Abstract
Objective
To determine the proportion of fertility in Pakistani infertile females and discover if there are considerable connection among BMP15 gene polymorphism, follicle maturation and hormonal regulation in Pakistani infertile females.
Methods
All selected participants were initially examined through follicle-stimulating hormones (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), Prolactin, and Trans-vaginal scan (TVS). BMP15 gene polymorphism among infertile and fertile females was done by extracted Genomic DNA from whole blood. Sanger sequencing was performed for the identification of mutation in exons-intron boundaries of the BMP15 gene. Bioinformatics tools were used to assess the protein structure.
Results
The total five mutations including two novel missense variants of BMP15 in exon 2, whereas three previously reported i.e. two cosmic mutations (c.615delC), (c.584InsG) and one frame shift mutations (c.635delA) were also observed. The first novel mutation was found at (c.1038InsGG) (p.346Gln < Gly) in which the insertion of GG at DNA position 1038 of exon 2 resulting in a substitution of glutamine into glycine at 346th amino acid of BMP15 protein. The second novel variant (c.1049delT) (p. Ser334Pro) was also observed in exon 2 of the BMP15 gene, which substituted serine into proline at 334th amino acid of the BMP15 protein.
Conclusion
It is concluded that there are various missense mutations present in exon 2 of the BMP15 gene of Pakistani infertile females, consequently expected function of protein changes due to change in codons of amino acids. Provean and SIFT suggest the two novel variants as potentially deleterious. Although three other variants were also found in Pakistani infertile females which were previously reported. These mutations may result in early blockage of folliculogenesis and ovaries become streaky. Further research is required to resolve the actual allusion of these variations in the BMP15 gene.
Keywords: Infertility, Mutation, BMP15 gene, Sequencing
1. Introduction
Infertility is a noticeable and most important fitness problem of the world due to countless reasons such as an abnormal function of testes and ovaries, maternal age, excess weight, an abnormal number of chromosomes, genetic and epigenetic mutations, polycystic ovarian syndrome, cystic fibrosis, and premature ovarian failure. Several genetic features from both males and females are also involved in the reproduction process (Zorrilla and Yatsenko 2013). Gene mutation is also a reason for infertility because it always creates a problem in the pubertal development of a female, hence she has to face the deficiency of pituitary hormones or disturbing the ovarian functions. Consequently, the patient suffers from hypogonadotropic hypogonadism, a situation of missing or lacking puberty due, their blood serum also show less amount of reproductive hormones such as follicle-stimulating hormone (FSH) or luteinizing hormone (LH) (Badar et al. 2018). These are two main hormones for a female reproductive function that assist the ovaries in regulating their function by the mutual action of the most important ovarian partners such as activins, inhibins, growth differentiation factor 9 (GDF9), and bone morphogenetic protein 15 (BMP15) (Dixit et al. 2010). BMP15 gene helps in follicle growth and continues to grow up the primary gonadotrophin-independent phases of folliculogenesis, this regulates the production of follicular granulosa cell (GC) and also protect the GC from the apoptosis process, and support oocyte maturity proficiency (Persani et al. 2014).
For the normal reproduction process, it is crucial that BMP15 and GDF9 genes function properly; an abnormal function of these two important genes can exhibit premature ovarian failure (POF), an ovarian deficiency in which ovaries become weakened to produce mature follicles in young females (Kumar et al. 2017). BMP15 gene has been revealed to play its role to excite the growth of granulosa cells and regulate the sequence of folliculogenesis in combination with the FSH dependent stage (Galloway et al., 2000, Chang et al., 2002, Moore and Shimasaki, 2005, Crawford et al., 2011, Kumar et al., 2017). For many years scientists are exploring the possible participation of the BMP15 gene in POI (Di Pasquale et al., 2004, Tiotiu et al., 2010). Bone morphogenetic protein (BMP) 15 gene (locus Xp11.2) determined in 1998 (Dube et al. 1998) is considered as an important part of the transforming growth factor (TGF)-b super family which helps in growth and differentiation of granulosa cells (GCs), which gives essential support to the oocyte for upcoming healthy embryo/ fetal growth(Gilchrist et al., 2008, Sugimura et al., 2014). BMP15 protein is composed of an N-terminal prodomain and a C-terminal mature domain. In the event of BMP15 molecule composition, the prodomain caused to folds and make dimmers of the mature growth factor (Shi et al. 2011). Later BMP15 protein is split into several parts by the furin enzyme-like proteases; this enzyme was released from the oocyte which is non-covalently attached with its prodomain. Normally BMP15 lacks the cysteine particles which helps to make an intermolecular disulfide bond, and that’s why function as a non-covalent dimer (Mottershead et al. 2013). Due to prodomain dislocation, human BMP (hBMP)15 connects to a group of gene-like type I (ALK6) and type II (BMPRII) receptors on the outside of GCs.
