Abstract
Fast and precise diagnosis of infectious and non-infectious animal diseases and their targeted treatments are of utmost importance for their clinical management. The existing biochemical, serological and molecular methods of disease diagnosis need improvement in their specificity, sensitivity and cost and, are generally not amenable for being used as points-of-care (POC) device. Further, with dramatic changes in environment and farm management practices, one should also arm ourselves and prepare for emerging and re-emerging animal diseases such as cancer, prion diseases, COVID-19, influenza etc. Aptamer – oligonucleotide or short peptides that can specifically bind to target molecules – have increasingly become popular in developing biosensors for sensitive detection of analytes, pathogens (bacteria, virus, fungus, prions), drug residues, toxins and, cancerous cells. They have also been proven successful in the cellular delivery of drugs and targeted therapy of infectious diseases and physiological disorders. However, the in vivo application of aptamer-mediated biosensing and therapy in animals has been limited. This paper reviews the existing reports on the application of aptamer-based biosensors and targeted therapy in animals. It also dissects the various modifications to aptamers that were found to be successful in in vivo application of the aptamers in diagnostics and therapeutics. Finally, it also highlights major challenges and future directions in the application of aptamers in the field of veterinary medicine.
Keywords: Aptamer, Aptasensor, Point-of-care, RNA Spiegelmers, Targeted therapy, Theranostics
1. Introduction
Infectious diseases of livestock, particularly highly infectious diseases, almost always cause major economic losses to the livestock owners in particular and the country as a whole (Raboisson et al., 2020). Transmissible livestock diseases caused by pathogenic microorganisms such as viruses and bacteria have a negative impact on their health and well-being, limit their production, and often result in mortality, resulting in significant economic losses in the respective field.
Prompt diagnosis and proper treatment of livestock diseases is an integral aspect of disease management and prevention, and it decreases economic risk to dairy farmers. Precise diagnosis of animal diseases is an important step between the cause and cure of disease. The role of animal disease diagnostics is important not only for the welfare of animals and farmers but also for consumers' health. There are various animal diseases that can transmit to humans such as anthrax, rabies, tuberculosis, swine/bird flu, foot and mouth disease (FMD) and so on. The zoonotic diseases continue to be a concern today with recent outbreaks of severe acute respiratory syndrome corona virus (SARS-CoV) and influenza (H1N1). The SARS-CoV-2 of the current COVID-19 (Coronavirus Disease 2019) pandemic has also been detected in dogs, ferrets, cats (Shi et al., 2020)), and tigers, though infection from animal to human has not yet been verified. Viral and bacterial infections can cause serious diseases to livestock and humans. Precise and early detection of the pathogen is often crucial for clinical diagnosis and effective treatment.
An early diagnosis of the disease is a pre-requisite for effective treatment and management of animal diseases. In most cases the diagnosis and differential diagnosis of animal diseases are done based on symptoms and basic biochemical, microbial, parasitological and serological tests of biological samples. Additional confirmatory tests include ELISA, PCR, DNA chips and chromatographic techniques, which require sophisticated instruments, professional operators, and costly chemicals. More recently, newer POC biosensors have emerged as newer diagnostic tools but many of them suffer from low sensitivity, specificity and reliability (Sheikhzadeh et al., 2021, Wang et al., 2021). Biosensors are rapidly becoming a cutting-edge novel technology in disease diagnosis and treatment. Biosensors are analytical devices that use biological molecules to generate signals that can be used to detect a specific disease condition (Duarte et al., 2015). Biosensors have two important features such as selectivity and sensitivity (Seo and Gu, 2017). With the accumulating knowledge on the development of aptamers and aptasensors, they have been increasingly investigated and tested for the detection of various infectious agents, including bacteria and viruses, as well as their toxins.
Aptamers are single-stranded nucleic acids (DNA, RNA) having molecular recognition properties similar to antibodies that are usually 22–100 nucleobases long and consist of a variable region edged by constant regions, which allow for amplification and identification of sequences (Gu, 2016, Kanwar et al., 2011, Simmons et al., 2012). Aptamers have several applications like monoclonal antibodies such as biomarker identification, therapeutics, diagnostics, and high throughput screening (Green et al., 2001, Shigdar et al., 2011). Yet, by virtue of their biophysical properties, aptamers compete with antibodies in many aspects (Proske et al., 2005). Literature for the present review was collected through searched in PubMed, google using key words, aptamers, biosensors, animals disease diagnosis, drug residue, COVID19 etc. and manually from the institute library.
1.1. Aptamers synthesis technique
Aptamers can be chemically synthesized and have shown specificity comparable to antibodies for antigen capture and identification, making them excellent alternatives to antibodies (Thiviyanathan and Gorenstein, 2012). Thus, they are occasionally referred as “synthetic antibodies” and have been competing with antibodies in the research of disease diagnosis, prophylactics, and therapeutics. They offer advantages of reduced cost, smaller size, ease of production and modification, and improved stability over antibodies (Song et al., 2008). Consequently, a number of aptamer-based platforms have been developed in the form of biosensors (called aptasensors) for detection of pathogen and target analytes, immunophenotyping and management of animal health (Table 1).
Table 1.
Summary of aptamers/biosensors in veterinary medicine.
| S.No. | Aptamer type/Biosensor type | Target/Disease condition | Reference |
|---|---|---|---|
| RNA aptamers | |||
| 1 | RNA aptamer | N protein/sensitive detection of SARS coronavirus nucleocapsid protein | Ahn et al. (2009) |
| 2 | RNA aptamer | Fluorescence-based detection of the influenza A H1N1 virus using a sandwich-based aptamer assay | Tseng et al. (2016) |
| 3 | RNA aptamer | Diagnosis of FMD virus | Ellingham et al. (2006) |
| 4 | RNA aptamer | Detection of Shiga toxins of E. coli. | Challa et al. (2014) |
| 5 | RNA aptamer | Detection of capsid protein of the Porcine circovirus type 2. | Yoon et al. (2010) |
| 6 | RNA aptamer | Development of aptamers which specifically targets ATR/TEM8 Von Willebrand factor type A (VWA) domain. | Lee et al. (2015) |
| 7 | 20′-F-RNA aptamer | Detection of E. coli strains | So et al. (2008) |
| 8 | 20′-F-RNA aptamer | Detection of E. coli using dual strategy | Lee et al. (2009) |
| 9 | RNA Spiegelmer | NOX-B11-3, a RNA aptamer can suppress appetite by specifically binding to orexigenic hormone ghrelin and blocking its activity. | Becskei et al. (2008) |
| 10 | RNA Spiegelmer | Used to block the activity of anorectic and dipsogenic pancreatic hormone amylin. | Bilik et al. (2007) |
| DNA aptamers | |||
| 11 | DNA aptamer | Detection of avian influenza viruses | Lee et al. (2020) |
| 12 | DNA aptamer | Detection of H5N1 avian influenza virus | Wu et al. (2008) |
| 13 | DNA aptamer | Detection of triple reassortant influenza A virus | Wongphatcharachai et al. (2013) |
| 14 | DNA aptamer | Diagnosis of Bovine herpesvirus 1 | Xu et al. (2019) |
| 15 | DNA aptamer | Detection of FMD virus | Vidic et al. (2017) |
| 16 | DNA aptamer | Competitive fluorescence resonance energy transfer (FRET)-aptamer-based strategy for foot-and-mouth disease (FMD) | Bruno et al. (2008) |
| 17 | DNA aptamer | Detection of E. coli strains K88 | Li et al. (2011) |
| 18 | DNA aptamer | Detection of Salmonella enterica serovar. | Joshi et al. (2009) |
| 19 | DNA aptamer | Nanoparticle-based two-stage aptasensing platform for Campylobacter jejuni and Campylobacter coli. | Kim et al. (2019) |
| 20 | DNA aptamer | Detection of a TB biomarker HspX in sputum. | Lavania et al. (2018) |
| 21 | DNA aptamer | Detection of prion diseases | Wang et al. (2011) |
| 22 | DNA aptamer | Detection of prion protein on a solid-phase membrane. | Hossain et al.(2010) |
| 23 | DNA aptamer | Detection of prion protein with very high sensitivity. | Wang et al. (2012) |
| 24 | DNA aptamer | Detected a prion protein of BSE in the CNS. | Antipin and Nadtochey (2019) |
| 25 | DNA aptamer | Prion proteins (PrP) detection through the formation of T-Hg(2+)-T configuration | Xiao et al. (2012) |
| 26 | DNA aptamer | Electrochemical aptasensor for the detection of sulfadimethoxine in veterinary medicine and milk. | Bai et al. (2019) |
| 27 | DNA aptamer | Competitive voltammetric aptasensor for azlocillin β-lactam antibiotic. | Chinnappan et al. (2020a) |
| 28 | DNA aptamer | Detection of Bovine herpesvirus 1 | Xu et al., 2019, Xu et al., 2017 |
| ssDNA aptamers | |||
| 29 | ssDNA aptamer | Detection of SARS coronavirus nucleocapsid protein | Cho et al. (2011) |
| 30 | ssDNA aptamer | HN protein of Bovine Parainfluenza Virus type 3 | Cheng et al. (2018) |
| 31 | ssDNA aptamer | Detection of avian influenza H5Nx whole viruses | Nguyen et al. (2016) |
| 32 | ssDNA aptamer | Detection of Muscovy duck parvovirus | Lu et al. (2018) |
| 33 | ssDNA aptamer | Detection of Staphylococcus aureus | Baumstummler et al. (2014) |
| 34 | ssDNA aptamer | An enzyme linked aptamer for the detection of P48 protein of M. bovis | Fu et al. (2014) |
| 35 | ssDNA aptamer | Detection of TB from sputum samples | Rotherham et al. (2012) |
| 36 | ssDNA aptamer | Analytical assay for detection of brevetoxin. | Tian et al. (2016) |
| 37 | ssDNA aptamer | Combined the aptamer with electrochemical SPR to develop an aptasensing platform for detection of ampicillin in real time. | Blidar et al. (2019) |
| 38 | ssDNA aptamer | Aptamer based enzyme immunoassay was developed for the determination of enroflaxacin | Ni et al. (2014) |
| 39 | ssDNA aptamer | Developed an aptameric biosensor for the sensitive detection of tropomyosin allergen. | Chinnappan et al. (2020b) |
| Others | |||
| 40 | Self-quenched probe based strategy | Sensitive detection of Pseudorabies virus | Shen et al. (2018) |
| 41 | Mannose-modified fluorescent carbon quantum dots (Man–CQDs) | Detection of bacteria using novel dual recognition strategy | Cheng et al. (2016) |
| 42 | NH2 based aptamer | Impedimetric aptasensor for ultrasensitive detection of Pseudomonas aeruginosa | Roushani et al. (2019) |
| 43 | |||
| 44 | Biotin tagged aptamer | Mastitis detection | Ashley and Li (2013) |
| 45 | Colorimetric biosensor | Detection of plasmin as biomarker in case of mastitis | Chinnappan et al. (2017) |
| 46 | Colorimetric aptasensor | Detection of E. coli strains | Wu et al. (2012) |
| 47 | Fluroscent probe system | Quantum dot-based aptamer beacon for tumor cells. | Hwang et al. (2016) |
| 48 | Electrochemical biosensor | Direct detection of E. coli O111. | Luo et al. (2012) |
| 49 | Photoelectrochemical (PEC) immunosensor | Nanoparticles based aptamer for detection of Ochratoxin A. | Chen et al. (2018) |
| 50 | SPR biosensor | Whole bacteria detection | Ahn et al. (2018) |
| 51 | Biotin labeled DNA | For detection of Salmonella enterica serovar Typhimurium | Dwivedi et al. (2013) |
| 52 | Prion protein aptamer | Determination of BoIFN-γ in TB | Crulhas et al. (2017) |
| 53 | Specific aptamers of antibiotics | Specific aptamers for detection of antibiotics such as Florfenicol, Cefquinome, Kenamycin, Oxytetracyclin, Tetracyclin and Chloramphenicol. | Sadeghi et al., 2018, Wang et al., 2018, Xu et al., 2015, Zhou et al., 2018, Yan et al., 2018 |
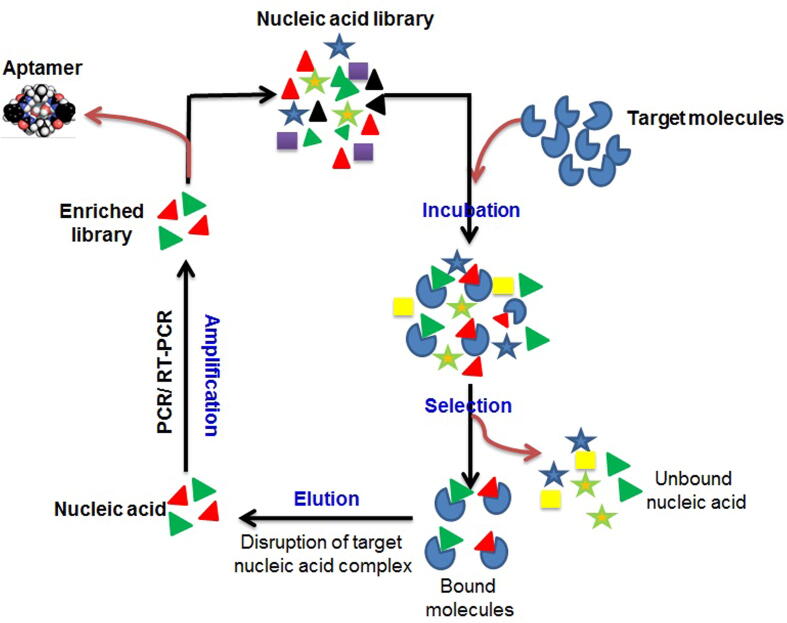
The conventional SELEX (Systematic Evolution of Ligands by Exponential Enrichment) technology was described by Tuerk and Gold (1990). Formation of aptamers by this method lasts from a few weeks up to a month. A library of oligonucleotides containing unique but random sequences was designed by chemical method used to select aptamers and usually used to hybridize primers during polymerase chain reaction (PCR). Many steps of selection introduced during the conventional method of SELEX solely depend on the type of the desired aptamer (Fig. 1).
