Highlights
-
•
Vicilins from Anadenanthera colubrina seeds (AcVs) interfered with the development of cowpea weevil (Callosobruchus maculatus) larvae.
-
•
AcVs present a strong effect at low concentrations on adult emergence (ED50 of 0.096%), on larvae, showing a marked reduction in mass (WD50 of 0.32%) and lethality (LD50 of 0.33%) (w:w).
-
•
AcV is a chitin-binding protein with approximately 230 kDa.
-
•
AcV chitin-binding fragments are associated with deleterious effects in larvae.
Keywords: Bioinsecticidal, Callosobruchus maculatus, Chitin-binding protein seeds
Abstract
Vicilins are seed proteins, and they constitute 70–80% of the total protein in leguminous seeds; with amolecular mass between 150 and 190 kDa, they are composed of subunits without disulfide bridges, with high affinity for chitin-binding. They are also associated with seed defense against insect pests. The chitin-binding vicilin from Anadenanthera colubrina seeds was purified by ammonium sulfate, followed by affinity chromatography on a chitin column, molecular exclusion on Superdex 75 Tricorn in FPLC system and Phenomenex C8 chromatography in HPLC system. The A. colubrina vicilin, named AcV, is a tetrameric glycoprotein composed of 1.55% carbohydrates and molecular weight determined by SDS-PAGE, consisting of 70, 73, 43 and 41 kDa. The AcV homogeneity was confirmed in native PAGE, where it was observed to be a unique band with slow mobility in this gel, with approximately 230 kDa. AcV added to the Callosobruchus maculatus diet in the bioassays resulted in a strong effect on adult emergence (ED50 of 0.096%), and in larvae caused a marked reduction in mass (WD50 of 0.32%) and lethality (LD50 of 0.33%) (w:w). The digestibility of AcV was evaluated in vitro with the digestive enzymes of larvae of C. maculatus of fourth instar, showing major fragments of 10 and 30 kDa. AcV showed reactivity against the anti-EvV antibody from Erythrina velutina vicilin. The deleterious effects of AcV are likely to be associated with the chitin-binding fragments generated by proteolysis in the bruchid gut, similarly to that found for vicilins from other leguminous plant species, Enterolobium contortisiliquum and Vigna unguiculata. AcV might be a candidate protein for a possible bioinsecticidal control of the bruchid weevil, C. maculatus.
1. Introduction
Weevils, like those of the genera Acanthoscelides, Zabrotes and Callosobruchus (Bruchinae), are beetles specialized in feeding on bean plants from different genera (Southgate, 1979, Tuda, 2007). The species Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae), endemic in Africa and Asia, is distributed through the tropical and subtropical world. This species is the main agricultural insect pest of Vigna unguiculate (L.) Walp. and Phaseoulus vulgaris L.. Both these beans represent the main nutritional source for different populations around the world, representing about 20–25% protein, 50–60% carbohydrates, and 1–2% lipids (Beck and Blumer, 2014, Gomez Carlos, 2004, Tuda et al., 2006).
The interaction between bruchids and legumes is highly specific, since an insect species feeds on few seed species (Somta et al., 2007). Legumes have developed a series of physiological and/or biochemical characteristics, which are associated with the co-evolutionary process with bruchids (Edwards and Singh, 2006). Secondary metabolites and antinutritional compounds affect the metabolic activity of bruchids (Somta et al., 2007, War et al., 2017).
One of the physiological characteristics present in the digestive tract of insect pests, and especially the genus Callosobruchus, is associated with the intestine. This in turn is divided into three portions (anterior, middle and posterior intestines), of which the middle intestine is the main site of absorption and digestion (Watanabe and Tokuda, 2010). The midgut is protected by the peritrophic membrane, more recently referred to as the peritrophic matrix (PM), a non-cellular and semipermeable layer (Lehane, 1997, Mehlhorn, 2016, Terra, 2001). This mucosal structure (Wang and Granados, 1997) has chitin incorporated [(poly(β-1.4-N-acetyl-D-glucosamine)] in its composition, which differs from animal mucus, resulting in a macromolecular complex formed by a protein core structure reinforced with chitin fibrils (Kramer et al., 1995, Lehane, 1997, Mehlhorn, 2016). The main functions of the peritrophic matrix are: as a physical barrier against microorganisms, as mechanical protection against lesions in the cells of the midgut, and as a selective barrier promoting the ecto-endoperitrophic circulation (Lehane, 1997, Terra, 2001). Several studies have demonstrated that alterations in peritrophic matrix permeability may lead to the death of insects due to malnutrition (Wang and Granados, 2001). Proteins that present chitin-binding capacity of the peritrophic matrix have been shown to be potential deleterious targets in artificial diets for the larval development stage of Plodia interpunctella, Ceratitis capitate, Z. Subfasciatus and C. maculatus using 7S globulins, also denominated vicilins (Amorim et al., 2008, Macedo et al., 2008, Teixeira et al., 2008). Investigating the insecticidal activity of variant vicilins of varieties resistant (IT81D-1053) to the cowpea beetle C. maculatus, Oliveira et al. (2014) observed that this genotype affects larval development, causing 100% mortality. In this study, larvae fed with a resistant variant vicilin and a non-resistant variant (FITC labelled) showed more variant vicilins accumulated on the surface of the midgut epithelium than non-variant vicilins, suggesting that these toxic effects are associated with interference with normal physiology, affecting larval development. Ferreira el a. (2021) showed that fractions derived from resistant cowpea cv. BRS Xiquexique containing vicillins have a strong chitin binding capacity and toxicity to the bruchid C. maculatus.
Vicilins comprise a well-known class of reserve proteins, which may constitute 70–80% of total protein in leguminous seeds. These proteins have a variable degree of glycosylation (1 to 2%) and molecular mass varying from 150 to 190 kDa, without disulfide bridges (Cândido et al., 2011, Derbyshire et al., 1976), with subunits of molecular weights between 12.5 and 33 kDa, as described for pea vicilin (Gatehouse et al., 1984). Vicilins are multifunctional proteins, and they are described as an energy source; they provide amino acids during germination, as well as the defense protein against fungi and insect pests (Cândido et al., 2011, Macedo et al., 1993). These processes directly impact the degradation, partial or complete, of the vicilins, produce fragments that can hinder the proteolytic digestion performed by insects and, therefore, characterize their additional function of constitutive application (Macedo et al., 1995, Uchôa et al., 2006).
Results obtained by computer simulations support the evidence that the ability to bind chitinous structures on the intestinal surface of insect larvae contributes to the bioinsecticidal potential of these proteins. Rocha et al (2018) revealed that vicilins (7S globulins) have 3 chitin-binding sites. These studies observed through three-dimensional models that the interactions of β-vignin-type vicilins with chito-oligosaccharides were stabilized by hydrogen bonds, a common property in carbohydrate-binding proteins.