The cause of mutation in the BMP15 gene and the relation of the gene with female infertility has still obscure, might be because of changes among contests and likely investigation inaccuracy. Until now, no report was published in Pakistani women on SNPs within the BMP15 gene. Therefore, this investigation was attempted to take a step ahead to resolve this problem and was intended to examine variations in the exons of bone morphogenetic protein (BMP) 15 gene in Pakistani infertile females.
2. Material and methods
2.1. Subjects
A total of 160 (110 cases and 50 control) individuals were analyzed. All obese infertile females who were aged between 18 and 40 years, suffering from disturbed hormones having amenorrhea were included whereas the females above the age of 40 years who were pregnant, had nephritis, cardiac and hypertensive conditions were excluded in this study. After identification of the patient, written consent was taken from all participants and their family partners and parents/guardians. An authenticated questionnaire had been prepared to save the information related to the causes of infertility, a period of infertility, diet, age, trans-vaginal scan (TVS) and lifestyles, besides anthropometric measurements. Clinical investigations for hormones; FSH, LH, TSH, and Prolactin, and genetic polymorphism of the BMP15 gene of blood samples was carried out. The ethics research committee and board of research and graduate studies of University of Sindh, Jamshoro, approved this study.
2.2. Blood sample collection
For hormonal investigation and DNA extraction, 6.0 ml venous blood was drawn from selected infertile and fertile females and stored as 3.0 ml in 50 ml falcon tubes containing 0.2 ml Ethylene diamine tetra acetic acid (EDTA) as anticoagulant for DNA extraction whereas3.0 ml of blood was collected in plain tubes and serum was separated for hormonal assays.
2.3. Hormones analysis
The hormones (FSH, LH, TSH, and Prolactin) concentrations in serum samples were determined in duplicate using the Cobas e411 immunoassay analyzer. The immunoassay sandwich principle technique was used for about 18 min. Results were obtained by a standard curve, which is instrument-specifically generated by 2-point calibration and a master curve provided through the reagent barcode on Elecsys 2010 MODULAR ANALYTICS E170.
2.4. Amplification of BMP15 gene and sequencing
Genomic DNA was extracted from whole blood with the genomic DNA preparation kit (Jena Bioscience, Germany). The polymerase chain reaction (PCR) of the BMP 15 gene was performed using the extracted genomic DNA with designed primers and PCR master mix in Eppendorf gradient thermo cycler as described by Mullis et al.(Mullis et al. 1986). PCR was performed in a 25 µl reaction volume containing 18.2 µl deionized distilled water, 2.5 µl of 10X Buffer, 1.5 µl of 25 mM MgCl2, 0.5 µl of 2.5 mM dNTPs mixture, 0.5 µl of each forward and reverse primers, 0.3 µl Taq polymerase (5U/µL), and 1 µl sample DNA. The designed sequences of primers of BMP15 gene exon 2 were F; 5′GGTTCTGGAATAACAAGGGAC3′, R; 5′CCACCAACTGATTGATAAGG 3′ with amplified product size 468 bp (Al-ajoury et al. 2015).
The cycling program was set as follows; initial denaturation at 94 °C for 4 min followed by 35cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 secs, and extension at 72 °C for 1 min and the final extension was done at 72 °C for 8 min. The PCR products were resolved by the electrophoresis with 2.5% agarose. PCR products were observed under ultraviolet trans-illumination in Gel Documentation equipment (Bio-Rad).
For direct mutation screening of PCR products of designated BMP15 gene exon 2, automated DNA Sequencer (ABI Genetic Analyzer) following Sanger's dideoxy chain termination method was used. Sequence alignment report was generated by chromas software and online BLAST sequence analyzer.
2.5. Statistical analysis
The statistical analysis was carried out by SPSS 20.0 (SPSS Inc., Chicago, II., USA). On the whole distributed data were articulated as mean ± SD, categorical variables are expressed as percentage, relative frequency. Provean (http://provean.jcvi.org/index.php) and SIFT (software implement fault tolerance) software were used to find out the possible harmful consequences of the amino acid alterations. Provean software calculates whether an amino acid substitution or deletion has any contact with the natural function of a protein (Choi 2012). The SIFT software check the effects of altered nucleotide bases in amino acid codons on protein function (Ng and Henikoff 2003).
