Abstract
Background
The prevalence of nonalcoholic fatty liver disease (NAFLD) in Saudi Arabia is predicted to exceed 30% by 2030. NAFLD leads to liver fibrosis, thus increasing morbidity and health care burden. Obesity and diabetes have been strongly associated with NAFLD in different cities in Saudi Arabia.
Objectives
Therefore, we aim to determine the prevalence rate of NAFLD and specific risk factors for NAFLD among patients of tertiary care hospital of Taif city.
Material and methods
We retrospectively analyzed the medical records of patients for two years, between Feb 2017 and Feb 2019, (n = 100) referred to the hepatology clinic at King Abdulaziz Specialist Hospital in Taif. The diagnosis of NAFLD was based on the radiology report for patients who were aged >20 years old. Other parameters including fasting blood glucose (FBG), platelets count, alanine transaminase (ALT), aspartate transaminase (AST) and total bilirubin were statistically analyzed.
Results
We found that 40% (P < 0.05) of all patients had NAFLD. The results revealed that a significantly high number of patients with NAFLD have high FBG levels (75%, P < 0.0001) and total bilirubin (P < 0.05). Meanwhile, platelet count was significantly reduced in patients with NAFLD (P < 0.05).
Conclusion
NAFLD can be a serious health problem in the Taif region. In addition, high FBG is a significant specific risk factor for NAFLD. Health care providers should pay more attention to limiting the prevalence of NAFLD and its risk factors.
Keywords: Nonalcoholic fatty liver disease, Obesity, Type 2 diabetes, Risk factors, Age, Fasting blood glucose
1. Introduction
Over the past few decades, the prevalence of nonalcoholic fatty liver disease (NAFLD) has significantly increased worldwide (Ge et al., 2020). reaching up to 24% (Younossi et al., 2018). The Middle East has been reported to have the second highest prevalence of NAFLD out of global regions. A recent observational study found that Saudi Arabia and other Gulf countries had the highest prevalence rate of NAFLD between 1990 and 2017 (Ge et al., 2020). Furthermore, Alswat et al. (2018) predicted that the prevalence rate of NAFLD among the Saudi Arabian population will reach 30% by 2030 (Alswat et al., 2018). Thus, there is an urgent need to investigate the incidence and prevalence of NAFLD in different areas of Saudi Arabia to promote different health sector services.
The major risk factors for NAFLD include type 2 diabetes mellitus and obesity (Camhi et al., 2011, Jimba et al., 2005, Younossi et al., 2019). The Middle East region has the second highest prevalence of overweight and obese individuals (web, 2017). Approximately 75% of Saudi Arabians are overweight or obese (Younossi, 2019). It has also been found that NAFLD is commonly present in type 2 diabetes patients (Younossi et al., 2018).
It is critical to diagnose NAFLD early in younger patients and initiate prompt treatment. As age advances, the risk factors, complications, and mortality are increasing. NAFLD is more common in middle-aged (50–60 years) and older adults (>60 years) (Bertolotti et al., 2014, Frith et al., 2009). Therefore, it is beneficial to look at the age groups in the study.
A consequence of NAFLD is an increase in morbidity and mortality (Ekstedt et al., 2015). NAFLD is a significant contributory factor to the development of death from cardiovascular diseases (Younossi et al., 2017). Annual death incidences from liver-related diseases, such as cardiovascular and NAFLD-related diseases, are expected to hit about 4,500 cases and 4.5 percent in Saudi Arabia by 2030 (Alswat et al., 2018).
For fatty to fibrotic liver diseases, abdominal ultrasonography remains the first noninvasive assessment process (Papagianni et al., 2015). While liver biopsy has been the gold standard for confirmation in the past, it is invasive and expensive (Ratziu et al., 2005). Furthermore, biochemical and hematological markers have been used as predictors for NAFLD diagnosis (Fracanzani et al., 2008), but they are not conclusive (Yoneda et al., 2011). Liver enzymes including alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and bilirubin have been linked to liver disease, although some studies haven't found them to be significant (Browning et al., 2004, Fracanzani et al., 2008).
