Abstract
Many factors affect the habitat selection for animal species, which in turn may greatly affect their distribution in different ecosystems. Understanding the processes that affect habitat selection is also critical for guiding and managing conservation initiatives. Our study aimed to assess the habitat selection by free-ranging Spiny-tailed lizard (Uromastyx aegyptia) by analyzing a geospatial data connecting its burrow parameters to different habitat characteristics within selected sites in Hail region, Saudi Arabia. We examined evidence and patterns of significant spatial clustering for (366) active burrows by linking their parameters (burrow entrance size, burrow entrance width and burrow entrance height), their reference geographical locations and, two habitat characteristics defined by soil type and vegetation cover. The objective of the analysis was to increase the understanding on the burrows aggregation process in the space and, to describe its possible relation to other spatial habitat configurations. Analysis of distances based on the Nearest Neighbor Index (NNI) and hotspots detection in Nearest neighbor hierarchical clustering (Nnh) suggested twelve (12) spatial clusters located within the study area. In addition, a spatial ordinary least square (OLS) and Poisson regression models revealed significant effects of soil type and vegetation cover on burrow parameters (OLS, p < 0.05; Poisson, p < 0.001), which indicate a strong association between burrows parameters and habitats characteristics. Findings from the study also suggest that other factors such as elevations, highways, and human settlement concentration spots could possibly play a major role in defining burrow spatial aggregation and furthermore have a significant impact on habitat selection.
Keywords: Spatial regression, Uromastyx aegyptia, Distance analysis, Burrow parameters, Hail region
1. Introduction
Animal species select their habitats from different spatial scales based on numerous environmental factors (Mackey and Lindenmayer, 2001, Rodríguez et al., 2007). Therefore, there is a great need to consider the habitat requirements of the species to establish successful management strategies that help prevent species extinction (Morrison et al., 2006). Such information will provide fundamental bases for restore species, identify important ecosystems and protected areas (Sato et al., 2014). However, this information is not always available and knowledge on how species select their habitats is limited for several species (IUCN, 2014).
Research on habitat selection is important for understanding the animal biological strategies they adapted to meet their needs. Additionally, information on pattern of habitat use or selection are critical for conservation and management purposes (Scott et al., 2002, Guisan and Thuiller, 2005). Understanding this behavioral process requires observation of both of the habits of animals in their natural habitats (e.g. burrow parameters) and how they make use of their surrounding environments. For this purpose, many statistical methods, including combination models, were developed to quantify habitat selection (see Manly et al., 2002, Fauchald and Tveraa, 2003, Strickland and McDonald, 2006, Freitas et al., 2008). Generally, these methods use resource selection function, which compare resource attributes in areas where animals are observed and areas where animals are known to be available. Here, we try to incorporate the spatial dimension for habitat selection and how this analysis may possibly yield a useful result that can be implemented in conservation strategies. It is also important to note that spatial data analysis is mainly concern about the data that are spatially located with consideration to their explicit importance in their spatial arrangement.
The key species used in this study, the spiny-tailed lizards of the genus Uromastyx, is spreading across North Africa, Arabia, and the Middle East deserts (Wilms et al., 2012, Tamar et al., 2018). Wilms and Böhme (2007) identified 15 sub-species known to inhabit the open free range of the Arabian Peninsula. The spiny-tailed lizards (Uromastyx aegyptia) had experienced the greatest decline in population size (Wilms et al., 2012). This decline in population could be attributed to several factors (local and global). For instance, there is a high demand from locals because it is edible in their cultures. Changing in land-use policies is an example of the global factors thought to affect the population size of Spiny-tailed lizards negatively. Moreover, excessive hunting has resulted in a significant decrease in their wild population (Wilms et al., 2012). The conservation status, however, clearly appears very unequal across its entire range (Cox et al., 2012).
In our study we used the spatial data analysis to infer habitat selection and demonstrates how this model analysis can: (1) identify habitat characteristics that reflect the burrows aggregation within the study area and, best explaining their differences in parameters; (2) evaluate the significant response to different habitat characteristics (i.e. soil type and vegetation cover) and; (3) how habitat selection can possibly be predicted through variations in burrow parameters. The overall aim of this study is to provide the decision-makers and conservation managers with the essential information required to implement a long-term conservation action plan and to assist in the creation of legislation designed to conserve the species across its natural range.