Therefore, we intend in this work to purify a vicilin from A. colubrina seeds and evaluate its bioinsecticidal potential against the pest C. maculatus in a bioassay system with artificial seeds, besides contributing to improving the understanding of toxicity to vicilins.
2. Methodology
2.1. Biological material: Insects and seeds
Larvae and adults of the bean bruchid, C. maculatus, used in this work came from the permanent insect colonies established in the Department of Cell Biology and Genetics, Federal University of Rio Grande do Norte - UFRN, Brazil. Insect colonies were maintained with bean seeds (commercial), at 28–30 °C and relative humidity of 60–70%. Seeds of cowpea, Vigna unguiculata, were infested with sexually mature females of C. maculatus for a period of 24 h, in the proportion of one female to 5 seeds. After this period, the females were removed and the infested seeds kept in sterilized plastic bottles and maintained at a constant temperature of 28 °C with 70% relative humidity, for a period of 28 to 30 days. The insects that emerged from the seeds were withdrawn daily from the flasks, transferred to empty vials and maintained for 48 h for sexual maturation and female fertilization. After this period, the fertilized females were used to infest new bean seeds to maintain a balanced insect population for the experiments.
The seeds of Anadenanthera colubrina Benth Brenan were obtained by the technical division - sowing sector of the Floresta Nacional (FLONA) de Nísia Floresta, Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio/MMA), Rio Grande do Norte state, Brazil.
2.2. Vicilin purification
For the extraction of proteins, the dried seeds were peeled of their tegument and their cotyledons were crushed in a cooled electric mill to obtain a fine-grained flour. The flour was subjected to homogenization in 0.02 M sodium tetraborate buffer with 0.15 M sodium chloride at pH 7.5 at the ratio of 1:10 (w:v), under constant stirring for 4 h at room temperature. The suspension was centrifuged at 12000 × g for 30 min at 4 °C. The precipitate was discarded and the supernatant filtered on cotton wool, after which it was considered crude extract (CE). The CE was fractionated with ammonium sulfate in three concentration ranges: 0–30, 30–60 and 60–90%. The 60–90% protein fraction was dialyzed against 0.02 M sodium tetraborate buffer with 0.15 M sodium chloride at pH 7.5 and stored under freezing, −20 °C for the next steps. After dialysis, the proteins were subjected to 3.7 × 3.0 cm chitin matrix affinity chromatography (Sigma-Aldrich), previously equilibrated with 0.02 M sodium tetraborate buffer with 0.15 M sodium chloride at pH 7.5. Chromatography was carried out at a flow rate of 2 mL.min−1 and protein monitored at 280 nm. After elution of the non-adsorbed proteins with the equilibration buffer, the retained proteins were eluted with 0.1 M HCl. The fractions corresponding to the retained peak were dialyzed against distilled water, lyophilized and denominated AcV. The chitin-retained peak was submitted to 10 × 300 mm Superdex 75 column molecular exclusion chromatography (Tricorn, GE Healthcare) in an AKTA purifier chromatographic system (GE Healthcare). The mobile phase, consisting of 0.02 M Tris-HCl buffer with 0.2 M NaCl at pH 8.0, was maintained at a flow rate of 0.5 mL.min−1. The isolated protein peak in the molecular exclusion column was analyzed on RP-HPLC column Phenomenex C8 (5 μm, St 4.6/250, 100 Å) pre-equilibrated with 0.1% trifluoroacetic acid. Proteins were eluted with a linear gradient 0–100% of acetonitrile in 0.1% trifluoroacetic acid. Elution profiles were monitored at 216 and 280 nm.
2.3. Vicilin characterization
The occurrence of subunits in AcV was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at room temperature (Laemmli, 1970) using a 12% polyacrylamide gel for separation and a 4% polyacrylamide gel for concentration. AcV homogeneity was observed on native polyacrylamide gel (PAGE) also as described by (Laemmli, 1970). The proteins used as molecular weight standards was obtained from Cytiva Life Sciences® (Brazil) with range of 14.4–97 kDa, containing phosphorylase (97.0 kDa), bovine serum albumin (66.0 kDa), ovalbumin (45.0 kDa), carbonic anhydrase (30.0 kDa), soybean trypsin inhibitor (20.1 kDa), and a-lactalbumin (14.4 kDa). Coomassie Blue G 250 at 1% was used for dying proteins (Bradford, 1976). After protein separation by SDS-PAGE, the gels were subjected to immunodetection of the proteins following the procedure described by (Towbin et al., 1979), using nitrocellulose membranes.
2.4. Quantification of protein and carbohydrate contents
The protein content was measured by the Bradford method, (1976), with bovine serum albumin as protein standard. Carbohydrate content was measured according to Dubois et al., (1956), with D-glucose as the carbohydrate standard. All assays were carried out in triplicate with standard deviation.
2.5. Detection of peptidases inhibitory activities
Globulin inhibitory assays against peptidase extracts from C. macutatus larvae were measured using 1% azocasein solution at pH 8.0 (serine proteinase activities) as described by Rufino et al., (2013) with some modifications. A 50 µL aliquot of AcV (2 mg.mL−1) was incubated with 50 µL of midgut homogenates and 400 µL of 50 mM Tris-HCl and 20 mM CaCl2 at pH 8.0 buffer at 37 °C for 15 min before adding the 500 µL of 1% azocasein, 50 mM Tris-HCl, and 20 mM CaCl2 at pH 8.0 substrate. After 30 min of incubation, the reaction was stopped by adding 150 µL of 20% TCA solution. After this step, the samples were centrifuged at 10000 × g for 10 min and supernatants were alkalinized with 0.2 N NaOH solution. The results were monitored by absorbance at 440 nm. Appropriate controls without AcV were prepared in the same conditions as the tests. One inhibitory unit (IU) was defined as the amount of inhibitor activity that decreased absorbance by 0.01 at 440 nm. All assays were performed in triplicate.
2.6. Evaluation of amylase inhibitory activities
Globulin inhibitory assays against midgut amylase from larvae (4th instar) were measured using 1% starch solution at pH 5.5. An aliquot of 40 µL from midgut homogenate was preincubated with 50 µL of globulin (2.0 mg.mL−1) at 30 °C for 15 min, prior to the addition of 2 mL of 1% soluble starch solution in 0.1 M acetate buffer, pH 5.5 containing 20 mM NaCl and 0.1 mM CaCl2 substrate solution. After 60 min at 30 °C, the reaction was interrupted adding an aliquot of 100 µL of a solution containing 1 mM iodine and 24 mM potassium iodide solution. Absorbances were read at 656 nm. Appropriate controls without globulin were included, and inhibitory activity was determined by measuring the remaining enzymatic activity at pH 5.5 after preincubation with samples. One inhibitory unit (IU) was defined as the amount of inhibitor activity that decreased absorbance by 0.01 at 656 nm. All assays were performed in triplicate (Teixeira et al., 2008).
