Abstract
The main aim of this study is to analyze antioxidant properties of Polygonatum odoratum fermented with bacteria, fungi and yeast. Antioxidant activities (1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging, hydroxyl radical scavenging, and anti-lipid peroxidation abilities) were assessed in samples of flavones isolated from fermented P. odoratum (Mill.) druce samples. Fermentations using Lactobacillus, yeast and Aspergillus were investigated. Results showed that the antioxidant ability of Polygonatum odoratum flavones was decreased by the fermentation of Lactobacillus and yeast. Aspergillus niger fermentation improved the antioxidant ability of P. odoratum flavones. In this study, effective antioxidant activity was achieved in flavones fermented with Aspergillus niger than yeast and Lactobacillus species.
Keywords: Polygonatum odoratum, Flavones, Fermentation, Antioxidant activity
1. Introduction
The high intake of various plant based products is mainly associated with a decreased risk of a number of diseases, such as, cancer and atherosclerosis (Arasu et al., 2014b, Arasu et al., 2015b). In most cases, these health benefits have been mainly attributed to secondary metabolites that show significant antioxidant activity (Kim et al., 2014a, Kim et al., 2014b, Lee et al., 2014a, Lee et al., 2014b). Highly harmful reactive oxygen species and free radicals have been found to play significant role in the causes of various chronic diseases, including, cancer, hypertension, and diabetes (Arasu et al., 2014b, Arasu et al., 2015b, Lee et al., 2014a, Lee et al., 2014b). Antioxidants such as vitamin E and C are very much important for effective protection against various reactive oxygen species (Seo et al., 2015, Arasu et al., 2014b, Arasu et al., 2015b, Arasu et al., 2014a, Arasu et al., 2015a). Moreover, the majority of various antioxidant properties of medicinal plants may be from active compounds such as, flavonoids and phenolic acids, than carotene and vitamins. Hence, antioxidant secondary metabolites are widely discussed in recent years (Al-Dhabi et al., 2014, Balachandran et al., 2014, Arasu et al., 2014b, Arasu et al., 2015b, Park et al., 2014a, Park et al., 2014b, Park et al., 2014c). Polygonatum odoratum (Mill.) druce (POD) is a Liliaceae perennial herb and its root serves a dual purpose as both a food source and a medicinal constituent. It is sweet and mild, clear lung and warm stomach (Choi and Park, 2002), nourishing Yin and moistening dryness, and promoting fluid to quench thirst (Zhou et al., 2015). POD contains a variety of active substances such as amino acids, polysaccharides, glycosides and flavones (Quan et al., 2015). Flavones are antioxidants with activities associated with anti-aging, anti-virus, bacteriostasis and anti-cancer activities (Bai et al., 2014, Jiang et al., 2013), and enhancement of the immune system (Guo et al., 2012). Flavones have been listed as a functional factor in health food (Lan et al., 2011). Some flavones have potent antioxidant activity and the antioxidant activity of flavones in different materials varies because of their different chemical structures (Khan et al., 2010, Goupy et al., 2003, Arasu et al., 2013). Flavones in POD are mainly classified as high iso-flavones (Qian et al., 2010, Arasu et al., 2014b, Arasu et al., 2015b). These high iso-flavones possess high antioxidant activity because they contain more phenolic hydroxyl groups. In recent years, POD has been used as raw material for the preparation of fermented foods, such as bread, cake, wine, beverage, sauce, tea, candy, etc. (Baek et al., 2012). Yeast is often used in the production of fermented food such as druce bread. Lactic acid bacterial fermentation processing may be used in the production of druce fermented beverages to simplify the complex starch molecules and thereby improve product quality and flavor. Aspergillus niger fermentation may be used in the production of sauces to improve their acid content. The activity of proteases increases the utilization rate of raw materials and the quality of products. The effects of different fermentation methods on the antioxidant activity of flavones from POD have seldom been studied. In this paper, the effects of three different fermentation methods on the antioxidant activity of POD flavones in vitro were studied in order to analyze industrial applications for the development and utilization of POD and the maintenance and augmentation of its biological activity.
2. Materials and methods
2.1. Materials and reagents
POD was purchased from Songjianghe City, Baishan, Jilin Province, China. Methanol (chromatographic grade), was purchased from Tedia Company, Inc, USA. Yeast was obtained from Angel Yeast Limited by Share Ltd. Lactobacillus was provided by Kunshan Moshengyou Biotechnology Ltd. Aspergillus niger was purchased from Shanghai Luwei Science and Technology Ltd. DPPH originated from Sigma, USA. Lecithin was bought from Beijing Aobo Biotechnology Ltd. Reagents involved in this study were analytical grade, and distilled water prepared on-site.
