in wheat would be dependent on the ability to maintain photosynthetic stability under drought stress. The present experiments were carried out to examine the effects of water stress on germination indices, biochemical, physiological, and yield attributes in two important wheat genotypes commonly used in Pakistani agriculture.
2. Materials and methods
The experiments were conducted in Crop Physiology Laboratory, Department of Agronomy, PMAS-Arid Agriculture University, Rawalpindi, Pakistan. Seeds of wheat (Triticum aestivum L.) genotypes (Chakwal-50 and Wafaq-2001) were obtained from the National Agricultural Research Center, Islamabad, Pakistan.
In the first experiment, wheat seeds of uniform size were surface sterilized for 8 min in 0.1% HgCl2 and subsequently washed three times in distilled water after fully flushing with tap water. Wheat seeds were sown in 9 cm Petri dishes and placed in a growth chamber under a constant day/night temperature regime of 25 °C/20 °C, 16 h/8h (light/dark) with a light intensity of 350 µ mol m−2 s−1. Wheat seeds were subjected to five stress levels of osmotic stress i.e. 0 bars (distilled water, control), −2 bars, −4 bars, −6 bars and −8 bars to test their germination percentage, mean germination time, coleoptile length, root-shoot length and their dry weight, germination inhibition percentage and root-shoot dry weight susceptibility index. Osmotic stress was created using different concentrations of polyethylene glycol (PEG-6000) following the method employed (Michel and Kaufmann, 1973) and the experiment was laid out in a two factorial fashion using completely randomized design with four replications. The data were recorded for eight days (i.e. until the germination process was completed). Germination was calculated every 24 h for eight days using the following formula:
| Germination (%) = Number of germinated seeds / Total No. of seeds germinated × 100 |
Mean germination time (MGT) was determined as described by Sadeghi et al. (2011).
| MGT = ΣDn / Σn |
Where Dn is the number of seeds that germinated on day D and n is the number of days from the beginning of the germination test to day D.
Root dry weight susceptibility index (RDSI) was recorded by using the following formula
| RDSI = Root Dry Weight (Stressed Plants) / Root Dry Weight (Controlled Plants) × 100 |
Shoot dry weight susceptibility index (SDSI) was recorded by using the following formula
| SDSI = Shoot Dry Weight (Stressed Plants) / Shoot Dry Weight (Controlled Plants) × 100 |
In the second experiment, seeds were sown in 9 cm Petri dishes and placed in a growth chamber under a constant day/night temperature regime of 25 °C/20 °C, 16 h/8h (light/dark) with a light intensity of 350 µ mol m−2 s−1. After seven days the seedlings were shifted to hydroponic pots (L 20 cm × W 12 × H 8 cm) having modified Murashige and Skoog (MS) medium solution (Murashige and Skoog, 1962) (Table 1). The seedlings were again placed in a growth chamber. Murashige and Skoog medium solution was replaced after 3 days and same concentration was applied again to seedling. Each pot contains 12 wheat seedlings and three pots were maintained for each replication. After one week, the modified MS medium was supplemented by PEG-6000 to induce osmotic stress of −2, −4, −6, and −8 bars. MS medium without PEG served as control. After two weeks, there was a visible effect of the treatments on growth, and plants were harvested. PEG-6000 solutions were made following Michel and Kaufmann (1973), and experiment was laid out in the Complete Randomized Design (CRD) Factorial design with three replicates. The data on free proline, total soluble sugars, total soluble protein, leaf membrane stability index, relative water content, superoxide dismutase, peroxidase, catalase, and malondialdehyde activities were recorded.
Table 1.
Composition of Murashige and Skoog (MS) medium solution.
| Sr.# | Salts | Concentration |
|---|---|---|
| Solution-A | ||
| 1 | NH4NO3 | 1.65 g/liter |
| 2 | KNO3 | 1.90 g/liter |
| Solution-B | ||
| 3 | CaCl2 | 0.44 g/liter |
| Solution-C | ||
| 4 | MgSO4·7H2O | 0.37 g/liter |
| 5 | KH2PO4 | 0.17 g/liter |
| Solution-D | ||
| 6 | FeSO4·7H2O | 27.80 mg/liter |
| 7 | Na2EDTA·2H2O | 33.60 mg/liter |
| Solution-E | ||
| 8 | H3BO3 | 6.20 mg/liter |
| 9 | ZnSO4·7H2O | 8.60 mg/liter |
| 10 | MnSO4·H2O | 16.9 mg/liter |
| Solution-F | ||
| 11 | Na2MoO4·2H2O | 0.25 mg/liter |
| 12 | CuSO4·5H2O | 0.025 mg/liter |
| 13 | CoCl2·6H2O | 0.025 mg/liter |
| 14 | KI | 0.83 mg/liter |
| Solution-G | ||
| 15 | Myoinositol | 100 mg/liter |
| 16 | Nicotinic acid | 0.5 mg/liter |
| 17 | Pyrodoxine HCl | 0.5 mg/liter |
| 18 | Thiamine HCl | 0.1 mg/liter |
| 19 | Glycine | 0.20 mg/liter |
2.1. Proline content (mg g−1 fresh weight):
Proline amounts were determined according to the method of Bates et al. (1973). A fresh sample weight of 0.1 g of leaves was added in 5 ml of 3% sulfosalicylic acid in test tubes, ground, and then allowed to settle. Then, 2 ml from the supernatant was mixed with 2 ml each of glacial acetic acid and ninhydrin reagent and was boiled for 1 h in a water bath at 100 °C. After one hour, the reaction was stopped in ice and 4 ml of toluene was added, vortexed, and the absorbance of the supernatant was read at 520 nm on the UV Spectrophotometer (UV-1900, Shimadzu, Japan). Toluene was used as blank sample.
2.2. Total soluble sugars content (mg g−1 fresh weight):
Total soluble sugars (TSS) were determined according to the method of Dubois et al. (1951). Fresh leaves (0.1 g) were added with 5 ml of 80% ethanol to test tubes, placed in a water bath, and heated for 1 h at 80 °C. Then, 1 ml of the sample extract was taken in another set of test tubes and mixed with 1 ml each of 18% phenol and distilled water, and then allowed to stand at room temperature for an hour. Finally, 5 ml of sulphuric acid was added and the whole mixture was vortexed. The absorbance was read at 490 nm wavelength on the UV spectrophotometer. Ethanol 80% was used as a blank sample.
2.3. Total soluble protein content (mg g−1 fresh weight):
Total soluble protein (TSP) was determined according to the method of Lowry et al. (1951) by using BSA as standard (Fresh). Fresh leaves (0.1 g) were added in test tubes having a 5 ml phosphate buffer. Samples of each test tube were ground with pestle and mortar around the ice. 0.5 ml of this sample extract was added in another set of test tubes in which 0.5 ml of distilled water were added and finally, 3 ml of bio-red color dye was added and vortexed for a while and the absorbance was read at 595 nm wavelength on the UV Spectrophotometer. Phosphate buffer was used as a blank sample.
2.4. Total free amino acid content (mg g−1 fresh weight):
Total free amino acids (AA) were measured according to the method of Hamilton and Van Slyke (1943). 1 ml of each sample extract was treated with 1 ml of 10% pyridine and 1 ml of 2% ninhydrin solution. The optical densities of these colored solutions were then read at 570 nm on the UV Spectrophotometer.
2.5. Chlorophyll content (mg g−1 fresh weight):
Chlorophyll analysis was determined according to the method of Arnon (1949). 80% Ethanol (5 ml) was taken in test tubes, and immediately weighed (0.1 g) fresh leaf samples were added, immersed in ethanol, and tubes were capped. Extract was kept in a water bath at 80 °C for 10 min. The extract was cooled in darkroom. The optical density was measured at 645 and 663 nm for chlorophyll “a” and “b” respectively, by exposing to lower light by using a UV Spectrophotometer. Ethanol 80% was used as a blank sample.
