Abstract
In marine ecosystems, fluctuations in surface-seawater carbon dioxide (CO2), significantly influence the whole metabolism of marine algae, especially during the early stages of macroalgal development. In this study, the response of the green alga Ulva fasciata for elevating ocean acidification was investigated using four levels of pCO2 ~ 280, 550, 750 and 1050 µatm. Maximum growth rate (6.6% day−1), protein (32.43 %DW) and pigment (2.9 mg/g) accumulation were observed at pCO2-550 with an increase of ~2-fold compared to control. On the other hand, lipid and carbohydrate contents recorded their maximum production (4.23 and 46.96 %DW, respectively) at pCO2-750 while control showed 3.70 and 42.37 %DW, respectively. SDS-PAGE showed the presence of unique bands in response to pCO2, especially at 550 µatm. Dominant associated bacteria was shifted from Halomonas hydrothermalis of control to Vibrio toranzoniae at pCO2-1050. These findings suggest that ocean acidification at 550 µatm might impose noticeable effects on growth, protein, pigments, and protein profile of U. fasciata, which could be a good source for fish farming. While, pCO2-750 was recommended for energetic purpose, due to its high lipid and carbohydrate contents.
Keywords: Ulva fasciata, pCO2 levels, Growth, Microbiota, Protein profile
1. Introduction
Future climate forecasts expect the continuous raising of atmospheric CO2 concentrations (pCO2) due to the anthropogenic activities and exceed ~600 ppm by the year 2100 under the “business as usual” scenario (Pachauri et al., 2014). Oceans are one of the leading sinks of this CO2 increase as they can absorb over 25 million tons of anthropogenic CO2 daily, causing unprecedented changes to ocean chemistry (IPCC, 2007). Raised ocean CO2 concentrations modify the speciation of dissolved inorganic carbon in seawater and reduce pH by the carbonate buffer system, along with varying abilities of macrophytes to use CO2 and HCO3. By the end of the millennium, the pH of the ocean surface from a pre-industrial value (8.2) to 7.4 (Caldeira and Wickett, 2003). When pH is shifted, the carbon speciation in seawater is changed, which has strong consequences for photosynthesis, respiration, and calcification metabolism.
Additionally, these same three metabolic processes themselves change the pH of the surrounding seawater of the algae. Therefore, these changes have serious pressure on algae, including macroalgae (Kinnby et al., 2021, Xiao et al., 2021) or microalgae (Pourjamshidian et al., 2019). According to the European Water Framework Directive (EWFD) 2000/60/CE, the algal community is considered an essential indicator of anthropogenic stresses in water ecosystems since it might alter the composition of their community, leading to the change or disappearance of some species (Baggini et al., 2014, Elshobary et al., 2020b, Han et al., 2020).
Seaweeds might benefit from rising CO2 through increased photosynthesis and carbon acquisition, with subsequent greater growth rates (Aires et al., 2018, Cornwall and Hurd, 2020, Mackey et al., 2015). Unlike photosynthesis, other metabolic processes, such as ion homeostasis, respiration, nutrient uptake and enzyme activity, are suppressed by ocean acidification conditions (Fernández et al., 2015, Gutow et al., 2014, Hofmann et al., 2013). This shifting in algal metabolism may promote modifications in seaweed chemistry and change the dietary quality of tissue for grazers. In addition, association microbiota can also be influenced by environmental changes, with feedback results (Aires et al., 2018).
The Egyptian coastline of the Mediterranean (Elshobary et al., 2020a, Khairy and El-Sheikh, 2015, Osman et al., 2012), and Red Sea coasts (El-Shenody et al., 2019, Madkour et al., 2019), has a diverse variation of naturally-growing seaweed that could harvested throughout the year. Ulva or sea lettuce species are among the most plentiful representatives, being ubiquitous in coastal benthic communities around the world. Several Ulva species have been traditionally used for food and feed supplements for its high growth rate and high protein content suitable for food application (Kazir et al., 2019). Moreover, it also has several pharmaceuticals uses such as antimicrobial (Osman et al., 2013, Osman et al., 2012), antioxidant (Khairy and El-Sheikh, 2015), anticancer activities (Abou El Azm et al., 2019), biostimulants (Ashour et al., 2021, Hassan et al., 2021) and biofuel (Osman et al., 2020). Nonetheless, Ulva remains generally understudied, where Ulva is regarded as an attractive model organism for studying algal response and improvement against mutualistic interactions under stress conditions (Wichard et al., 2015).
This study aimed to evaluate the effects of pCO2 induced ocean acidification on Ulva fasciata, which is widely distributed throughout the Alexandria coast, Egypt (Osman et al., 2020, Osman et al., 2010, Osman et al., 2012). U. fasciata has reared under control pCO2 conditions (280 µatm) and three different pCO2 levels (550, 750 and 1050 µatm), in order to determine how U. fasciata may respond to pCO2 levels by evaluating algal specific growth, biochemical constituents, protein profile, and its associated microbiota which could pave the way to improve their applications.
2. Materials and methods
2.1. Algal sampling
Samples of the green macroalga Ulva fasciata were collected from the submerged rocks and substrates in the shallow water of the boulders at the sea anchor of the National Institute of Oceanography and Fisheries (NIOF) at 31°12′35.9″N 29°52′58.4″E, Alexandria, Egypt. The specimens have been washed immediately with seawater to eliminate sand and rock debris. The sample was preserved in a polyethylene bag filled with filtered seawater and transported to the laboratory in an icebox (5 min away). Seaweed was gently scrubbed with running filtered seawater (Whatman® GF/C glass microfiber filters, 0.5 μm) to clean epibiota (other seaweed, zooplankton and bivalves). All cleaned algal fronds had been blotted on towel papers to get rid of extra water and then weighed about 5 g fresh weight (FW) to start the culture experiment. The sample was adapted to lighting, temperature, and flow laboratory conditions for 48 h before evaluating its growth and biochemical composition.
2.2. Algal culture conditions
Adapted thalli of U. fasciata (equivalent to 5 g FW) was once positioned in 4 L plastic jars in triplicates and filled with filtered (Whatman® GF/C filters, 0.5 μm) and autoclaved seawater (salinity 30 PSU). Jars were enriched with dissolved pCO2 ~ 1050, 750, 550, and control (2 8 0) µatm which organized in-stock solution then was brought in distinct concentrations to attain the desired pH values 7.2, 7.6, 7.86, 8.1 (control), respectively. Jars were covered with transparent nylon film to minimize gas exchange with the environment and subjected to the different pCO2 levels using a flowmeter gas system that mixed ambient air with 5% CO2 gas. The jars have been aerated gently via air blower to ensure a non-stop mixing of the water and preserve algal homogeneity with the experimental media. Alga used to be saved at a temperature of 25 ± 0.5 °C and underneath non-stop illumination through white fluorescence lamps at 200 µmol photons m−2 s−1.