2.2. Methods
2.2.1. Powder preparation of POD
POD was cleaned then placed in an air force oven to dry at 70 °C. Dried material was ground using a 425 μm pore-size screen.
2.2.2. Fermentation treatments of POD
2.2.2.1. Yeast fermentation
The yeast was placed in water at 26 ± 1 °C along with 1% sugar for activation. After 15 min, 5000 g of POD powder was placed in a glass tank. The pH value of the tank was adjusted to 6.0 and the yeast was inoculated at 1% concentration and small amount of distilled water was added in. After mixing, the tank was placed in an incubator at 28 °C for 24 h then moved to 70 °C for complete drying of the sample (Wang et al., 2018). The powder from this fermentation was ground and used for further analysis.
2.2.2.2. Lactobacillus fermentation
5000 g of POD powder was placed in a glass tank. A small amount of distilled water was added in to form homogenous slurry. The pH value was adjusted to 5.8 and Lactobacillus was inoculated at 1% concentration. The tank was subsequently placed in an incubator at 28 °C for 24 h and 70 °C for complete drying (Wei et al., 2018). Again the powder was grounded using a screener with a pore size of 425 μm after cooling to the room temperature for further experiment.
2.2.2.3. Aspergillus niger fermentation
5000 g of POD powder was placed in a glass tank. 400 g of blood powder, 40 g of KH2PO4, 1 g of MgSO4 and water were added to the tank. Aspergillus niger was inoculated at 0.25% concentration. The pH value was adjusted to 6.0 and the tank was placed in an oven at 32 °C for 5 days (Iyyappan et al., 2018), followed by drying at 70 °C. After cooling to the room temperature, the fermentation product was ground as described.
2.2.3. Extraction and purification of flavones
Each of the fermented POD powders was dissolved in ethyl alcohol (95%) at a ratio of 1:10 (weight to volume), mixed and ultrasonically extracted for 20 min. This procedure was repeated twice. The supernatant was rotary evaporated and decolorized 5 times using petroleum ether prior to filtration in treated macro-porous resin D101 columns at a flow rate of 1 mL/min. All samples were pumped into their respective columns and remained on the resin for 2 h. Columns were washed with different concentrations of ethanol. After washing, an 80% ethanol solution was collected, and most of the ethanol was removed from the samples by rotary evaporation. Residual alcohol was poured out and samples were brought to room temperature.
2.2.4. Isolation of flavones from extracted solution
The liquid sample obtained from resin purification contains both flavone and non-flavone components. The purity of flavone was improved by U3000 preparative high performance liquid chromatography (HPLC) (Semerfly Company, USA). After purification, the samples were filtered through 0.22 μm Millipore filters. Equipment conditions for HPLC enhancement of flavones were as follows: A single injection of 600 μL, at a column temperature of 25 °C was run. The detection wavelength was set at 296 nm and the flow rate was 3 mL/min. The elution profile is listed in Table 1. The flavonoid peaks of the effluent components were collected after qualitative analysis, and then were decompressed, steam-dried, suspended in a small amount of distilled water and transferred to a plate for lyophilization and subsequent storage (see Table 2.).
Table 1.
Elution of flavone using High Performance Liquid Chromatography.
| Elution program | Duration (min) | Flow rate (mL/min) | A methanol (%) | B water (%) |
|---|---|---|---|---|
| 0.000 | 3.000 | 50.0 | 50.0 | |
| 8.000 | 3.000 | 50.0 | 50.0 | |
| 10.000 | 3.000 | 80.0 | 20.0 | |
| 33.000 | 3.000 | 80.0 | 20.0 | |
| 37.000 | 3.000 | 50.0 | 50.0 | |
| 42.000 | 3.000 | 50.0 | 50.0 |
Table 2.