2.6. Relative water content (%):
Relative water content (RWC) of the leaf was determined according to the method of Barrs and Weatherley (1962). Completely extended leaves were excised, and the fresh weight of the leaves was taken immediately. The leaves were soaked in distilled water for 4 h under constant light at room temperature. The turgid weight of the leaf was calculated. The sample was dried at 80 °C for 24 hrs. The total dry mass of the sample was recorded. Finally, the relative water content of the leaf was calculated by employing the following formula:
| RWC = (Fresh Weight - Dry Weight) / (Turgid Weight - Dry Weight) × 100 |
2.7. Leaf membrane stability index:
Leaf membrane stability index (MSI) was determined according to the method of Premachandra et al. (1990) with slight modification (Sairam, 1994). Leaf discs (100 mg) were thoroughly washed with tap water followed by washing with double-distilled water thereafter the discs were heated in 10 ml of double distilled water at 40 °C for 30 min. Then electrical conductivity (C1) was recorded by EC (Electrical Conductivity) meter. Subsequently, the same samples were placed in a boiling water bath (100 °C) for 10 min and their electrical conductivity was also recorded (C2). The MSI was calculated as:
| MSI = [1 - (C1/ C2)] × 100 |
2.8. Enzyme extraction and assay procedure:
A leaf sample (0.5 g each) was homogenized in a waring blender at 4 °C. The grinding medium contained 0.05 M Potassium phosphate buffer (pH 7.8), 20 mM L-1 β- mercaptoethanol, 1 mM L-1 EDTA, and 0.1 mM L-1 phenyl methane sulfonyl fluoride (PMSF) (Baker et al., 1996). Polyvinyl pyrrolidone (PVP) (0.2 mg L-1) was added to the samples to scavenge leaf phenolics. Homogenates were centrifuged at 1700 rpm for 15 min at 4 °C. The supernatant fractions were carried out at 0–4 °C. All activities were determined at 25 °C. We preferred to express all enzyme activities on a protein basis. Protein concentrations were measured (Bradford, 1976), with BSA as a standard.
Superoxide dismutase activity (U g−1 of fresh weight) was determined by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) (Beauchamp and Fridovich, 1971). The 3 ml reaction mixture contained 2.4 × 10-6 M L-1 riboflavin and 0.013 M L-1 methionine phosphate at pH 7.8. One unit of enzyme activity was the amount of enzyme bringing about 50% inhibition of the photochemical reduction of NBT (Dhindsa and Matowe, 1981).
Catalase activity (U g−1 of fresh weight) was determined by measuring the decreasing rate in the absorbance of H2O2 at 240 nm (Aebi, 1984). One unit was defined as the amount of enzyme catalyzing the decomposition of 1 μM L-1 H2O2 per minute calculated from the extinction coefficient for H2O2 at 240 nm of 0.036 cm2 μM L-1 (Luck, 1963).
Peroxidase Activity (U g−1 of fresh weight) was determined by using guaiacol as the substrate (Volk and Feierabend, 1989). The molar extinction coefficient of tetraguaiacol (26.6 mM−1 cm−1) was employed in the calculation of the enzyme concentration.
Malondialdehyde activity (nM g−1 of fresh weight) was measured according to the method of Stewart and Bewley (1980). 0.5 g of leaf samples were homogenized in 5 ml of distilled water. An equal volume of 0.5% thiobarbituric acid (TBA) in 20% trichloroacetic acid solution was added and the sample incubated at 95 °C for 30 min. The reaction stopped by putting the reaction tubes in the ice bath. The samples then centrifuged at 10000 × g for 30 min. The supernatant removed, absorption read at 532 nm, and the amount of nonspecific absorption at 600 nm read and subtracted from this value. The amount of MDA present calculated from the extinction coefficient of 155 mM−1 cm−1.
In the third experiment, wheat seeds were grown in plastic pots (30 cm in diameter, 40 cm in depth) containing 10 kg loamy soil (80% sand, 15% silt and 5% clay). A fertilizer mixture containing 500 mg N, 300 mg P and 200 mg K as Urea, DAP and Muriate of potash was applied per plot. Eight seeds were sown in each plot. One week after emergence, the seedlings were thinned to four per pot. Two water regimes groups were set: control (80% potted soil water holding capacity) and water stress (60%, 40% and 20% potted soil water holding capacity) according to Hsiao (1973). Wheat plants were sown on 1st November and harvested on 15th April. Completely Randomize Design (CRD) in a factorial set up was laid with three replications and data was collected on various physiological and yield aspects.
2.9. Gas exchange measurements
Gas exchange measurements [(photosynthesis rate (μM m−2 sec-1), transpiration rate (M m−2 sec-1), stomatal conductance (M m−2 sec-1)] were carried out at flag leaf stage (120 days after sowing) by using Infrared Gas Analyzer (LI-6400XT Portable Photosynthesis System, USA) (Long and Bernacchi, 2003). Three readings of gas exchange measurements, from a single flag leaf were taken and averaged and in the same way data was recorded form four flag leaves from a single pot. The measurements were taken between 11 am and 3 pm. 2 cm−2 leaf chamber fluorometers were set to measure gas exchange measurements. Settings of the sensor heads were 1500 µ mol m−2 s−1 PFD for saturating light intensity, 400 ppm of CO2 concentration in the reference cell of the instruments, 25 °C block temperature, 300 µ mol s−1 flow rate, and 40–70% RH to optimize the microclimate for photosynthesis during the measurements.
Photosynthetic water use efficiency is the ratio of the rate of carbon assimilation (photosynthesis) to the rate of transpiration.
2.10. Leaf osmotic potential (-MPa)
The leaf osmotic potential of the cell sap from the flag leaves was measured with a freezing point osmometer (Osmomat 010 Gonatec GMBH.D 10823 Berlin Germany) (Capell and Doerffling, 1993). To obtain the cell sap, the frozen-thawed leaf material of leaf was enclosed in a 2 ml plastic syringe and was pressed to ooze out the sap (50 µL) to take readings (milliosmol) from the osmometer. The readings from the osmometer were transferred to MPa values.
2.11. Leaf water potential (-MPa)
The water potential of the leaf was recorded by a pressure bomb (Scholander et al., 1965). Fresh and fully expanded leaves were excised from the plants and were placed in a sealed sleeve. It was fixed through a specimen holder on the pressure vessel. After an hour of mounting, the required pressure was attained in pressure vessel until the sap appeared from the excised end of the leaf sample. At this point, the pressure reading was equivalent to the negative force with which the plant water is held within that particular sample.
2.12. Cuticular wax content (μg m−2)
Leaf area was measured and leaf samples (05) were washed three times in 10 ml chloroform for 30 sec per wash. After this, the extract was filtered, water was evaporated and the wax was weighed. It was expressed on the basis of leaf area only, i.e wax content μg m−2 (Silva-Fernandes et al., 1964).
2.13. Yield attributes
At maturity 5 plants of each wheat genotype per treatment were selected at random and data was recorded for number of grains per spike, 1000-grain weight and grain yield per plant. The spikes collected from plants were manually hand threshed and seeds obtained were weighed in grams with the help of an electric balance.
2.14. Statistical analysis
Data obtained were analyzed for analysis of variance (ANOVA) by two-factor factorial in completely randomized design and mean comparisons (p ≤ 0.05) was carried out by Duncan Multiple Range test using M−Stat−C Statistical software (Steel et al., 1997).
3. Results
3.1. Impact of water stress on germination indices and growth parameters
Significant differences were observed between both genotypes for germination percentage and mean germination time (Table 2). Germination percentage decreased from 100% (control, 0 bars) for both varieties, to 70% in Chakwal-50 and 52.5% in Wafaq-2001 at −8 bars of osmotic stress. Mean germination time (MGT) decreased from 17.6 (control) to 6.6 in Chakwal-50 and 15.5 (control) to 5.1 in Wafaq-2001 at −8 of bars osmotic stress. An increase in osmotic stress levels caused a linear decline in germination percentage and MGT in both genotypes.
Table 2.
Effect of different level of PEG-6000 induced osmotic stress on germination (%) and mean germination time of wheat genotypes.
| Osmotic Stress | Germination (%) |
Mean Germination Time |
||
|---|---|---|---|---|
| Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | |
| Control | 100 ± 0.00 a | 100 ± 0.00 a | 17.6 ± 0.13 a | 15.5 ± 0.15b |
| −2 Bars | 98.1 ± 0.31 a (1.9%) | 93.8 ± 0.36b (6.3%) | 13.9 ± 0.05c (20.9%) | 13.0 ± 0.08 d (16.1%) |
| −4 Bars | 90.0 ± 1.02b (10%) | 77.5 ± 1.02c (22.5%) | 11.6 ± 0.15 e (33.8%) | 9.8 ± 0.16f (36.5%) |
| −6 Bars | 77.5 ± 1.53c (22.5%) | 65.0 ± 1.53 e (35%) | 8.3 ± 0.17 g (52.9%) | 6.9 ± 0.15 h (55.6%) |
| −8 Bars | 70.0 ± 0.51 d (30%) | 52.5 ± 1.14f (47.5%) | 6.6 ± 0.04 h (62.2%) | 5.1 ± 0.10 I (67.4%) |
| LSD | Interaction (T × V) : 4.18 | Interaction (T × V) : 0.38 | ||
The values represent the averages (±SE) of four independent replicates followed by different letters within columns are significantly different at P ≤ 0.05, according to Duncan’s Multiple Range test. The data in parentheses indicate percent decrease in germination and mean germination time of wheat genotypes in comparison to their control values.