2. Materials and methods
2.1. Study area and data collection
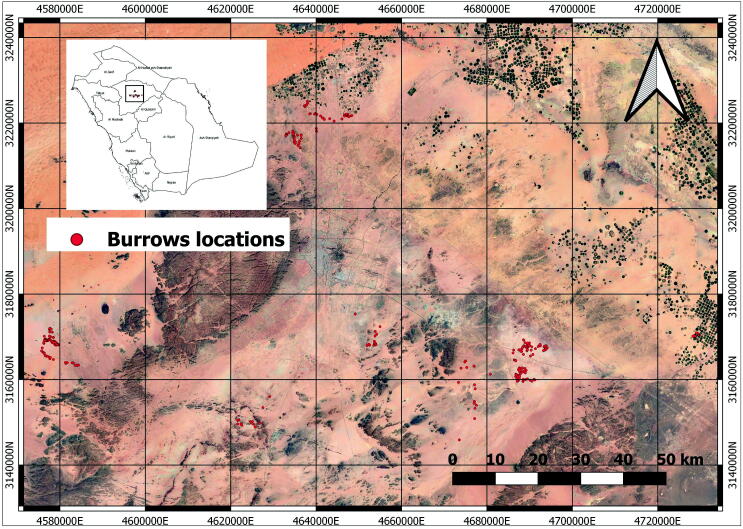
The study was conducted in Hail region, Saudi Arabia (Fig. 1), during the spring and summer of 2013, 2014, 2017, 2018, and 2019. A total of 366 active burrows were identified and recorded in each site locations. A sample route was made at each site by driving a four-wheel car at a speed of 20–30km/h. The detected active burrows were photographed, and their locations were recorded using a handheld GPS device. A burrow was considered active either the spiny-tailed lizard was directly observed, or its fresh marks/tracks were found at the entrance of the burrow. For each active burrow, the following parameters (burrow entrance width, burrow entrance height, soil type, and vegetation cover) were reported (Wilms et al., 2009, Wilms et al., 2010). The soil type was classified into three groups (coarse sand CS, fine gravel FG, and medium gravel MG). At the same time, the following categories of vegetation cover were measured around entrances (vegetation cover 0 < 25% (1), 25 < 50% cover (2), 50 < 75% cover (3) and > 75% cover (4)) within a radius of five meters. After that, the size of each burrow entrance was measured using the equation below:
Where: A = size of the burrow entrance in cm2, W = entrance width in cm, H = entrance height in cm (see Wilms et al., 2010).
Fig. 1.
Study areas and burrows locations in Hail region, Saudi Arabia.
2.2. Spatial data analysis
2.2.1. Assessing spatial dependence for burrow parameters
The spatial nature of each data value was evaluated by creating a spatial weight matrix based on data value locations. Since several methods for creating spatial weights exist (Anselin and Rey, 2014), the Queen’s Contiguity method was implemented in this analysis. Based on spatial weight matrix, hypothesis of spatial randomness in burrow parameter values was tested using global and local Moran’s I statistical tests. Global Moran's I (Moran, 1950) is widely used to examine the existence of spatial autocorrelation. As suggested by Moran (1948), the model will describe the statistics between a variable and its spatial lag, assuming that random and independent variations exist. Its values vary between −1 and 1. The meaning “1>” signifies perfect positive spatial autocorrelation, while “−1<” indicates perfect negative spatial autocorrelation, and “0” implies perfect spatial randomness (Tu and Xia, 2008). Local spatial autocorrelation was quantified using Local Indicators of Spatial Association (LISA), which calculates the degree of spatial autocorrelation at each particular position by utilizing local Moran's I (Anselin, 2010). LISA may also be used to determine the effect of specific locations on global statistical significance and classify ”outliers“ (see Levine, 2013).
Whilst the global Moran’s I was used to test whether burrows are clustering in across data values in space, local Moran’s I was used to detect locations of spatial patterns (hot and cold spots) and identify significant clusters. Anselin (2010) defined the local Moran statistics as an analysis of local clusters and outliers in space. The local cluster map describes the High and Low-Low spatial clusters where the outliers are labeled in Low-High and High-Low values. As mentioned, positive and negative global Moran’s I statistic indicates a clustering process where similar and dissimilar values are close to each other respectively.
2.2.2. Examining other habitat physical characteristics and processes
We discussed other physical factor such as possible effects of elevation, highways, and human settlement concentration spots as driving factors in habitat selection. In the last part, we also discussed the effect of habitat fragmentation as a possible ecological driver and process could shape the species habitat selection in different ways.
2.3. Data analysis
Spatial data analyses were performed using the functions implemented in GeoDa® (Anselin et al., 2006), CrimeStat IV® (Levine, 2013), QGIS v.3.16.1 (QGIS, 2020) and ArcGIS® Desktop v.10.1 (ESRI, 2011). Other spatial data used in this study were downloaded from DIVA-GIS (https://www.diva-gis.org/), including cities, villages, highways, and elevation values. To achieve our objective, we evaluated our dataset using the following methods that combine both burrow parameter and habitat characteristics: 1) we examined the First-order properties (general spatial distribution) by assessing the variation in burrow parameter values without regards to the location of each sample (test of global Moran's I), 2) Second-order properties (sub-regional patterns) by identifying clusters in Local Moran cluster map, 3) explaining the variation by performing regression analysis using – ordinary least squares OLS and Poisson to examine the spatial relationship and factors behind the observed spatial pattern and, 4) Distance analysis by running the Nearest Neighbor Index (NNI) and detection of hotspots in Nearest Neighbor Hierarchical Clustering (Nnh). Data analysis were performed using the burrow entrance size calculated from the parameters as response, soil type and vegetation cover as explanatory variables.
2.3.1. Model testing
2.3.1.1. Statistical test of robustness of the model
The statistical tests involved testing validity of ordinary least squares (OLS) standard assumptions and spatial dependence in regression residuals. The linear regression analysis is used to find a (linear) relationship between a dependent variable, 'burrow entrance size' and two explanatory (independent) variables: soil type and vegetation cover.
2.3.1.2. Analysis of Poisson regression
Assuming errors are distributed independently, discreetly, and following a Poisson distribution, the Poisson regression was used here to describe the actual distribution of probabilities. Assuming that the mean and variance of the errors are equal, the Maximum Likelihood Estimator (MLE) was used to evaluate Poisson regression using the method implemented in CrimeStat IV.