Fig. 1.
Schematic representation is showing traditional SELEX procedure. A random nucleic acid (RNA/DNA) library is incubated with a target molecules and then unbound molecules are separated from the bound molecules by the process of selection. Eluted nucleic acid are amplified by PCR and serve as an enriched library for the next cycle.
Nucleic-acid aptamers are paying attention towards intense interest and have found various applications in different areas. Extensive research is available on applying aptamers in the diagnosis of diseases in human beings, but very limited data is available in veterinary medicine. Therefore, this review is aimed to focus on the application of aptamers in the diagnosis of animal diseases, detection of drug residues and contaminants, and targeted drug delivery.
2. Application of aptamers in diagnosis of animal diseases
There are various bacterial (e.g. mastitis, tuberculosis, enteritis or colibacillosis, tuberculosis etc.) and viral diseases (e.g. SARS CoV, influenza, FMD etc.) that have a high prevalence rate in animals and are affecting farmers' economies in many ways (Mendes et al., 2020, Puerto et al., 2021, Tsouloufi et al., 2020). Other conditions, such as bovine spongiform encephalopathy (BSE), cancer, and toxins, play important roles in veterinary medicine in addition to infectious diseases.
2.1. Diagnosis of viral diseases
2.1.1. Severe acute respiratory syndrome corona virus (SARS-CoV)
2.1.1.1. Influenza
Influenza viruses have various subtypes such as H1N1, H2N2, H5N1, H7N2, H9N2, which has global concern, and there is a need to develop methods for the early detection of these viruses to prevent loss in poultry and other agricultural sectors. The use of aptamers for the diagnosis of the virus has been extensively studied. Several aptamers have been reported to be effective in several viruses such as Muscovy duck parvovirus (Lu et al., 2018), H5Hx influenza viruses (Nguyen et al., 2016), H5N1 avian influenza virus (Wu et al., 2008), Triple reassortant influenza A virus (Wongphatcharachai et al., 2013), Bovine herpesvirus 1 (Xu et al., 2019), pseudorabies virus (Shen et al., 2018) etc.
In this connection, Cheng et al. (2018) proposed an indirect enzyme-linked aptamer assay (ELAA) for the detection of viruses in cattle. They developed ssDNA aptamers that can rapidly detect HN protein of Bovine Para-influenza Virus type 3 with high affinity and specificity. The positive detection rate of the ELAA was higher than that of the commercial ELISA kits, and the coincidence rate of ELAA and ELISA was 88%. A real time immuno-polymerase chain reaction (RT-I-PCR) method has also been developed for detecting viruses such as H9N2 influenza virus in poultry. In the assay, aptamers are applied as ligands to capture and detect the virus with higher sensitivity than standard ELISA (Hmila et al., 2017).
The particular diagnosis of avian influenza H5N2 whole viral particles has also been possible using a cognate pair of sandwich-type aptamers. In this strategy, a pair of aptamers were selected for whole avian influenza virus particles of H5N2 as the analyte of interest by using graphene-oxide-based SELEX (GO-SELEX) (Kim et al., 2019). The lateral flow strips could detect the particles as low as 6 × 105 in the buffer and 1.2 × 106 in the duck's feces by direct examination. Such aptamer-based test strips can thus, be useful for rapid screening and preventing pandemics. Sandwich-type SPR- based aptasensor has also been used to diagnose avian influenza H5Nx whole viruses (Nguyen et al., 2016). Seo and Gu (2017) have also critically reviewed the literature on aptamer-based sandwich-type biosensors.
Several studies have also used electrochemical aptasensors for the detection of avian influenza viruses. To avoid the electrochemical signal-based errors due to external interferences, Lee et al. (2020) utilized a self-calibrating dual-electrode system based electrochemical aptasensor to detect avian influenza viruses. They fabricated two electrodes using tungsten rods, and methylene blue was used as an electron mediator. One electrode was capped with the virus-specific aptamer, whereas the second electrode was capped with a control aptamer. This dual-electrode platform exhibited excellent output-signal stability compared to a conventional single-electrode platform and did not require purification and washing steps. Thus, using such dual electrodes for the detection of electrochemical signals from aptasensors offers a reliable and robust platform for detecting molecules.
A new approach was adopted for fluorescence-based detection of H1N1 virus by sandwich-based aptamer assay (Tseng et al., 2016), which is comparatively much faster than the conventional viral culture.
2.1.2. Foot and mouth disease (FMD)
FMD mainly affects the animals such as swine, cattle, goats, deer, and sheep. FMD affects extensive areas worldwide enlisted in the notifiable diseases to the World Organization for Animal Health (http://www.oie.int/eng/en index.htm). Since it was recognized as a significant epidemic disease in 16th century to yet it is a major global animal health problem (Longjam et al., 2011). FMD has caused numerous outbreaks in the US, UK and Asia including India. Owing to significant economic losses there is an urgent need to develop a fast and most unswerving test for the detection of FMD virus. In order to detect this disease, DNA aptamers were successfully screened (Vidic et al., 2017).
Aptamers have also been used for selective detection of pathogenic viruses. Bruno et al. (2008) developed a FRET based novel aptamer for FMD and labelled it with Black Hole Quencher-2 and Alexa Fluor 546-14-dUTP dye. The detection principle was based on competitive fluorescence energy transfer (FRET) and could detect FMD within 10 min with a sensitivity of 25–250 ng/mL of a FMD peptide. Ellingham et al. (2006)have been selected RNA-dependent RNA polymerase (3Dpol) enzyme for RNA aptamers specific to FMD.
2.1.3. Severe acute respiratory syndrome corona virus (SARS-CoV)
The recent pandemics and epizootics in various parts of the world have highlighted the public health importance of viruses such COVID-19 (SARS-CoV-2), influenza virus, herpes viruses etc. At the beginning of January 2020, a novel coronavirus (COVID-19) was identified in Wuhan, China as a causal agent of an outbreak of respiratory disease. SARS-CoV-2 (Severe Acute Respiratory Syndrome - Coronavirus-2) is a member of Coronaviridae, which is the responsible agent for COVID-19. SARS-CoV-2 is an enveloped virus measuring approximately 50–200 nm in diameter, having single strand RNA genome consists of 26–32 kb in length (Lu et al., 2020, Xu et al., 2020). WHO declared this disease a pandemic on 11 March 2020. COVID-19 has presently spread in the 222 countries/territories with the death of 3.27 millions out of total positive cases, which accounts for more than 156.75 millions upto 07 May 2021. While in India alone, 2.15 millions are infected, and 0.234 millions deaths have been reported. As per reports, strains of SARS-CoV-2 are showing mutations in different countries. A recent study at Peking University in Beijing reported two types of strains: “L-type” and “S-type”. It has been suggested L-type strain is “more aggressive“ and jumped from animals to humans.
Few studies have reported the possible connections with animals in the origin of SARS-CoV-2 and reverse zoonosis. However, a recent study in China investigated the possibilities of the vulnerability of ferrets and animals in close connection with humans to SARS-CoV-2(Shi et al., 2020). They found that replication of SARS-CoV-2 is very less in dogs, pigs, chickens, and ducks but efficiently in ferrets and cats. It has also been reported that the virus spreads in cats via respiratory droplets. Wildlife Conservation Society releases one report, the USA on 05 April 2020 that a tiger at the Zoo found positive for COVID-19.
Recently, WHO conducted a two-day meeting on COVID-19 and highlighted various issues related to the importance or possible linkage of COVID-19 with animals (WHO, 2020). Various researchers have also tried to develop aptamers for the diagnosis of SARS-CoV. Ahn et al. (2009) have been developed a RNA aptamer, is capable to bind with N protein with high affinity with a K(d) of 1.65 nM (nano Molar), using a SELEX and recombinant N protein. K(d) value denotes the equilibrium dissociation constant between the antibody and its antigen. The strength of a given interaction can be judged through the association constant K or the dissociation constant Kd. The nucleocapsid protein binding ssDNA aptamer was developed with Kd value of 4.93 ± 0.30 nM by SELEX procedure (Cho et al., 2011).
The aptamer can potentially be used in the diagnosis of COVID19 in humans as well as animals. A recent paper on POC diagnostic devices for rapid detection of novel coronavirus (SARS-nCoV19) has been reviewed all possible recent diagnostic tests including aptamer/biosensors based tests for COVID19 (Rao, 2021). About 900 SARS-nCoV-2 test kits are in pipeline, among 395 test kits are molecular bested test kits and only few test kits are developed using Aptamer technology https://www.finddx.org/covid-19/pipeline/. (Rao, 2021).
Rapid microbiology has developed “Pinpoint's Low-Cost Handheld COVID-19 Aptamer-based Diagnostic Device” which is an accurate COVID-19 diagnostic test that non-clinicians can execute in human being. Moreover, there is no widespread of COVID19 in the farm and pet animals except few studies of possible transmission from humans to animals; hence research in this direction is still awaited. Chen et al. (2018) have developed a novel method for the detection of SRAS-CoV-2N protein using DNA based aptamers. However, the diagnostic performance, such as sensitivity and specificity needs further investigation.
2.2. Diagnosis of bacterial diseases
2.2.1. Mastitis
Bovine mastitis can be well-defined as ‘inflammation of mammary gland’ and is one of the most common and dreadly diseases of dairy cows and poses substantial economic loss to dairy industry. The estimated total economic loss of US$ 2 billion per year in the United States of America Viguier et al. (2009) due to mastitis. The estimated annual economic loss due to both subclinical and clinical mastitis in India was US$ 98,228 million [7165.51 crore Indian Rupees] (Bansal and Gupta, 2009). It is among the most critical diseases of cattle and buffalo because it causes numerous complications to milk production, processing of milk and quality of milk and its products, resulting in severe economic losses to the dairy industry. Mastitis is caused by more than 200 infectious microorganisms and commonest pathogens are Staphylococcus aureus, E. coli, Streptococcus spp., Pseudomonas spp., Mycoplasma spp. etc. (Sharma and Maiti, 2010, Sharma et al., 2012, Zhang et al., 2009).