Qualitative methods for flavones in POD.
| No. | Test methods | Qualitative features |
|---|---|---|
| 1 | Ultraviolet absorption diagram | Weak spectral Ⅰ between 300 and 400 nm; strong spectral Ⅱ between 260 and 300 nm; spectral Ⅰ is acromion of spectral Ⅱ. |
| 2 | AlCl3 reaction/AlCl3 + HCl reaction | When heated or placed for a long time, the solution is green, the ultraviolet fluorescence is enhanced and the absorption band is red-shifted. When hydrochloric acid is added, the absorption band is blue-shifted. |
| 3 | FeCl3 reaction | Solution turns green with black precipitation. |
| 4 | HCl-Mg reaction | Produce brown foam or solution turns light brown. |
| 5 | Strong alkali reaction | Solution yellow deepened or turned brown. |
2.2.5. Identification and purity analysis of flavones
2.2.5.1. Qualitative analysis of flavones
The flavones in the samples were identified by hydrochloric acid-magnesium powder coloration testing, aluminum chloride coloration testing and ultraviolet absorption band analysis (Arasu et al., 2014a, Arasu et al., 2015a, Lee et al., 2016).
2.2.5.2. Purity analyses
Purity analyses were carried out according to the method of Socha et al., 2009, Dorman et al., 2003. Rutin was used as the standard and the curve was drawn by the method of aluminum nitrate coloration (Fig. 1). The regression equation is y = 1.0526x − 0.0051 (R2 = 0.9989). POD flavones were determined by their OD value using the method of aluminum nitrate coloration. Purity was calculated using the equation obtained.
Fig. 1.
Rutin standard curve.
2.2.6. Antioxidant activity of POD flavones
Five different concentrations (0.1 mg/mL, 0.2 mg/mL, 0.3 mg/mL, 0.4 mg/mL and 0.5 mg/mL) were prepared from extracted fermentation samples by adding 95% ethanol solution.
2.2.6.1. DPPH activity
DPPH solution (2 mL) was added along with 1 mL of flavone preparation, and 1 mL 95% ethanol. The tubes were vortexed and kept in dark for 30 min. The OD value (A1) was read at 517 nm using a UV-visible spectrophotometer (Sharma et al., 2008, Chang et al., 2002, Qiao et al., 2009). Ethanol (95%) was used as the control, and its OD value is defined as A0. The DPPH scavenging rate is calculated according to the following equation (Zhao et al., 2015, Choi et al., 2015).
where A0 is OD value of control sample, A1 is OD value of samples to be measured.
2.2.6.2. Anti lipid peroxidase assay
Briefly, 1 mL of 0.4 mmol/L ferrous sulfate, 1 mL of flavonoid sample and 1 mL of lecithin were combined. The reaction was performed at 37 °C and incubated in dark for 60 min. A mixture of 2 mL trichloroacetic acid (TCA) - thiobarbituric acid (TBA) – hydrochloric acid (HCl) was added and incubated in water bath at 90–100 °C for 5 min. The supernatant was quickly cooled. After centrifugation (5000 rpm/5 min), the OD value was determined at 535 nm using the Uv–visible spectrophotometer (each respective preparation is read as A1) (Srigopalram et al., 2017). The blank (95% ethanol) was recorded as A0. The activity is calculated as according to the equation described in Section 2.2.6.1.
2.2.6.3. Hydroxyl free radical scavenging activity
Flavonoids were tested for their hydroxyl free radical scavenging ability. 2 mL of flavonoids, 2 mL 6 mmol/L FeSO4 and 2 mL 0.3% H2O2 were combined and stirred for 10 min. Then, 2 mL 6 mmol/L salicylic acid was added, and incubated at 30 °C for 30 min (Qiao et al., 2009, Kim et al., 2014a, Kim et al., 2014b). The OD value of flavonoids samples was read at 510 nm using UV–visible spectrophotometer and recorded as A1 values. The OD value of 95% ethanol (control sample) was recorded as A0. Calculations were carried out as defined.
3. Results
3.1. Qualitative analysis of flavones
After purification by macro-porous resin, there were 17 major peaks in the HPLC preparations of POD flavonoids. The chromatogram (Fig. 2) clearly indicates the qualitative peaks of flavones. The components of peaks 1–7 did not conform to the qualitative phenomena of flavonoids, while the compounds of peaks 8–17 (the components with retention time between 33 min and 42 min) confirmed the presence of flavonoids (Table 3).
Fig. 2.

Total flavones prepared from HPLC.
Table 3.