Root, shoot, and coleoptile length decreased severely with the increase in osmotic stress in both varieties (Table 3). Root length decreased from 14.1 cm (control) to 5.1 cm (63.8%) in Chakwal-50 and 11.4 cm (control) to 3.2 cm (71.9%) in Wafaq-2001 at −8 bars osmotic stress. Shoot length decreased from 12.5 cm (control) 3.9 cm (68.8%) in Chakwal-50 and 9.7 cm (control) to 2.1 cm (78.4%) in Wafaq-2001 at −8 bars osmotic stress. Shoot length continuously decreased with increasing exposure to more severe osmotic stress levels. PEG-induced osmotic stress also had a significant adverse effect on root and shoot dry weight in both varieties (Table 4), and root dry weight was more affected than shoot dry weight. Root dry weight decreased from 188 mg (control) to 90.0 mg (52.1%) in Chakwal-50 and 131.0 mg (control) to 28.0 mg (78.6%) in Wafaq-2001 at −8 bars osmotic stress. Drought stress drastically reduces the root dry weight in wheat seedlings. Significant differences were found between genotypes with respect to seedling growth subjected to osmotic stress. Shoot dry weight decreased from 328.0 mg (control) to 171.0 mg (47.9%) in Chakwal-50 and 282.0 mg (control) to 83.0 mg (70.6%) in Wafaq-2001 at −8 bars osmotic stress. Data pertaining to germination inhibition, root, and shoot dry weight susceptibility index is presented in Table 5. Germination inhibition was significantly increased with the increase in osmotic stress in both the genotypes. Maximum (46.9%) germination inhibition was recorded for Wafaq-2001, whereas minimum (30.6%) germination inhibition was recorded for Chakwal-50 at −8 bars of osmotic stress.
Table 3.
Effect of different level of PEG-6000 induced osmotic stress on root, shoot and coleoptile length (cm) of wheat genotypes.
| Osmotic Stress | Root Length (cm) |
Shoot Length (cm) |
Coleoptile Length (cm) |
|||
|---|---|---|---|---|---|---|
| Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | |
| Control | 14.1 ± 0.05 a | 11.4 ± 0.05b | 12.5 ± 0.05 a | 9.7 ± 0.07b | 4.9 ± 0.04 a | 3.8 ± 0.03c |
| −2 Bars | 11.7 ± 0.13b (17%) | 8.9 ± 0.05 d (21.9%) | 9.1 ± 0.07c (27.2%) | 8.3 ± 0.07 d (14.4%) | 4.4 ± 0.04b (9.8%) | 3.5 ± 0.04 d (7.9%) |
| −4 Bars | 9.8 ± 0.15c (30.5%) | 7 ± 0.05 e (38.6%) | 7.5 ± 0.05 e (40%) | 6.2 ± 0.05f (36.1%) | 3.8 ± 0.04c (22.1%) | 2.6 ± 0.02f (31.6%) |
| −6 Bars | 7.3 ± 0.07 e (48.2%) | 5 ± 0.05f (56.1%) | 6.2 ± 0.07f (50.4%) | 4.1 ± 0.05 g (57.7%) | 2.9 ± 0.04 e (41%) | 1.5 ± 0.05 h(60.5%) |
| −8 Bars | 5.1 ± 0.07f (63.8%) | 3.2 ± 0.07 g (71.9%) | 3.9 ± 0.06 g (68.8%) | 2.1 ± 0.06 h (78.4%) | 2 ± 0.04 g (59.4%) | 0.9 ± 0.04 I (76.3%) |
| LSD | Interaction (T × V) : 0.46 | Interaction (T × V) : 0.35 | Interaction (T × V) : 0.21 | |||
The values represent the averages (±SE) of four independent replicates followed by different letters within columns are significantly different at P ≤ 0.05, according to Duncan’s Multiple Range test. The data in parentheses indicate percent decrease in root length, shoot length and coleoptile length of wheat genotypes in comparison to their control values.
Table 4.
Effect of different level of PEG-6000 induced osmotic stress on root and shoot dry weight (mg) of wheat genotypes.
| Osmotic Stress | Root Dry Weight (mg) |
Shoot Dry Weight (mg) |
Root-Shoot Ratio |
|||
|---|---|---|---|---|---|---|
| Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | |
| Control | 188 ± 1.28 a | 131 ± 1.47c | 328 ± 3.04 a | 282 ± 2.16b | 0.574 ± 0.006c | 0.465 ± 0.007 d |
| −2 Bars | 161 ± 1.29b (14.4%) | 136 ± 1.29c (-3.8%) | 288 ± 2.11b (12.2%) | 203 ± 1.24 d (28.0%) | 0.560 ± 0.005c | 0.671 ± 0.010b |
| −4 Bars | 138 ± 0.98c (26.6%) | 116 ± 1.29 d (11.5%) | 239 ± 2.15c (27.1%) | 163 ± 2.04 e (42.2%) | 0.578 ± 0.005c | 0.713 ± 0.010b |
| −6 Bars | 113 ± 1.17 d (39.9%) | 93 ± 1.14 e (29.0%) | 202 ± 2.15 d (38.4%) | 104 ± 2.10f (63.1%) | 0.560 ± 0.006c | 0.901 ± 0.029 a |
| −8 Bars | 90 ± 1.24 e (52.1%) | 28 ± 0.65f (78.6%) | 171 ± 2.12 e (47.9%) | 83 ± 2.16 g (70.6%) | 0.527 ± 0.008 cd | 0.340 ± 0.013 e |
| LSD | Interaction (T × V) : 7.17 | Interaction (T × V) : 12.72 | Interaction (T × V) : 0.072 | |||
The values represent the averages (±SE) of four independent replicates followed by different letters within columns are significantly different at P ≤ 0.05, according to Duncan’s Multiple Range test. The data in parentheses indicate percent decrease in root dry weight, shoot dry weight and root-shoot ratio of wheat genotypes in comparison to their control values.
Table 5.
Germination inhibition percentage, root and shoot dry weight susceptibility index of wheat genotypes under different level of PEG-6000 induced osmotic stress.
| Osmotic Stress | Germination Inhibition Percentage |
Root Dry weight Susceptibility Index |
Shoot Dry Weight Susceptibility Index |
|||
|---|---|---|---|---|---|---|
| Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | |
| −2 Bars | 1.3 ± 0.31f | 5.6 ± 0.36f | 85.8 ± 0.95b | 104.3 ± 2.01 a | 88.0 ± 1.12 a | 72.0 ± 0.51b |
| −4 Bars | 11.3 ± 1.02 e | 23.8 ± 1.02 d | 73.3 ± 0.69c | 88.8 ± 1.94b | 73.3 ± 1.29b | 57.8 ± 0.31c |
| −6 Bars | 24.4 ± 1.53 d | 36.9 ± 1.53b | 60.3 ± 0.68 d | 71.0 ± 0.71c | 61.5 ± 0.34c | 37.0 ± 0.90 e |
| −8 Bars | 30.6 ± 0.51c | 46.9 ± 1.14 a | 48.3 ± 0.76 e | 21.3 ± 0.58f | 52.3 ± 1.00 d | 29.5 ± 0.91f |
| LSD | Interaction (T × V) : 4.67 | Interaction (T × V) : 6.67 | Interaction (T × V) : 5.15 | |||
The values represent the averages (±SE) of four independent replicates followed by different letters within columns are significantly different at P ≤ 0.05, according to Duncan’s Multiple Range test.