The prevalence of NAFLD among Taif patients has yet to be determined. As a result, the aim of this study was to find out how common NAFLD is among patients at King Abdulaziz Specialist Hospital in Taif, Saudi Arabia. In addition, an attempt was made to investigate the risk factors for NAFLD among them.
2. Materials and methods
This retrospective study was ethically approved by the directorate of health affairs in Taif (IRB registration number: HAP-02-T-067; approval number: 183).
We collected data from the medical records of patients for two years, between Feb 2017 and Feb 2019, (n = 100) who were referred to the hepatology clinic at King Abdulaziz Specialist Hospital (KASH) in Taif. On the basis of the radiologist’s reports, only 100 cases who were reported to be with fatty livers and aged >20 years were included in the study. Laboratory investigation reports for fasting blood glucose (FBG), ALT, AST, ALP, total bilirubin (TB), platelet count, hepatitis B virus (HBV), and hepatitis C virus (HCV) were collected (Table 1). Patients data with HBV, HCV positive, and/or confirmed hepatocarcinoma results were excluded from the study.
Table 1.
General characteristics of patients enrolled in this study.
| No. of patients | (%) Saudi | (%) Non-Saudi | (%) Male | (%) Female | Age | |
|---|---|---|---|---|---|---|
| Patients from hepatology clinic | 100 | 90 | 10 | 47 | 53 | 26–111 years |
| Patients with hepatocellular carcinoma | 3 | 3 | 0 | 2 | 1 | 65–87 years |
| Patients with Hepatitis A | 0 | 0 | 0 | 0 | 0 | _ |
| Patients with Hepatitis B | 4 | 4 | 0 | 3 | 1 | 37–100 years |
| Patients with Hepatitis C | 4 | 2 | 2 | 1 | 3 | 68–84 years |
| Patients with fasting blood glucose > 100 mg/dL | 64 | 60 | 4 | 27 | 37 | 22–111 years |
| Patients with AST > 34 U/L | 34 | 31 | 3 | 18 | 16 | 31–89 years |
| Patients with ALT > 55 U/L | 17 | 16 | 1 | 12 | 5 | 26–89 years |
2.1. Statistical analysis
A parametric unpaired t test with Welch’s correction was used to detect changes in the FBG in the NAFLD group and compare changes in TB, AST, ALT, ALP, and platelet count parameters between NAFLD and non-NAFLD groups. Post hoc two-tailed t-tests were used to test statistical significance (P < 0.05) for differences between the groups. Significance levels: P < 0.05 (*), P < 0.005 (**), P < 0.0005 (***), and P < 0.0001 (****). All data is presented as mean ± standard deviation of the group. The statistical analysis was performed using GraphPad Prism software (version 8.4.3).
3. Results
3.1. Prevalence of NAFLD among patients referred to the hepatology clinic
All of the data in this study was almost evenly distributed between male (47%) and female (53%) patients, with ages ranging from 22 to 111 years old and 90 percent of them being Saudi patients (Table 1). Notably, there is a direct relationship between liver disease and a rise in serum FBG levels (Table 1).
On the basis of the radiology reports, 43% of all patients had NAFLD, but none of them had liver biopsies to verify the radiology report. Furthermore, approximately 3% of patients were excluded from the study because they had hepatitis B or C. Therefore, only 40% of patients were considered as patients with NAFLD (P < 0.0189) and included in our study for further analysis (Fig. 1).
Fig. 1.