3. Results
In the present study, BMP15 gene was analyzed to check the variants concerning infertility in 110 infertile females and 50 control fertile females. The PCR and DNA sequencing was performed for all exon–intron boundaries of the BMP15 gene of infertile females. Results showed that 13.6% of infertile females were carrying different variants in the exon 2 of the BMP15 gene, resulting in nucleotide and amino acid changes in cDNA and protein respectively (Table 1).
Table 1.
BMP 15 gene exon 2 variants showing mutations identified in the present study.
| S. no | Gene | Exon | Nucleotide Variant | Protein Variant | Sift | Provean |
|---|---|---|---|---|---|---|
| 1 | BMP15 | 2 | c.615delC | p.L205L | Deleterious | Deleterious |
| 2 | BMP15 | 2 | c.584InsG | p.W195W | Neutral | Neutral |
| 3 | BMP15 | 2 | c.635delA | p.Q212K | Deleterious | Deleterious |
| 4 | BMP15 | 2 | c.1038InsGG | p.Q346G | Deleterious | Deleterious |
| 5 | BMP15 | 2 | c.1000delT | p.S334P | Deleterious | Deleterious |
The intron–exon boundaries of BMP15 gene was screened through sanger sequencing of all the participants of the study. Total five mutations were observed in exon 2 of BMP15 gene, two were newly identified missense variants c.1038InsGG(p.Q246G) and c.1000delT (p.S334P) and three were reported variants. Silico analysis was performed on novel variants which declared to be deleterious effect on phenotype of individual.
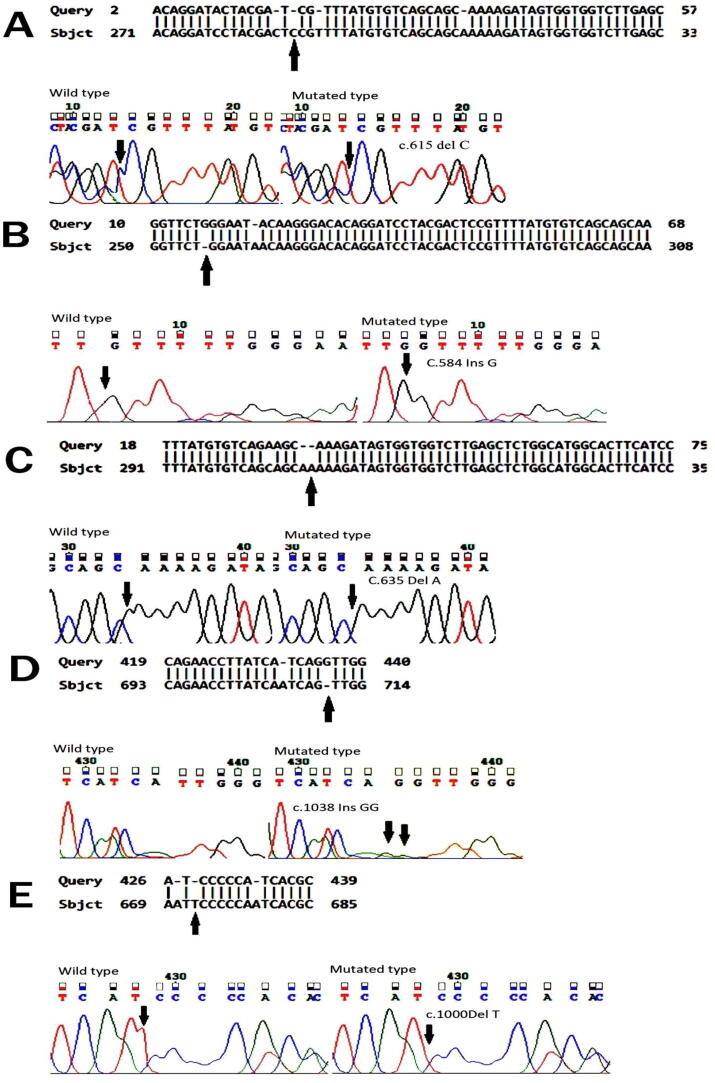
The direct sequencing of exon 2 and exon–intron boundaries of BMP15 gene in 110 infertile females revealed five mutations including two novel missense variants. The first novel mutation was found at (c.1038InsGG) (p.346Gln < Gly) in which the insertion of GG at DNA position 1038 of exon 2 resulting in a substitution of glutamine into glycine at 346th amino acid of BMP15 protein (Table 1, Fig. 1D). The score certified by the Provean software was deleterious (PROVEAN score is −4.172 and the cutoff value is −2.5). The second novel variant (c.1049delT)(p. Ser334Pro) was also observed in exon 2 of the BMP15 gene, which substituted serine into proline at 334th amino acid of the BMP15 protein (Table 1, Fig. 1E). This BMP15 gene variants acquire the potentially deleterious effect on female fertility, was confirmed via bioinformatics tool SIFT and PROVEAN (Provean score is −4.681), mentioned in Table 1. Three previously reported mutations in BMP15 gene exon 2 were also found in studied cases of infertile females including two were cosmic mutations and one was frame shift mutation. The previously studied frame shift variant (c.635delA)(p.212Gln < Lys)(Q212K), resulting in substitution of glutamine into lysine was observed in six affected (infertile) females which was confirmed deleterious by provean and SIFT with provean score −1.015 (Table 1, Fig. 1C). On the other hand, the cosmic mutation (c.584InsG)(p.W195W)was found in two infertile females (Table 1, Fig. 1B) in which insertion of G was observed and there was no change of amino acid occurred with the non-pathogenic effect of this variant confirmed through the bioinformatics tool Provean, however, the pathogenic effect of this variant is low with a neutral value as calculated via bioinformatics tool Provean. Another cosmic mutation (c.615delC)(p.L205L) deletion of C occurred in the BMP15 gene at nucleotide position 615, hence no amino acid change at this position but provean score is −8.350 showing the deleterious effect of this previously reported mutation (Table 1, Fig. 1A).