3.2. Impact of water stress on biochemical parameters
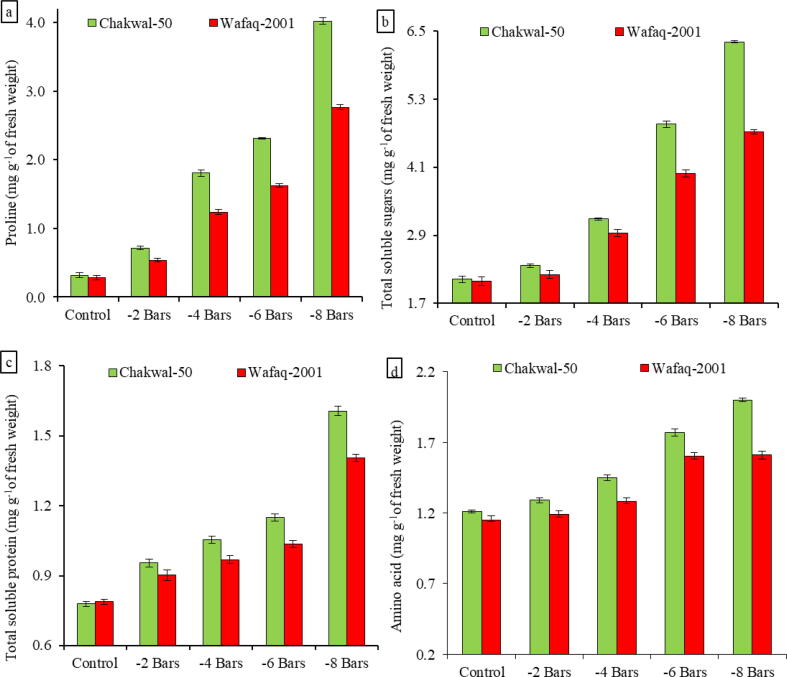
Proline content increased (92%) in Chakwal-50 and (90%) Wafaq-2001 at −8 bars of osmotic stress when compared with control (Fig. 1a). A strong increase in the levels of total soluble sugars was recorded in both genotypes. Chakwal-50 accumulated more sugar content (6.31 mg g−1) then Wafaq-2001 (4.73 mg g−1) at −8 bars of osmotic stress (Fig. 1b). Sensitive genotypes showed less increase in total soluble sugars than did tolerant genotypes. From the results, it can be concluded that Chakwal-50 is a more stress-tolerant genotype than Wafaq-2001. In the present study, an increase in total leaf protein under water stress was observed in both genotypes. Protein content increased 32% in Chakwal-50 and 25% in Wafaq-2001 at −8 bars of osmotic stress (Fig. 1c). The concentration of free amino acids increased under osmotic stress. The sensitive genotype (Wafaq-2001) showed a lower magnitude of an increase in amino acid than the tolerant one (Chakwal-50). Free amino acid content increased 40% in Chakwal-50 and 29% in Wafaq-2001 at −8 bars of osmotic stress (Fig. 1d).
Fig. 1.
Changes in (a) proline, (b) total soluble sugars, (c) total soluble protein and (d) amino acid content in Chakwal-50 and Wafaq-2001 as influenced by water deficit stress. Data are the means of three replicates with standard deviations shown by vertical bars.
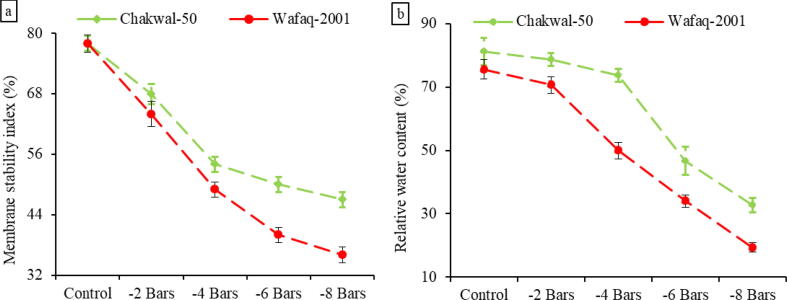
Osmotic stress caused a decline in leaf membrane stability index (MSI) and relative water content (RWC) in wheat genotypes during the stress period. (Fig. 2). Leaf membrane stability index was decreased by as much as 40% and 54% in Chakwal-50 and Wafaq-2001 respectively. During stress periods, the tolerant genotype (Chakwal-50) maintained significantly (P ≤ 0.05) higher MSI as compared to the sensitive genotype (Wafaq-2001).
Fig. 2.
Changes in (a) membrane stability index and (b) relative water content in Chakwal-50 and Wafaq-2001 as influenced by water deficit stress. Data are the means of three replicates with standard deviations shown by vertical bars.
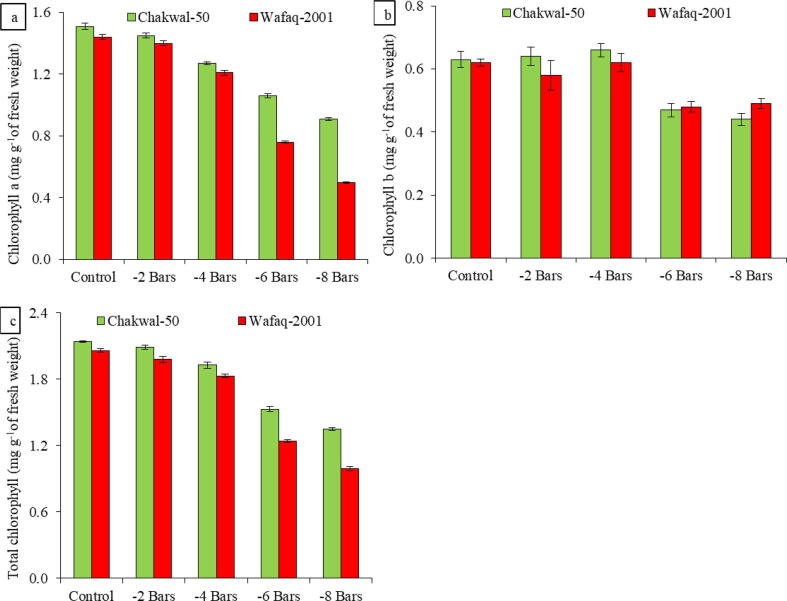
Water stress resulted in a decline in chlorophyll a, chlorophyll b, and total chlorophyll content in both wheat genotypes (Fig. 3). Chlorophyll a content decreased rapidly as osmotic stress approached −8 bars, and dropped more in Wafaq-2001 (188%) than in Chakwal-50 (66%) at that stress level, whereas chlorophyll b levels were more reduced in Chakwal-50 (43%) more than Wafaq-2001 (27%) at −8 bars. Overall total chlorophyll was reduced in both genotypes, but Wafaq-2001 exhibited more total chlorophyll reduction than Chakwal-50 at all osmotic stress levels.
Fig. 3.
Changes in (a) chlorophyll a, (b) chlorophyll b and (c) total chlorophyll content in Chakwal-50 and Wafaq-2001 as influenced by water deficit stress. Data are the means of three replicates with standard deviations shown by vertical bars.
3.3. Impact of water stress on antioxidant enzymes activity
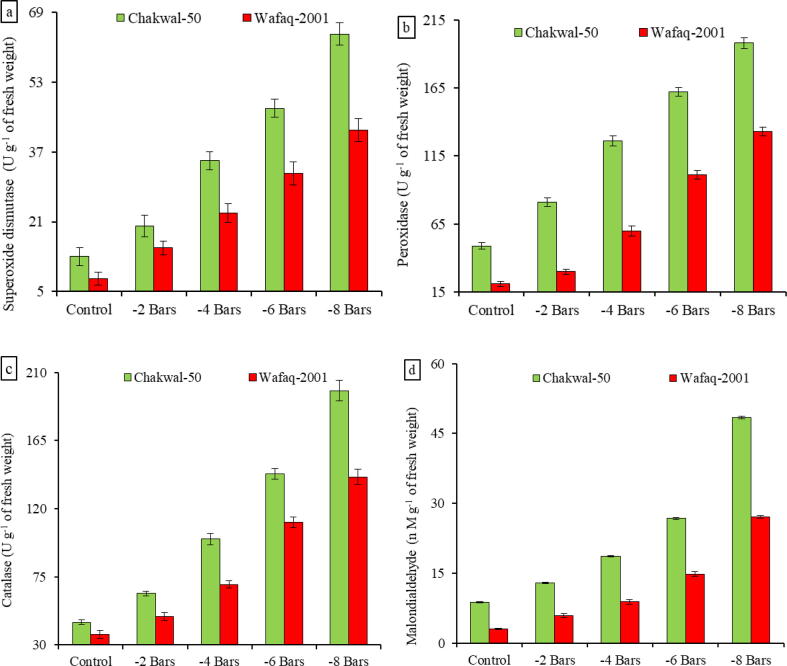
Experimental findings on antioxidant enzyme system; superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) activity and malondialdehyde (MDA) amount indicated that both wheat genotypes responded differently under control and water stress conditions (Fig. 4). The change in wheat leaves suggest that oxidative stress may be an influential component of osmotic stress. A rapid increase in the activities of SOD, POD, CAT, and MDA amounts was observed in both wheat genotypes. Superoxide dismutase activity increased by 80% in Chakwal-50 and 81% in Wafaq-2001. Mean comparisons indicated that POD content under water deficit conditions was higher than in the optimum non-stressed conditions. Peroxidase activities also increased by 61% in Chakwal-50 and 65% in Wafaq-2001. Water-stress induced an increase in CAT activities. Catalase activity increased by 58% in Chakwal-50 and 53% in Wafaq-2001. Malondialdehyde (MDA) content increased by 82% in Chakwal-50 and 89% in Wafaq-2001.