Qualitative test results of flavones in POD.
| Peak No. | UV spectrum | AlCl3 | AlCL3 + HCl | FeCl3 | HCl-Mg | Alkali |
|---|---|---|---|---|---|---|
| 1 | Abnormal | – | – | – | – | – |
| 2 | Abnormal | – | – | – | – | – |
| 3 | Abnormal | – | – | – | – | – |
| 4 | Abnormal | – | – | – | – | – |
| 5 | Abnormal | – | – | – | – | – |
| 6 | Abnormal | – | – | – | – | – |
| 7 | Abnormal | – | – | – | – | – |
| 8 | Normal | + | + | + | + | + |
| 9 | Normal | + | + | + | + | + |
| 10 | Normal | + | + | + | + | + |
| 11 | Normal | + | + | + | + | + |
| 12 | Normal | + | + | + | + | + |
| 13 | Normal | + | + | + | – | – |
| 14 | Normal | + | + | + | + | + |
| 15 | Normal | + | + | + | + | + |
| 16 | Normal | + | + | + | + | + |
| 17 | Normal | + | + | + | + | – |
This phenomenon is associated with the solution of hydrochloric acid-magnesium powder in sample tubes appearing as a light red color. The ultraviolet absorption band shifted to the right (Fig. 3) in these solutions, and the ultraviolet absorption intensity was higher than that of solutions without aluminum chloride, indicating that the effluent contained flavonoids.
Fig. 3.

Comparison of ultraviolet absorption before and after AlCl3 was added to prepared solutions.
Flavonoids in POD was determined by aluminum nitrate spectrophotometry method. The absorbance was incorporated into the Rutin standard curve equation in order to calculate the total flavonoids content. The experimental results were described in Table 4.
Table 4.
Flavone concentrations in POD powder samples.
| No. | Sample name | OD (A) | Flavones concentration (mg/mL) | Purity (%) |
|---|---|---|---|---|
| 1 | Control | 0.542 | 0.520 | 74.16 |
| 2 | Yeast fermented | 0.632 | 0.605 | 72.38 |
| 3 | Lactobacillus fermented | 0.625 | 0.599 | 70.76 |
| 4 | A. niger fermented | 0.871 | 0.832 | 86.65 |
Following separation of non-flavone components using preparative liquid chromatography, the total flavones content of fermented POD powder samples was assessed. Total flavones content fermented with yeast and Lactobacillus significantly decreased compared with the control sample. While, the total flavones content fermented with A. niger significantly increased.
3.2. Antioxidant activity
3.2.1. DPPH free radical scavenging ability
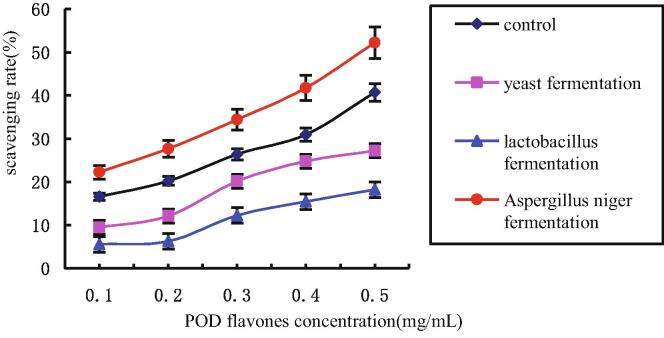
POD powder was fermented using yeast, Lactobacillus and A. niger respectively. The DPPH free radical scavenging ability of POD flavone was determined and results are shown in Fig. 4.
Fig. 4.
DPPH free radical scavenging activity of POD flavones treated by different fermentation methods.
All flavonoids tested have an ability to scavenge DPPH free radicals (Fig. 4). An increase in flavonoid concentration is associated with an increase in DPPH free radical scavenging rates. Compared with untreated control, the DPPH free radical scavenging ability of POD flavonoids after yeast and Lactobacillus fermentations decreased, while the activity increased after A. niger fermentation. The DPPH free radical scavenging ability effectively reflects the antioxidant capacity of flavonoids, and is related to their molecular structure. The number and position of phenolic hydroxyl groups in flavonoids are the key factors affecting their antioxidant activity. Lactobacillus fermentation and yeast fermentations could significantly decrease the DPPH free radical scavenging capacity of POD flavonoids, possibly due to changes in the molecular structure of the affected flavonoids. Alternatively, reaction of phenolic hydroxyl groups in POD with other components of POD may also be responsible for observed changes in activity. Tsangalis et al. (2004) found that Aspergillus niger secreted beta-glucosidase by which iso-flavones are transformed into aglycones, thereby improving the overall yield of flavones. DPPH free radical scavenging ability of POD flavonoids fermented by A. niger increased, which was also related to the increase of total flavonoids content. Because of the high content of polysaccharides in POD, a certain proportion of flavonoids in the total flavonoids of POD exist in the form of glycosides. The content of flavonoid aglycones therefore is expected to increase significantly after fermentation by Aspergillus niger. The increased antioxidant activity of POD flavones indicated positive correlation to aglycone levels in the samples.