2.3.1.3. Distance analysis: Nearest Neighbor Index (NNI)
The nearest neighbor index (Clark and Evans, 1954) attempts to compare the distances between nearest points and distances that would be predicted on the basis of chance. This model calculates the distance to the nearest other point (nearest neighbor) with an average of all other points, theoretically, and for each point. If the NNI is less than 1.0, this indicates that the observed average distance is smaller than the mean random distance (i.e., points are closer to each other than would be predicted by chance) and thus suggesting clustering evidence. The function for this analysis was also performed in CrimeStat IV.
2.3.1.4. Hotspot analysis: nearest neighbor hierarchical clustering (Nnh)
The nearest neighbor hierarchical clustering analysis (Nnh) attempts to classify the spatially similar points into groups according to a specific criterion (i.e., burrow parameters in this case). The (Nnh) function routine implemented in CrimeStat works by repeating clustering until all points are either clustered into a single cluster or the clustering fails.
3. Results
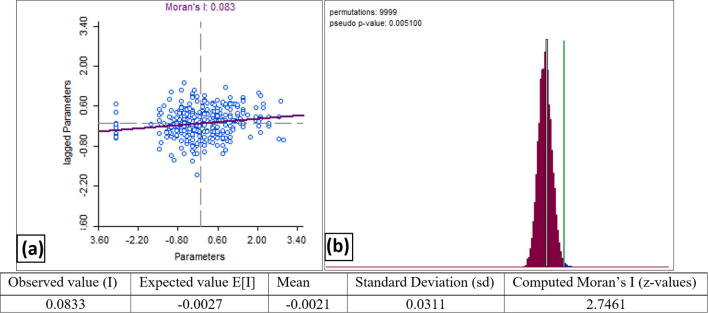
Burrow parameters were plotted in the horizontal (X) axis where their spatially lagged parts in the vertical (Y) axis as shown in Moran scatter plot (Fig. 2a). As shown in the figure, in the top of the plot which is (0.083) is indicated the linear slop that corresponds to global Moran's I value. Again, the scatter plot reveals some outliers which can be found at the high end of the slop line.
Fig. 2.
(a) Moran scatter plot and (b) reference distribution for Moran’s I.
3.1. Assessing significance by reference distribution
By running the randomization option with the number of permutations of (9999) in the Fig. 2a, a pseudo p-value have been generated (p = 0.005) indicating a rejection to our null hypothesis that there is no difference in burrow parameters (Fig. 2b). Right side to the reference distribution, the green line is showing the actual statistics of the data at (0.0.083).
3.2. Significance map
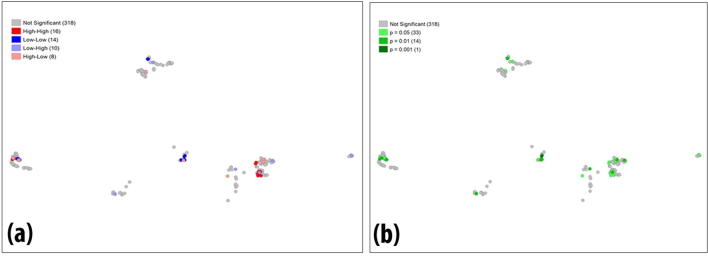
By running the model for (999) permutations and p-value (0.05) for both cluster maps (Fig. 3a), a new map (Fig. 3b) has identified groups of significances ranging from light to dark green corresponding to more spatial associations (dark green) where the p-value is (0.001).
Fig. 3.
LISA (a) cluster and (b) significant maps (Significance level is shown as *P < 0.05, **P < 0.01).
3.3. Regression analysis
3.3.1. Ordinary least squares (OLS) and Poisson regression
Results explained in (Table 1) showing that both soil type and vegetation cover have a significant effect on the parameters of the burrows (P < 0.05). Test of Poisson regression for the model also indicated a strong correlation (Table 1). Test with Maximum Likelihood Estimator (MLE) for both models can be found in (Table 2) respectively.
Table 1.
Regression analysis and Model diagnostics.
|
Regression analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | df | Coefficient | St. Err. | Tolerance | t-value | p | Model | *Model p-value | |
| Burrow parameters | Intercept | 1 | 34.280 | 3.402 | – | 10.077 | 0.001 | OLS | 0.002 |
| Soil type | 1 | 7.464 | 1.789 | 0.957 | 4.173 | 0.001 | |||
| Vegetation cover | 1 | 11.726 | 1.680 | 0.957 | 6.980 | 0.001 | |||
| Intercept | 1 | 3.645 | 0.020 | – | 189.948 | 0.001 | Poisson | 0.000 | |
| Soil type | 1 | 0.128 | 0.009 | 0.957 | 14.234 | 0.001 | |||
| Vegetation cover | 1 | 0.194 | 0.008 | 0.957 | 22.682 | 0.001 | |||
| Model diagnostics | |||||||||
| Test | df | value | Prob. | Type of diagnostic | |||||
| jarque-bera | 2 | 30.300 | 0.000 | Normality of errors | |||||
| breusch-pagan test | 2 | 3.577 | 0.1672 | Heteroscedasticity | |||||
| koenker-bassett test | 2 | 2.100 | 0.34990 | ||||||
| Moran's I (error) | 0.072 | 2.445 | 0.0145 | Spatial dependence | |||||
| Lagrange Multiplier (lag) | 1 | 6.155 | 0.0131 | ||||||
| Robust LM (lag) | 1 | 7.660 | 0.0056 | ||||||
| Lagrange Multiplier (error) | 1 | 5.350 | 0.0207 | ||||||
| Robust LM (error) | 1 | 6.855 | 0.0088 | ||||||
| Lagrange Multiplier (SARMA) | 2 | 13.010 | 0.0015 | ||||||
see (Table 2).