Fig. 4.
Changes in the enzyme activities of (a) superoxide dismutase = SOD, (b) peroxidase = POD, (c) catalase = CAT and (d) malondialdehyde = MDA content in Chakwal-50 and Wafaq-2001 as influenced by water deficit stress. Data are the means of three replicates with standard deviations shown by vertical bars.
3.4. Impact of water deficit stress on physiological parameters
In the study here, an increase in water-deficit stress was accompanied by a decrease in photosynthesis rate and transpiration rate in both genotypes (Table 6). Maximum photosynthesis rate (35.7 μM m−2 sec-1) was recorded in Chakwal-50 at 80% soil water holding capacity whereas minimum photosynthesis rate (21.8 μM m−2 sec-1) was recorded in Wafaq-2001 at 20% soil water holding capacity. In Chakwal-50 photosynthesis rate decreased from 35.7 μM m−2 sec-1 (80% soil water capacity) to 23.8 μM m−2 sec-1 (20% soil water capacity), whereas in Wafaq-2001 photosynthesis rate decreased from 31 μM m−2 sec-1 (80% soil water capacity) to 21.8 μM m−2 sec-1 (20% soil water capacity).
Table 6.
Photosynthesis rate, transpiration rate, photosynthetic water use efficiency and stomatal conductance under various levels of potted soil water holding capacity.
| Field Capacity Levels | Photosynthesis Rate (μM m−2sec-1) |
Transpiration Rate (mM m−2sec-1) |
Photosynthetic Water Use Efficiency |
Stomatal Conductance (M m−2sec-1) |
||||
|---|---|---|---|---|---|---|---|---|
| Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | |
| 80% | 35.7 ± 0.48 a | 31 ± 0.48 bc | 8.8 ± 0.12 a | 7.2 ± 0.12b | 4.1 ± 0.11b | 4.3 ± 0.14b | 0.93 ± 0.03 a | 0.82 ± 0.01f |
| 60% | 32.8 ± 0.45b | 28.8 ± 0.48 cd | 8.3 ± 0.09 a | 6.9 ± 0.08b | 3.9 ± 0.07b | 4.2 ± 0.03b | 0.82 ± 0.03 ab | 0.97 ± 0.01 cd |
| 40% | 27.8 ± 0.50 d | 24.1 ± 0.42 e | 7.5 ± 0.16b | 5.8 ± 0.17c | 3.7 ± 0.15b | 4.2 ± 0.20b | 0.68 ± 0.03b | 1.03 ± 0.01b |
| 20% | 23.8 ± 0.41 e | 21.8 ± 0.45 e | 5.4 ± 0.18c | 4.1 ± 0.17 d | 4.5 ± 0.22b | 5.4 ± 0.12 a | 0.42 ± 0.03c | 1.08 ± 0.01 a |
| LSD | Interaction (T × V) : 2.49 | Interaction (T × V) : 0.80 | Interaction (T × V) : 0.80 | Interaction (T × V) : 0.20 | ||||
The values represent the averages (±SE) of four independent replicates followed by different letters within columns are significantly different at P ≤ 0.05, according to Duncan’s Multiple Range test.
The transpiration rate in both genotypes was significantly different. Chakwal-50 maintained higher transpiration rates at all levels of soil water capacity when compared to Wafaq-2001. The maximum transpiration rate (8.8 mM m−2 sec-1) was recorded at 80% soil water capacity in Chakwal-50 whereas the minimum transpiration rate (4.1 mM m−2 sec-1) was recorded at 80% soil water holding capacity in Wafaq-2001.
In Chakwal-50, stomatal conductance decreased from 0.93 M m−2 sec-1 (80% soil water holding capacity) to 0.42 M m−2 sec-1 (20% soil water holding capacity), whereas in Wafaq-2001 stomatal conductance decreased from 0.71 M m−2 sec-1 (80% soil water holding capacity) to 0.22 M m−2 sec-1 (20% soil water holding capacity). Stomatal conductance and the uptake of CO2 are reduced by water deficit, which ultimately affects the growth and yield of crop plants (Scheuermann et al., 1991).
Changing levels of soil water-holding capacity significantly affected leaf osmotic potential, leaf water potential, leaf cuticular wax amounts, and relative water content of both genotypes (Table 7). Increases in water stress resulted in a decline in osmotic potential, water potential, and relative water contents of the leaves. Leaf osmotic potential in both varieties was significantly different. The highest leaf osmotic potential (-0.82 MPa) was recorded at 80% soil water holding capacity and the lowest (-1.08 MPa) was recorded at 20% sol water capacity in Chakwal-50. The highest leaf water potential (-0.29 MPa) was recorded at 80% soil water holding capacity and lowest leaf water potential (-1.51 MPa) was recorded at 20% field capacity in Wafaq-2001.
Table 7.
Leaf osmotic potential, leaf water potential, leaf cuticular wax and relative water content under various levels of potted soil water holding capacity.
| Field Capacity Levels | Leaf Osmotic Potential (-MPa) |
Leaf Water Potential (-MPa) |
Cuticular Wax Content (μg m−2) |
Relative Water Content (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | |
| 80% | 0.82 ± 0.01f | 0.80 ± 0.03f | 0.30 ± 0.03 g | 0.29 ± 0.03 g | 149 ± 5.29 e | 97.7 ± 8.04f | 87.7 ± 1.71 a | 81.7 ± 1.68b |
| 60% | 0.97 ± 0.01 cd | 0.89 ± 0.03 e | 0.61 ± 0.02f | 0.82 ± 0.02 e | 234 ± 7.86c | 145.7 ± 5.00 e | 81 ± 0.33b | 71 ± 0.33 cd |
| 40% | 1.02 ± 0.01b | 0.94 ± 0.03 de | 0.93 ± 0.02 d | 1.31 ± 0.01b | 326 ± 9.29b | 200.3 ± 5.00 d | 74 ± 0.33c | 62 ± 0.33 e |
| 20% | 1.08 ± 0.01 a | 1 ± 0.03 bc | 1.15 ± 0.02c | 1.51 ± 0.02 a | 400.7 ± 5.50 a | 298.3 ± 6.08b | 69 ± 0.33 d | 56 ± 0.33f |
| LSD | Interaction (T × V) : 0.05 | Interaction (T × V) : 0.07 | Interaction (T × V) : 33.01 | Interaction (T × V) : 4.56 | ||||
The values represent the averages (±SE) of four independent replicates followed by different letters within columns are significantly different at P ≤ 0.05, according to Duncan’s Multiple Range test.
An increase in water stress was accompanied by an increase in total cuticular wax amount. Maximum cuticular wax load (400.7 μg m−2) was recorded at 20% soil water holding capacity in Chakwal-50 and minimum cuticular wax load (97.7 μg m−2) was recorded at 80% soil water holding capacity in Wafaq-2001.
Different levels of soil water-holding capacity significantly affected relative water contents of these wheat genotypes, and an increase in water deficit stress was accompanied by linear decreases in RWC. Maximum RWC (87.7%) was recorded at 80% field capacity in Chakwal-50 and minimum RWC (56%) was recorded at 20% field capacity in Wafaq-2001.
3.5. Impact of water stress on yield and yield attributes
Water stress has a significant effect on the number of grains spike-1, 1000 grain weight and grain yield plant−1 (Table 8). Maximum number of grains spike-1 (29.7) was recorded in Chakwal-50 at 80% soil water holding capacity and minimum number of grain spike-1 (26.6) was recorded at 20% field capacity in Wafaq-2001. A decline in 1000 grain weight was also recorded with the increase in water stress levels. Highest 1000 grain weight (38.4 g) was recorded in Chakwal-50 at 80% soil water holding capacity and minimum 1000 grain weight (27.5 g) was recorded at 20% field capacity in Wafaq-2001. Grain yield plant−1 also decreased significantly with the increase in the water stress. Maximum grain yield plant−1 (4.1 g) was recorded in Chakwal-50 at 80% soil water holding capacity and minimum grain yield plant−1 (2.6 g) was recorded at 20% soil water holding capacity in Wafaq-2001.
Table 8.