2.7. Detection of hemagglutination and hemolytic activities
Human red blood cells (from blood bags generously donated by the Hemocentro Dalton Cunha, Rio Grande do Norte, Brazil) from different types (A, B and O) treated with papain or trypsin (both of them at 0.5 mg/mL) were incubated with serial dilutions of CB, F3, AcV FPLC and HPLC in saline solution (NaCl 0.15 M) in a 96-well plate, at a ratio of 1:1. The plate was incubated for 1 h (at pH 7.4 and 22 °C), and a negative control (saline solution and red blood cells) was performed for further comparison. The degree of agglutination was visually analyzed and the title expressed in hemagglutination units (U.H.), which are defined as the inverse of the highest dilution where Red Blood Cell (RBC) agglutination was observed (Santos et al., 2019).
RBCs were separated from the plasma by sedimentation and washed three times with saline solution. Then, 100 μL of the RBC suspension was incubated with 100 μL of the samples (CB, F3, AcV FPLC and HPLC) for 60 min at 25 °C. For positive control, 100 μL of RBC suspension was incubated with 100 μL of 1% Triton X-100, while 100 μL of saline was incubated with same volume of RBC suspension for negative control. After incubation, the reaction mixture was centrifuged (Hettich® MIKRO 200/200R) at 3.200 × g for 5 min at 25 °C. Aliquots of 100 μL of supernatants were transferred to 96-well plates and analyzed by spectrophotometry with readings at 405 nm (Pharmacia Biotec® Ultrospec 2100 pro). The mean and standard deviation was determined by three replicate assays (Santos et al., 2019).
2.8. In vivo bioassay
The experiment with C. maculatus larvae in an artificial seed system was performed according to the method described by Macedo et al., (1993). Increasing concentrations (0.02, 0.1, 0.2, 0.5, 1.0 and 2.0%) of AcV were incorporated into an artificial diet system (400 mg each) (w:w). The different AcV concentrations were mixed with a fine cowpea seed meal and then pressed using a cylindrical brass hammer and a hand press. Artificial seeds were offered for 24 h oviposition of fertilized females. After this period, the females were removed and the number of eggs reduced to three per seed, incubated for 20 days at 28° C with 60% relative humidity. At the end of this period, the seeds were opened to determine the WD50, a concentration of AcV capable of reducing the larvae weights by half and LD50, the AcV concentration responsible for decreasing the number of live larvae by half. To determine the ED50, the concentration of AcV responsible for halving the number of adults appearing, the life cycle was extended to 33 days and the experiment ended at 50 days after the infestation. The experiments were performed in four replicates and the mean was determined. Artificial control seeds were made with cowpea and bovine serum albumin (Sigma).
2.9. Digestibility with larval midgut homogenate
At 17 days after larvae hatched, the C. maculatus larvae had their intestine removed with the aid of a forceps and a stereomicroscope. A total of 200 intestines were removed and placed in 200 mL of saline solution (0.15 M NaCl). The intestines were soft stirred and maintained in 0.8 mL of 0.05 M sodium acetate buffer, pH 7.5 on ice for 10 min. The total intestinal extract was centrifuged at 10000 × g for 20 min at 4 °C and the supernatant, called midgut homogenate (MH), was collected and used for in vitro assays. For digestibility tests, an aliquot of AcV (0.192 mg. mL−1) in 0.05 M sodium acetate buffer, pH 7.5 was incubated with IH (0.048 mg. mL−1) at 37 °C for periods of 0, 15, 30 min and 1, 2, 4, 6, 8, 12 and 24 h. The ratio of vicilin to IH was 6:1. 10% SDS solution was added to stop the digestion. Protein hydrolysis was observed by 12% SDS-PAGE (Laemmli, 1970, Oliveira et al., 2007). Assays were performed in triplicate.
2.10. Statistical analysis
A normality test (distribution) of the data was made, and the data were not statistically parametric. Based on this, the analyses were made by the Kruskal-Wallis non-parametric analysis of variance; since we had five groups, thus comparing independent samples, the result was significant, that is, “p” <0.05%. To observe which concentration of AcV was most efficient in inhibiting larval development or promoting larval death, Dunn's hypothesis test was performed, which showed that the concentration of 0.5% was the one that best interfered in the biological cycle of C. maculatus. All statistical analyses were carried out using the GraphPad Prism program, available free.
3. Results
Saturation with ammonium sulfate at 60–90%, obtained from the raw extract of the seeds of A. colubrina, corresponded to the rich fraction with globuline proteins. This was subjected to a chromatography sequence to purify the vicilin. After the use of chitin-affinity chromatography (Fig. 1) and molecular exclusion chromatography (Superdex 75) on an FPLC system (Fig. 2), the sample obtained was named AcV, A. colubrina Vicilin, and was then submitted to Reverse phase HPLC, in a Phenomenex C8 column (Fig. 3), obtaining four peaks, which presented molecular masses of 73, 70, 43, 41 kDa. This standard of a tetrameric protein is observed in SDS-PAGE, in the three chromatographies performed (Fig. 4a). In non-denaturant conditions, the AcV is a homogeneous protein, with acid characteristic (Fig. 4B). Carbohydrate dosage was established at 1.55%, which classifies it as a glycoprotein. It was observed that AcV does not present biological activities of inhibition of the serine, cystein and α-amylase enzymes, and nor does it present hemagglutination or hemolytic activities, when tested (Table 1).
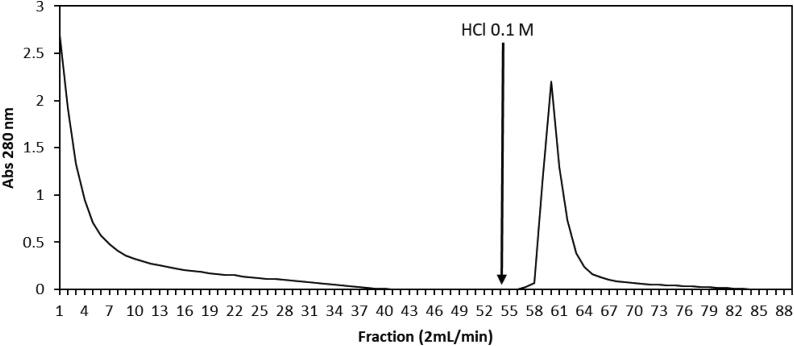
Fig. 1.
Elution profile on chitin affinity chromatography of F60-90 from A. colubrina. The column was pre-equilibrated with 0.02 M sodium tetraborate buffer, pH 7.5. Approximately 1.024 mg/mL of the F60-90 was applied. The adsorbed proteins, named AcV, were eluted with 0.1 M HCl, at a constant flow rate of 2 mL/min; the absorbance was monitored at 280 nm.