The experiment was performed at the National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt, during spring 2019. Ambient pH 8.1 global levels (280 pCO2, control) and elevated pCO2 levels (550, 750 and 1050 µatm) related to 7.86, 7.6, 7.2, respectively were applied using acid-base addition (±0.02 pH units) (Smithson, 2002). Acidification levels were measured in the jars continually along the day to be always controlled in the right tested pH values. Algal total biomass in DW/L was measured during the long-term CO2 enriched seawater along 12 days culturing at three days intervals.
2.3. Growth measurement
The rate of U. fasciata growth was expressed as the specific growth rate (SGR) that was expressed as a percentage of daily increase or decrease in algal biomass (% / days intervals) as described in (Korzen et al., 2015). SGR was calculated using the following formula:
| SGR=[ln (Wt/W0)]/t×100 |
Where Wt is the biomass (dry weight) in time per day culture and W0 is the initial biomass. t is time in days
2.4. Nutritional biochemical constituents
The biochemical compositions (protein, lipid, pigments and carbohydrates contents) of U. fasciata cultured on the different pCO2 concentrations were determined at the end of the exponential growth phase (9th day). About 5 g FW of algal sample was cut out and dried to a constant weight at 50 °C in an oven (approximately 0.5 g DW), ground to fine powder, and stored in a desiccator until further use.
2.4.1. Photosynthetic pigments
Three grams of U. fasciata fresh weight equal to 0.3 g DW were homogenized in 30 ml acetone (80%v/v) overnight in dark at 4 °C followed by centrifugation at 10,000 × g for 5 min (TDL-8 M, Luxiangyi, Hunan, China). Chlorophyll a (Chl a), chlorophyll b (Chl b) and total carotenoids were determined spectrophotometrically (Shimadzu UV- 2401PC, Kyoto, Japan) at wavelength 664, 470 and 450 nm, respectively, and expressed as mg/g DW according to (Lichtenthaler, 1987) and (Ismail and Osman, 2016).
2.4.2. Total carbohydrate content
Carbohydrate content was determined by a microplate phenol–sulfuric acid method (Masuko et al., 2005) and modified by Elshobary et al. (2015). Total soluble carbohydrate was measured at 490 nm against the blank and determined per DW using glucose as a standard.
2.4.3. Total lipid content
The lipid content of the macroalgal samples was measured by a solvent extraction method using Soxhlet, where petroleum ether was used as a solvent. The values are presented as a percent of the dry weight (DW) of the samples as described by (Elshobary et al., 2020a).
2.4.4. Total protein content
For protein analysis, algal powder (0.5 mg DW) were digested in 1 N NaOH for 24 h at room temperature and quantified, according to Bradford methods (Bradford, 1976) modified by (Kruger, 2009). The absorbance was measured by UV/ visible spectrophotometer at 595 nm against a blank. Bovine serum albumin was used as standard, where protein content was assessed as %DW.
2.5. Algal protein profile
The protein profile of the total soluble protein of U. fasciata under the four different acidification levels was detected via Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970). Ten μl of protein ladder (prestained dual-color standard, 14.4–116 kDa, Bio-Rad, USA) and 15 μg of protein from each treatment were loaded onto stacking gel of 5% and separating gel of 12% acrylamide in 25 mM Tris–HCl, pH 8.3, 0.18 M Glycine and 0.1% SDS. SDS PAGE was performed using a Protean II xi cell electrophoresis unit (Biorad, Hercules, CA, USA). The separation was carried out at 180 V for 2 h. Gels were stained for 30 min with 0.02% (w/v) Coomassie Brilliant Blue R-250 in 50% (v/v) methanol and 7.5% (v/v) acetic acid, followed by a distaining for 70 min with 50% (v/v) methanol and 7.5% (v/v) acetic acid. Pictures of the gels were taken with Gel documentation system (Geldoc-it, UVP, England), which was applied for data analysis using Totallab analysis software, ww.totallab.com, (Ver.1.0.1).
2.6. Isolation of the dominant associated bacteria
Within 2 h after collection, Ulva thallus was washed five times in autoclaved Petri dishes with sterilized and filtered (0.2 µm pore size) seawater to remove loosely attached bacteria, water was exchange after each step. Rinsed samples were placed in new sterilized Petri dishes. The whole surface of the thallus was vigorously swabbed with a sterile cotton swab. Subsequently, swab tips were spread on marine agar Zobell medium (Himedia) supplemented with 1.5% agar under aseptic conditions. The plates were incubated at 20℃ for three days, and the dominant colonies were purified on a fresh plate to obtain single colonies. Bacterial isolates were sustained at −20℃ for molecular identification.
2.7. Molecular identification of the dominant bacterial isolates
According to manufacturer protocol, DNA from bacterial isolates was obtained using GeneJET Genomic DNA Purification Kit (K0721/Thermo Fisher). PCR amplification of partial 16S rRNA gene sequences was carried out using the forward primer 27F (5′- AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′- CTACGGCTACCTTGTTACGA- 3′). Amplification protocol was carried out according to (Huo et al., 2020; Elshobary et al., 2015), using a thermal cycler (Applied Biosystems 2720, Foster City, CA, USA).
Agarose gel electrophoresis was used to detect the amplified products, which recovered and purified using E.Z.N.A.® Gel Extraction Kit (D2501-01). The purified DNA fragments have been sequenced directly by Macrogen Korea Company through ABI 3730XL sequencer. The sequences were blasted using the nBLAST search (http://www.ncbi.nlm.nih.gov/blast) to find the most homology isolates sequences available in the GenBank. The sequences have been aligned using Clustal W with the default parameters (MEGA X software) (www. megasoftware.net). A dendrogram was created using the neighbour-joining (NJ) algorithm based on the parameter distance (PD) using MEGA X software.
2.8. Statistical analysis
All experiments were carried out in triplicates, and results were analyzed using ANOVA. To evaluate the interactive effect of age and pCO2 on SGR, two-way ANOVA was used with experimental age and pCO2 as fixed factors. Whereas, one-way ANOVA was used to assess the effect of pCO2 (fixed factor) on all biochemical components followed by Duncan and LSD (least significant difference) comparisons to determine the significance level at P ≤ 0.05. The statistical analyses were done using SPSS software (version 23, SPSS Inc., USA), and data were presented as the mean ± SD.
3. Results
3.1. Algal growth rate
A long-term CO2 exposure test (12 days) was performed to obtain different pCO2 levels (280, 550, 750, and 1050 µatm) and investigate their impact on U. fasciata specific growth rate (SGR) (Fig. 1). In general, increasing CO2 has enhanced the growth rate than that recorded in the control treatment. The highest value (6.6% day−1) was recorded at pCO2-550 on the 6th day with an increase of 2.7 times than control. The results also showed that the optimum SGR was obtained approximately at the 6th day of culture, where all treatments have the same exponential phase period. Otherwise, they were varied in their stationary phase durations according to their response to different pCO2 levels. The shortest stationary phase duration was noted at the highest pCO2 level (1050 µatm), whereas in the other treatments, it lasted till the 9th day of culture, where all the analyses in this study have been performed. The statistical analysis of two-way ANOVA indicated that the variation in pCO2 levels, age, and interaction was significantly affected SGR (ANOVA p < 0.001) (Table S1).