Number of grains spike-1, 1000 grain weight and grain yield plant−1 under various levels of potted soil water holding capacity.
| Field Capacity Levels | Number of grains spike-1 |
1000 grain weight (g) |
Grain yield Plant−1 (g) |
|||
|---|---|---|---|---|---|---|
| Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | Chakwal-50 | Wafaq-2001 | |
| 80% | 29.7 ± 0.09 a | 28.7 ± 0.22 abc | 38.4 ± 0.11 a | 36.1 ± 0.12b | 4.1 ± 0.13 a | 3.8 ± 0.08 ab |
| 60% | 29.4 ± 0.15 ab | 28.5 ± 0.11 bc | 37.6 ± 0.19 a | 35 ± 0.16c | 3.9 ± 0.03 a | 3.5 ± 0.08 bc |
| 40% | 28.9 ± 0.22 ab | 27.8 ± 0.29 cd | 35 ± 0.17c | 33.2 ± 0.20 d | 3.4 ± 0.04 cd | 3.1 ± 0.07 de |
| 20% | 27.1 ± 0.16 de | 26.6 ± 0.20 e | 33.2 ± 0.23 d | 27.5 ± 0.22 e | 2.7 ± 0.08 ef | 2.6 ± 0.07f |
| LSD | Interaction (T × V) : 1.05 | Interaction (T × V) : 0.87 | Interaction (T × V) : 0.35 | |||
The values represent the averages (±SE) of four independent replicates followed by different letters within columns are significantly different at P ≤ 0.05, according to Duncan’s Multiple Range test.
4. Discussion
The two genotypes selected for this study are well-established varieties used in commercial wheat production, and they are known to have differences in their growth and drought response, with Chakwal-50 commonly reported as being more drought tolerant (21). However, research studies to verify its superior drought tolerance, and elucidate the basis for its excellent performance have not been conducted. Our results here show that for a variety of growth-related traits, increasing osmotic stress had a much more severe negative effect on Wafaq-2001 than on Chakwal-50 wheat, for traits such as germination percentage, root length, shoot length, root dry weight, shoot dry weight, and the inhibition of germination. Both Wafaq-2001 and Chakwal-50 plants exhibited osmotic adjustment by synthesizing and accumulating compatible solutes such as free amino acids, although Chakwal-50 expressed improved osmotic adjustment for the osmolytes free amino acid, soluble sugars, and free protein. Hammad and Ali (2014) also reported that free amino acid amounts increased under comparable water-deficit stress conditions, and that a higher osmotic response was associated with improved drought tolerance, and an important adaptive advantage.
Relative water content is probably the most meaningful measure of plant water status in terms of the physiological consequence of cellular water deficit. Relative water content significantly decreased with the increase in osmotic stress in both varieties, with relative water content decreasing as much as 29% in Chakwal-50 and 43% in Wafaq-2001, respectively, and this more severe response for Wafaq-2001 occurred at all levels of osmotic stress examined. Relative water content is an indicator of water status (or availability) within the plant, and any reduction in RWC can cause loss of turgidity, which in turn affects cell size and shape of plants, and itself induces many metabolic responses (Siddique et al., 2000). Our results are in line with the findings of Qaseem et al. (2019) that MSI and RWC decreased with the increase in water-deficit stress.
Chlorophyll is the main component of photosynthesis, and one of the physiological processes most sensitive to environmental stress (Hussain et al., 2019). Similar to high temperature, water deficiency may induce lipid peroxidase and electrolytic leakage from chloroplast and thylakoid membranes, leading to a loss of chlorophyll content (Djanaguiraman et al., 2010). The rate of decrease in chlorophyll content, and leaf membrane stability index, under water-deficient conditions differed significantly between the genotypes under study, indicating possible genetic variability for this trait such that Chakwal-50 demonstrated much more tolerance to osmotic stress than Wafaq-2001 wheat.
In general, the antioxidant enzyme content of leaves is increased with the decline in water availability, suggesting that the production of antioxidant enzymes is probably a common response of plants to drought stress conditions. The activity of antioxidant enzymes is generally increased during abiotic stress, and this is correlated with enhancing cellular protection. Increased activity of antioxidant enzymes may also be responsible delayed leaf senescence. Moreover, the active oxygen-induced damage to the cell may be minimized or prevented by increased antioxidant activities, but this protection may not be enough under more severe drought stress. The scarcity of water is a severe environmental constraint to plant productivity. Drought-induced loss in crop yield probably exceeds losses from all other causes annually, and both the severity and duration of the stress can be critical (Djanaguiraman et al., 2010). MDA is regarded as a marker for the evaluation of lipid peroxidation and/or damage to plasmalemma and organelle membranes, and this increases with environmental stress. In particular, lipid peroxidation is linked to the activity of antioxidant enzymes (e.g. with the increase of SOD, POD and CAT) (Esfandiari et al., 2007). While our results revealed only small differences between the lines examined, there was a trend toward a greater increase in antioxidants in Wafaq-2001 than in Chakwal-50. Whether this indicated a stronger stress response for this trait in the more drought susceptible Wafaq-50, and/or whether this more accurately indicates that Wafaq-50 is actually experiencing more stress in its tissues, is still uncertain.
Progressive reduction in soil water-holding capacity with depleting water supply increasingly impacts crop photosynthesis rate, transpiration rate, and photosynthetic water use efficiency (Ahmed et al., 2010). In this study, we observed reduced photosynthesis and transpiration rates in both genotypes, with both showing stomatal closure. However, the impacts were more severe on Wafaq-50, consistent with its higher susceptibility to drought. Drought is abiotic stress which can affect plant growth and development at various stages in its life cycle (Blum, 1996). The response of plants to drought stress is a highly complex phenomenon as it integrates the effects of various stress levels, and plant response at various levels of organization (i.e. cells and organs). The perception of drought stress at the plant level is indicated by a decline in photosynthesis rate and overall plant growth (Blum, 1996). In field crop production situations, another reason for decreased photosynthetic rate may be the decreased leaf water potential and relative water content that can occur under water deficit due to sub-optimal irrigation, which then has a pronounced effect on photosynthetic rate and yield.
Plant transpiration is a physical process in which part of the net radiation energy is converted into latent heat, under physiological control by changes in stomatal aperture (Jarvis and McNaughton, 1986). The effect of water stress on the transpiration rate may vary. The results presented herein are consistent with findings of other scientist who reported that transpiration rate decreases with increasing water stress (Olszewski et al., 2009, Ahmed et al., 2010). Researchers have previously reported that a decline in photosynthesis typically follows an increase in water deficit, and there are variations in the way stomatal and non-stomatal responses occur in different plant genotypes (Shangguan et al., 1999). The photosynthetic rate is determined by several factors of which the most important is the availability of water during different stages of the life cycle. As a short-term reduction in water availability, plants showed stomatal limitations whereas long-term stress from sub-optimal water availability causes non-stomatal limitation (Cornic and Briantais, 1991). The results were present here are similar to the findings reported by other scientists that stomatal conductance declines with a rise in water deficit (Olszewski et al., 2009, Ahmed et al., 2010). Whether active or passive, the accumulation of solutes has been shown to support expansion growth, maintain photosynthesis, and improve reproductive success under severe drought conditions in a number of plant systems (Westgate, 2008).
The role of the cuticle, or cuticle membrane, that cover the aerial portion of leaves and other plant tissues has been shown to inhibit water loss due to the presence of the cuticle’s hydrophobic intra- and epi-cuticular waxes (Mamrutha et al., 2010, Xu et al., 2014). Johnson et al. (1983) observed that elevated glaucousness (leaf waxiness) resulted in decreased transpiration with enhanced yield, as well as improved water use efficiency. Bengston et al. (1978) reported that elevated cuticular wax conferred drought tolerance in oat varieties. One of the important factors in determining plant drought tolerance is the genetic variations in cuticular wax amount, composition, and crystalline structure. Such variations in cuticular wax of wheat plants were reported (Johnson et al., 1983). Our findings that water deficit treatments led to increased wax amounts on wheat leaves are consistent with the works of other scientists (Johnson et al., 1983, Clarke and Richards, 1988).
Numerous studies have shown that the capacity to maintain high RWC during mild drought is indicative of drought tolerance, and may translate to high drought tolerance under more severe conditions (Colom and Vazzana, 2003). Relative water content is linked with cell size and may establish a balance between transpiration rate and water supply to the leaf (Fischer and Wood, 1979), and osmotic adjustment by plants is an influential mechanism which conserves cellular hydration under drought. The ability to maintain high RWC serves as a tolerance mechanism to tackle increasing climatological drought, which is often associated with osmotic regulation, increasing cuticular wax load (to conserve more water), and a reduced elasticity of tissue cell wall (Ritchie et al., 1990). Siddique et al. (2000) reported that relative water content reduced to 43% from 88% by moisture deficit stress in wheat cultivars, and these findings are in agreement with the findings presented here, and those of others (Keyvan, 2010).