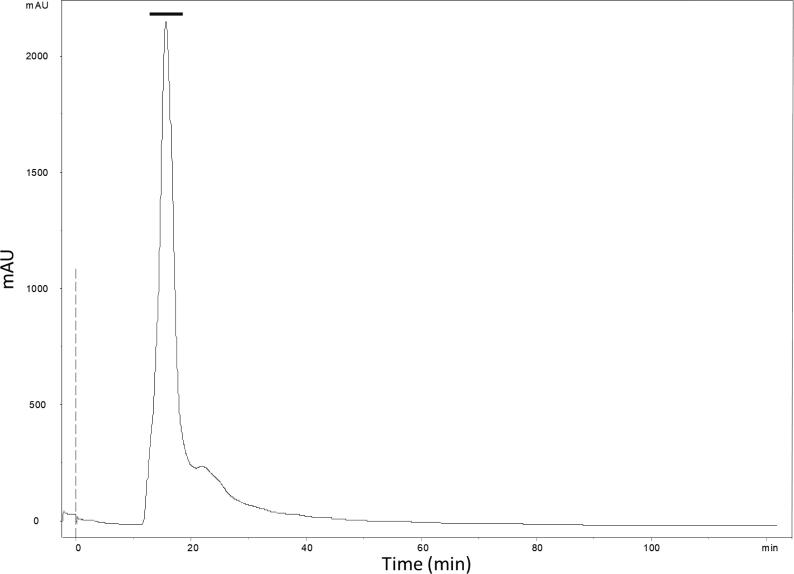
Fig. 2.
AcV elution profile on Superdex 75 Tricorn 10x300 mm chromatography in a FPLC/AKTA system. The column was equilibrated and eluted with 0.02 M Tris buffer with 0.2 M NaCl at pH 8.0 at constant flow rate of 0.5 mL/min. The absorbance was measured at 280 nm. (-) corresponds to fractions 11 to 16.
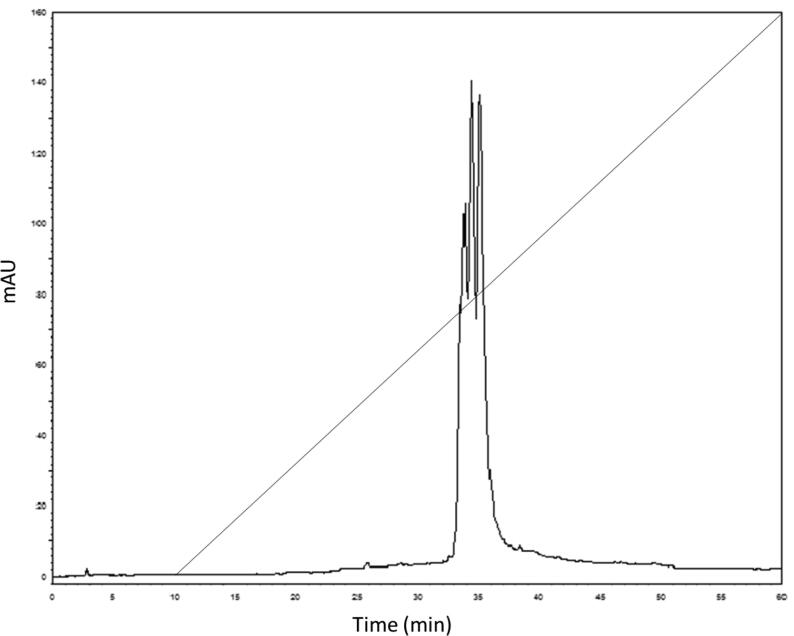
Fig. 3.
Elution profile of AcV on Phenomenex C8 (5 μm, st 4.6/250, 100 Å) chromatography in a HPLC system. The column was equilibrated with trifluoroacetic acid (0.1%) and eluted with a linear gradient (0–100%) of 100% acetonitrile in 0.1% trifluoroacetic acid. The elution profile was monitored at 214 and 280 nm.
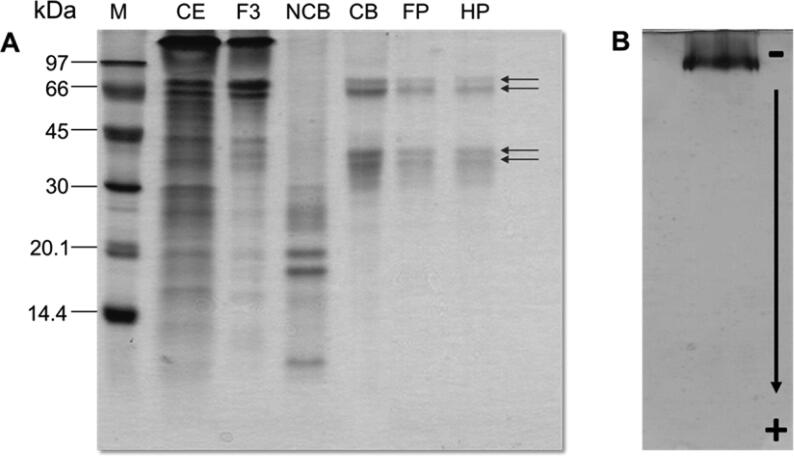
Fig. 4.
(A) SDS-PAGE (15%) of A. colubrina protein fractions stained with comassie bright blue. M: molecular mass markers (Phosphorylase (97.0 kDa); Bovine serum albumin (66.0 kDa); Ovalbumin (45.0 kDa); Carbonic anhydrase (30.0 kDa); Trypsin inhibitor (20.1 kDa) and α-lactobumin (14.4 kDa); EC – crude extract; F3 - precipitated proteins with (NH4)2SO4 at the 60–90% of saturation; NCB – chitin non-retained fraction in chitin column; FP - fraction obtained by Superdex 75 Tricorn in a FPLC/AKTA system; HP - fraction obtained by reversed-phase HPLC (Phenomenex C8); CB - chitin retained fraction (AcV). Arrows indicate the subunits of A. colubrina vicillin (AcV); (B) PAGE (15%) of AcV revealed with silver; Calibration curve indicating the molecular mass of the subunits of AcV (a, 73; b, 70; c, 43 and d, 41 kDa).
Table 1.
Detection of defensive proteins and biological activities throughout the AcV purification process.
| Anadenanthera colubrina |
DEFENSE PROTEINS |
||||
|---|---|---|---|---|---|
| α-amylase inhibitors | Serine inhibitors1 | Cysteine inhibitors2 | Hemolytic Activities | Lectins3 | |
| Crude extract | + | + | + | – | – |
| F3 (60–90%) | + | + | + | – | – |
| AcV | – | – | – | – | – |
| FPLC | – | – | – | – | – |
| HPLC | – | – | – | – | – |
(+) positive for activity; (-) negative for activity; the proteolytic enzymes tested, (1) serine protease (Trypsin, Chymotrypsin and Neutrophil Elastase) and (2) cysteine protease (papain); (3) Hemagglutinating activity, indicating the presence of lectins, was tested for (Type A, B, O) human erythrocytes treated and untreated with trypsin and papain.