3.2.2. Determination of anti-lipid peroxidation activity
Following fermentations with yeast, Lactobacillus or A. niger, flavones of POD samples were tested for anti-lipid peroxidation abilities. Lactobacillus fermented sample showed less scavenging activity than yeast fermented sample. A. niger fermented sample showed better scavenging activity and the results were shown in Fig. 5.
Fig. 5.
Anti-lipid peroxidation scavenging rates of POD flavones fermented by different organisms.
3.2.3. Hydroxyl free radical scavenging rate
Fermentation samples were tested for hydroxyl free radical scavenging rates. With the increase of flavone contents in prepared samples, the hydroxyl free radical scavenging rate increased (Fig. 6). Maximum hydroxyl free radical scavenging rate were measured in A. niger-fermented samples. Compared with control POD, the hydroxyl free radical scavenging rate of POD fermented by yeast or lactobacillus decreased, and yeast fermentated sample decreased significantly. Hydroxyl free radical scavenging rates of POD fermented by A. niger increased significantly.
Fig. 6.
Hydroxyl free radical scavenging rates of POD flavones treated by different fermentation methods.
4. Discussion
Plant phenolic compounds, including flavonoids showed potent antioxidant properties (Kim et al., 2013, Park et al., 2013). Glucosinolates, free amino acids, vitamin C and anthocyanins were were characterized from Brassica oleracea L. showed antioxidant properties (Park et al., 2014a, Park et al., 2014b, Park et al., 2014c). In this study antioxidant activity was analyzed from flavones sample. All POD flavone samples resulting from different fermentation methods have the ability to scavenge anti-lipid peroxidase free radicals. A significant trend was observed in the increase of flavones concentration and an associated increase in the scavenging rate of free radicals. Compared with untreated POD, yeast and Lactobacillus fermentation decreased the anti-lipid peroxidation free radical scavenging ability of the samples. However, A. niger fermentation increased the observed anti-lipid peroxidation free radical scavenging ability. POD flavones not only scavenge the free radicals in the initiation phase of oil chain, but also directly capture the free radicals in the free radical reaction chain (Cao et al., 2015), thus effectively blocking the chain auto-oxidation of oil free radicals and playing an anti-oxidation role. This blocking effect of flavones on free radicals in oils is closely related to the position of phenolic hydroxyl groups in the flavone molecules. The phenolic hydroxyl groups at 3 and 5 positions have strong antioxidant effect and are more likely to block the free chain reaction of oils. Lactobacillus fermentation and yeast fermentation may cause the structural changes of phenolic hydroxyl groups of POD flavones, which may lead to a significant decline in the anti-lipid peroxidation ability of POD flavones. Bučková et al. (2002) studied the antioxidant properties of total flavones of Glycyrrhiza uralensis. The results showed that the antioxidant capacity of flavones was significantly related to the dosage. Flavones could be used in combination with VC, citric acid and so on, playing a synergistic role. It can be seen that in the process of lactic acid bacterial fermentation and yeast fermentation of POD-derived foods, flavones can be used effectively in combinations with other additives to enhance the anti-lipid peroxidation ability. The hydroxyl free radical is an active oxygen species with a strong ability to capture electrons. The free radicals are comprehensive widely distributed in foods and the human body, and are capable of destroying red blood cells as well as degrading DNA, cell membranes and polysaccharides (Li et al., 2014). The treated POD flavones possess significant abilities to scavenge hydroxyl free radicals and useful for developing as a beneficial food supplement.