Table 2.
OLS and Poisson regression models: both tested with Maximum Likelihood Estimator (MLE).
|
OLS regression model |
Poisson regression model |
||
|---|---|---|---|
| Test | Value | Test | Value |
| Squared multiple R | 0.1735 | Likelihood statistics: | |
| Adjusted squared multiple R | 0.1695 | Log-likelihood | −3916.836 |
| F test of model | 40.801 | Per case | −10.0175 |
| P-value of model | 0.002 | AIC | 7841.671 |
| Mean absolute deviation: | 18.670 | Per case | 20.055 |
| 1st (highest) quartile | 30.003 | BIC/SC | 7857.546 |
| 2nd quartile | 9.048 | Per case | 20.096 |
| 3rd quartile | 8.596 | Deviance | 5686.654 |
| 4th (lowest) quartile | 26.774 | P-value of Deviance | 0.0001 |
| Mean squared predicted error: | 607.326 | Pearson Chi-Square | 4409.026 |
| 1st (highest) quartile | 1180.847 | P-value of Chi-Square | 0.000 |
| 2nd quartile | 118.590 | Model error estimates: | |
| 3rd quartile | 118.640 | Mean absolute deviation | 19.218 |
| 4th (lowest) quartile | 999.111 | 1st (highest) quartile | 30.565 |
| 2nd quartile | 10.049 | ||
| 3rd quartile | 8.529 | ||
| 4th (lowest) quartile | 27.473 | ||
| Mean squared predicted error: | 643.043 | ||
| 1st (highest) quartile | 1219.597 | ||
| 2nd quartile | 143.889 | ||
| 3rd quartile | 128.498 | ||
| 4th (lowest) quartile | 1067.074 | ||
| Dispersion tests | |||
| Adjusted deviance | 14.694 | ||
| P-value of Deviance | 0.000 | ||
| Adjusted Pearson Chi-Square | 11.393 | ||
| P-value of Chi-Square | 0.000 | ||
| Dispersion multiplier | 10.363 | ||
| Z | 6.562 | ||
| P-value of Z | n.s | ||
| Inverse dispersion multiplier | 0.096 | ||
3.3.2. Model diagnostics
Regression diagnostics test for both models as explained in (Table 1) showing the p-values for Jarque-Bera and indicating there is no problem with the normality of errors in selected model. Diagnostics for heteroscedasticity, as explained by Breusch-Pagan and Koenker-Bassett tests, are showing larger values than 0.05. Diagnostics test for spatial dependence of the model was performed using Moran’s I score which was significant (0.0725) and indicating strong spatial autocorrelation of the residuals.
3.4. Distance and hotspot analyses
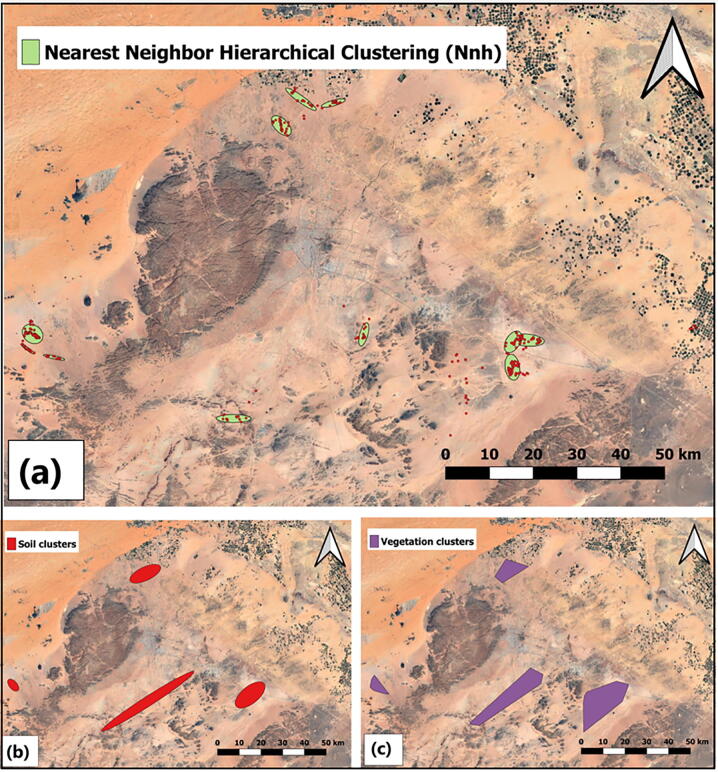
Analysis result of nearest neighbor index as described in (Table 3) indicates a NNI index of (0.0977). Results of Nearest Neighbor Hierarchical Clustering (Nnh) as shown also in (Table 3) indicate (12) clusters found among the areas of study. The results were transferred in QGIS to illustrate those cluster positions (Fig. 4a). We also reported the burrow clustering based on the soil type and vegetation cover within the study area (Fig. 4b&c).
Table 3.