Mastitis poses major problem in dairy farms regardless of various prevention and control methods (Sharma et al., 2018). Delay in diagnosis and treatment of mastitis are the major factors in the failure of treatment protocols (Down et al., 2017, Vasquez et al., 2017). It has been shown that timely and accurate diagnosis of mastitis is a major key for prevention and control of mastitis and its effective treatment (Down et al., 2016). Various methods of diagnosis of mastitis have been developed (El-Sayed et al., 2017). Current methods for detecting clinical and particularly subclinical mastitis (e.g., California mastitis test, somatic cell count, and bacteriological culture) (Sharma et al., 2018, Sharma et al., 2011) are either insufficient, have low accuracy, or have low specificity (Robles et al., 2021, Windria et al., 2021, Zecconi et al., 2021). The various conformist methods for detection and identification of pathogens are generally either time-consuming or expensive for the dairy farmers at their farms in field conditions. Recent advances and developments in micro- and nanotechnologies have opened the window for the development of biosensors (Martins et al., 2019).
The sensor-based diagnostic systems help in detecting the intramammary infection with minimal stress on the animal (Martins et al., 2019). The various emerging technologies, including transcriptome and proteome analyses and nano- and microfabrication of handy portable devices, offer promising, effective methods for early diagnosis of mastitis (Duarte et al., 2015). Change et al. (2016) developed aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters for the detection of bacteria. Baumstummler et al. (2014) identified SOMAmer (slow off-rate modified aptamer) to detect S. aureus. SOMAmers are made from ssDNA that contain pyrimidine residues and have quite long (>30 min) dissociation rates (Gold et al., 2010). They found that SpA and ClfASOMAmers are useful for the selective detection of S. aureus. Another study used ELONA (Enzyme-Linked OligoNucleotide Assay) to detect the Protein A-binding aptamer PA#2/8 in S. aureus (Stoltenburg et al., 2016). Roushani et al. (2019b) have also developed an impedimetric aptasensor for ultrasensitive detection of Pseudomonas aeruginosa using a glassy carbon electrode. The glassy carbon electrode was modified by electro-deposition of silver nanoparticles and NH2aptamer was covalently attached to its surface. Using such an aptamer-coated electrochemical probe, P. aeruginosa could be detected in the range of 102–107 CFU/mL with a detection limit of 33 CFU/mL in serum samples.
Mycoplasma bovis (M. bovis) is a disease causing agent that causes mastitis, respiratory diseases and arthritis in cattle. Mycoplasmas do not have cell wall, which is the reason that many antibiotics are not effective against Mycoplasma. Various methods such as culture, FAT, and PCR are used for the diagnosis of Mycoplasmosis, but recently developed biosensors can detect Mycoplasma infection rapidly, easily and with a high specificity level. In a study, ssDNA aptamers were selected against P48 protein of M. bovis with high affinity and specificity (Fu et al., 2014).
During the inflammation of mammary gland various enzymes are released from the inflammatory cells, which may be used as mastitis markers. Guliye et al. (2002) emphasized that high levels of enzyme activity related to quality of milk are associated with damaged udder cells and have been used as an evidence of the degree of udder inflammation. Catalase acts as an indicator of increased milk somatic cell count (SCC) and mastitis, and also works as an antioxidant in the milk. It was demonstrated that catalase activity can be used as a marker of mastitis (Fox and Kelly, 2006).
An aptamer-based surface plasmon resonance (SPR) biosensor with a detection limit of 20.5 nM has been developed for detecting catalase in milk as a mastitis marker (Ashley and Li, 2013). Aptamer tagged with biotin was immobilized onto a gold platform by affinity capture. While Ashley et al. (2012) developed catalase aptamer by non-SELEX method for detecting catalase in bovine milk with a KD value in the low micro molar range. A recent study of Chinnappan et al. (2017) developed magnetic nanoparticles-based colorimetric biosensor assay in which plasmin was used as biomarker with a high sensitivity (1 ng/mL).
2.2.2. Enteritis causing bacteria
Colibacillosisis considered as the most important problems faced in livestock production, causing significant losses related to economy in dairy farming, which is mainly caused by Escherichia coli (Bashahun and Amina, 2017). Some pathogenic strains of enteric bacteria cause various diseases in livestock and poultry such as gastrointestinal diseases, diarrhoea, mastitis, urinary tract infections, meningitis, septicaemia etc. In poultry, E. coli causes colibacillosis, which is categorized by the relocation of the virulent strains (e.g. O78:K80, O1:K1, O2:K1) from intestinal tract to other organ systems such as respiratory or urinary tracts. This infection in poultry has negative impact on egg production, chicken growth and increases mortality of birds and causes severe economic losses (Vidic et al., 2017). An important challenge in the diagnosis of E. coli is to differentiate between the closely related pathogenic and non-pathogenic species.
There are many conventional methods to detect the presence of E. coli, and various assays have been developed to detect these strains at the herald of the diseases. The conventional method to detect E. coli usually takes two to three days (Liu et al., 2020, Rani et al., 2021). This method is not enough to characterize the bacteria, and additionally requires bacterial identification either by PCR (Rajendhran and Gunasekaran, 2011), 16S rRNA sequencing, or by using biological probes, including antibodies and aptamers (Gopinath et al., 2014). These methods show drawbacks in terms of long identification time (typically few days), high labor and costly reagents (Fournier-Wirth et al., 2006).
Screening of E. coli strains using aptamer –functionalised Single-Walled carbon nano tube field effect transistors have been reported (So et al., 2008). Dual strategy combining immunomagnetic separation and real time PCR amplification of aptamers to detect E. coli was manufactured (Lee et al., 2009). New method for competitive FRET- aptamers applied to E. coli assay development (Bruno et al., 2010). Another group developed electrochemical based aptasensor for the detection of E. coli directly and calorimetrically (Wu et al., 2012). The DNA aptamers have also been developed for detection of E. coli strains K88 through binding with fimbriae protein of enteropathogenic E. coli (Li et al., 2011). Luo et al. (2012) have developed a sensitive and specific electrochemical biosensor for direct detection of E. coli O111, while showed less sensitivity to other bacteria such as Salmonella typhimurium, S. aureus and non-pathogenic E. coli.
Ahn et al. (2018) adopted whole-bacteria SELEX (WB-SELEX) strategy to isolate specific aptamer and develop surface plasmon resonance (SPR) biosensor. The SPR is highly sensitive to various molecular binding processes occurring onto planar metal surface or metal nanoparticles and is the basis of many label-free biosensors and lab-on-chip sensors. When combined with specific aptamers, the developed biosensor could specifically and selectively discriminate pathogenic Vibrio parahaemolyticusbacteria from other enteric species such as Escherichia coli, Listeria monocytogenes, Sigellasonnei, and Vibrio fischeri. Such SPR biosensors demonstrate the feasibility of using DNA aptamer platform for developing POC diagnostics. The WB-SELEX strategy has also been used for development of aptamers to capture and detect Salmonella enterica serovar Typhimurium (Dwivedi et al., 2013). The aptamer showed high binding affinity with Kd value of 1.73 ± 0.54 μM and low cross-reactivity with other food borne bacteria. In another study, Joshi et al. (2009) developed a DNA-based aptamer to detect Salmonella enterica serovar Typhimurium by selective enrichment against outer membrane proteins (OMPs).
A number of efforts have also been made to make the aptamer-based technologies label free and capable of being used at POC. Kim et al. (2019) developed a gold nanoparticle-based two-stage aptasensing platform for fast and on-site detection of Campylobacter jejuni and Campylobacter coli and detected by the change of colour from red to purple. The aptasensor were found to be highly specific when tested on chicken samples. Such calorimetric detection methods can provide an excellent and rapid screening tool for on-site detection of bacteria and may possibly be extended to other bacterial species. A novel aptamer-based strategy has recently been developed for label-free, in situ detection of bacteria by utilizing surface-enhanced Raman scattering (SERS). The SERS signals generated by bacteria-aptamer on silver nanoparticles should linear dependence on bacterial concentration and could be used to recognize bacteria quickly with a detection limit of 1.5 CFU/mL (Gao et al., 2017). Thus, this strategy offers an opportunity to create SERS-based biochips for quick in-situ detection of bacterial.
2.2.3. Tuberculosis
Bovine tuberculosis (BT) is a slow progressing disease and primarily caused by the Mycobacterium bovis (M. bovis), and to a lesser extent by M. caprae and M. tuberculosis. Bovine tuberculosis is affecting the livelihoods and is hampering efforts to control and/or elimination by 2030 (WHO, 2018). The traditional diagnostic test i.e. tuberculin skin test, is a standard and the most popular test in the livestock. However, the bacterial culture of M. bovis is a time-consuming process and can take eight weeks or more. Aptamers are hopeful tools for developing POC diagnostic assays for TB. Mozioglu et al. (2016) developed ssDNA aptamers that recognize M. tuberculosis H37Ra using SELEX procedure.
Bovine tuberculosis caused by M. tuberculosis is difficult to be identified by the pathological symptoms. Release of bovine interferon gamma (BoIFN-γ) by T-cells can offers an diagnostic marker of M. tuberculosis (Crulhas et al., 2017). They developed an electrochemical aptasensor for sensitive and specific determination of BoIFN-γ. In this study, this biosensor can be used for on-site detection bovine TB (Crulhas et al., 2017). In another study, Rotherham et al. (2012) developed ssDNA aptamers to detect TB from sputum samples. It has reported that CSIR 2.11 aptamer have affinity to bind with CFP-10.ESAT-6 was developed to detect BT (Rotherham et al., 2012). Lavania et al. (2018) developed two DNA aptamer-based diagnostictests, namely aptamer linked immobilized sorbent assay (Aptamer ALISA) and electrochemical sensor (ECS), for the direct detection of a TB biomarker HspX in sputum.
2.3. Diagnosis prions diseases
Prion disease is a lethal and tragic neurodegenerative condition that affects both humans and animals. Transmissible spongiform encephalopathy is the common term assigned to all known prion diseases. Prions are infectious particles that lead Gerstmann-Straussler-Scheinker syndrome in humans, bovine spongiform encephalopathy (BSE) in cattle and there is a link between the transmission of prion diseases in humans and cattle (MacGregor, 2001). Prion diseases are neurodegenerative in nature and there is a great demand for sensitive and rapid diagnostic tools for these particles.
DNA aptamers have been used to detect transmissible spongiform encephalopathies in animals. The assay is based on their ability to detect conformationaly normal prion protein from abnormal isoforms in prion diseases. Using SELEX enrichment, Wang et al. (2011) identified DNA aptamers, which can distinguish normal and abnormal PrP isoforms. The aptamer can even distinguish different strains of prions in blood samples, which are usually not detectable by antibody-based detection procedure. Researchers have developed a high-throughput, inexpensive and convenient method for a specific and sensitive determination of prion protein on a solid-phase membrane (Hossain et al., 2010).
In another approach, a cellular prion protein (rhPrPC) based detection platform was developed using magnetic and gold-coated magnetic nanoparticles as support material (Kouassi et al., 2007) biotinylated aptamer. An effective aptamer-mediated chemiluminesence detection of prion protein has been developed (Zhang et al., 2014). More research led to the development of robust aptasensor for the detection of prion protein with very high sensitivity (Wang et al., 2012). Antipin and Nadtochey (2019) detected a prion protein of BSE in the CNS of BSE affected cow by using DNA aptamers specifically binded with amyloid epitops. A study established a sensitive method for the development of DNA aptamer for prion proteins (PrP) detection through the formation of T-Hg(2+)-T configuration (Xiao et al., 2012).
2.4. Diagnosis of cancer
In recent year, efforts have also been made to use aptsensors for diagnosis of cancer. Hwang et al. (2016) have applied quantum dot-based aptamer beacon for fluorescent imaging of circulating tumor cells. In this strategy, aptamers were designed for binding sequences of EpCAM/MUC1, which are known to be expressed in circulating metastasizing cancerous cells. The aptamers were labelled with Black Hole Quencher-2 and Cy5.5 fluorescent dye and, conjugated to quantum dots as aptamer linker beacon. The binding of aptamer to the EpCAM/MUC1 of circulating cancerous cells triggers the dissociation of quencher and generates fluorescence signal of Cy5.5 for its detection. The aptamer beacon was successfully tested in Panc02-implanted mouse model for the rapid monitoring of the circulating tumor cells.
Aptamers have also been explored for detection of blood analytes and replace biochemical analytical methods. One such example is the development of RNA aptasensor for detecting cyclic adenosine monophosphate (cAMP), which is related with the healthy state of the animal. Zhao et al. (2015) developed an RNA aptamer having principle of electrochemical impedance spectroscopy- enhanced by gold nanoparticles electrodeposited on the surface of gold electrode which can detect cAMP in a wide range with a detection limit of 50 nM in serum. Importantly, the aptasensor could be fabricated in cost effective way and could be used multiple times thereby demonstrating great potential as POC device for clinical test.