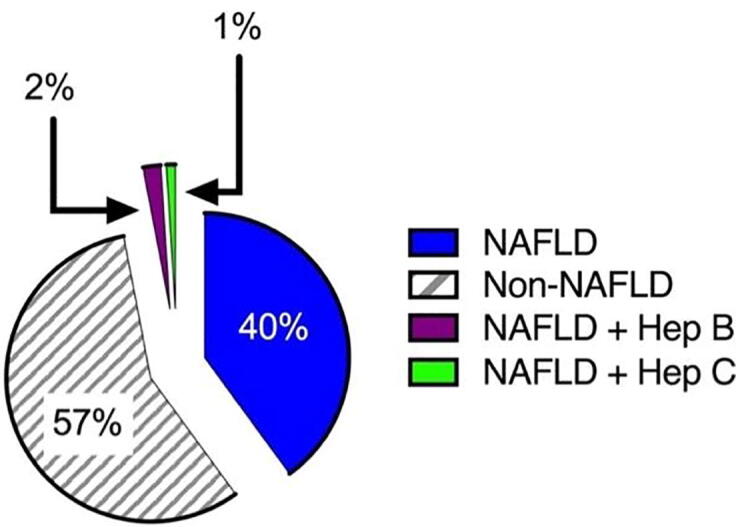
Proportions of patients with and without NAFLD.
3.2. Prevalence of NAFLD with age
Our results indicate that age can be a risk factor for NAFLD. A significant (P < 0.001) relationship was observed between age of the participants and occurrence of NAFLD. Approximately 90% of patients with NAFLD were above 30 years old (Fig. 2). This suggests that NAFLD prevalence increases with an increase in age.
Fig. 2.
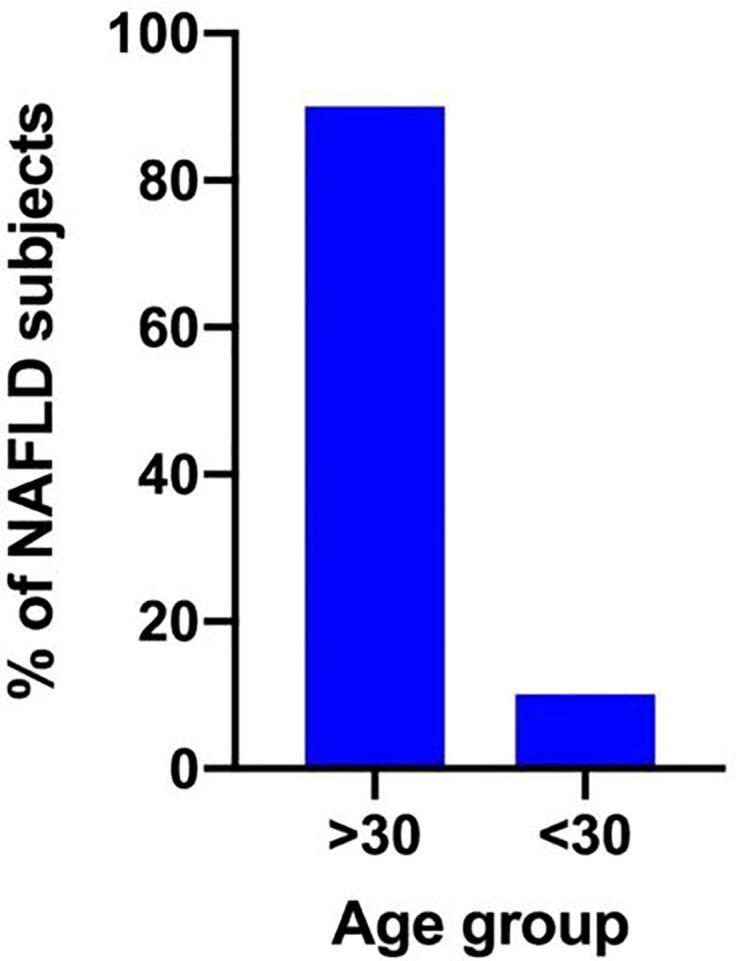
Proportion of NAFLD patients with age > 30 and < 30 years.
3.3. Proportion of patients with NAFLD with high FBG
Our results revealed that a very high proportion (75%) of patients with NAFLD have high FBG (P < 0.001). On the other hand, only 25% of NAFLD cases were not reported with high levels of FBG. Therefore, these results suggest that people with high levels of FBG are at higher risk of NAFLD (Fig. 3).
Fig. 3.