Nearest Neighbor Index (NNI) and Nearest Neighbor Hierarchical Clustering (Nnh) analysis.
| Nearest Neighbor Index (NNI) |
Nearest Neighbor Hierarchical Clustering (Nnh) |
||
|---|---|---|---|
| Test | Value | Test | Value |
| Mean Nearest Neighbor Distance | 249.70 m | Likelihood of grouping pair of points by chance | 0.500 (50.000%) |
| Standard Dev of Nearest: | Z-value for confidence interval | 0.000 | |
| Neighbor Distance | 609.31 m | Standard Deviations | 1.0 |
| Minimum Distance | 2.43 m | Clusters found | 12 |
| Maximum Distance | 136471.90 m | ||
| Mean Random Distance | 2555.74 m | ||
| Mean Dispersed Distance | 5492.44 m | ||
| Nearest Neighbor Index | 0.0977 | ||
| Standard Error | 69.83 m | ||
| Test Statistic (Z) | −33.023 | ||
| p-value (one tail) | 0.000 | ||
| p-value (two tail) | 0.000 | ||
Fig. 4.
Clusters as revealed by (a) Nearest Neighbor Hierarchical Clustering (Nnh) method and, the burrow clustering based on (b) soil type and (c) vegetation cover as determined by nearest neighbor analysis.
4. Discussion
Numerous factors contribute to the loss of the species, including habitat destruction, grazing, hunting, and degradation (Sodhi et al., 2009). Rapid increase in human population, urban sprawl and the transition to marginal agricultural and grazing land have been known to change the natural ecosystems and have contributed to the extinction of many species across the globe (Sala et al., 2000, Cardillo et al., 2004). Reptiles are among the most threatened species; nevertheless, limited scientific interest has been given reptile species, particularly in the field of conservation (Böhm et al., 2013, Gibbon et al., 2000). This can be due to that reptiles are considered elusive and inhabit different and difficult habitat types. In the Arabian Peninsula the reptiles were from the least studied species until recently where more species had been described (Sindaco et al., 2013, Sindaco et al., 2014, Šmíd et al., 2013, Badiane et al., 2014, Metallinou et al., 2015, Carranza et al., 2018).
Addressing data gaps is essential to reverse species loss for conservation managers, decision-makers, and management strategies (Morrison et al., 2006). Research in conservation planning in areas where there are still threatening processes working on wild species is therefore highly needed.
This study addressed the question on how animal species choose their habitat based on surrounding environment. We investigated whether burrow parameters and its location preferences vary depending on habitat characteristics, particularly, soil type and vegetation cover at different sites in Hail region, Saudi Arabia. We used three different types of cluster statistics: global, local and distance to scrutinize our hypothesis regarding burrow clustering patterns. Furthermore, we incorporated a spatial dependency analysis by testing the spatial regression using OLS and Poisson regression models.
4.1. Cluster statistics
4.1.1. Global Moran’s I and LISA
Global statistics can define whether spatial structure exists, but cannot identify which cluster it is, nor can it decide whether the clusters differ from one location to another. Earlier spatial pattern tests (like Moran’s I) were global in nature, and provided one statistic, such as a global autocorrelation coefficient, that summarized spatial pattern over the entire sampling sites. Moran’s I can give a global statistic indicating there is clustering going on within the study area but, cannot explain exactly where or how. This pattern can explain the emerged outliers in the scatter plot shown in (Fig. 2).
To investigate deeper, we run local cluster statistics by testing the Local Indicators of Spatial Association (LISA). Local cluster statistics such like LISA (Anselin, 2010) tends to quantify spatial autocorrelation and clustering within the small areas that together comprise the whole study area. It can also reveal the presence of significant spatial clusters or outliers and a testing for regional clustering. Results obtained in (Fig. 3a&b) indicated significant clustering going on at different sites within the study area and supporting our hypothesis.
4.1.2. NNI and Nnh
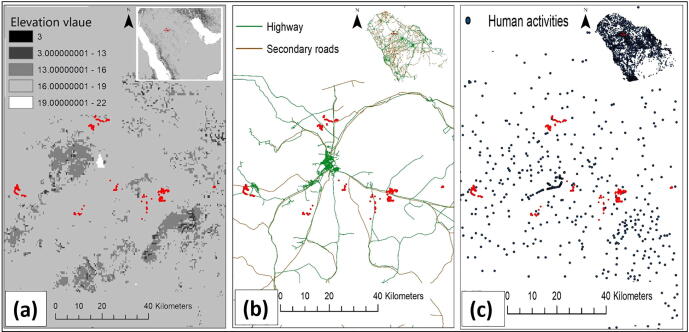
Cluster analysis is a classification method that group objects based on their properties. It recognizes the similarities between observations to group related observations together. As an example, hierarchical clustering method can categorize all observations into groups, and then aligning the groups based on how similar they are. The nearest neighbor distance is also used as an indicator of dissimilarity in the hierarchical clustering (Bailey and Gatrell, 1995). A comparison of the distances between two points (or group of points) and the average distance of all points is the closest neighbor measure, when the distance fulfills a prior criterion (i.e. probabilities of threshold distance) observes are linked into a new class. This process is repeated until all points in a first-order cluster have been assigned. The results of the nearest neighbor index analysis (NNI) and the nearest neighbor hierarchical clustering (Nnh) show strong clustering evidence, suggesting 12 possible clusters within the study area. Here we will drive two potential reasons for such form of clustering pattern observed in our study area based on physical characteristics and human activities within the study area. Physical features such as elevation variations may play a significant deterministic role in habitat selection (Fischer and Lindenmayer 2005). As shown in (Fig. 5a), burrows were located at two different elevation levels, indicating that lower elevation could allow more aggregation and better habitat characteristics (combined with the 'Nnh' results). This can be described in terms of other related elevation variables such as temperature and relative humidity (Wilms et al., 2009, Wilms et al., 2010).
Fig. 5.