Fig. 1.
Specific growth rate of U. fasciata (%/ day) cultured for 12 days on different pCO2 levels.
3.2. Biochemical constituents and pigment contents of U. Fasciata
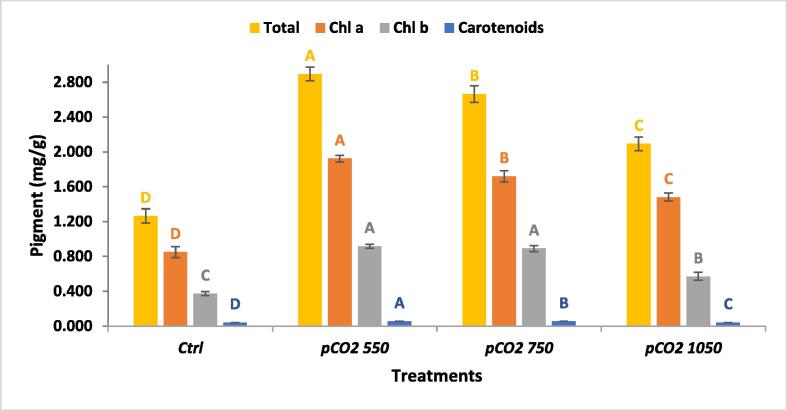
The photosynthetic pigments of U. fasciata were significantly affected by pCO2 levels (ANOVA p < 0.05). The pigment content of U. fasciata thalli was increased dramatically than ambient condition. The maximum Chl a, Chl b, carotenoids and total pigments (1.95, 0.93, 0.054, 2.9 mg/g respectively) were recorded at pCO2-550 (Fig. 2), with an increase of 107%, 55% and 1%, respectively than recorded in control. While rising pCO2 over 550 causes a reduction in pigment content. In general, raising pCO2 enhanced 550 µatm attained the maximum pigment production.
Fig. 2.
Pigment content of U. fasciata under different pCO2 concentrations.
Total protein, lipid and carbohydrate contents of the U. fasciata were significantly improved by applying different pCO2 levels (ANOVA p < 0.05). Higher protein accumulation was found at pCO2-550 µatm (32.43%DW) with an increase of 1.2-fold than recoded in control. In contrast, the protein content was decreased by increasing pCO2 levels over 550 µatm (Table 1). On the other hand, the lipid and carbohydrate contents of U. fasciata were improved significantly by elevated pCO2. Their highest values (4.23 and 46.96 %DW, respectively) were recorded at pCO2-750 (Table 1). Even though the most elevated PCO2 of 1050 µatm recorded lipid and carbohydrate yield below 750 µatm, they were still higher than those observed in the control treatment. Table 2.
Table 1.
Biochemical constituents of U. fasciata cultured on different pCO2 concentrations.
| Treatment pCO2 | Protein (%DW) | Lipid (%DW) | Carbohydrate (%DW) |
|---|---|---|---|
| Control (pCO2 280) | 26.99 ± 0.23c | 3.70 ± 0.04c | 42.34 ± 0.21c |
| pCO2 550 | 32.43 ± 0.27a | 3.90 ± 0.03b | 46.04 ± 0.17b |
| pCO2 750 | 28.70 ± 0.48b | 4.23 ± 0.05a | 46.96 ± 0.09a |
| pCO2 1050 | 21.53 ± 0.20d | 3.93 ± 0.04b | 46.13 ± 0.11b |
| F- value | 610.00* | 126.68* | 559.68* |
| LSD | 0.26 | 0.28 | 0.12 |
Table 2.
Sequence identity of the dominate associated bacteria within U. fasciata thallus using a comparison with most identity GenBank accessions.
| Treatments | Identified strain with the highest identity | Max Score | Total Score | Query Cover | E value | Identity % | Accession number |
|---|---|---|---|---|---|---|---|
| Control (pCO2-280) | Halomonas hydrothermalis | 1105 | 6610 | 100% | 0.0 | 100.00% | AP022843 |
| pCO2-550 | Halomonas venusta | 761 | 761 | 100% | 0.0 | 99.76% | MT510186 |
| pCO2-750 | Halomonas venusta | 981 | 981 | 100% | 0.0 | 100.00% | MT299647 |
| pCO2-1050 | Vibrio campbellii | 761 | 761 | 100% | 0.0 | 99.76% | MT510186 |
3.3. Protein profile analysis
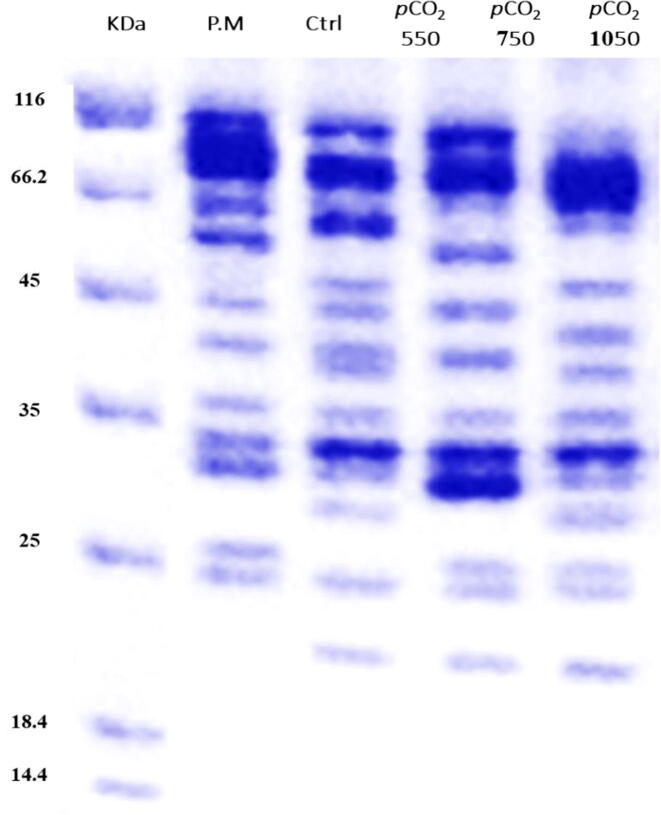
Protein profile analysis was determined using SDS-PAGE. In general, the most abundant protein bands had weights of 35, 32.5, 31, 22.4 kDa. Comparing to control, different unfamiliar and unique protein bands were expressed and reflected by different pCO2 values of U. fasciata culturing media (Fig. 3). Treating with pCO2 550 µatm reflected the highest number of bands (13 bands) with unique five bands at 59, 42, 26.5, 26 and 20 KDa. Interestingly, all pCO2 treatments showed a unique band at 20 KDa that was not observed in control. However, the other treatment and control reflected only 11 bands but at different molecular weights. Only one unique fraction with 27 KDa was distinguished in medium with pCO2-750. Two unique fractions with 48 and 28 KDa were reflected by pCO2-1050 (Fig. 3).