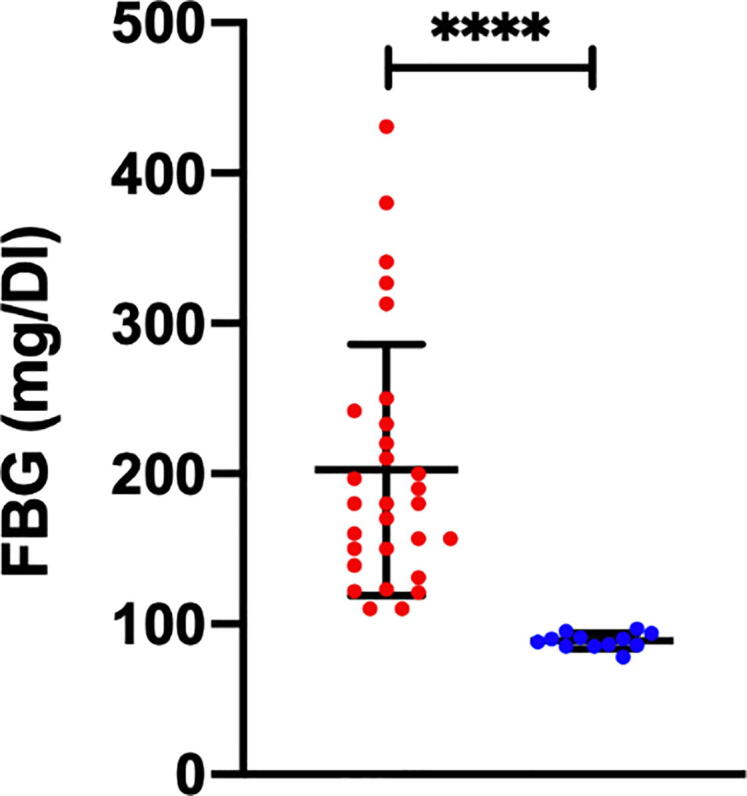
A significant proportion of NAFLD patients reported with a high FBG level (red label) and another proportion reported with a normal FBG (blue label). (P < 0.001).
3.4. Differences in liver markers between patients with and without NAFLD
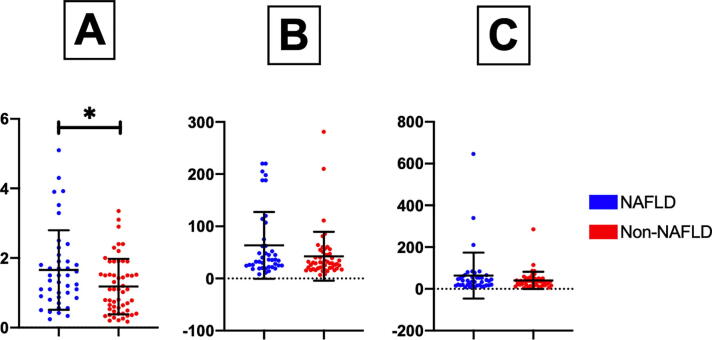
A significant increase in the serum level of TB was noted in patients with NAFLD compared to those without NAFLD (P < 0.01). On the other hand, there were no statistical differences in AST, ALT, and ALP between patients with and without NAFLD. These findings suggest that these biochemical markers may not always be indicators of NAFLD (Fig. 4).
Fig. 4.
Differences in liver markers between patients with NAFLD (blue label) and those without NAFLD (red label): (A) total bilirubin, (B) AST, and (C) ALT. * P < 0.01.
3.5. Differences in platelet count between patients with and without NAFLD
Our results indicate that patients with NAFLD are at increased risk of reduction in platelet counts compared to those without NALFD. Our data indicated that patients with NAFLD have a significantly lower platelet count (P < 0.0397) (Fig. 5) than those without NAFLD. Thus, a low platelet count may be an indicator of NAFLD.
Fig. 5.