A map showing the burrow locations within the study area and across (a) different elevation values, (b) roads and highways and (c) towns and villages.
4.2. Spatial dependency analysis
Based on the results of OLS and Poisson tested models, the burrow parameters can be explained by the soil type and vegetation cover. However, the two explanatory variables used in the model cannot explain the outliers shown in (Fig. 2) (i.e. Moran scatter plot), indicating that other biotic or abiotic factors may shape habitat selection or burrow preference for the spiny-tailed lizards (Jablonski, 2008, Peñalver-Alcázar et al., 2016).
4.3. Habitat fragmentation
As ecological driver and influential factor, habitat fragmentation is a process involving the splitting and discontinuity of preferred habitat for many species (Fahrig, 2003, Rogan and Lacher, 2018). This process is mainly driven by human activities (Barnosky et al., 2012, Elias, 2013). As illustrated in (Fig. 5b&c) combined with Fig. 3a&b, we assumed that the expansion of human activities, roads, cities, villages and highways may have a significant effect on a species' natural habitat (Brehme et al., 2013). Changes in habitat due to fragmentation may shift the range of species by dividing the natural ecosystems into small isolated patches, therefore, we expected these isolate patches to have a potential effect on our species selection for habitat. We suggest a fragmentation-based study that includes genetic information about the animal to better examine this hypothesis and, to highlight the dispersal mechanism and testing the gene flow between and among different sites.
5. Conclusion
In this study we addressed two ecological questions: 1) Where are the hotspots with higher-than-expected proportion of burrows within the study area? and, 2) What is/are the factor(s) contributing to higher-than-expected aggregation. In order to ensure the survival of our key species, further studies are needed to evaluate more variables that might influence Spiny-tailed lizards' habitat selection/preference. Key suggested studies that incorporate genetic investigation, capture-recapture and population assessment could further highlight the current situation of the species within its range. Since the survival of our species depends on their patchy habitats, a solid action plan for long-term conservation is urgently needed. These measures may include: 1) Annual surveys to get the latest information on status and distribution. 2) Inclusion of land use management into land use planning. 3) Conservation needs to understand that spiny-tailed lizards can only thrive in the socio-economic climate of human societies where they share wild terrestrial ecosystems.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to acknowledge the support of the University of Ha’il represented by the Deanship of Scientific Research through a grant number (150464). We are thankful to Taif University, Taif, Saudi Arabia, for support under Researcher Supporting project number (TURSP-2020/06).
Footnotes
Peer review under responsibility of King Saud University.
References
- Anselin L. Local Indicators of Spatial Association—LISA. Geogr. Analy. 2010;27:93–115. doi: 10.1111/J.1538-4632.1995.TB00338.X. [DOI] [Google Scholar]
- Anselin L., Syabri I., Kho Y. GeoDa: An Introduction to Spatial Data Analysis. Geogr. Analy. 2006;38:5–22. doi: 10.1111/J.0016-7363.2005.00671.X. [DOI] [Google Scholar]
- Anselin, L., Rey, S., 2014. Modern Spatial Econometrics in Practice: A Guide to GeoDa, GeoDaSpace and PySAL.
- Badiane A., Garcia-Porta J., Červenka J., Kratochvíl L., Sindaco R., Robinson M.D., Morales H.E., Mazuch T., Price T., Amat F., Shobrak M., Wilms T.M., Simó-Riudalbas M., Ahmadzadeh F., Papenfuss T., Cluchier A., Viglione J., Carranza S. Phylogenetic relationships of Semaphore geckos (Squamata: Sphaerodactylidae: Pristurus) with an assessment of the taxonomy of Pristurus rupestris. Zootaxa. 2014;3835(1):33–58. doi: 10.11646/zootaxa.3835.1.2. [DOI] [PubMed] [Google Scholar]
- Bailey, T., Gatrell, A., 1995. Interactive Spatial Data Analysis. https://doi.org/10.2307/2265559.
- Barnosky A.D., Hadly E., Bascompte J., Berlow E., Brown J., Fortelius M., Getz W., Harte J., Hastings A., Marquet P., Martinez N.D., Mooers A., Roopnarine P., Vermeij G., Williams J.W., Gillespie R., Kitzes J., Marshall C., Matzke N., Mindell D.P., Revilla E., Smith A. Approaching a state shift in Earth’s biosphere. Nature. 2012;486:52–58. doi: 10.1038/nature11018. [DOI] [PubMed] [Google Scholar]
- Böhm M., Collen B. The conservation status of the world's reptiles. Biol. Conserv. 2013;157:372–385. doi: 10.1016/j.biocon.2012.07.015. [DOI] [Google Scholar]
- Brehme C.S., Tracey J., Mcclenaghan L., Fisher R. Permeability of roads to movement of scrubland lizards and small mammals. Conservation Biol.: J. Soc. Conservation Biol. 2013;27(4):710–720. doi: 10.1111/cobi.12081. [DOI] [PubMed] [Google Scholar]
- Cardillo M., Purvis A., Sechrest W., Gittleman J.L., Bielby J., Mace G. Human Population Density and Extinction Risk in the World's Carnivores. PLoS Biol. 2004;2 doi: 10.1371/journal.pbio.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza S., Xipell M., Tarroso P., Gardner A., Arnold E.N., Robinson M., Simó-Riudalbas M., Vasconcelos R., Pous P.D., Amat F., Šmíd J., Sindaco R., Metallinou M., Els J., Pleguezuelos J., Machado L., Donaire D., Martinez G., Garcia-Porta J., Mazuch T., Wilms T., Gebhart J., Aznar J., Gallego J., Zwanzig B., Fernández-Guiberteau D., Papenfuss T.L., Saadi S.A., Alghafri A., Khalifa S., Farqani H.A., Bilal S.B., Alazri I.S., Adhoobi A.S., Omairi Z.S., Shariani M.A., Kiyumi A.A., Sariri T.A., Shukaili A.S., Akhzami S.N. Diversity, distribution and conservation of the terrestrial reptiles of Oman (Sauropsida, Squamata) PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0190389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, N.A., Mallon, D., Bowles, P., Els, J., Tognelli, M.F., 2012. The Conservation Status and Distribution of Reptiles of the Arabian Peninsula. Cambridge, UK and Gland, Switzerland: IUCN, and Sharjah, UAE: Environment and Protected Areas Authority. https://portals.iucn.org/library/sites/library/files/documents/RL-53-002.pdf.