3. Sensing of toxins
Several approaches have also been made to find out selective aptamers for detecting bacterial, fungal and plant toxins. In a study, Challa et al. (2014) developed a RNA aptamer against Shiga toxins of by E. coli, which is known to cause life threatening hemolytic uremic syndrome. The RNA aptamers could block the binding of Shiga toxins to cells. Unfortunately, the developed aptamers could not neutralize the toxin-mediated cytotoxicity and death of the cells. An aptamer-based aptasensor for detecting Ochratoxin A has also been developed. Chen et al. (2018) hybridized Ochratoxin A aptamer onto dual gold nanoparticles and immobilized onto an electrode for electrochemical signal reporting. Due to high surface-to-volume ratio and excellent electro-conductivity of gold nanoparticles, the developed aptasensor was ultrasensitive to as low target concentration as 0.001–5000 ppb over six orders of magnitude. Tian et al. (2016) developed an aptamer-based analytical assay for detection of brevetoxin. The aptamers had a dissociation constant of 4.83 μM and IC50 of 73.81 ng/mL. The assay was as efficient as commercial ELISA.
Toxins present in feed and fodders are occasional cause of toxicity and death in animals. One such example is Ricin toxin resulting from the castor bean plant. The Ricin toxin is highly thermostable, active at acidic and alkaline pHs and is known to cause animal death due to toxicity. Aptamers against the B-chain of ricin was shown to detect ricin B in several liquid food and outcompeted ELISA kit with a detection limit of 25 ng/mL (Lamont et al., 2011). The aptamer could also be combined with surface enhanced Raman scattering technique for label free detection. Thus, such aptamers can serve as pre-analytical tool for screening of toxins in animal feed and fodder. Zhang et al. (2018) developed a fast and cost-effective ssDNA aptamer to detect zearalenone in animal feed with a detection limit of 12.5 nM.
4. Application of aptamers on detection of drug residues and contaminants
Aptamers have also been used for the purpose of detecting drug remains in milk, meat and farm waste. They have also been used successfully for detection of contaminants in drugs, allergens or environmental residues in various animal products. In particular, the rampant usage of antibiotics in animal feeds and veterinary medicine had led to their widespread presence in various animal products and environment. Entry of these antibiotic residues into food chain has resulted in development of several new bacterial strains with multiple antimicrobial resistance and allergic reactions. Thus, identification of antibacterial residues is an important research area for animal industry to ensure consumer safety. A number of rapid screening tests have been developed for detection antibiotic residues in foods of animal origin and are based on immunological tests or physico-chemical methods. However, each of these tests suffers from disadvantages such as cost, specificity and reliability. Thus, several studies have investigated the applications of aptamers as possible tool for prompt and specific detection of antibiotic residues. Bai et al. (2019) have prepared a DNA aptamer-based electrochemical aptasensor to detect sulfadimethoxine in veterinary drugs and milk. In their unique approach, a sulfadimethoxine- specific DNA aptamer was developed, which upon binding with sulfadimethoxine, triggers the cleavage activity of nuclease PI to cause an electrochemical change that an electrode can detect. The developed aptasensor had a detection limit of 0.038 nmol/L and could specifically detect the sulfadimethoxine can with linear range of 0.1–500 nmol/L. A graphene-doped Bismuth sulphide nanorods has also been used as a visible-light photoelectrochemical aptasensing platform for detecting sulfadimethoxine in veterinary medicine and milk with a detection limit of 0.55 nM (Okoth et al., 2016). Blidar et al. (2019) combined the aptamer with electrochemical SPR to develop an aptasensing platform for detection of ampicillin in real time. The technique could detect ampicillin in the range of 2.5–1000 μmol/L with a limit of detection of 1 μmol/L. Such aptasensors could be useful for identifying the presence of ampicillin residues in the food and environment.
Specific aptamers have also been developed and used for detection of other antibiotics such as Florfenicol (Sadeghi et al., 2018), Cefquinome (Wang et al., 2018), Kenamycin (Xu et al., 2015), Oxytetracyclin (Xu et al., 2018), Tetracyclin (Zhou et al., 2018) and, Chloramphenicol (Yan et al., 2018), which allowed rapid and sensitive colorimetric detection by naked eye or using a colorimeter with detection limit of 0.0031 ng/mL. In order to detect chloramphenicol residues, Roushani et al. (2019a) developed an aptasensor by coating 3-aminomethyl pyridine functionalized GO on the surface of glassy carbon electrod, which was again coated with silver nanoparticle and chloramphenicol specific aptamer. The aptasensor allowed impedimetric ultrasensitive detection of chloramphenicol by glassy carbon electrode in the linear range of 1.0 pM to 1.0 nM with the detection limit of 0.3 pM in milk samples. DNA aptamer has also been used to construct a competitive voltammetric aptasensor for azlocillin β-lactam antibiotic (Chinnappan et al., 2020a). The developed aptasensor was highly selective, sensitive and cost-effective with a limit of detection of 1.2 pg/mL. Antibiotics such as enrofloxacin are widely used fluoroquinolone in poultry and are the main causative agent of bacterial drug resistance. A single stranded DNA aptamer based enzyme immunoassay was developed for the determination of enroflaxacin (Ni et al., 2014).
High doses of quinolones antibiotics are regularly used in livestock farming and they are one of the most commonly observed antibiotic residues in animal products and water bodies. Aptamer-based technologies have been shown to be a sensitive and effecting monitoring tool for quinolones. Reinemann et al. (2016) selected an aptamer pool, which could detect various quinolones such as ofloxacin with high specificity using the Capture-SELEX procedure. The aptamers were demonstrated to be effective in detecting ofloxacin in water and in effluents of sewage plants.
Components of animal products can cause allergic reaction to consumers and therefore, quick and sensitive methods for detecting various allergens in animal foods are essential to assist patients in managing their allergies. Currently, immunoassays, polymerase chain reaction and mass spectrometry are employed for analysing allergens. However, these methods are complex, costly and time consuming. Thus, aptamers are being investigated for rapid and specific detection of food allergen. Chinnappan et al. (2020b) developed anaptameric biosensor for the sensitive detection of tropomyosin allergen. The aptamer-based sensor detected the allergen within 30 min with a detection limit of 2 nM.
5. Application of aptamers in the targeted drug delivery and treatment of animal diseases
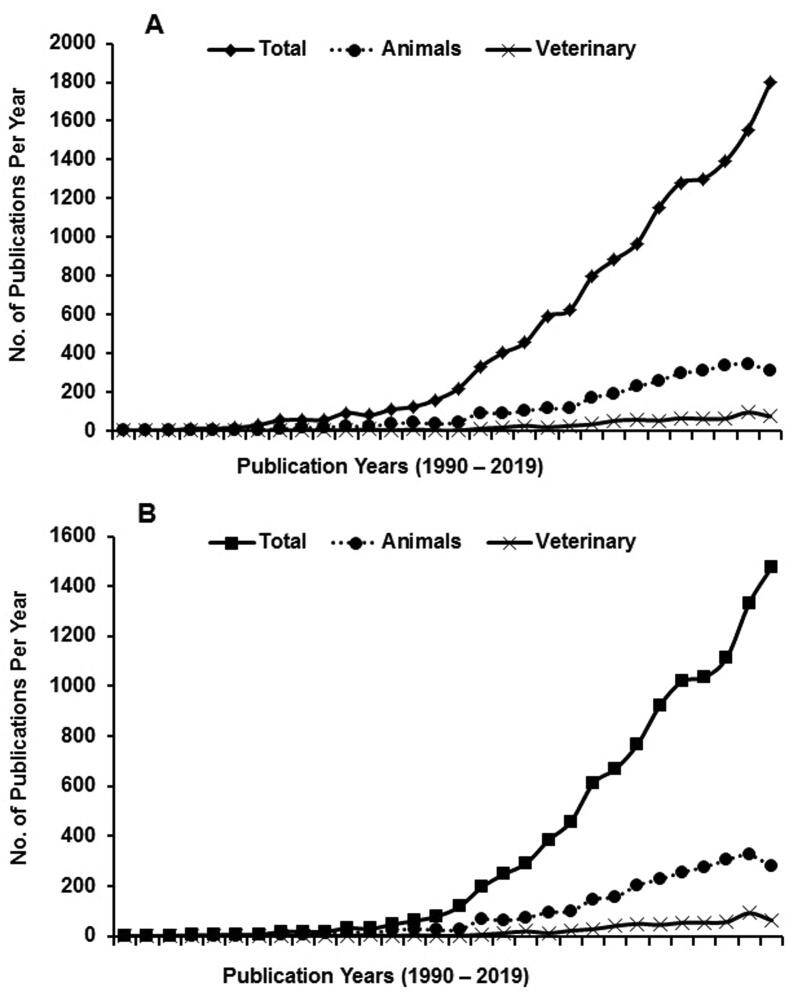
Current therapeutic strategies such as use of antibiotic or antiviral agents are ineffective in many cases and may even result in development of resistance upon their prolonged usage (Aminov, 2021, Caudell et al., 2020, Palma et al., 2020). Consequently, newer alternative therapeutic approaches are actively explored world-wide. In recent years, several oligonucleotide-based therapies such as short interfering RNAs (siRNA), microRNAs, aptamers, DNAzymes, and ribozymes have been used for diagnosis and/or treatment of viral diseases (Kim and Lee, 2021, Lundstrom, 2020). Among these nuclei-acid based approaches, DNA or RNA aptamers have been increasingly gaining wide attention due to their efficacy and specificity to pathogens and ease in their production by complete chemical synthesis methods. The first report on the use of aptamers for clinical application appeared in 1992 and since then it has been widely explored for human application using animal models (Fig. 2). Unfortunately, the development and application of aptamer-based diagnostics and therapeutics in veterinary field has been slow. Nevertheless, after successful demonstration on animal models, efforts are being made for application of aptamers on diagnosis and treatment of livestock and pet animals (Kim et al., 2020, Kim and Lee, 2021, Vidic et al., 2017). There are many studies of aptamers are going on in the different stages of testing and have provided researchers to address some of the disadvantages of antibodies (Table 2).
Fig. 2.
Number of publications on aptamers published per year during 1990–2019. A: Overall publications on aptamers; B: Publications on the use of aptamers for diagnosis or therapy. Total number of publications (any species) and publications exclusively on animals or veterinary science are shown separately. First publication on aptamer appeared on 1992 whereas its application in animal or veterinary science appeared first on 1993.
Table 2.