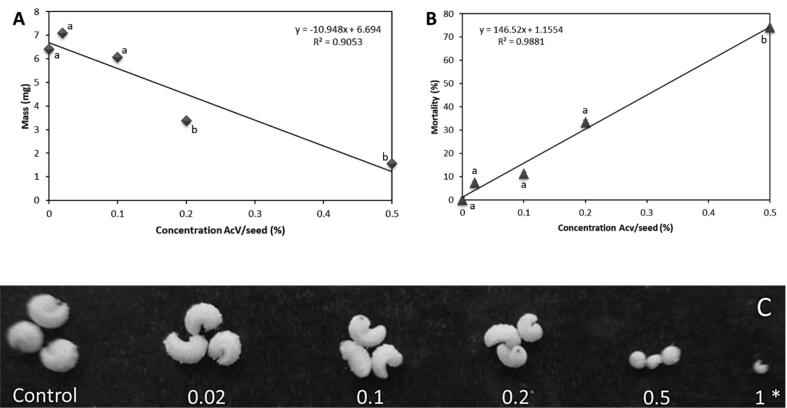
The artificial diet containing AcV caused a decrease in the development of C. maculatus larvae in a dose-dependent manner up to 0.5% (Fig. 5C); above that, all larvae were found dead. Based on these parameters, the WD50 and LD50 were determined. The AcV dose–effect curves showed efficiency in defending the seed, affecting the larval mass, determining the WD50 at 0.32% and the number of larvae, LD50, at 0.33% during the pest development cycle (Fig. 5A and 5B).
Fig. 5.
Effect of AcV on mass and mortality in C. maculatus. (A and B) dose effect curves on larval mass and mortality, respectively and BSA as a positive control. Different letters represent significant difference between concentrations according to Kruskal-Wallis test, where, p < 0.05. (C) C. maculatus larvae fed with artificial diet at increasing concentrations of 0; 0.02; 0.1; 0.2; 0.5 and 1% (w/w) of AcV. (*) only this single larva was observed after artificial diet assay at concentration of 1%.
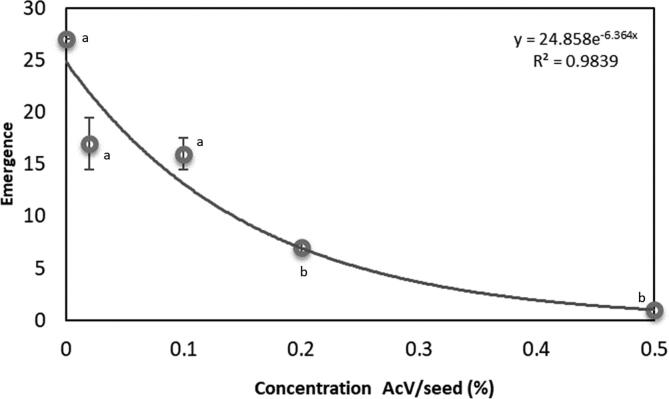
For the ED50, we observed a gradual delay in the emergence of the insect according to the increase in AcV concentrations (29 days to 0.02%, 30 days to 0.1%, 32 days to 0.2%, 33 days to 0.5% with the appearance of a single specimen with a mass of 7 mg). At concentrations of 1 and 2%, there was no emergence of adults until the end of the experiment, 50 days after the infestation (Fig. 6). The adult control group underwent, as expected, a normal 28-day life cycle to emerge.
Fig. 6.
Effect of AcV (0.02, 0.1, 0.2, 0.5, 1.0 and 2.0%, w/w) different concentrations on emergence adults of C. maculatus. Different letters represent significant difference between the concentrations according to Kruskal-Wallis test, where, p < 0.05.
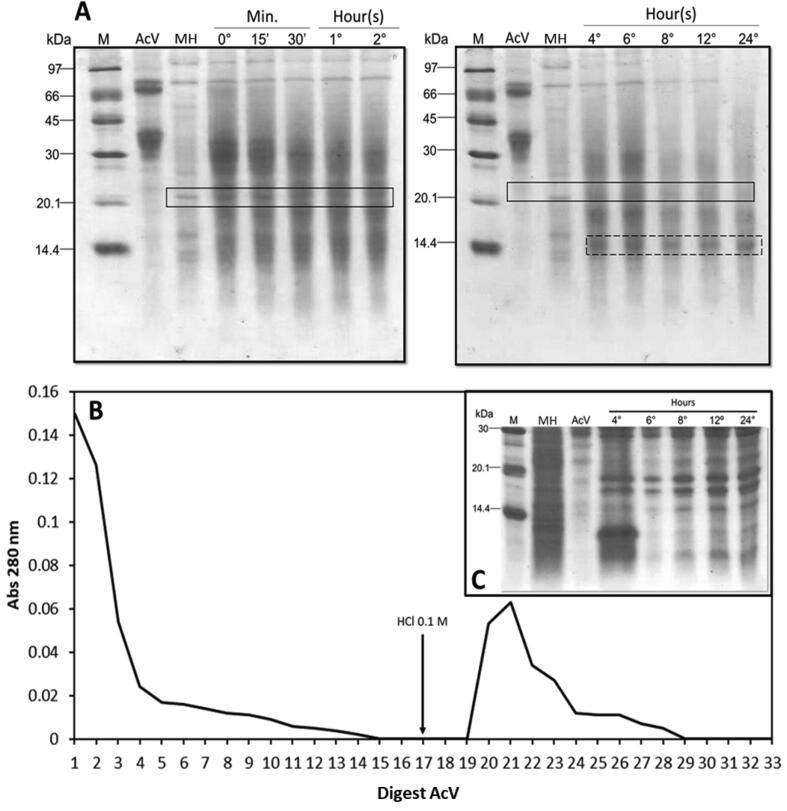
AcV digestibility was evaluated in vitro using the total homogenate of the digestion enzymes from the fourth instar larvae of C. maculatus (Fig. 7). After digestion, at different times, the samples were submitted to SDS-PAGE. In both techniques the presence of AcV fragments with molecular mass around 10 to 30 kDa was observed (Fig. 7A). Then, the AcV fragments, obtained within 24 h, were discovered by affinity chromatography on the chitin matrix, where it was possible to verify that they still maintained the affinity for the chitinous matrix, and a molecular mass range (Fig. 7C) close to that observed in Fig. 7A.
Fig. 7.