- Elias, S., 2013. Global Change Impacts on the Biosphere. Reference Module in Earth Systems and Environmental Sciences, Elsevier. https://doi.org/10.1016/B978-0-12-409548-9.09532-4.
- Clark, Evans Distance to Nearest Neighbor as a Measure of Spatial Relationships in Populations. Ecology. 1954;35:445–453. [Google Scholar]
- ESRI . Environmental Systems Research Institute; Redlands, CA: 2011. ArcGIS Desktop: Release 10.1. [Google Scholar]
- Fahrig L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003;34:487–515. doi: 10.1146/ANNUREV.ECOLSYS.34.011802.132419. [DOI] [Google Scholar]
- Fauchald P., Tveraa T. Using first-passage time in the analysis of area-restricted search and habitat selection. Ecology. 2003;84:282–288. doi: 10.1890/0012-9658%282003%29084%5B0282%3AUFPTIT%5D2.0.CO%3B2. [DOI] [Google Scholar]
- Fischer J., Lindenmayer D. The sensitivity of lizards to elevation: A case study from south-eastern Australia. Divers. Distrib. 2005;11:225–233. doi: 10.1111/J.1366-9516.2005.00139.X. [DOI] [Google Scholar]
- Freitas C., Kovacs K., Lydersen C., Ims R.A. A novel method for quantifying habitat selection and predicting habitat use. J. Appl. Ecol. 2008;45:1213–1220. doi: 10.1111/J.1365-2664.2008.01505.X. [DOI] [Google Scholar]
- Gibbon J., Scott D., Ryan T., Buhlmann K., Tuberville T.D., Metts B.S., Greene J., Mills T., Leiden Y., Poppy S., Winne C.T. The Global Decline of Reptiles. Déjà Vu Amphibians. 2000 doi: 10.1641/0006-3568%282000%29050%5B0653%3ATGDORD%5D2.0.CO%3B2. [DOI] [Google Scholar]
- Guisan A., Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 2005;8:993–1009. doi: 10.1111/J.1461-0248.2005.00792.X. [DOI] [PubMed] [Google Scholar]
- IUCN . International Union for Conservation of Nature and Natural Resource; 2014. 160,000 species by 2020 – will you help? [Google Scholar]
- Jablonski D. Biotic Interactions and Macroevolution: Extensions and Mismatches Across Scales and Levels. Evolut.; Int. J. Organic Evolution. 2008 doi: 10.1111/j.1558-5646.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- Levine N. CrimeStat IV: A Spatial Statistics Program for the Analysis of Crime Incident Locations. Version. 2013;4 [Google Scholar]
- Mackey B., Lindenmayer D. Towards a hierarchical framework for modelling the spatial distribution of animals. J. Biogeogr. 2001;28:1147–1166. doi: 10.1046/J.1365-2699.2001.00626.X. [DOI] [Google Scholar]
- Manly B.F.J., McDonald L.L., Thomas D.L., McDonald T.L., Erikson W.P. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. Resource selection by animals: statistical design and analysis for field studies. [Google Scholar]
- Metallinou M., Červenka J., Crochet P., Kratochvíl L., Wilms T.M., Geniez P., Shobrak M., Brito J.C., Carranza S. Species on the rocks: Systematics and biogeography of the rock-dwelling Ptyodactylus geckos (Squamata: Phyllodactylidae) in North Africa and Arabia. Mol. Phylogenet. Evol. 2015;85:208–220. doi: 10.1016/j.ympev.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Moran P. The Interpretation of Statistical Maps. J. Royal Statist. Soc. Ser. B-Methodol. 1948;10:243–251. doi: 10.1111/J.2517-6161.1948.TB00012.X. [DOI] [Google Scholar]
- Moran P. Notes on continuous stochastic phenomena. Biometrika. 1950;37(1–2):17–23. doi: 10.1093/BIOMET%2F37.1-2.17. [DOI] [PubMed] [Google Scholar]
- Morrison M.L., Marcot B.G., Mannan R.W. Island Press; Washington, DC, USA: 2006. Wildlife-Habitat Relationships: Concepts and Applications. [Google Scholar]
- Peñalver-Alcázar M., Aragón P., Breedveld M., Fitze P. Microhabitat selection in the common lizard: implications of biotic interactions, age, sex, local processes, and model transferability among populations. Ecol. Evol. 2016;6:3594–3607. doi: 10.1002/ece3.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QGIS Development Team, 2020. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.orG.