Important patents on the development and application of aptamers in veterinary diagnostics and therapeutics.
| No | Title | Inventors [Country] | Publication Number | Priority Date |
|---|---|---|---|---|
| 1 | Switch mode nucleic acid aptamer probe and uses thereof in detection of tumor living cell and living body | Guo Qiuping et al., [China] | WO2011134328A1 | 2010-04-26 |
| 2 | Nucleic acid aptamer-based diagnostic methods with novel techniques for signal enhancement | Nussbaum Ofer [USA] | WO2012004790A2 | 2010-07-06 |
| 3 | Carcinoembryonic antigen detection kit based on nucleic acid aptamer autocatalysis effect, and preparation method thereof | Zou Mingjing [China] | CN107064505A | 2016-02-18 |
| 4 | Aptamer-directed drug delivery | Bagalkot Vaishali et al., [USA] | WO2007137117A2 | 2006-05-17 |
| 5 | Method for detecting N-hydroxyacetylneuraminic acid content based on aptamer | Gong Sheng et al., [China] | CN108709989A | 2018-05-29 |
| 6 | Nucleic acid aptamer specifically binding to enrofloxacin or ciprofloxacin | Jeong Sang Hee et al., [South Korea] | KR101526921B1 | 2013-06-28 |
| 7 | Aptamer for binding bifidobacterium breve as well as screening method and application of aptamer | Chen Wei et al., [China] | CN105838719A | 2016-04-13 |
| 8 | Serum IgG antibody aptamer of tuberculosis patients and preparation method thereof | Cai Jiangli et al., [China] | CN103045600A | 2011-10-11 |
| 9 | Gene regulation with aptamer and modulator complexes for gene therapy | BUEHLER BERND et al., [UK] | US2005260164A1 | 2002-10-10 |
| 10 | Aptamers for prion diagnostics and aptamer binding detection system | Mohanty Sarina [USA] | WO2014008440A2 | 2012-07-04 |
| 11 | DNA aptamer binding to glyphosate with specificity | Gu Man Bock et al., [South Korea] | KR101338520B1 | 2011-10-25 |
| 12 | Aptamer ELISA detection kit, preparation method, and application thereof | DONG ZAIZAI et al., [China] | CN108957001A | 2018-06-27 |
| 13 | Research and development of aptamer-based rapid detection technology for agricultural veterinary drug residues | Ling Qingyun | CN109596605A | 2017-09-30 |
| 14 | Aptamer specifically binding to nonylphenol and detecting method using thereof | Kim So Youn [South Korea] and Ren Shuo [China] | KR101822881B1 | 2014-03-31 |
| 15 | Biosensing probe kit for detecting sulfadiazine on basis of nucleotide aptamer specificity, and application thereof | Sun Qi et al., [China] | CN107119054A | 2017-05-04 |
| 16 | Aptamer-based multiplexed assays | Katilius Evaldas et al, [USA] | SG10201701361WA | 2012-06-07 |
| 17 | DNA Insulin receptor-specific DNA aptamer and uses thereof | Ahn Ji Young et al., [South Korea] | KR101850793B1 | 2016-07-14 |
| 18 | Target substance detection method using aptamer | Kim So Youn [South Korea] | WO2010093223A2 | 2009-02-16 |
| 19 | Amine-terminated aptamer functionalized surface plasmon resonanace sensors, methods of making and methods of using same | Cameron Brent D et al., [USA] | US9562266B1 | 2012-01-14 |
| 20 | Nucleic acids aptamer binding specifically to cucurbitacin | Choi Tae Young et al., [South Korea] | KR101953994B1 | 2017-09-26 |
| 21 | Use of nucleic acid aptamer in alkaline phosphatase heterodimer recognition and binding or in tumor detection | Bing Tao et al., [China] | WO2019149115A1 | 2018-02-02 |
| 22 | Application of nucleic acid aptamer in recognizing and binding alkaline phosphatase heterodimer | Bing Tao et al., [China] | CN109554369A | 2018-02-02 |
| 23 | Nucleic acid aptamer capable of specifically binding to tetracyclines compound and use thereof | Gu Man Bock et al., [South Korea] | KR101342710B1 | 2012-02-14 |
| 24 | Aptamer nucleic acid probe kit for detecting doxycycline residue as well as preparation method and application thereof | He Hongqiu et al., [China] | CN104360077A | 2014-11-25 |
| 25 | Real-time and continuous measurement in vivo using aptamer-based biosensors | Arroyo Curras Netzahualcoyotl et al., [USA] | WO2017164982A1 | 2016-03-21 |
| 26 | Nucleic acids aptamer binding specifically to Ingenol mebutate | Choi Tae Young et al., [South Korea] | KR101987591B1 | 2018-08-06 |
| 27 | Antigen-binding fragment and/or aptamer for binding to an extracellular part of cd9 and therapeutic uses | Corbeil Denis et al., [USA] | WO2020044285A1 | 2018-08-29 |
| 28 | Escherichia coli o157:h7 aptamer and applications thereof | Amraee Masoum et al.,[Iran] | WO2018189639A1 | 2017-04-15 |
| 29 | Nucleic acid aptamer which specifically binds to bisphenol A | Kim So Youn [South Korea] | EP2397550A2 | 2009-02-16 |
| 30 | Method for detecting mycoplasma gallisepticum antibody of enzyme-linked nucleic acid aptamer and kit special for mycoplasma gallisepticum antibody | Wang Feng and Wu Wenxue [China] | CN109762825A | 2019-03-25 |
| 31 | Target substance detection method using aptamer | Kim So Youn [South Korea] | US2015212038A1 | 2009-02-16 |
| 32 | Nucleic acid aptamer capable of specifically binding to tebuconazole, mefenacet, and inabenfide, and use therof | Gu Man Bock et al., [South Korea] | WO2015056980A1 | 2013-10-16 |
| 33 | Aptamer inhibition of thrombus formation | Li Minyong et al., [China] | AU2009281857A1 | 2008-08-15 |
| 34 | Aptamer-based rapid kanamycin detection test paper as well as preparation method and application thereof | Liu Jing et al., [China] | CN106990237A | 2017-05-10 |
| 35 | Aptamer of enrofloxacin and preparation method and application thereof | Yu Xiaoyan et al., [China] | CN104450725A | 2015-01-05 |
| 36 | Aptamer-coated implant, process of production, and uses | Borck Alexander [Germany] | US2011150964A1 | 2009-12-21 |
| 37 | DNA EGFR-specific DNA aptamer as incubation additives for promotion of mammalian cell growth and uses thereof | Ahn Ji Young et al., [South Korea] | KR101859615B1 | 2016-07-14 |
| 38 | Aptamer-based device for detection of cancer markers and methods of use | Datta Bhaskar et al., [USA] | US2012088232A1 | 2010-09-30 |
| 39 | DNA PDGFR-specific DNA aptamer as incubation additives for promotion of mammalian cell growth and uses thereof | Ahn Ji Young [South Korea] | KR101833231B1 | 2016-07-14 |
Various aptamers have been shown to successfully treat various infectious and non-infectious diseases by specifically targeting the pathogen, hormone or, enzymes or by allowing targeted delivery of another drug. In recent years, antimicrobial peptides such as beta-defensin have emerged as a promising new class of antibacterial and antiviral drugs. Unfortunately, most exogenously administered antimicrobial peptides are unstable in biological fluids and have low penetrating ability into mammalian cells. Several researchers have used DNA aptamers for effective and rapid delivery of antimicrobial peptides into the cells.
Aptamers such as FO24 and FO21 have been tested as preventive and/or therapeutic antiviral therapy against viruses. Studies have shown that injection of FO24 and FO21 ssDNA aptamers for 24 h prior to inoculation with street rabies virus FJ strain into mice resulted in 60–80% survival rate (Liang et al., 2014a, Liang et al., 2013). The aptamers could be used for both pre-exposure as well as post-exposure prophylaxis but the pre-exposure prophylaxis was superior to post-exposure prophylaxis. These aptamers were specific to street rabies virus and did not the replication of other viruses such as canine distemper virus and canine parvovirus (Liang et al., 2014b). However, clinical trials have not yet been done to use them as therapeutic molecules. Similarly, Xu et al., 2019, Xu et al., 2017 demonstrated that specific DNA aptamers can inhibit the production of Bovine herpesvirus 1 through blocking nucleocytoplasmic shuttling and inhibiting the infectivity of the virus by blocking viral entry. The developed aptamers exhibited high affinity and specificity for Bovine herpesvirus 1 with a Kd value of 3.519 nM and significantly reduced viral replication. Thus, DNA aptamers could be used as a novel tool for the treatment of Bovine herpesvirus 1 infection in cattle. In yet another study, Yoon et al. (2010) developed RNA aptamers that bound to the capsid protein of the Porcine circovirus type 2 with nanomole affinity and neutralized the viral infectivity in PK-15 cells in dose dependent manner.
Aptamers have also been investigated as novel anti-prion compounds. It was shown that peptide aptamers binding to PrP(C) prion protein can abrogate prion propagation (Corda et al., 2018). Binding of the designed anti-prion aptamer with PrP(C) prion protein increased α-cleavage and interfered with its internalization and inhibited prior replication. Aptamers can also used for neutralization of bacterial, viral, fungal and plant toxins. Lee et al. (2015) developed RNA aptamers which specifically targets ATR/TEM8 Von Willebrand factor type A (VWA) domain. Such RNA aptamers were shown to have an inhibitor efficiency (IC50) of 5 μM and could block the binding of Protective Antigen (PA) to its receptors in order to neutralize anthrax toxin.
Aptamers have also been used for non-infections conditions such as hormonal imbalance, intestinal motility, modifying apatite or thirst etc. Becskei et al. (2008) showed that NOX-B11-3, a RNA aptamer (called RNA Spiegelmers) can suppress appetite during food deprivation by specifically binding to orexigenic hormone ghrelin and blocking its activity. The NOX-B11-3 could effectively block the effect of endogenous ghrelin on neuronal activity in the hypothalamic arcuate nucleus. Interestingly, the NOX-B11-3 acted only on animal under fasting and not on normal-fed animals. Similarly, RNA Spiegelmer has been used to block the activity of anorectic and dipsogenic pancreatic hormone amylin (Bilik et al., 2007). Such RNA Spiegelmer may be beneficial for the treatment of complications arises due to high amount of plasma amylin, as in cancer anorexia.
6. Conclusion
This review paper has put forward an outline of novel progresses in developing aptamer-based sensors to diagnose and treat major animal diseases. In the light of current limitations in common detection techniques of early disease diagnosis, researchers and scientists are turning their attention towards the development of aptamer based diagnosis for efficient rapid non-invasive detection of diseases in veterinary medicine. Aptamer based biosensors are attaining importance over other biosensors due to its several advantages. However, the development of biosensors for multiple disease detection is still a big challenge to the scientist and researchers. Integration of high multidisciplinary approaches and the use of nanomaterials in the development of aptamer biosensors will enhance the sensitivity of these devices and make it more efficient for early detection of disease, which is the need of the hour. In conclusion, while there are still some gaps in developing aptamers for clinical applications in veterinary medicine, aptamers will be widely used in early and most accurate disease diagnosis with the improvement of the relevant technologies.
7. Future perspectives and challenges in the application of aptamers
In veterinary medicine, there is still lot to be done for the developments in the diagnostic techniques to meet the demand of dairy farmers. We expect that this new field of aptamer-based biosensors in the diagnosis and treatment of animal diseases will eventually become a real-world tool. This would help in encountering challenges that could be difficult with currently available conventional technologies.
The success of aptamers in clinics depends on their stability in blood and other biological fluids upon injection into the body. The general use of aptamers is also hindered by their incapability of intracellular accumulation at the site of action. While several studies have shown success with the use of aptamers under in vitro conditions, their use in animal system requires special considerations. One such approach could be encapsulation of the aptamers for protecting them against nuclease activity of biological fluids. Bedi et al. (2013) established that fusion phage coat protein fpVIII, exhibiting cancer-targeting peptides, can commendably encapsulate siRNAs and could be used as nanophages to transport them into the cells leading to specific silencing of the model gene.
A yet another challenge in the application of aptamers is the complex optimization process of SELEX in screening and identifying the monoclonal aptamer candidate for target molecule of interest. Target molecules occasionally have isoforms or variants which may not be targeted by a single monoclonal aptamer, as is the case with FMD virus. The FMD virus has several strains and a monoclonal aptamer developed against conserved peptide region could selective identify few strains but were did not match the sensitivity of the polyclonal aptamer. Similarly, many toxins such as Shiga toxins have two antigenically distinct forms, which could not be identified by a single aptamer (Kaur et al., 2020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Department of Biotechnology, Government of India, New Delhi, India under the project grant No. BT/PR26666/AAQ/1/713/2017 and National Research Foundation project grant No.: 2017R1A2B2012125, Republic of Korea.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Neelesh Sharma, Email: drneelesh_sharma@yahoo.co.in.
Sung Jin Lee, Email: sjlee@kangwon.ac.kr.