Fig. 1.
1A Sequence chromatogram of affected individual in which deletion of C is taking place but no change into amino acid leucine remain same L205L Protein structure in Fig. 1A shows mutation with deleterious effect by SIFT and provean. Fig. 1B. Sequence chromatogram of affected individual in which insertion of G is taking place but no change into amino acid tryptophan remain same W195W protein fig shows mutation with neutral effect by SIFT and provean. Fig. 1C. Sequence chromatogram of affected individual into which glutamine change into lysine at the position 212 due to deletion of Adinine. Protein Model via SWISS-MODEL bioinformatics tool showing the glutamine change into lysine at the position of 212. Fig. 1D. Sequence chromatogram of the affected individual in which insertion of GG, change the amino acid glutamine to glycine at 346 positions. Protein model via SWISS-MODEL, showing the change of amino acids of glutamine to glycine at the position of 346. Fig. 1E. Sequence chromatogram of individual in which deletion of T is mentioned which alters the amino acid Serine into Proline at the position of 334. Protein models via SWISS-MODEL in that amino acids serine change into proline at the position of 334.
According to Table 2, the total of 15 infertile, out of 110 females was carrying different gene mutations. All the females were near to 30 or above 30 years of their age, the menstrual cycle was also found disturbed or need to induce by medicines, hormonal secretion and follicle size were measured significantly lower, all three hormones, FSH, LH and Prolactin were found abnormal in range the comparison of infertile hormones and follicle with control females were shown in Table 3, according to present study all control females were mostly have normal hormonal secretion and their TVS were also shown their mature follicle at 14 day of cycle (Table 3). All the infertile females were frequently eating junk food, broiler chicken, meat, the follicles of these females were also measured either multiple small size (polycystic) or immature in growth through TVS ultrasound (Fig. 2, B & C, Table 2). These findings indicate that an improper diet might be one of the reason for disturbed hormonal secretion and an immature follicle in the infertile females. Changes in amino acid codon can cause hormonal disturbance due to which follicles do not reach their desired size and also affect the menstrual cycle. TVS Fig. 2D is showing fully grown mature fertile follicle, ready to fertilize with sperm. The follicle average size is (4 cm).
Table 2.
The changes in hormonal levels and follicle size in the infertile females showing BMP15 gene exon 2 variants.