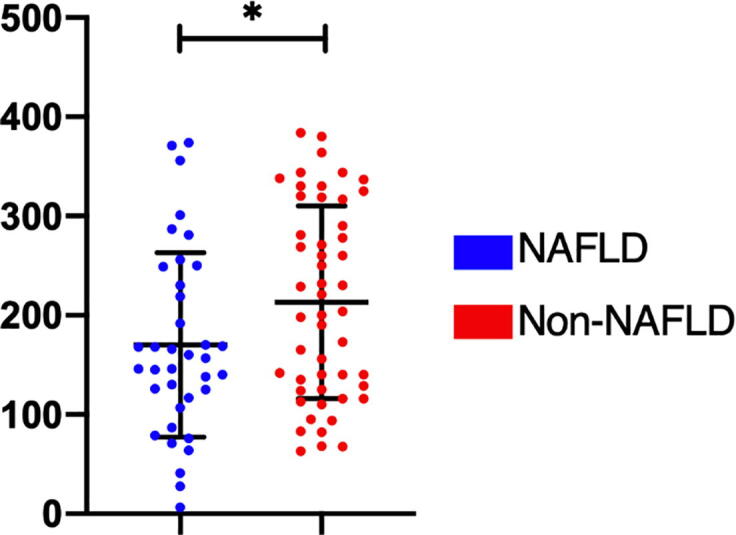
Statistical difference in platelet counts between the NAFLD (blue) and non-NAFLD groups (red) (P < 0.0397).
4. Discussion
Our data indicated that 40% of patients referred to the hepatology clinic at King Abdulaziz Specialist Hospital (KASH) had NAFLD. A high fasting blood glucose (FBG) level was significantly associated with non-alcoholic fatty liver disease (NAFLD) at 75%, which indicates that a high level of FBG is an important risk factor for NAFLD. Also, advanced age was a vital factor in NAFLD.
Many studies have looked into the prevalence of NAFLD in the general population in different countries. NAFLD was found to be 30 percent prevalent in a population-based survey in the United States (Farrell and Larter, 2006, Le et al., 2017). Using imaging reporting systems in various world regions, a meta-analysis found that the prevalence of NAFLD was about 25% (Younossi et al., 2016). A study published in 2020 reported a staggering rise in the global prevalence of NAFLD (Ge et al., 2020). NAFLD prevalence escalated from 391.2 million in 1990 to 882.1 million in 2017, with the prevalence rate increasing from 8.2 percent to 10.9 percent during the same time span. Men accounted for roughly two-thirds of all cases; however, women had a higher rate of NAFLD prevalence than men. The current study's findings revealed a similar upward trend in Taif hospital, implying that this may be due to a rise in risk factor proportions. Therefore, understanding the prevalence of NAFLD and detecting it early are critical. Otherwise, it could lead to end-stage liver disease, which is associated with a higher mortality rate (Ekstedt et al., 2015). According to the findings of this report, a large proportion of NAFLD patients were over 30 years old. This emphasizes the importance of early detection and treatment of this problem in the Taif region.
Several studies have proposed that the starting age for high onset NAFLD is at the age of 20, so we chose 20 as the eligibility age for sample inclusion. NAFLD prevalence rises with age in the general population, from 1 to 3 percent in children (Tominaga et al., 2009), 5 percent in adolescents (Tsuruta et al., 2010), 18 percent between 20 and 40 years, 39 percent between 40 and 50 years, and over 40 percent in those over 70 years (Kagansky et al., 2004, Dahshan et al., 2009). Besides, NAFLD’s higher prevalence rate in patients of advanced age more than 40 years old, the mortality rate was reported in patients aged above 60 years (Frith et al., 2009). The present study found that 90% of patients with NAFLD were older than 30 years. This could be explained by the fact that older people are at significant risk of NAFLD-related diseases such as cardiovascular disease, diabetes, and obesity (Frith et al., 2009).