We observed that drought caused a reduction in spike length, number of grains spike-1, 1000 grain weight and grain yield plant−1 in both of the wheat varieties examined here, results that are in agreement with the findings of Bayoumi et al. (2008). In a field study, Blum and Pnuel (1990) reported that yield and yield components of wheat varieties were significantly decreased when they received suboptimal annual precipitation. The reason for lower grain yield under stressed conditions was mainly due to reduction in the number of spikes plant−1 and number of grains spike-1. Drought stress at any growth stage was shown to reduce grain yield in these experiments. The decrease in 1000-grains weight under stressed conditions may be due to photosynthetic translocation within the plant (Iqbal et al., 1999), conditions that produced shriveled grains due to hastened maturity. This is possibly due to the shortage of moisture which induced the plants to complete their grain formation in relatively shorter time period (Riaz and Chowdhry, 2003). Under drought conditions, the availability of current assimilates for extending seed filling will often be severely reduced. In such circumstances, the variety that can better mobilize reserves of carbohydrates from the stem to filling seed will be able to produce a higher yield.
5. Conclusion
Water deficit treatments had substantial effects on germination and vegetative growth traits of wheat genotypes. Proline, total soluble sugar, proteins, and amino acids had a direct association, whereas total chlorophyll content, seedling fresh and dry weight had an inverse association with water deficit stress. Photosynthesis rate, transpiration rate, stomatal conductance, relative water content, and osmotic potential declines with rise in water stress, whereas wax load accumulated on water stress wheat, presumably these as part of a response to increase drought tolerance. Ample genetic dissimilarity existed in both wheat genotypes for drought tolerance. However, Chakwal-50 may be considered a superior cultivar for low rainfall and irrigation cropping systems.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to acknowledge the support of Prince Sultan University for paying the Article Processing Charges of this publication.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abdul Qayyum, Email: aqayyum@uoh.edu.pk.
Ahmad Sher, Email: ahmad.sher@bzu.edu.pk.
References
- Aebi H. Catalase In vitro. Methods in Enzymology. 1984;105:121–127. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Hassan F.U., Aslam M., Akram M.N., Aslam M.A. Photosynthesis of spring wheat (Triticum aestivum) in rainfed ecology of Pakistan. African Journal of. Biotechnology. 2010;9(44):7495–7503. [Google Scholar]
- Ali M.A., Abbas A., Niaz S., Zulkiffal M., Ali S. Morpho-physiological criteria for drought tolerance in Sorghum (Sorghum bicolor) at seedling and post-anthesis stages. International Journal of Agriculture and Biology. 2009;11(6):674–680. [Google Scholar]
- Allen R.D. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiology. 1995;107:1049–1057. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D.I. Copper, enzyme in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R., Irigoyen J.J., Sánchez-Díaz M. Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiologia Plantarum. 2003;117(4):540–549. doi: 10.1034/j.1399-3054.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- Asseng S., Ewert F., Martre P., Rötter R.P., Lobell D.B., Cammarano D., Kimball B.A., Ottman M.J., Wall G.W., White J.W., Reynolds M.P. Rising temperatures reduce global wheat production. Nature climate change. 2015;5(2):143–147. [Google Scholar]
- Baker A.T., Brichl M.M., Dorr R., Powis G. Decreased antioxidant defence and increased oxidant stress during dexamethasone-induced apoptosis: bcl-2 prevents the loss of antioxidant enzyme activity. Cell Death Differentiation. 1996;3:207–213. [PubMed] [Google Scholar]
- Barrs H.D., Weatherley P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Australian journal of biological. 1962 sciences15:413–428. [Google Scholar]
- Bartoli C.G., Simontacchi M., Tambussi E., Beltrano J., Montaldi E., Puntarulo S. Drought and watering dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. Journal of Experimental Botany. 1999;50:375–383. [Google Scholar]
- Bates L.S., Waldran R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant and Soil. 1973;39:205–208. [Google Scholar]
- Bayoumi T.Y., Eid M.H., Metwali E.M. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. African Journal of Biotechnology. 2008;7(14):2341–2352. [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: Improved assay and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bengston C., Larsson S., Lilienberg C. Effects of water stress on cuticular transpiration rate and amount and composition of epicuticular wax in seedlings of six oat varieties. Physiologia Plantarum. 1978;44:319–324. [Google Scholar]
- Blum A. Crop response to drought and the interpretation of adaptation. Plant Growth Regulation. 1996;20:135–148. [Google Scholar]
- Blum A., Pnuel Y. Physiological attributes associated with drought resistance on wheat cultivars in a Mediterranean environment. Australian Journal of Agricultural Research. 1990;41:799–810. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;172:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke J.J., Mahan J.R. Environmental regulation of cellular protection systems. In Casadebaig, P., P. Debaeke, J. Lecoeur. 2008. Thresholds for leaf expansion and transpiration response to soil water deficit in a range of sunflower genotypes. European Journal of Agronomy. 1991;28:646–654. [Google Scholar]
- Çakir R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crop Res. 2004;89:1–16. [Google Scholar]
- Capell, B. and Doerffling. 1993. Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiologia Plantarum 88:638-646. [DOI] [PubMed]
- Clarke J.M., Richards R.A. The effect of glaucousness, epicuticular wax, leaf age, plant height and growth environment on water loss rates of excised wheat leaves. Canadian Journal of Plant Science. 1988;68:975–982. [Google Scholar]
- Colom M.R., Vazzana C. Photosynthesis and PS-II functionality of drought-resistant and drought sensitive weeping love grass plants. Environmental and Experimental Botany. 2003;49:135–144. [Google Scholar]
- Cornic G., Briantais J.M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta. 1991;183:178–184. doi: 10.1007/BF00197786. [DOI] [PubMed] [Google Scholar]
- Dhindsa R.S., Matowe W. Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. Journal of Experimental Botany. 1981;32:79–91. [Google Scholar]
- Djanaguiraman M., Prasad P.V.V., Seppanen M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiology and Biochemistry. 2010;48:999–1007. doi: 10.1016/j.plaphy.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Dubois M., Gilles K., Hammiltron J.K., Robers P.A., Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167–168. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- ElBasyoni I., Saadalla M., Baenziger S., Bockelman H., Morsy S. Cell Membrane Stability and Association Mapping for Drought and Heat Tolerance in a Worldwide Wheat Collection. Sustainability. 2017;9:1606. [Google Scholar]
- Esfandiari, E., F. Shekari1, F. Shekari and M. Esfandiari1. 2007. The effect of salt stress on antioxidant enzymes activity and lipid peroxidation on the wheat seedling. Notulae Botanicae Horti Agrobotanici Cluj 31(1):48-56.
- Fischer R.A., Wood J.T. Drought resistance in spring wheat cultivars. III. Yield association with morpho-physiological traits. Australian Journal of Agricultural Research. 1979;30:1001–1020. [Google Scholar]
- Hamilton P.B., Van Slyke D.D. Amino acid determination with Ninhydrin. Journal of Biological Chemistry. 1943;150:231–233. [Google Scholar]
- Hammad S.A.R., Ali O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Annals of Agricultural Sciences. 2014;59(1):133–145. [Google Scholar]
- Han H., Tian Z., Fan Y., Cui Y., Cai J. Water-deficit treatment followed by re-watering stimulates seminal root growth associated with hormone balance and photosynthesis in wheat (Triticum aestivum L.) seedlings. Plant Growth Regulation. 2015;77:201–210. [Google Scholar]
- Herbinger K., Tausz M., Wonisch A., Soja G., Sorger A., Grill D. Complex interactive effects of drought and ozone stress on the antioxidant defense systems of two wheat cultivars. Plant Physiology and Biochemistry. 2002;40:691–696. [Google Scholar]
- Hsiao T.C. Plant responses to water stress. Annual Review of Plant Physiology. 1973;24:519–570. [Google Scholar]
- Hussain, H.A., S. Men, S.Y. Hussain, S. Chen, S. Ali, K. Zhang, Y. Zhang, Q. Li, C. Xu, Liao and L. Wang. 2019. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Scientific Reports 9:3890. https://doi.org/10.1038/s41598-019-40362-7 [DOI] [PMC free article] [PubMed]
- Iqbal M., Ahmed K., Ahmed I., Sadiq M., Ashraf M. Yield and yield components of durum wheat as influenced by water stress at various growth stages. Pakistan Journal of Biological Sciences. 1999;2:11–14. [Google Scholar]
- Izanloo A., Condon A.G., Langridge P., Tester M., Schnurbusch T. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. Journal of Experimental Botany. 2008;59:3327–3346. doi: 10.1093/jxb/ern199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P.J., McNaughton K.G. Vol. 15. Academic Press; London: 1986. Stomatal control of transpiration: scaling up from leaf to region; pp. 86–112. (Advances in Ecological Research). [Google Scholar]
- Johnson D.A., Richards R.A., Turner N.C. Yield, water relations and surface reflectance of near isogenic wheat lines differing in glaucousness. Crop Science. 1983;23:318–325. [Google Scholar]
- Keyvan S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. Journal of Animal and Plant Science. 2010;8(3):1051–1060. [Google Scholar]
- Long S.P., Bernacchi C.J. Gas exchange measurements, what they can tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany. 2003;54:2393–2401. doi: 10.1093/jxb/erg262. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Robebrogh N.J., Farr A.L., Randall R.J. Protein measurement with the folin-phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Luck, H. 1963. Catalase. In: Methods of Enzymatic Analysis. (Ed.): H.U. Bergmeyer. Verlag Chemie: Wernheim, Germany, pp. 885-894.