5. Conclusions
Antioxidant activities were assessed in fermented POD samples by three different methods. Flavones isolated from the fermentation samples were assessed for 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging ability, hydroxyl radical scavenging ability and anti-lipid peroxidation abilities. Results showed improved antioxidant activity in A. niger fermented sample. The practical recommendation could be the use of A. niger and avoid the use of lactobacillus and yeast.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This experiment was funded by Jilin Provincial Department of education “13th Five-Year” science and technology project JJKH20170437KJ.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Dhabi N.A., Arasu M.V., Park C.H., Park S.U. Recent studies on rosmarinic acid and its biological and pharmacological activities. EXCLI J. 2014;13:1192. [PMC free article] [PubMed] [Google Scholar]
- Arasu M.V., Jung M.W., Ilavenil S., Jane M., Kim D.H., Lee K.D., Park H.S., Hur T.Y., Choi G.J., Lim Y.C., Al-Dhabi N.A. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013;115(5):1172–1185. doi: 10.1111/jam.12319. [DOI] [PubMed] [Google Scholar]
- Arasu M.V., Ilavenil S., Kim D.H., Roh S.G., Lee J.C., Choi K.C. In vitro and in vivo enhancement of adipogenesis by Italian ryegrass (Lolium multiflorum) in 3T3-L1 cells and mice. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasu M.V., Kim S.J., Al-Dhabi N.A., Suzuki T., Yamauchi H., Lee S.W. Comparison of Flavonoid Contents between Common and Tartary Buckwheat (Fagopyrum) Sprouts Cultured with/without Soil. Asian J. Chem. 2014;26(18):5985. [Google Scholar]
- Arasu M.V., Al-Dhabi N.A., Rejiniemon T.S., Lee K.D., Huxley V.A.J., Kim D.H., Duraipandiyan V., Karuppiah P., Choi K.C. Identification and characterization of Lactobacillus brevis P68 with antifungal, antioxidant and probiotic functional properties. Ind. J. Microbiol. 2015;55(1):19–28. [Google Scholar]
- Arasu M.V., Jung M.W., Kim D.H., Park H.S., Ilavenil S., Al-Dhabi N.A., Choi K.C. Identification and phylogenetic characterization of novel Lactobacillus plantarum species and their metabolite profiles in grass silage. Ann. Microbiol. 2015;65(1):15–25. [Google Scholar]
- Baek S.H., Lee J.G., Park S.Y., Piao X.L., Kim H.Y., Bae O.N., Park J.H. Gas chromatographic determination of azetidine-2-carboxylic acid in rhizomes of Polygonatum sibiricum and Polygonatum odoratum. J. Food Comp. Anal. 2012;25(2):137–141. [Google Scholar]
- Bai H., Li W., Zhao H., Anzai Y., Li H., Guo H., Kato F., Koike K. Isolation and structural elucidation of novel cholestane glycosides and spirostane saponins from Polygonatum odoratum. Steroids. 2014;80:7–14. doi: 10.1016/j.steroids.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Balachandran C., Sangeetha B., Duraipandiyan V., Raj M.K., Ignacimuthu S., Al-Dhabi N.A., Balakrishna K., Parthasarathy K., Arulmozhi N.M., Arasu M.V. A flavonoid isolated from Streptomyces sp. (ERINLG-4) induces apoptosis in human lung cancer A549 cells through p53 and cytochrome c release caspase dependant pathway. Chemico-Biol. Interact. 2014;224:24–35. doi: 10.1016/j.cbi.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Bučková M., Labuda J., Šandula J., Križková L., Štěpánek I., Ďuračková Z. Detection of damage to DNA and antioxidative activity of yeast polysaccharides at the DNA-modified screen-printed electrode. Talanta. 2002;56(5):939–947. doi: 10.1016/s0039-9140(01)00654-3. [DOI] [PubMed] [Google Scholar]
- Cao J., Zheng Y., Xia X., Wang Q., Xiao J. Total flavonoid contents, antioxidant potential and acetylcholinesterase inhibition activity of the extracts from 15 ferns in China. Ind. Crop. Prod. 2015;75:135–140. [Google Scholar]
- Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food. Drug Anal. 2002;10(3):178–182. [Google Scholar]
- Choi K.C., Ilavenil S., Arasu M.V., Park H.S., Kim W.H. Effect of novel Lactobacillus plantarum KCC-10 and KCC-19 on fermentation characterization of alfalfa silage. J. Korean Soc. Grass. Forage Sci. 2015;35(2):166–170. [Google Scholar]