Fig. 3.
Protein fingerprinting patterns for U. fasciata under different pCO2. PM, protein marker.
3.4. Bacterial identification
As shown in Table (2), bacterial consortium associated with U. fasciata in control and different pCO2 concentrations were identified by amplifying the 16S rDNA gene and aligned with the close related strains (Fig. 4). The dominant bacterium was identified in each treatment. The 16S rRNA gene phylogeny was inferred from about 500 bp nucleotide sequences (PCR-based) that originated from four predominant bacterial isolates. From the maximum neighbor joint phylogenetic tree based on the 16S rRNA gene, the 16S gene region was aligned with 16S nucleotide sequences of 29 bacterial strains in the NCBI Ribosomal RNA sequence. The phylogenetic tree consisted of two main clades the first clade of Halomonas sp., where the dominant strain of moderate pCO2 level (550 and 750 µatm) was grouped with Halomonas venusta (MK357745, MF928305, MF928305 and LN995436) with identity percentages reached up to 99%, respectively and bootstrap of 77. While, the dominant strain of control was grouped with Halomonas hydrothermalis (AP022843) with 100% similarity percentage and 91 bootstraps. The second clade was grouped the dominate species of the highest pCO2 level (1050 µatm) with Vibrio toranzoniae (MT510186, LR722816 and MN945290) that showed identity percentage reached to 99% and bootstrap of 92%. From the above results, pCO2 variation has a significant effect on U. fasciata associated bacteria consortium.
Fig. 4.
Neighbour-joining (NJ) dendrogram was constructed for the dominant bacterial strains based on 16S rRNA nucleotide sequences. Bootstrap values higher than 70 are shown on the trees.
4. Discussion
In marine ecosystems, enrichment of different CO2 in seawater levels consequently affects the acidification of seawater, which influences marine algae metabolism. The overgrowth of Ulva sp. in response to elevated pCO2 in eutrophic estuaries can be directly promoted by acidification (Young and Gobler, 2016a). However, Reidenbach et al. (2017) detected no changes in U. australis growth by decreasing pCO2, which influenced the carbon and nitrogen metabolisms. Also, Chen et al. found that both lowered and increased seawater pH exert significant physiological stress on U. lactuca germlings (Chen et al., 2017).
In this study, CO2 enrichment enhanced the growth rate of Ulva fasciata than recorded in the control and the highest value (6.6% day−1) was recorded at pCO2-550 on the 6th day with an increase of more than double folds than control. In this regard, Young and Gobler (2016) reported that Gracilaria and Ulva's growth rates were significantly boosted by an average of 70% and 30%, respectively, beyond control treatment when exposed to raised levels of pCO2. Furthermore, Gracilaria and Ulva show a physiological shift from near-exclusive use of HCO3– to mainly CO2 use when subjected to elevated pCO2 via detecting δ13C isotopes. This shift in carbon dependence coupled with growth rate increased in response to increased pCO2, proposed that these seaweeds' photosynthesis depended on their inorganic carbon source (Young and Gobler, 2016a). Semesi et al. (2009) demonstrated that increasing dissolved CO2 concentration to a specific level of ~ 26 µmol kg−1 caused enhancing the photosynthetic rates of Hydrolithon sp by 13%. Additionally, the negative impact of the higher pCO2 on the growth of U. fasciata in the present study may contribute to the lowered pH. Elevated pCO2 reduces pH at the surface of the cell, which could modify both extracellular and intracellular acid-base balance (Flynn et al., 2012). Intracellular metabolic activities, including photosynthesis and development, may be impaired by disrupted homeostasis (Z. Xu et al., 2017). A reduced growth rate caused by decreased pH was also found in different seaweed such as Pyropia yezoensis (G. Gao et al., 2019), P. haitanensis (K. Xu et al., 2017) and U. lactuca (Olischläger et al., 2013).
The marine macroalga Ulva sp. is a candidate raw biomass with a high growth rate and high protein, lipid, carbohydrate yield suitable for food application (Kazir et al., 2019, Khairy and El-Shafay, 2013). A recent study showed that pH-shift of Ulva sp improves the nutrient contents and gives a high grade of food applications (Harrysson et al., 2019).
Color and flavor are essential criteria in determining seaweed feed and food qualities and thus, market value. Seaweeds with a darker color and more protein are favorable by consumers and have a higher value in the market. The color of seaweeds is mainly determined by photosynthetic pigments, such as chlorophyll and carotenoids (Niwa and Harada, 2013). Regarding algal pigments, chlorophyll a, b and carotenoids contents of U. fasciata thalli were significantly differentiated at a different level of pCO2, the maximum Chl a, Chl b and carotenoids were recorded at pCO2-550 and declined by elevating the pCO2 level. This finding was in accordance (G. Gao et al., 2019; K. Gao et al., 2019) who recorded that increasing pCO2 concentration improved the color and flavor of edible red algae Pyropia yezoensis by boosting pigments, and amino acids contents. In brown seaweed Sargassum vulgare, photosynthetic pigments have improved at the acidified site than alkaline one (Kumar et al., 2020). On contrary, raising pCO2 to 750 µatm, the pigment content was decreased. The same finding was recorded in U. lactuca, where both Chl a and Chl b content were reduced at 750 µatm at the end of the experiment (Olischläger et al., 2013).
Carbohydrate is the main calorific compound in seaweeds that are utilized in metabolism as a source of energy needed for respiration and other important processes (Sudhakar et al., 2019) as well as soluble carbohydrates can serve as precursors for bioactive metabolites (Kumar et al., 2020). Seaweeds carbohydrate is a valuable and sustainable source for pharmaceutical, cosmeceutical, and traditional applications (Ahmed et al., 2014) and bioethanol feedstock (Elshobary et al., 2020a, Osman et al., 2020). The results showed that the carbohydrate was the most dominant component in U. fasciata. This finding is agreed with results of (Osman et al., 2020) who observed that the carbohydrate content of U. fasciata ranged from 37.1%DW during winter to 40.46 %DW during summer, while U. rigida sp. showed lower trends of carbohydrates 28.6% when cultivated under fish effluents compared to the control site (Korzen et al., 2015). Different levels of pCO2 showed a significant effect on the carbohydrate content and the highest carbohydrate content (46.96%DW) was demonstrated at 750 µatm. In a recent study, the content of fucoidan and alginate polysaccharides were higher in the algal community of acidified environment (Kumar et al., 2020). Rogers et al., 1998, Webber et al., 1994 found that elevating CO2 can decrease RuBisCO concentrations; however, it can lead to an increase in soluble carbohydrate content, which can increase the total carbon content of algal tissue. In contrast, pCO2 did not affect carbohydrate content in U. rigida (Gao et al., 2017). These differences may be depending on acclimation ability of each species to the different degrees of pCO2. Although there is no clear metabolic understanding of the relationship between CO2 concentrations and cell wall carbohydrates, it has been documented that elevated CO2 concentrations may enhance the activity of enzymes responsible for the synthesis of cell wall uronic acid, resulting in increased cell wall carbohydrate synthesis (Cheng et al., 2015). Another research discovered that under elevated acidification level, genes encoding enzymes involved in cell wall formation and structure, as well as carbon storage, were expressed at higher levels in Sargassum vulgare than under control conditions (Kumar et al., 2017).