- Mamrutha H.M., Mogili T., Lakshmi K.J., Rama N., Kosma D., Kumar M.U., Jenks M.A., Nataraja K.N. Leaf cuticular wax amount and crystal morphology regulate post-harvest water loss in mulberry (Morus species) Plant Physiology and Biochemistry. 2010;48(8):690–696. doi: 10.1016/j.plaphy.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Michel B.E., Kaufmann M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiology. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Y.I.J., Jian Hua Z. Abscisic acid and antioxidant defense in plant cells. Acta Botanica Sinica. 2004;46:1–9. [Google Scholar]
- Mundree S.G., Baker B., Mowla S., Peters S., Marais S., Willigen C.V., Govender K., Maredza A., Muyanga S., Farrant J.M., Thomson J.A. Physiological and molecular insights into drought tolerance. African Journal of Biotechnology. 2002;1(2):28–38. [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology. 1962;15:473–497. [Google Scholar]
- Olszewski J., Pszczółkowska A., Makowska M., Kulik T., Okorski A. Effect of water deficit on gas exchange parameters, productivity and grain wholesomeness of spring wheat. Polish Journal of Natural Science. 2009;24(2):85–92. [Google Scholar]
- Premachandra G.S., Saneoka H., Ogata S. Cell membrane stability, an indicator of drought tolerance as affected by applied nitrogen in soybean. Journal of Agricultural Science Cambridge. 1990;115:63–66. [Google Scholar]
- Qaseem M.F., Qureshi R., Shaheen H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse Wheat (Triticum aestivum L.). genotypes varying in sensitivity to heat and drought stress. Scientific Reports. 2019;9(6955) doi: 10.1038/s41598-019-43477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayyum A., Razzaq A., Ahmad M., Jenks M.A. Water stress causes differential effects on germination indices, total soluble sugar and proline content in wheat (Triticum aestivum L.) genotypes. African Journal of Biotechnology. 2011;10(64):14038–14045. [Google Scholar]
- Reddy A.R., Chaitanya K.V., Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Riaz R., Chowdhry M. Genetic analysis of some economic traits of wheat under drought condition. Asian Journal of Plant Sciences. 2003;2:790–796. [Google Scholar]
- Ritchie S.W., Nguyan H.T., Holaday A.S. Leaf water content and gas exchange parameters of two wheat genotypes differing in drought resistance. Crop Science. 1990;30:105–111. [Google Scholar]
- Robinson J.M., Bunce J.A. Influence of drought-induced water stress on soybean and spinach leaf ascorbate-dehydroascorbate level and redox status. International Journal of Plant Sciences. 2000;161:271–279. doi: 10.1086/314257. [DOI] [PubMed] [Google Scholar]
- Sadeghi H., Khazaei F., Yari L., Sheidaei S. Effect of seed osmopriming on seed germination behavior and vigor of soybean (Glycine max) ARPN Journal of Agricultural and Biological Science. 2011;6(1):39–43. [Google Scholar]
- Sairam R.K. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian Journal of Experimental Biology. 1994;32:594–597. [Google Scholar]
- Sallam A., Alqudah A.M., Dawood M.F.A., Baenziger P.S., Börner A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. International Journal of Molecular Sciences. 2019;20(13):3137. doi: 10.3390/ijms20133137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann R., Biehler K., Stuhlfauth T., Fock H.P. Simultaneous gas exchange and fluorescence measurements indicate differences in the responses of sunflower, bean and maize to water stress. Photosynthesis Research. 1991;27:189–197. doi: 10.1007/BF00035840. [DOI] [PubMed] [Google Scholar]
- Scholander P.F., Hammel H.T., Bradstreet E.D., Hemmingsen E.A. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Shangguan Z., Shao M., Dyckmans J. Interaction of osmotic adjustment and photosynthesis in winter wheat under soil drought. Journal of Plant Physiology. 1999;154:753–758. [Google Scholar]
- Shiferaw B., Smale M., Braun H.J., Duveiller E., Reynolds M., Muricho G. Crops that feed the world 10 past successes and future challenges to the role played by wheat in global food security. Food Security. 2013;5:291–317. [Google Scholar]
- Siddique M.R., Hamid B.A., Islam M.S. Drought stress effects on water relations of wheat. Botanical Bulletin of Academia Sinica. 2000;41:35–39. [Google Scholar]
- Silva-Fernandes A.M., Baker E.A., Martin J.T. Studies on plant cuticle 1V. The isolation and fractionation of cuticular waxes. Annals of Applied Biology. 1964;53:43–58. [Google Scholar]
- Soltys-Kalina D., Plich J., Strzelczyk-Żyta D., Śliwka J., Marczewski W. The Effect of Drought Stress on the Leaf Relative Water Content and Tuber Yield of a Half-Sib Family of 'Katahdin'-derived Potato Cultivars. Breeding Science. 2016;66(2):328–331. doi: 10.1270/jsbbs.66.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza R.P., Machado E.C., Silva J.A.B., Lagôa A.M.M.A., Silveira J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environmental and Experimental Botany. 2004;51:45–56. [Google Scholar]
- Steel R.G.D., Torrie J.H., Dickey D.A. 3rd ed. McGraw Hill Book Co.; Inc. New York: 1997. Principles and procedures of statistics: A biometrical approach; pp. 400–428. [Google Scholar]
- Stewart R.R.C., Bewley J.D. Lipid peroxidation associated aging of soybean axes. Plant Physiology. 1980;65:245–248. doi: 10.1104/pp.65.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk S., Feierabend J. Photoinactivation of catalase at low temperature and its relevance to photosynthetic and peroxide metabolism in leaves. Plant, Cell & Environment. 1989;12:701–712. [Google Scholar]
- Westgate M.E. Plants Osmotic Potential. Encyclopedia of Water Science. 2nd Edition. 2008;1(1) [Google Scholar]
- Xu X., Feng J., Lü S., Lohrey G.T., An H., Zhou Y., Jenks M.A. Leaf cuticular lipids on the Shandong and Yukon ecotypes of saltwater cress, Eutrema salsugineum, and their response to water deficiency and impact on cuticle. Physiologia Plantarum. 2014;151(4):446–458. doi: 10.1111/ppl.12127. [DOI] [PubMed] [Google Scholar]
- Yi X.P., Zhang Y.L., Yao H.S., Luo H.H., Gou L., Chow W.S., Zhang W.F. Rapid recovery of photosynthetic rate following soil water deficit and re-watering in cotton plants (Gossypium herbaceum L.) is related to the stability of the photosystems. Journal of Plant Physiology. 2016;193:23–34. doi: 10.1016/j.jplph.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Yu T.F., Xu Z.S., Guo J.K., Wang Y.X., Abernathy B., Fu J.D., Chen X., Zhou Y.B., Chen M., Guo X.G., Ma Y.Z. Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Scientific Reports. 2017;7:44050. doi: 10.1038/srep44050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.X., Kirkham M.B. Water relations of water stressed, splitroot C4 (Sorghum bicolor; Poaceae) and C3 (Helianthus annuus; Asteraceae) plants. American Journal of Botany. 1995;82:1220–1229. [Google Scholar]