| Sr. no |
P. ID | BMP15 variants | Age | Menstrual cycle | Hormonal level (In follicular phase) |
Diet | Follicle size (cm) | ||
|---|---|---|---|---|---|---|---|---|---|
| FSH mIU/ml | LH mIU/ml |
Prolactin ng/ml | |||||||
| 1. | 2 s |
615del C |
35 | Regular | 5.21 | 15.67 | 0.858 | Veg + meat | 0.8 |
| 2. | 40 s | 29 | Irregular | 4.51 | 12.43 | 20.46 | Chi + meat | 1.2 | |
| 3. | 16 s | 33 | Irregular | 6.83 | 10.45 | 19.12 | veg + chi | 1.0 | |
| 4. | 17 s |
584InsG |
29 | Regular | 4.21 | 8.63 | 40.16 | Chi + meat | Multi 0.5 |
| 5. | 11 s | 30 | Regular | 8.43 | 2.46 | 21.22 | Veg + chi | Multi 0.5 | |
| 6. | 20 s |
635delA |
35 | Irregular | 5.65 | 18.84 | 24.16 | Veg + chi | 1.0 |
| 7. | 33 s | 30 | Regular | 10.67 | 7.84 | 10.14 | Chi + meat | 0.9 | |
| 8. | 41 s | 29 | Regular | 8.78 | 2.86 | 20.34 | Veg + meat | 1.4 | |
| 9. | 10 s | 31 | Regular | 9.34 | 4.67 | 23.45 | Chi + meat | Less than 0.5 | |
| 10. | 12 s | 29 | Induce | 7.45 | 3.21 | 0.986 | Chi mostly | PCO | |
| 11. | 6 s | 30 | Regular | 6.83 | 15.48 | 19.18 | Veg + chi | 1.3 | |
| 12. | 7 s | 1038Ins GG | 33 | Induce | 7.21 | 9.73 | 20.76 | Chi + meat | Less than 0.5 |
| 13. | 19 s | 34 | Irregular | 4.21 | 14.67 | 23.32 | Chi + meat | PCO | |
| 14. | 27 s |
1000delT |
30 | Irregular | 6.21 | 5.38 | 23.35 | veg + meat | 1.2 |
| 15. | 21 s | 35 | Irregular | 6.56 | 12.43 | 18.67 | J. food + meat | PCO | |
Results described the information about those infertile females who got the genetic polymorphism in exon 2 of BMP15 gene. Total 15 individual’s bmp15 gene was found mutated out of 110 infertile females. All 15 infertile females were about to or cross 30 years, they were mostly facing irregular menstrual cycle or need to induce after a long interval, their reproductive hormones were not in normal. These females were frequently eating junk food, chicken and meat and they were not producing mature follicle as their trans vaginal scan showed.
Table 3.
Comparison of menstrual regularity, hormonal secretion and follicle size between control and infertile female.
| Sr. no | Attributes (Hormones) |
Groups | Control Freq. (50) |
Control % | P-value | Chi –sq | Infertile Freq. (1 1 0) |
Infertile % | P-value | Chi –sq |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Periods | Regular Irregular |
50 0 |
100 0 |
0.000 | 100 | 64 46 |
58.2 41.8 |
0.086 | 2.94545 |
| 2. | FSH | Normal Borderline Abnormal |
45 00 05 |
90 00 10 |
0.001 |
11.428 |
33 50 27 |
30 45.5 24.5 |
0.021 | 7.76364 |
| 3. | LH | Normal Borderline Abnormal |
50 00 00 |
100 00 00 |
0.000 | 16.667 | 36 43 31 |
32.7 39.1 28.2 |
0.371 | 1.98182 |
| 4. | Prolactin | Normal Borderline Abnormal |
41 3 6 |
82 6 12 |
0.005 |
7.757 |
63 27 20 |
57.3 24.5 18.2 |
0.000 | 29.0364 |
| 5. | Follicle size | Normal Abnormal |
50 00 |
100 | 0.000 | 100 | 15 95 |
13.6 86.4 |
0.000 | 58.1818 |
Showing the comparison between control and infertile females in accordance of periods regularity, normal hormonal secretion and follicle size. Results shows the significant differences in all parameters in control whereas P-value shows the non significant differences in periods regularity, FSH and LH secretion in infertile females, although prolactin and follicle size shows significant results.
Fig. 2.
2A was taken from affected individual which shows the mature follicle, another affected individual TVS showed the immature follicle in Fig 2B whereas some individual’s TVS were showing the polycystic ovary Fig 2C but Fig. 2D is showing the fully grown mature follicle which were ready to fertilize, this scan was taken from fertile female at 14 days of her menstrual cycle.
4. Discussion
Infertility may occur due to various health issues. In this study we have demonstrated that most of the female were having irregular menstrual cycle (41.8%) and the trans vaginal scan (TVS) showed that majority of patients (86.4%) have immature small size follicles ranged from 0.5 cm to 1.2 cm which fail to achieve either ovulation or fertilization as compared to control group. It was also confirmed by two other studies that the follicles those are either too small or too large are not considered as mature oocytes and also not suitable for fertilization (Revelli et al., 2014, Karavani et al., 2019). Shimasaki et al., 2004, Hashimoto et al., 2005 also mentioned the role of BMP15 gene in follicle development as it play an important role in folliculogenesis and GC (granulose cell) activities, the gene important function can be highlighted as under; a) this gene helps to promote maturity of follicles from the primary stage of follicles development to final folliculogenesis step; b) instruct follicular GC to react on FSH action; c) help to stop GC apoptosis; d) enhance oocyte growth ability; and e) regulates the process of ovulation.