- Rodríguez J., Brotons L., Bustamante J., Seoane J. The application of predictive modelling of species distribution to biodiversity conservation. Divers. Distrib. 2007;13:243–251. doi: 10.1111/J.1472-4642.2007.00356.X. [DOI] [Google Scholar]
- Rogan J., Lacher T. Impacts of Habitat Loss and Fragmentation on Terrestrial Biodiversity. Reference Module in Earth Systems and Environmental Sciences, Elsevier. 2018 doi: 10.1016/B978-0-12-409548-9.10913-3. [DOI] [Google Scholar]
- Sala O., Chapin F., Armesto J.J., Berlow E., Bloomfield J., Dirzo R., Huber-Sanwald E., Huenneke L.F., Jackson R.B., Kinzig A., Leemans R., Lodge D., Mooney H., Oesterheld M., Poff N., Sykes M., Walker B., Walker M., Wall D. Global biodiversity scenarios for the year 2100. Science. 2000;287(5459):1770–1774. doi: 10.1126/SCIENCE.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Sato C.F., Wood J., Schroder M., Green K., Michael D., Lindenmayer D. The impacts of ski resorts on reptiles: a natural experiment. Anim. Conserv. 2014;17:313–322. doi: 10.1111/ACV.12095. [DOI] [Google Scholar]
- Scott, J.M., Heglund, P.J., Haufler, J.B., Morrison, M., Raphael, M.G., Wall, W.B., 2002. Predicting Species Occurrences: Issues of Accuracy and Scale, Island Press, Covelo, CA, USA. https://doi.org/10.1644/1545-1542%282003%29084%3C0319%3AR%3E2.0.CO%3B2
- Sindaco, R., Nincheri, R., Lanza, B., 2014. Catalogue of Arabian reptiles in the collections of the “La Specola” Museum, Florence. Scripta Herpetologica. Studies on Amphibians and Reptiles in honour of Benedetto Lanza, 137-164.
- Sindaco, R., Venchi, A., Grieco, C., 2013. The Reptiles of the Western Palearctic, Volume 2: Annotated Checklist and Distributional Atlas of the Snakes of Europe, North Africa, Middle East and Central Asia, with an Update to Volume 1. Edizioni Belvedere, Latina.
- Šmíd J., Moravec J., Kratochvíl L., Gvoždík V., Nasher A.K., Busais S.M., Wilms T.M., Shobrak M., Carranza S. Two newly recognized species of Hemidactylus (Squamata, Gekkonidae) from the Arabian Peninsula and Sinai. Egypt. ZooKeys. 2013;79–107 doi: 10.3897/zookeys.355.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi N.S., Brook B.W., Bradshaw C.J.A. Causes and consequences of species extinctions. In: Levin S.A., editor. The Princeton Guide to Ecology. 1st ed. Princeton University Press; 2009. pp. 514–520. [Google Scholar]
- Strickland M.D., McDonald L.L. Introduction to the special section on resource selection. J. Wildl. Manage. 2006;70:321–323. doi: 10.2193/0022-541X%282006%2970%5B321%3AITTSSO%5D2.0.CO%3B2. [DOI] [Google Scholar]
- Tamar K., Metallinou M., Wilms T., Schmitz A., Crochet P., Geniez P., Carranza S. Evolutionary history of spiny-tailed lizards (Agamidae: Uromastyx) from the Saharo-Arabian region. Zoolog. Scr. 2018;47:159–173. doi: 10.1111/zsc.12266. [DOI] [Google Scholar]
- Tu J., Xia Z. Examining spatially varying relationships between land use and water quality using geographically weighted regression I: model design and evaluation. Sci. Total Environ. 2008;407(1):358–378. doi: 10.1016/j.scitotenv.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Wilms T., Böhme W. A review of the taxonomy of the spiny-tailed lizards of Arabia (Reptilia: Agamidae: Leiolepidinae: Uromastyx) Fauna Arabia. 2007;23:435–468. [Google Scholar]
- Wilms T., Wagner P., Shobrak M., Böhme W. Activity profiles, habitat selection and seasonality of body weight in a population of Arabian spiny-tailed lizards (Uromastyx aegyptia microlepis Blanford, 1875; Sauria: Agamidae) in Saudi Arabia. Bonn. Zool. Beitr. 2009;56:259–272. https://www.zoologicalbulletin.de/BzB_Volumes/Volume_56_4/259_272_BZB56_4_Wilms_Thomas_et_al.PDF [Google Scholar]
- Wilms T., Wagner P., Shobak M., Lutzmann N., Böhme W. Aspect of the ecology of the Arabian Spinytailed lizard (Uromastyx aeyptia microlepis Blandford 1875) at Mahazat asSayd protected Area. Salamandra. 2010;46:131–140. https://static1.squarespace.com/static/52d4deeae4b0f9fd2aedf3ef/t/52e53b80e4b00ef8382be9d6/1390754688101/Wilms_U.a.microlepis.pdf [Google Scholar]
- Wilms, T., Eid, E.K.A., Al Johany, A.M.H., Amr, Z.S.S., Els, J., Baha El Din, S., et al., 2012. Uromastyx aegyptia (errata version published in 2017). The IUCN Red List of Threatened Species 2012: e.T164729A115304711. https://dx.doi.org/10.2305/IUCN.UK.2012.RLTS.T164729A1071308.en.
Further Reading
- Dinsmore, S., 2007. Wildlife-habitat Relationships: Concepts and Applications. https://doi.org/10.1650/0010-5422%282007%29109%5B980%3AWRCAA%5D2.0.CO%3B2.