(A) SDS-PAGE of the in vitro digestion products of A. colubrina vicillin by the homogenate of C. maculatus larvae intestinal enzymes: M - molecular mass marker; A. colubrina vicillin; IH - Intestinal homogenate of larvae; (0 to 24), time of exposure of AcV, 30 mg, to intestinal homogenate of larvae in hours in vitro. (B) elution profile of 24-hour digested AcV fragments with C. maculatus intestinal homogenate on chitin affinity chromatography. The column was pre-equilibrated with 0.02 M sodium tetraborate buffer, pH 7.5. The adsorbed fragments were eluted with 0.1 M HCl, in a constant flow of 1 mL/min, the absorbance was measured at 280 nm. (C) SDS-PAGE electrophoresis of the adsorbed fragments from the aforementioned chromatography: M - molecular mass marker; A. colubrina vicillin; MH - midgut homogenate of larvae; (4 to 24), time of exposure of AcV, 30 mg, to intestinal homogenate of larvae in hours in vitro. The fragments highlighted in box are more likely to be those ones found in Fig. 7 (A and C).
4. Discussion
In the present work, we isolated and purified a vicilin (denominated AcV) from A. colubrina. Combined analysis of electrophoresis under native conditions and SDS-PAGE revealed AcV is a tetrameric protein, composed of 41, 43, 70, and 73 kDa subunits. This multimeric pattern is commonly observed in other leguminous vicilins, as described by Orruño and Morgan (2007) and Yunes et al., (1998). Scholz et al. (1983) and Miranda et al. (2020) also found the presence of lower molecular weight subunits from post-translational proteolytic processing of vicilin subunits, which is one of the characteristics of this class of oligomeric proteins. The homogeneity of AcV analyzed by native electrophoresis, PAGE, also revealed the presence of a single band with acidic character, which corresponds to the properties found for several 7S globulins described by Cândido et al., (2011). On average, vicilins are described with glycosylation levels around 1 to 2% of their mass (Coelho et al., 2010, Derbyshire et al., 1976). AcV is a glycoprotein, with about 1.55% of its mass composed of carbohydrates.
Considering that the non-cultivated legume A. colubrina has an arsenal of defense proteins that protect its embryo, the vicilin from AcV seeds was isolated and its toxic effect was tested against the larvae and adults of C. maculatus, an insect pest of stored legume seeds, evaluated by bioassay on artificial seeds. The WD50 calculated from the larval bioassay was 0.32. This result for AcV showed a greater toxicity for mass loss in larvae of C. maculatus, when compared with the results of Yunes et al., (1998) in Vigna Unguiculata - IT81D 1045 cultivar (1.07%), Glycine max (1.66%) Phaseolus lunatus (1.74%), Canavalha ensiformes (2.15%), P. vulgaris (2.22%) V. angulares (5.40%) and V. unguiculata – CE-31 cultivar (6.25%).
The AcV was efficient in causing mortality of the larvae when we analyzed the LD50, which was 0.33%. Results obtained by Amorim, et al. (2008), in a study that evaluated another kind of lepidopteran, Plodia interpunctella (Indian-meal moth) in relation to a vicilin from the seed of another legume, Erythrina velutina (EvV) at 0.23% promoted the mortality of 50% of the population. Macedo et al., (2008) demonstrated that EvV presented toxicity to the larvae of the dipteran Ceratitis capitata (fruit fly) at an LD50 of 0.14%. These findings demonstrate the potential and versatility of vicilins in the management of different pests.
In contrast to Macedo et al., (1993), in a study that observed satisfactory results in the concentration of 4% of the tested vicilins, AcV, on the other hand, was efficient at a concentration of 1%, with only one living larva found, which had larval development characteristic of the second stage at the end of the 28 days of the experiment.
The amount of AcV that reduces the emergence of adults by half, ED50, was 0.096% (w:w). From the dose–response curve, it was observed that AcV in concentrations higher than 0.5% caused 100% mortality of adults in the test up to the 50th day after the infestation, thus corroborating the result observed for the LD50. These data demonstrate the potent effect of AcV on disrupting the development and survival of C. maculatus adults. The low effective concentration of the emergence inhibition of adults demonstrates the potential of vicilins as a control tool for pests.
The digestion of vicilins and subsequent reuse of amino acids from these fragments is of paramount importance for maintaining the development of C. maculatus in its life cycle (Uchôa et al., 2006). It has been described that the absorption of these fragments in the larval enterocytes may be involved in cell surface recognition and vesicular trafficking in the cytoplasm (Oliveira et al., 2014). These fragments of resistant vicilins accumulate in the cells of the enterocytes and adipose body (Alexandre et al., 2011) in higher concentrations than those of susceptible vicilins, which results in the chronic effects observed in the larvae of C. maculatus. The fragments of the digestion of the AcV in vitro, which were between 10 and 30 kDa, corroborate results obtained for vicilin from V. unguiculata - IT81D 1045 Cultivar (Sales et al., 1992) and Albizia Lebbeck (Souza et al., 2012). Similar to that found by Uchôa et al., 2009, Oliveira et al., 2014, AcV maintained its ability to connect the chitin matrix, giving indications of its toxicity. These portions, which remain integrated with the ability to bind to the peritrophic matrix of the average gut of larvae, prevent the absorption of food and promote death due to the low nutrition of C. Maculatus. This toxic action of vicilins may explain the prolongation of its life cycle and/or the low rate of emergence of adults after contact with AcV. The cytotoxic effect of C. maculatus vicilins depends on the glycoprotein binding in the microvilli on the surface of the intestinal epithelium, mediated by caveolin, which takes the fragments of vicilin to the hemolymph and mainly deposits them in the adipose body (Alexandre et al., 2011, Kunz et al., 2017). Miranda et al. (2020), showed a strong link in C. maculatus between the vicilin of V. unguiculata IT81D 1045 (cultivar) and acetylated chitin and that chemical modifications in the amino acid residues tryptophan, lysine and tyrosine decreased this affinity to chitin binding, consequently attenuating the toxicity of vicilin in relation to the insect. Potential chitin-binding regions were identified between the amino acids Arg208 and Lys216 or regions involving the amino acids Lys223 and/or Trp316. This information sheds light on the relationship between AcV and its toxicity in C. maculatus.
5. Conclusion
A. colubrina vicilin is an oligomeric glycoprotein with a molecular mass of approximately 228 kDa, composed of subunits with a molecular mass of 43, 41, 73 and 70 kDa. AcV and its peptide fragments show high affinity for chitin, with potent larvicidal activity and emergence of adults, with few fully completing their biological cycle. The binding of AcV, either whole or as fragments, to chitinous structures present in the insect’s intestine is probably closely associated with weight loss and insect mortality, indicating that the fragments generated of AcV digestion are still bioactive. This makes them potential sources of useful molecules for integrated pest management programs, such as the development of bioinsecticides, the production of toxic bait or the implementation of plant breeding programs, in monocultures of agricultural interest for the human population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
In this work, our special thanks go to Maurício Pereira de Sales (in memoriam) and José Xavier Filho (in memoriam), for their brilliant ideas and contributions to the study of vicilins and their mechanisms of bioinsecticidal activity. The authors acknowledge the support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Finance Code 001.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
A.F.J. França, Email: andersonfjf@ufrn.edu.br.