There are high death proportions of NAFLD patients from other related diseases, such as cardiovascular diseases, cirrhosis or hepatocellular carcinoma (Younossi and Henry, 2016). Also, long-term NAFLD-related mortality was mostly associated with liver fibrosis based on a histological reporting system. Therefore, liver fibrosis score system assessment has been an independent predictive factor of liver disease–related mortality (Younossi et al., 2011). Metabolic syndrome is closely associated with NAFLD. Patients with metabolic syndrome, such as type 2 diabetes, obesity, and dyslipidemia, are at high risk of NAFLD. A study found that 80% of fat content on the liver tissue was present in patients with type 2 diabetes (Kotronen et al., 2008). NAFLD has been highly prevalent in adults with type 2 diabetes (Jimba et al., 2005, Targher et al., 2007). Moreover, previous studies found that there is a significant association between NAFLD and high level of FBG (Hsu et al., 2019, Zou et al., 2020). The present study found that 75% of patients with NAFLD have a high FBG level. This finding is in accordance with earlier reports.
Biochemical markers are early noninvasive predictor markers for NAFLD (Papagianni et al., 2015, Zhou et al., 2019). However, there is a high proportion of patients with NAFLD with normal liver function tests, such as AST and ALT (Browning et al., 2004). On the other hand, platelet counts have been predicted as an ideal parameter to assess liver fibrosis in patients with NAFLD (Yoneda et al., 2011). Several studies have found a significant reduction in platelet counts among NAFLD patients compared to those without NAFLD (Milovanovic Alempijevic et al., 2017, Yoneda et al., 2011). The results of the current study are consistent with the findings of previous studies. This suggests that patients with NAFLD with low platelet counts are more likely to progress to liver cirrhosis.
5. Limitations
Our study has some limitations. First, our sample size falls short of the proposed target number required for this research. In the future, we hope to see at least 400 patients. The low sample size also reduced the statistical power. Second, none of the patients in our cohort had liver biopsy, so we do not have histology reports.
6. Conclusion
Our data indicate that the prevalence of NAFLD among the Taif population is at 40%. In addition, type 2 diabetes is an important high-risk factor for NAFLD. This led to urgent warnings that should be taken to control type 2 diabetes in primary care centers to avoid the increased prevalence of NAFLD.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to thank King Abdulaziz specialist hospital administration for providing research administrative support. Also, thank the deanship of scientific research for supporting this study.
The author Abdulhakeem S. Alamri supported by Taif University with number (TURSP-2020/288), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abdulhakeem S. Alamri, Email: a.alamri@tu.edu.sa.
Syed Mohammed Basheeruddin Asdaq, Email: sasdaq@gmail.com.
References
- Alswat K., Aljumah A., Sanai F., Abaalkhail F., Alghamdi M., Al Hamoudi W., Razavi H. Nonalcoholic fatty liver disease burden & #8211; Saudi Arabia and United Arab Emirates, 2017–2030. Saudi J. Gastroenterol. 2018;24(4):211–219. doi: 10.4103/sjg.SJG_122_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti M., Lonardo A., Mussi C., Baldelli E., Pellegrini E., Ballestri S., Loria P. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J. Gastroenterol. 2014;20(39):14185–14204. doi: 10.3748/wjg.v20.i39.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C., Hobbs H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Camhi S.M., Bray G.A., Bouchard C., Greenway F.L., Johnson W.D., Newton R.L., Katzmarzyk P.T. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity. 2011;19(2):402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahshan A., Chalmers L.J., Tolia V. Nonalcoholic fatty liver disease in children. Therapy. 2009;6(1):83–91. [Google Scholar]
- Ekstedt M., Hagström H., Nasr P., Fredrikson M., Stål P., Kechagias S., Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–s112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Fracanzani A.L., Valenti L., Bugianesi E., Andreoletti M., Colli A., Vanni E., Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology. 2008;48(3):792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- Frith J., Day C.P., Henderson E., Burt A.D., Newton J.L. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55(6):607–613. doi: 10.1159/000235677. [DOI] [PubMed] [Google Scholar]