In macroalgae, the lipids constitute a suitable storage material widely distributed, in different macroalgal classes (Sudhakar et al., 2019). Lipid is a calorific component that can be used for aquaculture (El‐Khodary et al., 2020) or feedstock for biodiesel (Essa et al., 2018; Ashour et al., 2019, Elshobary et al., 2019, Huo et al., 2020). Despite the current study observed low lipid content (4.23 %DW), this is comparable with other reports, which showed the lipid content of macroalgae were<5% of dry weight (Elshobary et al., 2020a, Khairy and El-Shafay, 2013, Osman et al., 2020). The differences in lipid reported quantities could be due to several factors such as seasonal and geographical factors, climate change, and the development stage of the macroalgae (Osman et al., 2020). Noteworthy, the increase in ocean acidity combined with an increase in the lipid content, showing the maximum value at pCO2-750 with a rise of 54% than that were found in control. Gao et al. (2017) observed that increasing pCO2 up to pH 7.95 increased lipid content in U. rigida under the high temperature by 22.55% than low pCO2 treatments. Gordillo et al. (2001a) detected that under different pCO2 levels, significant changes were observed in total lipid content as well as its classes in U. rigida. Triglycerides accumulated at high CO2 and under nitrogen deficient, while chloroplast-related lipids recorded an inverse response In general, high pCO2 concentration of 1000 ppm showed a negative impact on total lipid accumulation.
Protein plays crucial roles in all algal biological processes; their activities can be described by transport and storage, enzymatic catalysis, and mechanical sustentative control (Sudhakar et al., 2019). The current study revealed that U. fasciata accumulated the highest protein content of 32.43%DW, at pCO2-550 compared with high pCO2 levels and control synchronized with the growth rate. A recent study showed that ocean acidification of Ulva sp. improves the protein yield to 29% by 2.3-fold higher than recorded by control and gives a high grade of food applications (Harrysson et al., 2019). Gao et al., (2017) reported that protein levels were increased in U. rigida in response to pH-shifting. In contrast, high CO2 upto10,000 ppm reduced total soluble protein compared to the ambient CO2 level of 350 ppm (Gordillo et al., 2001b, Gordillo et al., 2001a). The reduction in protein content under high CO2 level may be attributed to the algal species tends to accumulate some biochemical such carbohydrate and lipid over the others (protein) under high CO2 concentration. In this regard, Chen et al. observed that, carbohydrate content increased in Pyropia haitanensis while protein content decreased due to higher dissolved inorganic carbon in highly acidified seawater (Chen et al., 2019b). Several studies observed the same results (Chen et al., 2019a, Duarte et al., 2016, Gao et al., 2018b, Gao et al., 2018a). This may be explained by increasing CO2 concentration increases the CO2 passive diffusion , resulting in a reduction in active transport proteins inside the seaweed’s cell and allocating more energy for growth (Young and Gobler, 2016b). These results are also consistent with the SDS-PAGE which showed the largest number of unique protein bands were detected in the moderate pCO2 level (550 µatm) and these bands reduced by increasing pCO2 level.
The ability of seaweed to different biochemical stresses may be due to symbiosis with microbiota (Dominguez and Loret, 2019). Interactions between Ulva spp. and their associated bacteria have been well-characterized over the last decade, where, bacterial colonization has been defined based on 16S rRNA gene phylogeny (Wichard et al., 2015). The interconnected evolutionary history of algae and bacteria allowed a wide range of associations to be established, characterized by the coordinated exchange of nutrients and mutual support for growth factors (Cirri and Pohnert, 2019, Huo et al., 2020). Many studies stated that different compositions of bacterial communities could enable Ulva species to support the ‘competitive lottery’ theory for how symbiotic bacteria help algae in either ambient or harsh conditions (Comba-González et al., 2016, Ghaderiardakani et al., 2017, Kessler et al., 2018, Spoerner et al., 2012). As a result of acidification, the seaweed-associated bacterial community had changed, where, elevating CO2 altered the dominant associated bacteria from Halomonas sp. of ambient condition (280 µatm) and moderate pCO2° (550–750 µatm) to Vibrio toranzoniae at the highest pCO2 level (1050 µatm). This finding is in accordance with (Aires et al., 2018) who observed that the bacterial community of Sargassum muticum was changed, where Oceanospirillales and Vibrionales significantly increased their abundance in acidified conditions. Vibrionales, usually associated with diseased seaweeds, proposing that acidification may facilitate opportunistic/pathogenic bacteria. Moreover, Alpha diversity of total bacteria communities and Cyanobacteria communities was significantly variated among different pH/CO2 sites (Taylor et al., 2014). Coral microbiomes contribute to seaweed adaptation to environmental change, especially pH/CO2 levels (Biagi et al., 2020).
5. Conclusion
From the above results, it could be concluded that a considerable influence of pCO2 on the whole performance of U. fasciata, including growth rate, protein, pigment, lipid and carbohydrate, and associated microbiota. Ocean acidification at pCO2-550 µatm is the optimum concentration to improving growth, protein and pigment contents and protein profile which could be a good source for alimentary fish source. While elevating ocean acidification to pCO2-750 µatm could be preferable for bioenergy production by stimulating energetic compounds of lipid and carbohydrate.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors kindly acknowledge the support of the Saudi Biological Society, King Saud University, Riyadh, Saudi Arabia of this research.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.05.029.
Contributor Information
Hanan M. Khairy, Email: hanan_khairy@yahoo.com.