References
- Ahn D.G., Jeon I.J., Kim J.D., Song M.S., Han S.R., Lee S.W., Jung H., Oh J.W. RNA aptamer-based sensitive detection of SARS coronavirus nucleocapsid protein. Analyst. 2009;134:1896–1901. doi: 10.1039/b906788d. [DOI] [PubMed] [Google Scholar]
- Ahn J.Y., Lee K.A., Lee M.J., Sekhon S.S., Rhee S.K., Cho S.J., Ko J.H., Lee L., Han J., Kim S.Y., Min J., Kim Y.H. Surface plasmon resonance aptamer biosensor for discriminating pathogenic bacteria vibrio parahaemolyticus. J. Nanosci. Nanotechnol. 2018;18:1599–1605. doi: 10.1166/jnn.2018.14212. [DOI] [PubMed] [Google Scholar]
- Aminov R. Acquisition and spread of antimicrobial resistance: a tet(X) case study. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22083905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antipin A.A., Nadtochey G.A. Detection of prion protein, the infection agent of bovine spongiform encephalopathy, by using aptamers. Russian Veterinary J. 2019;6:5–8. [Google Scholar]
- Ashley J., Ji K., Li S.F. Selection of bovine catalase aptamers using non-SELEX. Electrophoresis. 2012;33:2783–2789. doi: 10.1002/elps.201200032. [DOI] [PubMed] [Google Scholar]
- Ashley J., Li S.F. An aptamer based surface plasmon resonance biosensor for the detection of bovine catalase in milk. Biosens. Bioelectron. 2013;48:126–131. doi: 10.1016/j.bios.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Bai Z., Chen Y., Li F., Zhou Y., Yin H., Ai S. Electrochemical aptasensor for sulfadimethoxine detection based on the triggered cleavage activity of nuclease P1 by aptamer-target complex. Talanta. 2019;204:409–414. doi: 10.1016/j.talanta.2019.06.035. [DOI] [PubMed] [Google Scholar]
- Bansal B.K., Gupta D.K. Economic analysis of bovine mastitis in India and Punjab – a review. Indian J. Dairy Sci. 2009;62:337–345. [Google Scholar]
- Bashahun G.M., Amina A. In calves: a review of literature. J. Anim. Sci. Vety Med. 2017;2:62–71. [Google Scholar]
- Baumstummler A., Lehmann D., Janjic N., Ochsner U.A. Specific capture and detection of Staphylococcus aureus with high-affinity modified aptamers to cell surface components. Lett. Appl. Microbiol. 2014;59:422–431. doi: 10.1111/lam.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becskei C., Bilik K.U., Klussmann S., Jarosch F., Lutz T.A., Riediger T. The anti-ghrelin Spiegelmer NOX-B11-3 blocks ghrelin- but not fasting-induced neuronal activation in the hypothalamic arcuate nucleus. J. Neuroendocrinol. 2008;20:85–92. doi: 10.1111/j.1365-2826.2007.01619.x. [DOI] [PubMed] [Google Scholar]
- Bedi D., Gillespie J.W., Petrenko V.A., Jr., Ebner A., Leitner M., Hinterdorfer P., Petrenko V.A. Targeted delivery of siRNA into breast cancer cells via phage fusion proteins. Mol. Pharm. 2013;10:551–559. doi: 10.1021/mp3006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilik K.U., Ergüven E., Klussmann S., Jarosch F., Wielinga P.Y., Lutz T.A., Riediger T. In-vitro and in-vivo antagonistic action of an anti-amylin Spiegelmer. NeuroReport. 2007;18:1855–1859. doi: 10.1097/WNR.0b013e3282f1ab04. [DOI] [PubMed] [Google Scholar]
- Blidar A., Feier B., Tertis M., Galatus R., Cristea C. Electrochemical surface plasmon resonance (EC-SPR) aptasensor for ampicillin detection. Anal. Bioanal. Chem. 2019;411:1053–1065. doi: 10.1007/s00216-018-1533-5. [DOI] [PubMed] [Google Scholar]
- Bruno J.G., Carrillo M.P., Phillips T. Development of DNA aptamers to a foot-and-mouth disease peptide for competitive FRET-based detection. J. Biomol. Tech. 2008;19:109–115. [PMC free article] [PubMed] [Google Scholar]
- Bruno J.G., Carrillo M.P., Phillips T., Andrews C.J. A novel screening method for competitive FRET-aptamers applied to E. coli assay development. J. Fluoresc. 2010;20:1211–1223. doi: 10.1007/s10895-010-0670-9. [DOI] [PubMed] [Google Scholar]
- Caudell M.A., Dorado-Garcia A., Eckford S., Creese C., Byarugaba D.K., Afakye K., Chansa-Kabali T., Fasina F.O., Kabali E., Kiambi S., Kimani T., Mainda G., Mangesho P.E., Chimpangu F., Dube K., Kikimoto B.B., Koka E., Mugara T., Rubegwa B., Swiswa S. Towards a bottom-up understanding of antimicrobial use and resistance on the farm: a knowledge, attitudes, and practices survey across livestock systems in five African countries. PLoS ONE. 2020;15:e0220274. doi: 10.1371/journal.pone.0220274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa S., Tzipori S., Sheoran A. Selective evolution of ligands by exponential enrichment to identify RNA aptamers against shiga toxins. J. Nucl. Acids. 2014;2014:214929. doi: 10.1155/2014/214929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Yan C., Cheng L., Yao L., Xue F., Xu J. An ultrasensitive signal-on electrochemical aptasensor for ochratoxin A determination based on DNA controlled layer-by-layer assembly of dual gold nanoparticle conjugates. Biosens. Bioelectron. 2018;117:845–851. doi: 10.1016/j.bios.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Cheng D., Yu M., Fu F., Han W., Li G., Xie J., Song Y., Swihart M.T., Song E. Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Anal. Chem. 2016;88:820–825. doi: 10.1021/acs.analchem.5b03320. [DOI] [PubMed] [Google Scholar]
- Cheng J., Wang J., Liu Y., Wu Q., Wang Z. Screening and identification of ssDNA aptamers against HN protein for detection of bovine parainfluenza virus type 3 antibodies in Serum. Curr. Pharm. Biotechnol. 2018;19:896–901. doi: 10.2174/1389201019666181031154046. [DOI] [PubMed] [Google Scholar]
- Chinnappan R., Al Attas S., Kaman W.E., Bikker F.J., Zourob M. Development of magnetic nanoparticle based calorimetric assay for the detection of bovine mastitis in cow milk. Anal. Biochem. 2017;523:58–64. doi: 10.1016/j.ab.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Chinnappan R., Eissa S., Alotaibi A., Siddiqua A., Alsager O.A., Zourob M. In vitro selection of DNA aptamers and their integration in a competitive voltammetric biosensor for azlocillin determination in waste water. Anal. Chim. Acta. 2020;1101:149–156. doi: 10.1016/j.aca.2019.12.023. [DOI] [PubMed] [Google Scholar]
- Chinnappan R., Rahamn A.A., AlZabn R., Kamath S., Lopata A.L., Abu-Salah K.M., Zourob M. Aptameric biosensor for the sensitive detection of major shrimp allergen, tropomyosin. Food Chem. 2020;314:126133. doi: 10.1016/j.foodchem.2019.126133. [DOI] [PubMed] [Google Scholar]
- Cho S.J., Woo H.M., Kim K.S., Oh J.W., Jeong Y.J. Novel system for detecting SARS coronavirus nucleocapsid protein using an ssDNA aptamer. J. Biosci. Bioeng. 2011;112:535–540. doi: 10.1016/j.jbiosc.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda E., Du X., Shim S.Y., Klein A.N., Siltberg-Liberles J., Gilch S. Interaction of peptide aptamers with prion protein central domain promotes α-cleavage of PrP(C) Mol. Neurobiol. 2018;55:7758–7774. doi: 10.1007/s12035-018-0944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crulhas B.P., Hadley D.J., Liu Y., Shin D., Stybayeva G., Imanbekova M., Hill A.E., Pedrosa V.A., Revzin A. An electrochemical aptasensor for detection of bovine interferon gamma. Anal. Methods. 2017;9 [Google Scholar]
- Down P.M., Bradley A.J., Breen J.E., Green M.J. Factors affecting the cost-effectiveness of on-farm culture prior to the treatment of clinical mastitis in dairy cows. Prev. Vet. Med. 2017;145:91–99. doi: 10.1016/j.prevetmed.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Down P.M., Bradley A.J., Breen J.E., Hudson C.D., Green M.J. Current management practices and interventions prioritised as part of a nationwide mastitis control plan. Vet. Rec. 2016;178:449. doi: 10.1136/vr.103203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte C.M., Freitas P.P., Bexiga R. Technological advances in bovine mastitis diagnosis: an overview. J. Vet. Diagn. Invest. 2015;27:665–672. doi: 10.1177/1040638715603087. [DOI] [PubMed] [Google Scholar]
- Dwivedi H.P., Smiley R.D., Jaykus L.A. Selection of DNA aptamers for capture and detection of Salmonella Typhimurium using a whole-cell SELEX approach in conjunction with cell sorting. Appl. Microbiol. Biotechnol. 2013;97:3677–3686. doi: 10.1007/s00253-013-4766-4. [DOI] [PubMed] [Google Scholar]
- El-Sayed A., Awad W., Abdou N.E., Castañeda Vázquez H. Molecular biological tools applied for identification of mastitis causing pathogens. Int. J. Vet. Sci. Med. 2017;5:89–97. doi: 10.1016/j.ijvsm.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingham M., Bunka D.H., Rowlands D.J., Stonehouse N.J. Selection and characterization of RNA aptamers to the RNA-dependent RNA polymerase from foot-and-mouth disease virus. RNA. 2006;12:1970–1979. doi: 10.1261/rna.161006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier-Wirth C., Deschaseaux M., Defer C., Godreuil S., Carrière C., Bertrand X., Tunez V., Schneider T., Coste J., Morel P. Evaluation of the enhanced bacterial detection system for screening of contaminated platelets. Transfusion. 2006;46:220–224. doi: 10.1111/j.1537-2995.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- Fox P.F., Kelly A.L. Indigenous enzymes in milk: overview and historical aspects-Part 2. Int. Dairy J. 2006;16:517–532. [Google Scholar]
- Fu P., Sun Z., Yu Z., Zhang Y., Shen J., Zhang H., Xu W., Jiang F., Chen H., Wu W. Enzyme linked aptamer assay: based on a competition format for sensitive detection of antibodies to Mycoplasma bovis in serum. Anal. Chem. 2014;86:1701–1709. doi: 10.1021/ac4042203. [DOI] [PubMed] [Google Scholar]
- Gao W., Li B., Yao R., Li Z., Wang X., Dong X., Qu H., Li Q., Li N., Chi H., Zhou B., Xia Z. Intuitive label-Free SERS detection of bacteria using aptamer-based in situ silver nanoparticles synthesis. Anal. Chem. 2017;89:9836–9842. doi: 10.1021/acs.analchem.7b01813. [DOI] [PubMed] [Google Scholar]
- Gold L., Ayers D., Bertino J., Bock C., Bock A., Brody E.N., Carter J., Dalby A.B., Eaton B.E., Fitzwater T., Flather D., Forbes A., Foreman T., Fowler C., Gawande B., Goss M., Gunn M., Gupta S., Halladay D., Heil J., Heilig J., Hicke B., Husar G., Janjic N., Jarvis T., Jennings S., Katilius E., Keeney T.R., Kim N., Koch T.H., Kraemer S., Kroiss L., Le N., Levine D., Lindsey W., Lollo B., Mayfield W., Mehan M., Mehler R., Nelson S.K., Nelson M., Nieuwlandt D., Nikrad M., Ochsner U., Ostroff R.M., Otis M., Parker T., Pietrasiewicz S., Resnicow D.I., Rohloff J., Sanders G., Sattin S., Schneider D., Singer B., Stanton M., Sterkel A., Stewart A., Stratford S., Vaught J.D., Vrkljan M., Walker J.J., Watrobka M., Waugh S., Weiss A., Wilcox S.K., Wolfson A., Wolk S.K., Zhang C., Zichi D. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 2010;5:e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S.C., Tang T.H., Chen Y., Citartan M., Lakshmipriya T. Bacterial detection: from microscope to smartphone. Biosens. Bioelectron. 2014;60:332–342. doi: 10.1016/j.bios.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Green Louis S., Bell Carol, Janjic Nebojsa. Aptamers as reagents for high-throughput screening. Biotechniques. 2001;30(5):1094–1110. doi: 10.2144/01305dd02. [DOI] [PubMed] [Google Scholar]
- Gu M.B. Highly sensitive detection of pandemic viruses, biomarkers, and small molecules by using Aptamer nanobiosensors. Nanomed-Nanotechnol. 2016;12:452. [Google Scholar]