- Ge X., Zheng L., Wang M., Du Y., Jiang J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: a population-based observational study. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2019-036663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.-L., Wu F.-Z., Lin K.-H., Chen Y.-H., Wu P.-C., Chen Y.-H., Yu H.-C. Role of fatty liver index and metabolic factors in the prediction of nonalcoholic fatty liver disease in a lean population receiving health checkup. Clin. Transl. Gastroenterol. 2019;10(5) doi: 10.14309/ctg.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimba S., Nakagami T., Takahashi M., Wakamatsu T., Hirota Y., Iwamoto Y., Wasada T. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22(9):1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- Kagansky N., Levy S., Keter D. Non-alcoholic fatty liver disease—a common and benign finding in octogenarian patients. Liver Int. 2004;24(6):588–594. doi: 10.1111/j.1478-3231.2004.0969.x. [DOI] [PubMed] [Google Scholar]
- Kotronen A., Juurinen L., Hakkarainen A., Westerbacka J., Corner A., Bergholm R., Yki-Jarvinen H. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care. 2008;31(1):165–169. doi: 10.2337/dc07-1463. [DOI] [PubMed] [Google Scholar]
- Le M.H., Devaki P., Ha N.B., Jun D.W., Te H.S., Cheung R.C., Nguyen M.H. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLOS ONE. 2017;12(3) doi: 10.1371/journal.pone.0173499. e0173499–e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic Alempijevic T., Stojkovic Lalosevic M., Dumic I., Jocic N., Pavlovic Markovic A., Dragasevic S., Milosavljevic T. Diagnostic Accuracy of Platelet Count and Platelet Indices in Noninvasive Assessment of Fibrosis in Nonalcoholic Fatty Liver Disease Patients. Canadian J. Gastroenterol. Hepatol. 2017;2017 doi: 10.1155/2017/6070135. 6070135–6070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagianni M., Sofogianni A., Tziomalos K. Non-invasive methods for the diagnosis of nonalcoholic fatty liver disease. World J. Hepatol. 2015;7(4):638–648. doi: 10.4254/wjh.v7.i4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E., Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- Targher G., Bertolini L., Padovani R., Rodella S., Tessari R., Zenari L., Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- Tominaga K., Fujimoto E., Suzuki K., Hayashi M., Ichikawa M., Inaba Y. Prevalence of non-alcoholic fatty liver disease in children and relationship to metabolic syndrome, insulin resistance, and waist circumference. Environ. Health Prev. Med. 2009;14(2):142–149. doi: 10.1007/s12199-008-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta G., Tanaka N., Hongo M. Nonalcoholic fatty liver disease in Japanese junior high school students: its prevalence and relationship to lifestyle habits. J. Gastroenterol. 2010;45(6):666–672. doi: 10.1007/s00535-009-0198-4. [DOI] [PubMed] [Google Scholar]
- web, W. w. (2017). World Obesity Facts. Obesity Rates by Country – 2017. Retrieved from https://renewbariatrics.com/obesity-rank-by-countries/
- Yoneda, M., Fujii, H., Sumida, Y., Hyogo, H., Itoh, Y., Ono, M., Japan Study Group of Nonalcoholic Fatty Liver, D., et al., 2011. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J. Gastroenterol. 46(11), 1300–1306. doi: 10.1007/s00535-011-0436-4. [DOI] [PubMed]
- Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150(8):1778–1785. doi: 10.1053/j.gastro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Tacke F., Arrese M., Chander Sharma B., Mostafa I., Bugianesi E., Vos M.B. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M. Non-alcoholic fatty liver disease & #x2013; A global public health perspective. J. Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Stepanova M., Rafiq N., Henry L., Loomba R., Makhlouf H., Goodman Z. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol. Commun. 2017;1(5):421–428. doi: 10.1002/hep4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z.M., Stepanova M., Rafiq N., Makhlouf H., Younoszai Z., Agrawal R., Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53(6):1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- Zhou J.-H., Cai J.-J., She Z.-G., Li H.-L. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J. Gastroenterol. 2019;25(11):1307–1326. doi: 10.3748/wjg.v25.i11.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Yu M., Sheng G. Association between fasting plasma glucose and nonalcoholic fatty liver disease in a nonobese Chinese population with normal blood lipid levels: a prospective cohort study. Lipids Health Dis. 2020;19(1):145. doi: 10.1186/s12944-020-01326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]