J.N. Araújo, Email: jonalsonn@yahoo.com.br.
Y.Q. Santos, Email: yago.queiroz@ifce.edu.br.
G.S.C. Carelli, Email: gabicarelli@hotmail.com.
D.A. Silva, Email: delanoanibal@gmail.com.
T.M.L. Amorim, Email: ticianaamorim@ufpi.edu.br.
L. Migliolo, Email: rf4900@ucdb.br.
E.A. Santos, Email: elizeu@cb.ufrn.br.
A.S. Oliveira, Email: cisteana@yahoo.com.br.
A.F. Uchôa, Email: afucho@ufrnet.br.
References
- Alexandre D., Linhares R.T., Queiroz B., Fontoura L., Uchôa A.F., Samuels R.I., Macedo M.L.R., Bezerra C.S., Oliveira E.M., Demartini D.R., Carlini C.R., Silva C.P. Vicilin-derived peptides are transferred from males to females as seminal nuptial gift in the seed-feeding beetle Callosobruchus maculatus. J. Insect Physiol. 2011;57:801–808. doi: 10.1016/j.jinsphys.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Amorim T.M.L., Macedo L.L.P., Uchoa A.F., Oliveira A.S., Pitanga J.C.M., Macedo F.P., Santos E.A., De Sales M.P. Proteolytic digestive enzymes and peritrophic membranes during the development of Plodia interpunctella (Lepidoptera: Piralidae): Targets for the action of Soybean Trypsin Inhibitor (SBTI) and Chitin-Binding Vicilin (EvV) J. Agric. Food Chem. 2008;56:7738–7745. doi: 10.1021/jf801224d. [DOI] [PubMed] [Google Scholar]
- Beck C.W., Blumer L.S. A Handbook on Bean Beetles. Callosobruchus maculatus. Natl. Sci. Found. 2014:1–17. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1088/1751-8113/44/8/085201. [DOI] [PubMed] [Google Scholar]
- Cândido E.D.S., Pinto M.F.S., Pelegrini P.B., Lima T.B., Silva O.N., Pogue R., Grossi-de-Sá M.F., Franco O.L. Plant storage proteins with antimicrobial activity: novel insights into plant defense mechanisms. FASEB J. 2011;25:3290–3305. doi: 10.1096/fj.11-184291. [DOI] [PubMed] [Google Scholar]
- Coelho M.B., Macedo M.L.R., Marangoni S., Da Silva D.S., Cesarino I., Mazzafera P. Purification of legumin-like proteins from coffea arabica and coffea racemosa seeds and their insecticidal properties toward cowpea weevil (Callosobruchus maculatus) (Coleoptera: Bruchidae) J. Agric. Food Chem. 2010;58:3050–3055. doi: 10.1021/jf9037216. [DOI] [PubMed] [Google Scholar]
- Derbyshire E., Wright D.J., Boulter D. Legumin and vicilin, storage proteins of legume seeds. Phytochemistry. 1976 doi: 10.1016/S0031-9422(00)89046-9. [DOI] [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Edwards O., Singh K.B. Resistance to insect pests: What do legumes have to offer? Euphytica. 2006;147:273–285. doi: 10.1007/s10681-006-3608-1. [DOI] [Google Scholar]
- Gatehouse J.A., Croy R.R.D., Boulter D., Shewry P.R. The synthesis and structure of pea storage proteins. CRC. Crit. Rev. Plant Sci. 1984;1:287–314. doi: 10.1080/07352688409382182. [DOI] [Google Scholar]
- Gomez Carlos P. COWPEA Post-harvest Operations. Food Agric. Organ. United Nations. 2004:1–70. [Google Scholar]
- Kramer K.J., Hopkins T.L., Schaefer J. Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem. Mol. Biol. 1995 doi: 10.1016/0965-1748(95)00053-4. [DOI] [Google Scholar]
- Kunz D., Oliveira G.B., Uchôa A.F., Samuels R.I., Macedo M.L.R., Silva C.P. Receptor mediated endocytosis of vicilin in Callosobruchus maculatus (Coleoptera: Chrysomelidae) larval midgut epithelial cells. Comp. Biochem. Physiol. Part - B Biochem. Mol. Biol. 2017;210:39–47. doi: 10.1016/j.cbpb.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehane M.J. Peritrophic matrix structure and function. Annu. Rev. Entomol. 1997;42:525–550. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- Macedo, L.L.P., Amorim, T.M.L., Uchôa, A.F., Oliveira, A.S., Ribeiro, J.K.C., De MacEdo, F.P., Santos, E.A., De Sales, M.P., 2008. Larvicidal effects of a chitin-binding vicilin from erythrina velutina seeds on the mediterranean fruit fly ceratitis capitata, in: Journal of Agricultural and Food Chemistry. American Chemical Society, pp. 802–808. https://doi.org/10.1021/jf072746n [DOI] [PubMed]
- Macedo M.L., Fernandes K.V., Sales M.P., Xavier-Filho J. Purification and properties of storage proteins (vicilins) from cowpea (Vigna unguiculata) seeds which are susceptible or resistant to the bruchid beetle Callosobruchus maculatus. Braz. J. Med. Biol. Res. 1995;28:183–190. [PubMed] [Google Scholar]
- Macedo M.L.R., Lúcia L.B., Moraes R.A., Xavier-Filho J. Vicilin variants and the resistance of cowpea (Vigna unguiculata) seeds to the cowpea weevil (Callosobruchus maculatus). Comp. Biochem. Physiol. Part C, Comp. 1993;105:89–94. doi: 10.1016/0742-8413(93)90063-Q. [DOI] [Google Scholar]
- Mehlhorn, H., 2016. Peritrophic Membranes, in: Encyclopedia of Parasitology, Zoophysiology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 2131–2132. https://doi.org/10.1007/978-3-662-43978-4_2374
- Miranda M.R.A., Uchôa A.F., Ferreira S.R., Ventury K.E., Costa E.P., Carmo P.R.L., Machado O.L.T., Fernandes K.V.S., Amancio Oliveira A.E. Chemical Modifications of Vicilins Interfere with Chitin-Binding Affinity and Toxicity to Callosobruchus maculatus (Coleoptera: Chrysomelidae) Insect: A Combined in Vitro and in Silico Analysis. J. Agric. Food Chem. 2020;68:5596–5605. doi: 10.1021/acs.jafc.9b08034. [DOI] [PubMed] [Google Scholar]