- Guliye A.Y., Van Creveld C., Yagil R. Detection of subclinical mastitis in dromedary camels (Camelus dromedarius) using somatic cell counts and the N-acetyl-beta-D-glucosaminidase test. Trop. Anim. Health Prod. 2002;34:95–104. doi: 10.1023/a:1014324421258. [DOI] [PubMed] [Google Scholar]
- Hmila I., Wongphatcharachai M., Laamiri N., Aouini R., Marnissi B., Arbi M., Sreevatsan S., Ghram A. A novel method for detection of H9N2 influenza viruses by an aptamer-real time-PCR. J. Virol. Methods. 2017;243:83–91. doi: 10.1016/j.jviromet.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Hossain M.T., Shibata T., Kabashima T., Kai M. Aptamer-mediated chemiluminescence detection of prion protein on a membrane using trimethoxyphenylglyoxal. Anal. Sci. 2010;26:645–647. doi: 10.2116/analsci.26.645. [DOI] [PubMed] [Google Scholar]
- Hwang J.Y., Kim S.T., Han H.S., Kim K., Han J.S. Optical aptamer probes of fluorescent imaging to rapid monitoring of circulating tumor cell. Sensors (Basel) 2016;16 doi: 10.3390/s16111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R., Janagama H., Dwivedi H.P., Senthil Kumar T.M., Jaykus L.A., Schefers J., Sreevatsan S. Selection, characterization, and application of DNA aptamers for the capture and detection of Salmonella enterica serovars. Mol. Cell. Probes. 2009;23:20–28. doi: 10.1016/j.mcp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Kanwar J.R., Roy K., Kanwar R.K. Chimeric aptamers in cancer cell-targeted drug delivery. Crit. Rev. Biochem. Mol. Biol. 2011;46:459–477. doi: 10.3109/10409238.2011.614592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Shorie M., Sabherwal P. Biolayer interferometry-SELEX for Shiga toxin antigenic-peptide aptamers & detection via chitosan-WSe(2) aptasensor. Biosens. Bioelectron. 2020;167:112498. doi: 10.1016/j.bios.2020.112498. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Choi J.W., Kim A.R., Lee S.C., Yoon M.Y. Development of ssDNA aptamers for diagnosis and inhibition of the highly pathogenic avian influenza virus subtype H5N1. Biomolecules. 2020;10 doi: 10.3390/biom10081116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Lee J., Lee B.H., Song C.S., Gu M.B. Specific detection of avian influenza H5N2 whole virus particles on lateral flow strips using a pair of sandwich-type aptamers. Biosens. Bioelectron. 2019;134:123–129. doi: 10.1016/j.bios.2019.03.061. [DOI] [PubMed] [Google Scholar]
- Kim T.H., Lee S.W. Aptamers for anti-viral therapeutics and diagnostics. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22084168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouassi G.K., Wang P., Sreevatan S., Irudayaraj J. Aptamer-mediated magnetic and gold-coated magnetic nanoparticles as detection assay for prion protein assessment. Biotechnol. Prog. 2007;23:1239–1244. doi: 10.1021/bp0602101. [DOI] [PubMed] [Google Scholar]
- Lamont E.A., He L., Warriner K., Labuza T.P., Sreevatsan S. A single DNA aptamer functions as a biosensor for ricin. Analyst. 2011;136:3884–3895. doi: 10.1039/c1an15352h. [DOI] [PubMed] [Google Scholar]
- Lavania S., Das R., Dhiman A., Myneedu V.P., Verma A., Singh N., Sharma T.K., Tyagi J.S. Aptamer-based TB antigen tests for the rapid diagnosis of pulmonary tuberculosis: potential utility in screening for tuberculosis. ACS Infect. Dis. 2018;4:1718–1726. doi: 10.1021/acsinfecdis.8b00201. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Lee S.H., Kim J.H., Noh Y.H., Noh G.J., Lee S.W. Pharmacokinetics of a cholesterol-conjugated aptamer against the hepatitis C virus (HCV) NS5B protein. Mol. Ther. Nucleic Acids. 2015;4:e254. doi: 10.1038/mtna.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Kim B.C., Kim K.W., Kim Y.K., Kim J., Oh M.K. A sensitive method to detect Escherichia coli based on immunomagnetic separation and real-time PCR amplification of aptamers. Biosens. Bioelectron. 2009;24:3550–3555. doi: 10.1016/j.bios.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Lee I., Kim S.E., Lee J., Woo D.H., Lee S., Pyo H., Song C.S. A self-calibrating electrochemical aptasensing platform: correcting external interference errors for the reliable and stable detection of avian influenza viruses. Biosens. Bioelectron. 2020;152:112010. doi: 10.1016/j.bios.2020.112010. [DOI] [PubMed] [Google Scholar]
- Li H., Ding X., Peng Z., Deng L., Wang D., Chen H., He Q. Aptamer selection for the detection of Escherichia coli K88. Can. J. Microbiol. 2011;57:453–459. doi: 10.1139/w11-030. [DOI] [PubMed] [Google Scholar]
- Liang H.R., Hu G.Q., Li L., Gao Y.W., Yang S.T., Xia X.Z. Aptamers targeting rabies virus-infected cells inhibit street rabies virus in vivo. Int. Immunopharmacol. 2014;21:432–438. doi: 10.1016/j.intimp.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Liang H.R., Hu G.Q., Xue X.H., Li L., Zheng X.X., Gao Y.W., Yang S.T., Xia X.Z. Selection of an aptamer against rabies virus: a new class of molecules with antiviral activity. Virus Res. 2014;184:7–13. doi: 10.1016/j.virusres.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Liang H.R., Liu Q., Zheng X.X., Gai W.W., Xue X.H., Hu G.Q., Wu H.X., Wang H.L., Yang S.T., Xia X.Z. Aptamers targeting rabies virus-infected cells inhibit viral replication both in vitro and in vivo. Virus Res. 2013;173:398–403. doi: 10.1016/j.virusres.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen P., Yuan S., Sun B., Sun R., Meng X. A novel method for sensitive detection of Escherichia coli O157:H7 based on an aptamer and hybridization chain reaction. Anal. Methods. 2020;12:3734–3740. doi: 10.1039/d0ay00708k. [DOI] [PubMed] [Google Scholar]
- Longjam N., Deb R., Sarmah A.K., Tayo T., Awachat V.B., Saxena V.K. A brief review on diagnosis of foot-and-mouth disease of livestock: conventional to molecular tools. Vet. Med. Int. 2011;2011:905768. doi: 10.4061/2011/905768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Ma Q., Yan W., Wang Y., Zhang Y., Zhao L., Chen H. Selection of an aptamer against Muscovy duck parvovirus for highly sensitive rapid visual detection by label-free aptasensor. Talanta. 2018;176:214–220. doi: 10.1016/j.talanta.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Lundstrom K. Viral vectors applied for RNAi-based antiviral therapy. Viruses. 2020;12 doi: 10.3390/v12090924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Lei Y., Yan L., Yu T., Li Q., Zhang D., Ding S., Ju H. A rapid and sensitive aptamer-based electrochemical biosensorfor direct detection of Escherichia coli O111. Electroanalysis. 2012;24:1186–1191. [Google Scholar]
- MacGregor I. Prion protein and developments in its detection. Transfus. Med. 2001;11:3–14. doi: 10.1046/j.1365-3148.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- Martins S.A.M., Martins V.C., Cardoso F.A., Germano J., Rodrigues M., Duarte C., Bexiga R., Cardoso S., Freitas P.P. Biosensors for on-farm diagnosis of mastitis. Front. Bioeng. Biotechnol. 2019;7:186. doi: 10.3389/fbioe.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A.J., Haydon D.T., McIntosh E., Hanley N., Halliday J.E.B. Socially vs. privately optimal control of livestock diseases: a case for integration of epidemiology and economics. Front. Vet. Sci. 2020;7:558409. doi: 10.3389/fvets.2020.558409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozioglu E., Gokmen O., Tamerler C., Kocagoz Z.T., Akgoz M. Selection of nucleic acid aptamers specific for mycobacterium tuberculosis. Appl. Biochem. Biotechnol. 2016;178:849–864. doi: 10.1007/s12010-015-1913-7. [DOI] [PubMed] [Google Scholar]
- Nguyen V.T., Seo H.B., Kim B.C., Kim S.K., Song C.S., Gu M.B. Highly sensitive sandwich-type SPR based detection of whole H5Nx viruses using a pair of aptamers. Biosens. Bioelectron. 2016;86:293–300. doi: 10.1016/j.bios.2016.06.064. [DOI] [PubMed] [Google Scholar]
- Ni H., Zhang Z.P., Ding X., Mi T., Wang Z., Liu M. Determination of enrofloxacin in bovine milk by a novel single-stranded DNA aptamer chemiluminescent enzyme immunoassay. Anal. Lett. 2014;47:2844–2856. [Google Scholar]
- Okoth O.K., Yan K., Liu Y., Zhang J. Graphene-doped Bi2S3 nanorods as visible-light photoelectrochemical aptasensing platform for sulfadimethoxine detection. Biosens. Bioelectron. 2016;86:636–642. doi: 10.1016/j.bios.2016.07.037. [DOI] [PubMed] [Google Scholar]
- Palma E., Tilocca B., Roncada P. Antimicrobial resistance in veterinary medicine: an overview. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21061914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske D., Blank M., Buhmann R., Resch A. Aptamers–basic research, drug development, and clinical applications. Appl. Microbiol. Biotechnol. 2005;69:367–374. doi: 10.1007/s00253-005-0193-5. [DOI] [PubMed] [Google Scholar]
- Puerto M.A., Shepley E., Cue R.I., Warner D., Dubuc J., Vasseur E. The hidden cost of disease: I. Impact of the first incidence of mastitis on production and economic indicators of primiparous dairy cows. J. Dairy Sci. 2021 doi: 10.3168/jds.2020-19584. [DOI] [PubMed] [Google Scholar]
- Raboisson D., Ferchiou A., Pinior B., Gautier T., Sans P., Lhermie G. The use of meta-analysis for the measurement of animal disease burden: losses due to clinical mastitis as an example. Front. Vet. Sci. 2020;7:149. doi: 10.3389/fvets.2020.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendhran J., Gunasekaran P. Microbial phylogeny and diversity: small subunit ribosomal RNA sequence analysis and beyond. Microbiol. Res. 2011;166:99–110. doi: 10.1016/j.micres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Rani A., Ravindran V.B., Surapaneni A., Shahsavari E., Haleyur N., Mantri N., Ball A.S. Evaluation and comparison of recombinase polymerase amplification coupled with lateral-flow bioassay for Escherichia coli O157:H7 detection using diifeerent genes. Sci. Rep. 2021;11:1881. doi: 10.1038/s41598-021-81312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V.K. Point of care diagnostic devices for rapid detection of novel coronavirus (SARS-nCoV19) Pandemic: a review. Front. Nanotechnol. 2021;2:593619. [Google Scholar]
- Reinemann C., Freiin von Fritsch U., Rudolph S., Strehlitz B. Generation and characterization of quinolone-specific DNA aptamers suitable for water monitoring. Biosens. Bioelectron. 2016;77:1039–1047. doi: 10.1016/j.bios.2015.10.069. [DOI] [PubMed] [Google Scholar]
- Robles I., Nolan D.T., Fendley C.A., Stokley H.L., France T.L., Ferrell J.L., Costa J.H.C. Technical note: Evaluation of a commercial on-farm milk leukocyte differential tester to identify subclinical mastitis cases in dairy cows. J. Dairy Sci. 2021;104:4942–4949. doi: 10.3168/jds.2020-19299. [DOI] [PubMed] [Google Scholar]
- Rotherham L.S., Maserumule C., Dheda K., Theron J., Khati M. Selection and application of ssDNA aptamers to detect active TB from sputum samples. PLoS ONE. 2012;7:e46862. doi: 10.1371/journal.pone.0046862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roushani M., Rahmati Z., Hoseini S.J., Hashemi Fath R. Impedimetric ultrasensitive detection of chloramphenicol based on aptamer MIP using a glassy carbon electrode modified by 3-ampy-RGO and silver nanoparticle. Colloids Surf B Biointerf. 2019;183:110451. doi: 10.1016/j.colsurfb.2019.110451. [DOI] [PubMed] [Google Scholar]