Liu’s research specified that FSH concentration increase with increasing age, which confirms the ovarian dysfunction and female does not able to conceive (Liu et al. 2015). It is reported that normal level of FSH is generally 10–13 mIU/ml if FSH level drops down, it decreases pregnancy rates (Anwar and Anwar 2016). The results of Table 2 demonstrated that many of the infertile females were showing normal range of reproductive hormones according to the standard reference value, even they were unable to conceive due to follicles immaturity because of BMP15 gene mutation, (Sudha and Reddy 2013) have also the same opinion. Since last 20 years it has been accepted that older age women face more problem in conception as compare to younger age women, as ability of reproduction decreases, the efficiency of ovary decreases, sexual craving reduces and chromosomal abnormalities and miscarriage chances increases with increasing age (Collins, et al., 2005, Abic and Yılmaz, 2019).
Many studies have been conducted in order to check the connection between BMP15 gene variants and infertility, not in human but also in animals as well. As it has been proved by many scientists that BMP15 gene actively takes part in the maturation of the oocytes and normal growth of follicles, all the way through granulosa cells activation (Database resources of the national center for biotechnology information, 2018). This gene is located on the X chromosome (Xp11.22), consisting of 2 exons; 17 amino acids are encoded by exon 1 in the first portion of the polypeptide region, whereas the remaining peptide region contains 125 amino acids encoded by exon 2 (Persani et al. 2014). Ovarian dysgenesis and premature ovarian failure can be caused due to imperfection in this gene. Laissue et al. (2006) also proved by his study that change in amino acid codon (L148P) in exon 2 of BMP-15 gene can cause POF, in French women, but this mutation cannot be considered as a main cause of ovarian insufficiency. Another study revealed that sufficient fluid secretion from BMP15 gene in the follicular phase is important for female fertility. However Heterozygous mutations in BMP15 gene may cause defect in the production of bioactive protein which may cause primary ovarian insufficiency in humans (Rossetti et al. 2009).
Any change in the codon of BMP15gene can become a reason for primary ovarian insufficiency (POI) in result very small amount of mature protein produces, which are less active or make a weak connection to work with GDF9. As BMP15 protein and GDF-9 works together for oocyte-specific growth, hence confirm the regulation of follicle production and the process of ovulation (Patino et al., 2017).
According to Di Pasquale et al. (2004), the first heterozygous BMP15 mutation (p.Cys235Tyr) linked with POF was found in the protein’s pro-domain, region and causing the reduction of granulosa cell multiplication by governing deleterious effect. Many variants showed the polymorphism in the BMP15gene with poor functional consequences in women diagnosed with physiological menopause at an early young age (Moron et al., 2006, Rossetti et al., 2009). In this study, we also observed some patients with menopause in early young age and need medication to induce menstrual cycle, among them two females also showed the deletion of A at 635th nucleotide position with QK Gln < Lys at 212th amino acid of the protein. Another mutation was found by the addition of GG at 1038, 1039 nucleotide positions of BMP15 gene which was not reported before with Q < G 346th amino acid in protein and substituted Gln < Gly.
Variations in BMP15 gene nucleotides can affect ovarian function and can cause female infertility. There are many interesting scientific interrelation characters of BMP15 gene which are supposed to be a valuable marker in IVF techniques (Persani et al. 2014). According to Karavani et al. (2019), both the quality and quantity of oocytes must be perfectly measured before going to in vitro fertilization. Present results demonstrated that many of the infertile females were showing a normal range of reproductive hormones, even their follicles were not found mature enough for fertilization or females got polycystic ovaries. Sudha and Reddy (2013) have also reported same finding.
Some changes in the BMP15 gene are favorable such as AG in contrast to AA codon may produce defensive results that help to avoid anovulation and infertility (Gonzalez et al., 2008), whereas on the other side, a frequent increase of TC and TT nucleotide in the BMP15 genotype can cause risk factors for premature ovarian insufficiency (POI) and menopause (Tiotiu et al. 2010). Other researchers also suggested that single nucleotide deletion (c.-8 del C) does not hamper the reading frame, but may involve in early steps of the translation (Rossetti et al. 2009). Based on these pieces of evidence, several mechanisms were checked in light of previous findings in animal species. It was observed that BMP15 works as a powerful stimulator of granulosa cell growth and supports the sequence of follicles formation and growth from the primary stage to the FSH-dependent stage reported by Otsuka (Otsuka et al. 2000).