Mostafa E. Elshobary, Email: mostafa_elshobary@science.tanta.edu.eg.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abou El Azm N., Fleita D., Rifaat D., Mpingirika E.Z., Amleh A., El-Sayed M.M.H. Production of bioactive compounds from the sulfated polysaccharides extracts of ulva lactuca: Post-extraction enzymatic hydrolysis followed by ion-exchange chromatographic fractionation. Molecules. 2019;24:2132. doi: 10.3390/molecules24112132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires T., Serebryakova A., Viard F., Serrão E.A., Engelen A.H. Acidification increases abundances of Vibrionales and Planctomycetia associated to a seaweed-grazer system: potential consequences for disease and prey digestion efficiency. PeerJ. 2018;6 doi: 10.7717/peerj.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour M., Elshobary M.E., El-Shenody R., Kamil A.W.A., Abomohra A.E. Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass and Bioenergy. 2019;120:439–447. doi: 10.1016/j.biombioe.2018.12.009. [DOI] [Google Scholar]
- Ashour Mohamed, Hassan Shimaa M., Elshobary Mostafa E., Ammar Gamal A.G., Gaber Ahmed, Alsanie Walaa F., Mansour Abdallah Tageldein, El‐Shenody Rania. Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum) Plants. 2021;10(1045) doi: 10.3390/plants10061045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggini C., Salomidi M., Voutsinas E., Bray L., Krasakopoulou E., Hall-Spencer J.M. Seasonality affects macroalgal community response to increases in pCO2. PLoS One. 2014;9:1–13. doi: 10.1371/journal.pone.0106520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E., Caroselli E., Barone M., Pezzimenti M., Teixido N., Soverini M., Rampelli S., Turroni S., Gambi M.C., Brigidi P., Goffredo S., Candela M. Patterns in microbiome composition differ with ocean acidification in anatomic compartments of the Mediterranean coral Astroides calycularis living at CO2 vents. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138048. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caldeira K., Wickett M.E. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Chen B., Lin L., Ma Z., Zhang T., Chen W., Zou D. Carbon and nitrogen accumulation and interspecific competition in two algae species, Pyropia haitanensis and Ulva lactuca, under ocean acidification conditions. Aquac. Int. 2019;27:721–733. doi: 10.1007/s10499-019-00360-y. [DOI] [Google Scholar]
- Chen B., Xia J., Zou D., Zhang X. Responses to ocean acidification and diurnal temperature variation in a commercially farmed seaweed, Pyropia haitanensis (Rhodophyta) Eur. J. Phycol. 2019;54:184–192. doi: 10.1080/09670262.2018.1539250. [DOI] [Google Scholar]
- Chen B., Zou D., Zhu M. Growth and photosynthetic responses of Ulva lactuca (Ulvales, Chlorophyta) germlings to different pH levels. Mar. Biol. Res. 2017;13:351–357. doi: 10.1080/17451000.2016.1267367. [DOI] [Google Scholar]
- Cheng Y.-S., Labavitch J.M., VanderGheynst J.S. Elevated CO 2 concentration impacts cell wall polysaccharide composition of green microalgae of the genus Chlorella. Lett. Appl. Microbiol. 2015;60:1–7. doi: 10.1111/lam.12320. [DOI] [PubMed] [Google Scholar]
- Cirri E., Pohnert G. Algae−bacteria interactions that balance the planktonic microbiome. New Phytol. 2019;223:100–106. doi: 10.1111/nph.15765. [DOI] [PubMed] [Google Scholar]
- Comba-González N.B., Ruiz-Toquica J.S., Lopez-Kleine L., Montoya-Castano D. Epiphytic bacteria of macroalgae of the genus Ulva and their potential in producing enzymes having biotechnological interest. J. Mar. Biol. 2016:2–9. [Google Scholar]
- Cornwall C.E., Hurd C.L. Variability in the benefits of ocean acidification to photosynthetic rates of macroalgae without CO2-concentrating mechanisms. Mar. Freshw. Res. 2020;71:275–280. doi: 10.1071/MF19134. [DOI] [Google Scholar]
- Dominguez H., Loret E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs. 2019;17:1–20. doi: 10.3390/md17060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte C., López J., Benítez S., Manríquez P.H., Navarro J.M., Bonta C.C., Torres R., Quijón P. Ocean acidification induces changes in algal palatability and herbivore feeding behavior and performance. Oecologia. 2016;180:453–462. doi: 10.1007/s00442-015-3459-3. [DOI] [PubMed] [Google Scholar]
- El-Khodary G.M., El-Sayed H.S., Khairy H.M., El-Sheikh M.A., Qi X., Elshobary M.E. Comparative study on growth, survival and pigmentation of Solea aegyptiaca larvae by using four different microalgal species with emphasize on water quality and nutritional value. Aquac. Nutr. 2020;anu.13211 doi: 10.1111/anu.13211. [DOI] [Google Scholar]
- El-Shenody RA, Ashour M, Ghobara MME. Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds: Dictyota dichotoma, Turbinaria decurrens, and Laurencia obtusa. Brazilian Journal of Food Technology. 2019;22 doi: 10.1590/1981-6723.20318. [DOI] [Google Scholar]
- Elshobary M.E., Abo-Shady A.M., Khairy H.M., Essa D., Zabed H.M., Qi X., Abomohra A.E. Influence of nutrient supplementation and starvation conditions on the biomass and lipid productivities of Micractinium reisseri grown in wastewater for biodiesel production. J. Environ. Manage. 2019;250 doi: 10.1016/j.jenvman.2019.109529. [DOI] [PubMed] [Google Scholar]
- Elshobary M.E., El-Shenody R., Abomohra A.E. Sequential biofuel production from seaweeds enhances the energy recovery : A case study for biodiesel and bioethanol production. Int. J. Energy Res. 2020;1–11 doi: 10.1002/er.6181. [DOI] [Google Scholar]
- Elshobary M.E., Essa D.I., Attiah A.M., Salem Z.E., Qi X. Algal community and pollution indicators for the assessment of water quality of Ismailia canal. Egypt. Stoch. Environ. Res. Risk Assess. 2020;34:1089–1103. doi: 10.1007/s00477-020-01809-w. [DOI] [Google Scholar]
- Elshobary M.E., Osman M.E.H., Abushady A.M., Piercey-Normore M.D. Comparison of lichen-forming cyanobacterial and green algal photobionts with free-living algae. Cryptogam. Algol. 2015;36:81–100. doi: 10.7872/crya.v36.iss1.2015.81. [DOI] [Google Scholar]
- Essa Dorya, Abo-Shady Atef, Khairy Hanan, Abomohra Abd El-Fatah, Elshobary Mostafa. Potential cultivation of halophilic oleaginous microalgae on industrial wastewater. Egyptian Journal of Botany. 2018;58(2):205–216. doi: 10.21608/ejbo.2018.809.1054. [DOI] [Google Scholar]