- Choi S.B., Park S. A steroidal glycoside from Polygonatum odoratum (Mill.) Druce. improves insulin resistance but does not alter insulin secretion in 90% pancreatectomized rats. Biosci. Biotechnol. Biochem. 2002;66(10):2036–2043. doi: 10.1271/bbb.66.2036. [DOI] [PubMed] [Google Scholar]
- Dorman H.J.D., Peltoketo A., Hiltunen R., Tikkanen M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83(2):255–262. [Google Scholar]
- Goupy P., Dufour C., Loonis M., Dangles O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003;51(3):615–622. doi: 10.1021/jf025938l. [DOI] [PubMed] [Google Scholar]
- Guo H.J., Zhao H.X., Bai H. Spectroscopic characteristics of steroidal saponins and homoisoflavonoids from Polygonatum odoratum. Acta Chinese Med. Pharmacol. 2012;5:41–46. [Google Scholar]
- Iyyappan J., Baskar G., Bharathiraja B., Saravanathamizhan R. Malic acid production from biodiesel derived crude glycerol using morphologically controlled Aspergillus niger in batch fermentation. Bioresour. Technol. 2018;269:393–399. doi: 10.1016/j.biortech.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Lv Y., Dai W., Miao X., Zhong D. Extraction and bioactivity of Polygonatum polysaccharides. Int. J. Biol. Macromol. 2013;54:131–135. doi: 10.1016/j.ijbiomac.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Khan M.K., Abert-Vian M., Fabiano-Tixier A.S., Dangles O., Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119(2):851–858. [Google Scholar]
- Kim S.J., Arasu M.V., Al-Dhabi N.A., Yoo D.S., Park M.H., Shin Y.S., Lee S.W. Quantitative Determination of Triterpenoidal Saponins in Platycodi radix and Its Variation in Different Regions of Korean Peninsula: A Herbal Plant Used as Traditional Medicine. Asian J. Chem. 2013;25(13):7093–7097. [Google Scholar]
- Kim S.J., Rahman M.M., Lee M.K., Seo J.M., Arasu M.V., Suzuki T., Al-Dhabi N.A., Yoon Y.H., Shim J.H. Identification and quantification of volatile and phenolic compounds composition in Buck wheat Sprouts by GC/MS and HPLC. Asian J. Chem. 2014;26(3):777. [Google Scholar]
- Kim Y.B., Thwe A.A., Kim Y., Li X., Cho J.W., Park P.B., Valan Arasu M., Abdullah Al-Dhabi N., Kim S.J., Suzuki T., Hyun Jho K. Transcripts of anthocyanidin reductase and leucoanthocyanidin reductase and measurement of catechin and epicatechin in tartary buckwheat. Sci. World J. 2014;27 doi: 10.1155/2014/726567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G., Chen H., Wang Z., Zhang W., Zhang L. Extraction of Polygonatum odoratum polysaccharides using response surface methodology and preparation of a compound beverage. Carbohy. Polymer. 2011;86(3):1175–1180. [Google Scholar]
- Lee M.K., Chun J.H., Byeon D.H., Chung S.O., Park S.U., Park S., Arasu M.V., Al-Dhabi N.A., Lim Y.P., Kim S.J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT-Food. Sci. Technol. 2014;58(1):93–101. [Google Scholar]
- Lee S.W., Seo J.M., Lee M.K., Chun J.H., Antonisamy P., Arasu M.V., Suzuki T., Al-Dhabi N.A., Kim S.J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crop. Prod. 2014;54:320–326. [Google Scholar]
- Lee S.Y., Yan Y.Z., Arasu M.V., Al-Dhabi N.A., Park S.U. Seasonal variation of saponin contents in Platycodon grandiflorum. Biosci. Biotechnol. Res. Asia. 2016;13(1):119–122. [Google Scholar]
- Li A.N., Li S., Zhang Y.J., Xu X.R., Chen Y.M., Li H.B. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–6047. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.H., Arasu M.V., Park N.Y., Choi Y.J., Lee S.W., Al-Dhabi N.A., Kim J.B., Kim S.J. Variation of glucoraphanin and glucobrassicin: anticancer components in Brassica during processing. Food Sci. Technol. 2013;33(4):624–631. [Google Scholar]
- Park S., Arasu M.V., Jiang N., Choi S.H., Lim Y.P., Park J.T., Al-Dhabi N.A., Kim S.J. Metabolite profiling of phenolics, anthocyanins and flavonols in cabbage (Brassica oleracea var. capitata) Ind. Crop. Prod. 2014;60:8–14. [Google Scholar]