In the present study, five different variants were found in fifteen patients, out of which two were novel variants, whereas other three different variants were previously reported. In earlier research scientists reported another variant c. 538G > A (p.Aln180Thr) in the same exon 2 of BMP 15 which has no harmful effect on the biological function of the protein (Laissue et al., 2006, Tiotiu et al., 2010). Another study showed one of the other variant 788 in TCT was most commonly found in POF patients and considered as polymorphism, this insertion was also found in many control, as previously reported (Kumar et al. 2017). An early study identified BMP15 gene mutations in basic regions of the prodomain (Coulam et al. 1986) which play a role in suppression of the expression of BMP15, either reduced the activity of BMP15 (Nelson 2009) or inhibited BMP15 synergy with GDF9 (Di Pasqualeet al. 2004).
Funding/Sponsorship
None
Authors’ contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be submitted for publication and agree to be accountable for all aspects of work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are highly thankful for the ISRA University hospital, Hyderabad, Pakistan for providing hormonal test facilities. Department of Gynecology, Liaquat University of Medical & Health Science, Jamshoro, Pakistan and center of Reproductive Medicines of Rehman Medical Institute, Peshawar, Pakistan are highly acknowledged for support during the collection of data and blood from infertile women.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Muhammad Rafiq, Email: m.rafiq@usindh.edu.pk.
Anoshiya Ali Khan, Email: anoshiya@scholars.usindh.edu.pk.
Nadir Ali Rind, Email: nadirali.rind@sbbusba.edu.pk.
Syed Habib Ahmed Naqvi, Email: habib.naqvi@usindh.edu.pk.
References
- Abic A., Yılmaz D.V. Infertility risk factors and nurse’s role. Int. J. Emerg. Trends Health Sci. 2019;3(1):1–8. doi: 10.18844/ijeths.v3i1.4073. [DOI] [Google Scholar]
- Al-Ajoury R., Kassem E., Al-halabi B. Investigation of some genetic variations in BMP15 accompanied with premature ovarian failure (POF) in Syrian women. Middle East Fertil. Soc. J. 2015;20(2):91–96. doi: 10.1016/j.mefs.2014.02.005. [DOI] [Google Scholar]
- Anwar S., Anwar A. Infertility: A review on causes, treatment and management. Womens Health Gynecol. 2016;5:2–5. [Google Scholar]
- Badar A., Ansari A.S., Lohyia N.K. An overview on the genetic determinants of infertility. Biomedical Journal of Scientific & Technical Research. 2018;10(4):7960–7964. doi: 10.26717/BJSTR.2018.10.001984. [DOI] [Google Scholar]
- Chang H., Brown C.W., Matzuk M.M. Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr. Rev. 2002;23(6):787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Choi Y. Proceedings of the ACM Conference on Bioinformatics, Computational Biology and Biomedicine. 2012. A fast computation of pairwise sequence alignment scores between a protein and a set of single-locus variants of another protein. [Google Scholar]
- Collins, J., Crosignani, P., & Group, E. C. W. 2005. Fertility and ageing. Hum. Reprod. Update 11(3), 261-276. doi:10.1093/humupd/dmi006. [DOI] [PubMed]
- Coulam C.B., Adamson S.C., Annegers J.F. Incidence of premature ovarian failure. Obstet. Gynecol. 1986;67(4):604–606. [PubMed] [Google Scholar]
- Crawford J.L., Heath D.A., Reader K.L. Oocytes in sheep homozygous for a mutation in bone morphogenetic protein receptor 1B express lower mRNA levels of bone morphogenetic protein 15 but not growth differentiation factor 9. Reproduction. 2011;142(1):53–61. doi: 10.1530/REP-10-0485. [DOI] [PubMed] [Google Scholar]
- Database resources of the national center for biotechnology information. 2018. Nucleic acids research. 46(D1):D8-D13. [DOI] [PMC free article] [PubMed]
- Di Pasquale E., Beck-Peccoz P., Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am. J. Hum. Genet. 2004;75(1):106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit H., Rao L., Padmalatha V., Raseswari T. Genes governing premature ovarian failure. Reprod. Biomed. Online. 2010;20(6):724–740. doi: 10.1016/j.rbmo.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Dube J.L., Wang P., Elvin J. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol. Endocrinol.. 1998;12(12):1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- Galloway S.M., McNatty K.P., Cambridge L.M. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 2000;25(3):279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- Gilchrist R.B., Lane M., Thompson J.G. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update. 2008;14(2):159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Ramirez-Lorca R., Calatayud C. Association of genetic markers within the BMP15 gene with anovulation and infertility in women with polycystic ovary syndrome. Fertil. Steril.. 2008;90(2):447–449. doi: 10.1016/j.fertnstert.2007.06.083. [DOI] [PubMed] [Google Scholar]