- Fernández P.A., Roleda M.Y., Hurd C.L. Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. Photosynth. Res. 2015;124:293–304. doi: 10.1007/s11120-015-0138-5. [DOI] [PubMed] [Google Scholar]
- Flynn K.J., Blackford J.C., Baird M.E., Raven J.A., Clark D.R., Beardall J., Brownlee C., Fabian H., Wheeler G.L. Changes in pH at the exterior surface of plankton with ocean acidification. Nat. Clim. Chang. 2012;2:510–513. [Google Scholar]
- Gao G., Clare A.S., Chatzidimitriou E., Rose C., Caldwell G. Effects of ocean warming and acidification, combined with nutrient enrichment, on chemical composition and functional properties of Ulva rigida. Food Chem. 2018;258:71–78. doi: 10.1016/j.foodchem.2018.03.040. [DOI] [PubMed] [Google Scholar]
- Gao G., Clare A.S., Rose C., Caldwell G.S. Ulva rigida in the future ocean: potential for carbon capture, bioremediation and biomethane production. GCB Bioenergy. 2018;10:39–51. doi: 10.1111/gcbb.12465. [DOI] [Google Scholar]
- Gao G., Clare A.S., Rose C., Caldwell G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017;114:439–447. doi: 10.1016/j.marpolbul.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Gao G., Gao Q., Bao M., Xu J., Li X. Nitrogen availability modulates the effects of ocean acidification on biomass yield and food quality of a marine crop Pyropia yezoensis. Food Chem. 2019;271:623–629. doi: 10.1016/j.foodchem.2018.07.090. [DOI] [PubMed] [Google Scholar]
- Gao K., Beardall J., Häder D.-P., Hall-Spencer J.M., Gao G., Hutchins D.A. Effects of ocean acidification on marine photosynthetic organisms under the concurrent influences of warming, UV radiation, and deoxygenation. Front. Mar. Sci. 2019;6:322. [Google Scholar]
- Ghaderiardakani, F., Coates, J.C., Wichard, T., 2017. Bacteria-induced morphogenesis of Ulva intestinalis and Ulva mutabilis (Chlorophyta): a contribution to the lottery theory. FEMS Microbiol. Ecol. 93. [DOI] [PMC free article] [PubMed]
- Gordillo F.J.L., Jiménez C., Goutx M., Niell X. Effects of CO2 and nitrogen supply on the biochemical composition of Ulva rigida with especial emphasis on lipid class analysis. J. Plant Physiol. 2001;158:367–373. [Google Scholar]
- Gordillo F.J.L., Niell F.X., Figueroa F.L. Non-photosynthetic enhancement of growth by high CO 2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta) Planta. 2001;213:64–70. doi: 10.1007/s004250000468. [DOI] [PubMed] [Google Scholar]
- Gutow L., Rahman M.M., Bartl K., Saborowski R., Bartsch I., Wiencke C. Ocean acidification affects growth but not nutritional quality of the seaweed Fucus vesiculosus (Phaeophyceae, Fucales) J. Exp. Mar. Bio. Ecol. 2014;453:84–90. doi: 10.1016/j.jembe.2014.01.005. [DOI] [Google Scholar]
- Han T., Shi R., Qi Z., Huang H., Wu F., Gong X. Biogenic acidification of Portuguese oyster Magallana angulata mariculture can be mediated through introducing brown seaweed Sargassum hemiphyllum. Aquaculture. 2020;520 [Google Scholar]
- Harrysson H., Konasani V.R., Toth G.B., Pavia H., Albers E., Undeland I. Strategies for Improving the Protein Yield in pH-Shift Processing of Ulva lactuca Linnaeus: Effects of Ulvan Lyases, pH-Exposure Time, and Temperature. ACS Sustain. Chem. Eng. 2019;7:12688–12691. [Google Scholar]
- Hassan Shimaa M, Ashour Mohamed, Soliman Ahmed A F, Hassanien Hesham A, Alsanie Walaa F, Gaber Ahmed, Elshobary Mostafa E. The potential of a new commercial seaweed extract in stimulating morpho-agronomic and bioactive properties of Eruca vesicaria (L.) Cav. Sustainability. 2021;13(8) doi: 10.3390/su13084485. [DOI] [Google Scholar]
- Hofmann L.C., Straub S., Bischof K. Elevated CO2 levels affect the activity of nitrate reductase and carbonic anhydrase in the calcifying rhodophyte Corallina officinalis. J. Exp. Bot. 2013;64:899–908. doi: 10.1093/jxb/ers369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo S., Basheer S., Liu F., Elshobary M., Zhang C., Qian J., Xu L., Arslan M., Cui F., Zan X., Zhu F., Zou B., Ding Q., Ma H. Bacterial intervention on the growth, nutrient removal and lipid production of filamentous oleaginous microalgae Tribonema sp. Algal Res. 2020;52 doi: 10.1016/j.algal.2020.102088. [DOI] [Google Scholar]
- IPCC, 2007. (Intergovernmental Panel on Climate Change) Climate Change 2007 Synthesis Report [WWW Document]. Cambridge Univ. Press. New York.
- Ismail M.M., Osman M.E.H. Seasonal fluctuation of photosynthetic pigments of most common red seaweeds species collected from Abu Qir, Alexandria. Egypt. Rev. Biol. Mar. Oceanogr. 2016;51:515–525. doi: 10.4067/S0718-19572016000300004. [DOI] [Google Scholar]
- Kazir M., Abuhassira Y., Robin A., Nahor O., Luo J., Israel A., Golberg A., Livney Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019;87:194–203. [Google Scholar]
- Kessler R.W., Weiss A., Kuegler S., Hermes C., Wichard T. Macroalgal–bacterial interactions: role of dimethylsulfoniopropionate in microbial gardening by Ulva (Chlorophyta) Mol. Ecol. 2018;27:1808–1819. doi: 10.1111/mec.14472. [DOI] [PubMed] [Google Scholar]
- Khairy H.M., El-Shafay S.M. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria. Egypt. Oceanologia. 2013;55:435–452. [Google Scholar]
- Khairy H.M., El-Sheikh M.A. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay. Egypt. Saudi J. Biol. Sci. 2015;22:623–630. doi: 10.1016/j.sjbs.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairy HM, El-Sheikh MA. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi Journal of Biological Sciences. 2015;22(2):623–630. doi: 10.1016/j.sjbs.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnby A., White J.C.B., Toth G.B., Pavia H. Ocean acidification decreases grazing pressure but alters morphological structure in a dominant coastal seaweed. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzen L., Pulidindi I.N., Israel A., Abelson A., Gedanken A. Single step production of bioethanol from the seaweed Ulva rigida using sonication. RSC Adv. 2015;5:16223–16229. [Google Scholar]
- Kruger N.J. The Bradford method for protein quantitation. protein Protoc. Handb. 2009;17–24 [Google Scholar]
- Kumar A., Buia M.C., Palumbo A., Mohany M., Wadaan M.A.M., Hozzein W.N., Beemster G.T.S., AbdElgawad H. Ocean acidification affects biological activities of seaweeds: A case study of Sargassum vulgare from Ischia volcanic CO2 vents. Environ. Pollut. 2020;259 doi: 10.1016/j.envpol.2019.113765. [DOI] [PubMed] [Google Scholar]