- Roushani M., Sarabaegi M., Pourahmad F. Impedimetric aptasensor for Pseudomonas aeruginosa by using a glassy carbon electrode modified with silver nanoparticles. Mikrochim. Acta. 2019;186:725. doi: 10.1007/s00604-019-3858-y. [DOI] [PubMed] [Google Scholar]
- Sadeghi A.S., Mohsenzadeh M., Abnous K., Taghdisi S.M., Ramezani M. Development and characterization of DNA aptamers against florfenicol: Fabrication of a sensitive fluorescent aptasensor for specific detection of florfenicol in milk. Talanta. 2018;182:193–201. doi: 10.1016/j.talanta.2018.01.083. [DOI] [PubMed] [Google Scholar]
- Seo H.B., Gu M.B. Aptamer-based sandwich-type biosensors. J. Biol. Eng. 2017;11:11. doi: 10.1186/s13036-017-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Huma Z.L., Singh S.G., Navjot, Sharma S., Gupta S.K., Upadhyay S.R. Prevalence of clinical and subclinical mastitis in buffaloes of Jammu region. Int. J. Agri. Environ. Biotech. 2018;11:415–420. [Google Scholar]
- Sharma N., Maiti S.K. Incidence, etiology and antibiogram of sub clinical mastitis in cows in Durg, Chhattisgarh. Indian J. Vet. Res. 2010;19:45–54. [Google Scholar]
- Sharma N., Rho G.J., Hong Y.H., Kang T.Y., Lee H.K., Hur T.Y., Jeong D.K. Bovine mastitis: an Asian perspective. Asian J. Anim. Vet. Adv. 2012;7:454–476. [Google Scholar]
- Sharma S., SIngh N.K., Bhadwal M.S. Relationship of somatic cell count and mastitis: an overview. Asian-Austra. J. Anim. Sci. 2011;24:429–438. [Google Scholar]
- Sheikhzadeh E., Beni V., Zourob M. Nanomaterial application in bio/sensors for the detection of infectious diseases. Talanta. 2021;230:122026. doi: 10.1016/j.talanta.2020.122026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Chen H., Cheng Z., Ma L., Huang L., Xiao M., Xiao W., Xie K., Tang Y. A novel fluorescent immunochromatographic strip combined with pocket fluorescence observation instrument for rapid detection of PRV. Anal. Bioanal. Chem. 2018;410:7655–7661. doi: 10.1007/s00216-018-1379-x. [DOI] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020 doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigdar S., Lin J., Yu Y., Pastuovic M., Wei M., Duan W. RNA aptamer against a cancer stem cell marker epithelial cell adhesion molecule. Cancer Sci. 2011;102:991–998. doi: 10.1111/j.1349-7006.2011.01897.x. [DOI] [PubMed] [Google Scholar]
- Simmons S.C., McKenzie E.A., Harris L.K., Aplin J.D., Brenchley P.E., Velasco-Garcia M.N., Missailidis S. Development of novel single-stranded nucleic acid aptamers against the pro-angiogenic and metastatic enzyme heparanase (HPSE1) PLoS ONE. 2012;7:e37938. doi: 10.1371/journal.pone.0037938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So H.M., Park D.W., Jeon E.K., Kim Y.H., Kim B.S., Lee C.K., Choi S.Y., Kim S.C., Chang H., Lee J.O. Detection and titer estimation of Escherichia coli using aptamer-functionalized single-walled carbon-nanotube field-effect transistors. Small. 2008;4:197–201. doi: 10.1002/smll.200700664. [DOI] [PubMed] [Google Scholar]
- Song S.H., Wang L., Li J., Zhao J., Fan C. Aptamer-based biosensors. Trends Analyt. Chem. 2008;27:108–117. [Google Scholar]
- Stoltenburg R., Krafčiková P., Víglaský V., Strehlitz B. G-quadruplex aptamer targeting Protein A and its capability to detect Staphylococcus aureus demonstrated by ELONA. Sci. Rep. 2016;6:33812. doi: 10.1038/srep33812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiviyanathan V., Gorenstein D.G. Aptamers and the next generation of diagnostic reagents. Proteomics Clin. Appl. 2012;6:563–573. doi: 10.1002/prca.201200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R.Y., Lin C., Yu S.Y., Gong S., Hu P., Li Y.S., Wu Z.C., Gao Y., Zhou Y., Liu Z.S., Ren H.L., Lu S.Y. Preparation of a specific ssDNA aptamer for brevetoxin-2 using SELEX. J. Anal. Methods Chem. 2016;2016:9241860. doi: 10.1155/2016/9241860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y.T., Wang C.H., Chang C.P., Lee G.B. Integrated microfluidic system for rapid detection of influenza H1N1 virus using a sandwich-based aptamer assay. Biosens. Bioelectron. 2016;82:105–111. doi: 10.1016/j.bios.2016.03.073. [DOI] [PubMed] [Google Scholar]
- Tsouloufi T.K., Donnison L.M., Smyth K.L., Peters A.R. Development of a systematic mapping review protocol for the most recent evidence on ruminant infectious disease frequency and disease-associated mortality: ethiopia as a case study. Anim. Health Res. Rev. 2020;21:96–102. doi: 10.1017/S1466252319000203. [DOI] [PubMed] [Google Scholar]
- Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Vasquez A.K., Nydam D.V., Capel M.B., Eicker S., Virkler P.D. Clinical outcome comparison of immediate blanket treatment versus a delayed pathogen-based treatment protocol for clinical mastitis in a New York dairy herd. J. Dairy Sci. 2017;100:2992–3003. doi: 10.3168/jds.2016-11614. [DOI] [PubMed] [Google Scholar]
- Vidic J., Manzano M., Chang C.M., Jaffrezic-Renault N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017;48:11. doi: 10.1186/s13567-017-0418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguier C., Arora S., Gilmartin N., Welbeck N., O’Kennedy R. Mastitis detection: current trends and future perspectives. Trends Biotechnol. 2009;27:486–493. doi: 10.1016/j.tibtech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu M., Wang Z., Li S., Deng Y., He N. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today. 2021;37:101092. doi: 10.1016/j.nantod.2021.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang C., Li H. Selection of DNA aptamers and establishment of an effective aptasensor for highly sensitive detection of cefquinome residues in milk. Analyst. 2018;143:3202–3208. doi: 10.1039/c8an00709h. [DOI] [PubMed] [Google Scholar]
- Wang P., Hatcher K.L., Bartz J.C., Chen S.G., Skinner P., Richt J., Liu H., Sreevatsan S. Selection and characterization of DNA aptamers against PrP(Sc) Exp. Biol. Med. (Maywood) 2011;236:466–476. doi: 10.1258/ebm.2011.010323. [DOI] [PubMed] [Google Scholar]
- Wang Y.X., Ye Z.Z., Si C.Y., Ying Y.B. Application of aptamer based biosensors for detection of pathogenic microorganisms. Chinese J. Analyt. Chem. 2012;40:634–642. [Google Scholar]
- WHO The challenges of preventing bovine tuberculosis. Bull. World Health Organ. 2018;96:82–83. doi: 10.2471/BLT.18.020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. A Coordinated Global Research Roadmap: 2019 Novel Coronavirus, vol. 2020. WHO Headquarters, Geneva.
- Windria S., Salasia S.I.O., Nugroho W., Widayanti R., Indarjulianto S. Development of ELISA against milk haptoglobin for diagnosis of subclinical mastitis in goats. Heliyon. 2021;7:e06314. doi: 10.1016/j.heliyon.2021.e06314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongphatcharachai M., Wang P., Enomoto S., Webby R.J., Gramer M.R., Amonsin A., Sreevatsan S. Neutralizing DNA aptamers against swine influenza H3N2 viruses. J. Clin. Microbiol. 2013;51:46–54. doi: 10.1128/JCM.02118-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.H., Li M., Wang Y., Ouyang H.X., Wang L., Li C.X., Cao Y.C., Meng Q.H., Lu J.X. Aptasensors for rapid detection of Escherichia coli O157:H7 and Salmonella typhimurium. Nanoscale Res. Lett. 2012;7:658. doi: 10.1186/1556-276X-7-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhang G., Li Y., Jin Y., Dale R., Sun L.Q., Wang M. Inhibition of highly pathogenic avian H5N1 influenza virus replication by RNA oligonucleotides targeting NS1 gene. Biochem. Biophys. Res. Commun. 2008;365:369–374. doi: 10.1016/j.bbrc.2007.10.196. [DOI] [PubMed] [Google Scholar]
- Xiao S.J., Hu P.P., Xiao G.F., Wang Y., Liu Y., Huang C.Z. Label-free detection of prion protein with its DNA aptamer through the formation of T-Hg2+-T configuration. J. Phys. Chem. B. 2012;116:9565–9569. doi: 10.1021/jp302522b. [DOI] [PubMed] [Google Scholar]
- Xu J., Cai Y., Jiang B., Li X., Jin H., Liu W., Kong Z., Hong J., Sealy J.E., Iqbal M., Li Y. An optimized aptamer-binding viral tegument protein VP8 inhibits the production of Bovine Herpesvirus-1 through blocking nucleocytoplasmic shuttling. Int. J. Biol. Macromol. 2019;140:1226–1238. doi: 10.1016/j.ijbiomac.2019.08.165. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhang X., Zhou S., Shen J., Yang D., Wu J., Li X., Li M., Huang X., Sealy J.E., Iqbal M., Li Y. A DNA aptamer efficiently inhibits the infectivity of Bovine herpesvirus 1 by blocking viral entry. Sci. Rep. 2017;7:11796. doi: 10.1038/s41598-017-10070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Han T., Li X., Sun L., Zhang Y. Colorimetric detection of kanamycin based on analyte-protected silver nanoparticles and aptamer-selective sensing mechanism. Anal. Chim. Acta. 2015;891:298–303. doi: 10.1016/j.aca.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lu C., Sun Y., Shao Y., Cai Y., Zhang Y., Miao J., Miao P. A colorimetric aptasensor for the antibiotics oxytetracycline and kanamycin based on the use of magnetic beads and gold nanoparticles. Mikrochim. Acta. 2018;185:548. doi: 10.1007/s00604-018-3077-y. [DOI] [PubMed] [Google Scholar]
- Yan C., Zhang J., Yao L., Xue F., Lu J., Li B., Chen W. Aptamer-mediated colorimetric method for rapid and sensitive detection of chloramphenicol in food. Food Chem. 2018;260:208–212. doi: 10.1016/j.foodchem.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Yoon S., Lee G., Han D., Song J.Y., Kang K.S., Lee Y.S. Neutralization of infectivity of porcine circovirus type 2 (PCV2) by capsid-binding 2'F-RNA aptamers. Antiviral Res. 2010;88:19–24. doi: 10.1016/j.antiviral.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Zecconi A., Meroni G., Sora V., Mattina R., Cipolla M., Zanini L. Total and differential cell counts as a tool to identify intramammary infections in cows after calving. Animals (Basel) 2021;11 doi: 10.3390/ani11030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.J., Lu Y.H., Long Y.J., Wang Q.L., Huang X.X., Zhu R., Wang X.L., Liang L.P., Teng P., Zhen H.Z. Anaptamer-functionalized gold nanoparticle biosensor for the detection of prion protein. Anal. Methods. 2014;6:2982–2987. [Google Scholar]
- Zhang Y., Jia X.F., Ya M.S., Siquinqimuge N., Liu B.Z., Suyila Y.C., Gao X.S., Liu Q.Y. Isolation and identification of pathogens from mastitis cow and drug sensitivity test. Anim. Husb. Vet. Med. 2009:2009–2107. [Google Scholar]
- Zhang Y., Lu T., Wang Y., Diao C., Zhou Y., Zhao L., Chen H. Selection of a DNA aptamer against zearalenone and docking analysis for highly sensitive rapid visual detection with label-free aptasensor. J. Agric. Food Chem. 2018;66:12102–12110. doi: 10.1021/acs.jafc.8b03963. [DOI] [PubMed] [Google Scholar]
- Zhao F., Xie Q., Xu M., Wang S., Zhou J., Liu F. RNA aptamer based electrochemical biosensor for sensitive and selective detection of cAMP. Biosens. Bioelectron. 2015;66:238–243. doi: 10.1016/j.bios.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Zhou C., Zou H., Sun C., Ren D., Xiong W., Li Y. Fluorescent aptasensor for detection of four tetracycline veterinary drugs in milk based on catalytic hairpin assembly reaction and displacement of G-quadruplex. Anal. Bioanal. Chem. 2018;410:2981–2989. doi: 10.1007/s00216-018-0981-2. [DOI] [PubMed] [Google Scholar]