- Hashimoto O., Moore R.K., Shimasaki S. Posttranslational processing of mouse and human BMP-15: potential implication in the determination of ovulation quota. Proc. Natl. Acad. Sci. 2005;102(15):5426–5431. doi: 10.1073/pnas.0409533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavani G., Schachter-Safrai N., Revel A. In vitro maturation rates in young premenarche patients. Fertil. Steril. 2019;112(2):315–322. doi: 10.1016/j.fertnstert.2019.03.026. [DOI] [PubMed] [Google Scholar]
- Kumar R., Alwani M., Kosta S. BMP15 and GDF9 gene mutations in premature ovarian failure. J. Reprod. Infertil. 2017;18(1):185–189. [PMC free article] [PubMed] [Google Scholar]
- Laissue P., Christin-Maitre S., Touraine P. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur. J. Endocrinol. 2006;154(5):739–744. doi: 10.1530/eje.1.02135. [DOI] [PubMed] [Google Scholar]
- Liu X.M., Chan H.C., Ding G.L. FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca2+/CREB pathway. Aging Cell. 2015;14(3):409–420. doi: 10.1111/acel.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.K., Shimasaki S. Molecular biology and physiological role of the oocyte factor, BMP-15. Mol. Cell. Endocrinol. 2005;234(1–2):67–73. doi: 10.1016/j.mce.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Moron F.J., de Castro F., Royo J.L. Bone morphogenetic protein 15 (BMP15) alleles predict over-response to recombinant follicle stimulation hormone and iatrogenic ovarian hyperstimulation syndrome (OHSS) Pharmacogenet. Genom. 2006;16(7):485–495. doi: 10.1097/01.fpc.0000215073.44589.96. [DOI] [PubMed] [Google Scholar]
- Mottershead, D. G., Harrison, C. A., & Mueller, T. D., et al., 2013. Growth differentiation factor 9: bone morphogenetic protein 15 (GDF9: BMP15) synergism and protein heterodimerization. Proc Natl Acad Sci. 110(25), E2257-E2257. doi: 10.1073/pnas.1303459110 [DOI] [PMC free article] [PubMed]
- Mullis K.B., Faloona F., Scharf S. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor symposia on quantitative biology, Cold Spring Harbor Laboratory Press. 1986 doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Nelson L.M. Primary ovarian insufficiency. N. Engl. J. Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P.C., Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F., Yao Z., Lee T.H. Bone morphogenetic protein-15 identification of target cells and biological functions. J. Biol. Chem. 2000;275(50):39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- Patino L.C., Walton K.L., Mueller T.D. BMP15 mutations associated with primary ovarian insufficiency reduce expression, activity, or synergy with GDF9. J. Clin. Endocrinol. Metab. 2017;102(3):1009–1019. doi: 10.1210/jc.2016-3503. [DOI] [PubMed] [Google Scholar]
- Persani L., Rossetti R., Di Pasquale E. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum. Reprod. Update. 2014;20(6):869–883. doi: 10.1093/humupd/dmu036. [DOI] [PubMed] [Google Scholar]
- Revelli A., Martiny G., Delle Piane L. A critical review of bi-dimensional and three-dimensional ultrasound techniques to monitor follicle growth: do they help improving IVF outcome? Reprod. Biol. Endocrinol. 2014;12(1):107. doi: 10.1186/1477-7827-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti R., Di Pasquale E., Marozzi A. BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum. Mutat. 2009;30(5):804–810. doi: 10.1002/humu.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Zhu J., Wang R. Latent TGF-β structure and activation.“. Nature. 2011;474(7351):343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S., Moore R.K., Otsuka F., Erickson G.F. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004;25(1):72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Sudha G., Reddy K. Causes of female infertility: a crosssectional study. I. J. Lat. Res. Sci. Technol. 2013;2(6):119–123. [Google Scholar]
- Sugimura S., Ritter L.J., Sutton-McDowall M.L. Amphiregulin co-operates with bone morphogenetic protein 15 to increase bovine oocyte developmental competence: effects on gap junction-mediated metabolite supply. Mol. Hum. Reprod. 2014;20(6):499–513. doi: 10.1093/molehr/gau013. [DOI] [PubMed] [Google Scholar]
- Tiotiu D., Alvaro Mercadal B., Imbert R. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum. Reprod. 2010;25(6):1581–1587. doi: 10.1093/humrep/deq073. [DOI] [PubMed] [Google Scholar]
- Zorrilla M., Yatsenko A.N. The genetics of infertility: current status of the field. Curr. Genet. Med. Rep. 2013;1(4):247–260. doi: 10.1007/s40142-013-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]