- Kumar A., Castellano I., Patti F.P., Delledonne M., Abdelgawad H., Beemster G.T.S., Asard H., Palumbo A., Buia M.C. Molecular response of Sargassum vulgare to acidification at volcanic CO 2 vents: insights from de novo transcriptomic analysis. Mol. Ecol. 2017;26:2276–2290. doi: 10.1111/mec.14034. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H.K. [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Mackey K., Morris J.J., Morel F., Kranz S. Response of Photosynthesis to Ocean Acidification. Oceanography. 2015;25:74–91. doi: 10.5670/oceanog.2015.33. [DOI] [Google Scholar]
- Madkour Fedekar, El-Shoubaky Gihan, Ebada Mohamed A. Antibacterial activity of some seaweeds from the Red Sea coast of Egypt. Egyptian Journal of Aquatic Biology and Fisheries. 2019;23(2):265–274. doi: 10.21608/ejabf.2019.31016. [DOI] [Google Scholar]
- Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S.-I., Lee Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Niwa K., Harada K. Physiological responses to nitrogen deficiency and resupply in different blade portions of Pyropia yezoensis f. narawaensis (Bangiales, Rhodophyta) J. Exp. Mar. Bio. Ecol. 2013;439:113–118. [Google Scholar]
- Olischläger M., Bartsch I., Gutow L., Wiencke C. Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycol. Res. 2013;61:180–190. doi: 10.1111/pre.12006. [DOI] [Google Scholar]
- Osman M.E.H., Abo-Shady A.M., Elshobary M.E., Abd El-Ghafar M.O., Abomohra A.E. Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ. Sci. Pollut. Res. 2020;27:32481–32493. doi: 10.1007/s11356-020-09534-1. [DOI] [PubMed] [Google Scholar]
- Osman M.E.H., Aboshady A.M., Elshobary M.E. Production and characterization of antimicrobial active substance from some macroalgae collected from Abu- Qir bay (Alexandria) Egypt. African J. Biotechnol. 2013;12:6847–6858. doi: 10.5897/AJB10.2150. [DOI] [Google Scholar]
- Osman M.E.H., Abushady A.M., Elshobary M.E. In vitro screening of antimicrobial activity of extracts of some macroalgae collected from Abu- Qir bay Alexandria. Egypt. African J. Biotechnol. 2010;9:7203–7208. doi: 10.5897/AJB09.1242. [DOI] [Google Scholar]
- Pachauri R.K., Allen M.R., Barros V.R., Broome J., Cramer W., Christ R., Church J.A., Clarke L., Dahe Q., Dasgupta P. Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Ipcc. 2014 [Google Scholar]
- Pourjamshidian R., Abolghasemi H., Esmaili M., Amrei H.D., Parsa M., Rezaei S. Carbon dioxide biofixation by Chlorella sp. In a bubble column reactor at different flow rates and CO2 concentrations. Brazilian J. Chem. Eng. 2019;36:639–645. doi: 10.1590/0104-6632.20190362s20180151. [DOI] [Google Scholar]
- Reidenbach L.B., Fernandez P.A., Leal P.P., Noisette F., McGraw C.M., Revill A.T., Hurd C.L., Kübler J.E. Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A., Fischer B.U., Bryant J., Frehner M., Blum H., Raines C.A., Long S.P. Acclimation of Photosynthesis to Elevated CO2under Low-Nitrogen Nutrition Is Affected by the Capacity for Assimilate Utilization. Perennial Ryegrass under Free-Air CO2 Enrichment. Plant Physiol. 1998;118:683–689. doi: 10.1104/pp.118.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semesi I.S., Kangwe J., Björk M. Alterations in seawater pH and CO2 affect calcification and photosynthesis in the tropical coralline alga, Hydrolithon sp. (Rhodophyta) Estuar. Coast. Shelf Sci. 2009;84:337–341. doi: 10.1016/j.ecss.2009.03.038. [DOI] [Google Scholar]
- Smithson, P.A., 2002. IPCC, 2001: climate change 2001: the scientific basis. Contribution of Working Group 1 to the Third Assessment Report of the Intergovernmental Panel on Climate Change, edited by J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Da. Int. J. Climatol. 22, 1144–1144. https://doi.org/10.1002/joc.763
- Spoerner M., Wichard T., Bachhuber T., Stratmann J., Oertel W. Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. J. Phycol. 2012;48:1433–1447. doi: 10.1111/j.1529-8817.2012.01231.x. [DOI] [PubMed] [Google Scholar]
- Sudhakar M.P., Kumar B.R., Mathimani T., Arunkumar K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019;228:1320–1333. doi: 10.1016/j.jclepro.2019.04.287. [DOI] [Google Scholar]
- Taylor J.D., Ellis R., Milazzo M., Hall-Spencer J.M., Cunliffe M. Intertidal epilithic bacteria diversity changes along a naturally occurring carbon dioxide and pH gradient. FEMS Microbiol. Ecol. 2014;89:670–678. doi: 10.1111/1574-6941.12368. [DOI] [PubMed] [Google Scholar]
- Webber A.N., Nie G.-Y., Long S.P. Acclimation of photosynthetic proteins to rising atmospheric CO2. Photosynth. Res. 1994;39:413–425. doi: 10.1007/BF00014595. [DOI] [PubMed] [Google Scholar]
- Wichard T., Charrier B.B., Mineur F.F.F., Bothwell J.H., Clerck O. De, Coates J.C., De Clerck O., Coates J.C., Clerck O. De, Coates J.C. The green seaweed Ulva: a model system to study morphogenesis. Front. Plant Sci. 2015;6:1–8. doi: 10.3389/fpls.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Agustí S., Yu Y., Huang Y., Chen W., Hu J., Li C., Li K., Wei F., Lu Y. Seaweed farms provide refugia from ocean acidification. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145192. [DOI] [PubMed] [Google Scholar]
- Xu K., Chen H., Wang W., Xu Y., Ji D., Chen C., Xie C. Responses of photosynthesis and CO2 concentrating mechanisms of marine crop Pyropia haitanensis thalli to large pH variations at different time scales. Algal Res. 2017;28:200–210. [Google Scholar]
- Xu Z., Gao G., Xu J., Wu H. Physiological response of a golden tide alga (Sargassum muticum) to the interaction of ocean acidification and phosphorus enrichment. Biogeosciences. 2017;14:671–681. [Google Scholar]
- Young C.S., Gobler C.J. Ocean acidification accelerates the growth of two bloom-forming macroalgae. PLoS One. 2016;11:1–21. doi: 10.1371/journal.pone.0155152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C.S., Gobler C.J. Ocean Acidification Accelerates the Growth of Two Bloom-Forming Macroalgae. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman, M.E.H., Abu-Shady, A.M., Elshobary, M.E., 2012. The Seasonal Fluctuation of the Antimicrobial Activity of Some Macroalgae Collected from Alexandria Coast, Egypt, in: Salmonella - Distribution, Adaptation, Control Measures and Molecular Technologies. InTech, pp. 173–186. https://doi.org/10.5772/31907
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.