- Oliveira A.S., Migliolo L., Aquino R.O., Ribeiro J.K.C., Macedo L.L.P., Andrade L.B.S., Bemquerer M.P., Santos E.A., Kiyota S., de Sales M.P. Purification and characterization of a trypsin-papain inhibitor from Pithecelobium dumosum seeds and its in vitro effects towards digestive enzymes from insect pests. Plant Physiol. Biochem. 2007;45:858–865. doi: 10.1016/j.plaphy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Oliveira G.B., Kunz D., Peres T.V., Leal R.B., Uchôa A.F., Samuels R.I., Macedo M.L.R., Carlini C.R., Ribeiro A.F., Grangeiro T.B., Terra W.R., Xavier-Filho J., Silva C.P. Variant vicilins from a resistant Vigna unguiculata lineage (IT81D-1053) accumulate inside Callosobruchus maculatus larval midgut epithelium. Comp. Biochem. Physiol. - B Biochem. Mol. Biol. 2014;168:45–52. doi: 10.1016/j.cbpb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Rufino F.P.S., Pedroso V.M.A., Araujo J.N., França A.F.J., Rabêlo L.M.A., Migliolo L., Kiyota S., Santos E.A., Franco O.L., Oliveira A.S. Inhibitory effects of a Kunitz-type inhibitor from Pithecellobium dumosum (Benth) seeds against insect-pests’ digestive proteinases. Plant Physiol. Biochem. 2013;63:70–76. doi: 10.1016/j.plaphy.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Sales M.P., Maria M.L., Xavier-Filho J. Digestibility of cowpea (Vigna unguiculata) vicilins by pepsin, papain and bruchid (insect) midgut proteinases. Comp. Biochem. Physiol. – Part B Biochem. 1992;103:945–950. doi: 10.1016/0305-0491(92)90220-L. [DOI] [Google Scholar]
- Santos, Y.Q. dos, Carelli, G.S.C., Veras, B.O. de, Batista, V.C., França, A.F.J. de, Silva, M.V. da, Santos, E.A. dos, 2019. Antitryptical, anticoagulant and hemagglutinating activities of Eucalyptus sp. seeds. F1000Research 8, 28. https://doi.org/10.12688/F1000RESEARCH.17080.1
- Somta P., Ammaranan C., Ooi P.A.C., Srinives P. Inheritance of seed resistance to bruchids in cultivated mungbean (Vigna radiata, L. Wilczek) Euphytica. 2007;155:47–55. doi: 10.1007/s10681-006-9299-9. [DOI] [Google Scholar]
- Southgate B.J. Biology of the Bruchidae. Annu. Rev. Entomol. 1979;24:449–473. doi: 10.1146/annurev.en.24.010179.002313. [DOI] [Google Scholar]
- Souza A.J., Ferreira A.T.S., Perales J., Beghini D.G., Fernandes K.V.S., Xavier-Filho J., Venancio T.M., Oliveira A.E.A. Identification of Albizia lebbeck seed coat chitin-binding vicilins (7S globulins) with high toxicity to the larvae of the bruchid Callosobruchus maculatus. Brazilian J. Med. Biol. Res. 2012;45:118–124. doi: 10.1590/S0100-879X2012007500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira F., Oliveira A., Macedo L., Santos E., de Sales M. Effects of a Chitin Binding Vicilin from Erythrina velutina Seeds on Bean Bruchid Pests (Callosobruchus maculatus and Zabrotes subfasciatus) Protein Pept. Lett. 2008;15:270–274. doi: 10.2174/092986608783744171. [DOI] [PubMed] [Google Scholar]
- Terra W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 2001;47:47–61. doi: 10.1002/arch.1036. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuda M. Applied evolutionary ecology of insects of the subfamily Bruchinae (Coleoptera: Chrysomelidae) Appl. Entomol. Zool. 2007;42:337–346. doi: 10.1303/aez.2007.337. [DOI] [Google Scholar]
- Tuda M., Rönn J., Buranapanichpan S., Wasano N., Arnqvist G. Evolutionary diversification of the bean beetle genus Callosobruchus (Coleoptera: Bruchidae): Traits associated with stored-product pest status. Mol. Ecol. 2006;15:3541–3551. doi: 10.1111/j.1365-294X.2006.03030.x. [DOI] [PubMed] [Google Scholar]
- Uchôa A.F., DaMatta R.A., Retamal C.A., Albuquerque-Cunha J.M., Souza S.M., Samuels R.I., Silva C.P., Xavier-Filho J. Presence of the storage seed protein vicilin in internal organs of larval Callosobruchus maculatus (Coleoptera: Bruchidae) J. Insect Physiol. 2006;52:169–178. doi: 10.1016/j.jinsphys.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Uchôa A.F., De Miranda M.R.A., De Souza A.J., Gomes V.M., Fernandes K.V.S., Lemos F.J.A., Oliveira A.E.A., Xavier-Filho J. Toxicity of hydrolyzed vicilins toward callosobruchus maculatus and phytopathogenic fungi. J. Agric. Food Chem. 2009;57:8056–8061. doi: 10.1021/jf900999m. [DOI] [PubMed] [Google Scholar]
- Wang P., Granados R.R. Molecular structure of the peritrophic membrane (PM): Identification of potential PM target sites for insect control. Arch. Insect Biochem. Physiol. 2001;47:110–118. doi: 10.1002/arch.1041. [DOI] [PubMed] [Google Scholar]
- Wang P., Granados R.R. An intestinal mucin is the target substrate for a baculovirus enhancin. Proc. Natl. Acad. Sci. U. S. A. 1997;94:6977–6982. doi: 10.1073/PNAS.94.13.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- War A.R., Murugesan S., Boddepalli V.N., Srinivasan R., Nair R.M. Mechanism of resistance in mungbean [Vigna radiata (L.) R. Wilczek var. radiata] to bruchids, callosobruchus spp. (Coleoptera: Bruchidae). Front. Plant Sci. 2017 doi: 10.3389/fpls.2017.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Tokuda G. Cellulolytic systems in insects. Annu. Rev. Entomol. 2010;55:609–632. doi: 10.1146/annurev-ento-112408-085319. [DOI] [PubMed] [Google Scholar]
- Yunes A.N.A., de Andrade M.T., Sales M.P., Morais R.A., Fernandes K.V.S., Gomes V.M., Xavier-Filho J. Legume seed vicilins (7S storage proteins) interfere with the development of the cowpea weevil (Callosobruchus maculatus (F)) J. Sci. Food Agric. 1998;76:111–116. doi: 10.1002/(sici)1097-0010(199801)76:1<111::aid-jsfa932>3.0.co;2-4. [DOI] [Google Scholar]