- Park S., Arasu M.V., Lee M.K., Chun J.H., Seo J.M., Al-Dhabi N.A., Kim S.J. Analysis and metabolite profiling of glucosinolates, anthocyanins and free amino acids in inbred lines of green and red cabbage (Brassica oleracea L.) LWT-Food Sci. Technol. 2014;58(1):203–213. [Google Scholar]
- Park S., Arasu M.V., Lee M.K., Chun J.H., Seo J.M., Lee S.W., Al-Dhabi N.A., Kim S.J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.) Food Chem. 2014;145:77–85. doi: 10.1016/j.foodchem.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Qian Y., Liang J.Y., Qu W., Che Y. Two new homoisoflavanones from Polygonatum odoratum (Mill.) Druce. Chinese Chem. Lett. 2010;21(6):706–708. [Google Scholar]
- Qiao D., Ke C., Hu B., Luo J., Ye H., Sun Y., Yan X., Zeng X. Antioxidant activities of polysaccharides from Hyriopsis cumingii. Carbohy. Polymer. 2009;78(2):199–204. [Google Scholar]
- Quan L.T., Wang S.C., Zhang J. Chemical constituents from Polygonatum odoratum. Biochem. Systemat. Ecol. 2015;58:281–284. [Google Scholar]
- Seo J.M., Arasu M.V., Kim Y.B., Park S.U., Kim S.J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2015;177:204–213. doi: 10.1016/j.foodchem.2014.12.094. [DOI] [PubMed] [Google Scholar]
- Sharma V., Kumar H.V., Rao L.J.M. Influence of milk and sugar on antioxidant potential of black tea. Food Res. Int. 2008;41(2):124–129. [Google Scholar]
- Socha R., Juszczak L., Pietrzyk S., Fortuna T. Antioxidant activity and phenolic composition of herb honeys. Food Chem. 2009;113(2):568–574. [Google Scholar]
- Srigopalram S., Park H.S., Ilavenil S., Kim D.H., Arasu M.V., Kuppusamy P., Lee K.D., Choi K.C. Isolation, in vitro probiotic characterization of Lactobacillus plantarum and its role on italian ryegrass silage quality enhancement. Int. J. Agric. Biol. 2017;19(1), 19:164–170. [Google Scholar]
- Tsangalis D., Ashton J.F., Stojanovska L., Wilcox G., Shah N.P. Development of an isoflavone aglycone-enriched soymilk using soy germ, soy protein isolate and bifidobacteria. Food Res. Int. 2004;37(4):301–312. [Google Scholar]
- Wang S., Chelikani V., Serventi L. Evaluation of chickpea as alternative to soy in plant-based beverages, fresh and fermented. LWT. 2018;97:570–572. [Google Scholar]
- Wei C., Liu G., Zhang J., Bao J. Elevating fermentation yield of cellulosic lactic acid in calcium lactate form from corn stover feedstock. Ind. Crop. Prod. 2018;126:415–420. [Google Scholar]
- Zhao P., Qi C., Wang G., Dai X., Hou X. Enrichment and purification of total flavonoids from Cortex juglandis M and shuricae extracts and their suppressive effect on carbon tetrachloride-induced hepatic injury in Mice. J. Chromatograph. B. 2015;1007:8–17. doi: 10.1016/j.jchromb.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Zhou X., Yuping Z., Zhao H., Liang J., Zhang Y., Shi S. Antioxidant homoisoflavonoids from Polygonatum odoratum. Food Chem. 2015;186:63–68. doi: 10.1016/j.foodchem.2015.02.058. [DOI] [PubMed] [Google Scholar]
Further Reading
- Rejiniemon T.S., Arasu M.V., Duraipandiyan V., Ponmurugan K., Al-Dhabi N.A., Arokiyaraj S., Agastian P., Choi K.C. In-vitro antimicrobial, antibiofilm, cytotoxic, antifeedant and larvicidal properties of novel quinone isolated from Aegle marmelos (Linn.) Correa. Ann. Clin. Microbiol. Antimicrob. 2014;13(1):48. doi: 10.1186/s12941-014-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valan Arasu M., Jung M.W., Ilavenil S., Kim D.H., Park H.S., Park J.W., Al-Dhabi N.A., Choi K.C. Characterization, phylogenetic affiliation and probiotic properties of high cell density Lactobacillus strains recovered from silage. J. Sci. Food Agri. 2014;94(12):2429–2440. doi: 10.1002/jsfa.6573. [DOI] [PubMed] [Google Scholar]
- Yang S.C., Arasu M.V., Chun J.H., Jang Y.S., Lee Y.H., Kim I.H., Lee K.T., Hong S.T., Kim S.J. Identification and determination of phenolic compounds in rapeseed meals (Brassica napus L.) J. Agric. Chem. Environ. 2015;4(01):14. [Google Scholar]