Abstract
Biological control using rhizosphere bacteria, Pseudomonas spp. and Serratia spp. is a prospective alternative technique to overcome plant parasitic nematodes infection. So, the current study was conducted in vitro on five egg-masses, 100 free eggs and 100 infective juveniles (IJs) of Meloidogyne incognita as well as greenhouse treatments on Luffa aegyptiaca L. to evaluate the nematicidal potential of six strains belong to Pseudomonas spp. and Serratia spp. as compared to oxamyl.
Results showed that the inhibitory effect and juvenile mortality varied according to bacteria species, strains and exposure time. All the tested bacteria significantly (P ≤ 0.05) inhibited egg hatching and increased juvenile mortality in vitro. After 3 days of treatment, Pseudomonas spp. were more effective against eggs (48.31to 55.15%) and IJs (20.98 to 25.30%) than Serratia spp. (44.55 to 49.75% with eggs) and (19.06 to 21.61% with IJs), respectively. In the pot experiment, Luffa aegyptiaca L. treated with Serratia spp. and Pseudomonas spp. displayed significantly higher (P ≤ 0.05) levels of growth (as indicated by root length, fresh roots weight and fresh shoots weight) compared to control plants and significantly (P ≤ 0.05) suppressed galling (number of galls) and reproduction (as indicated by number of egg-masses on roots and number of eggs and juveniles in pot soil). Meanwhile, among the treated plants, Serratia spp. and Pseudomonas spp. gave the best results in shoot weight of pots infected by eggs of M. incognita than those infected with IJs as compared with positive control. While, oxamyl treatment gave the best results in pots infected by eggs and IJs.
The lowest galling (gall index), number of eggs and juveniles in soil was observed in the treatment with mixture of Serratia spp. and Pseudomonas spp. as well as, enhanced growth of sponge gourd more than application each of them alone. Pots treated with oxamyl overwhelmed those treated with mixture of Serratia spp. and Pseudomonas spp.
Keywords: Meloidogyne incognita, Pseudomonas spp., Serratia spp., Egg hatching, Juvenile’s motility, Luffa aegyptiaca, Oxamyl
1. Introduction
Species of the root- knot nematodes (Meloidogyne spp.) are among the most damaging nematodes in light agriculture soils, causing an estimated $100 billion loss/year worldwide (Oka et al., 2002) and they have high reproductive potential and protected in plant tissues of wide host range. Therefore, control of such nematodes is partly difficult. Meloidogyne incognita (Kofoid and White) Chitwood is the most destructive species in most agricultural crops and nearby one hundred valid species are in the genus Meloidogyne (Trinh et al.,2019) which cause serious damage worldwide (Moens et al.,2009). In Egypt, this species is one of the most common and economically important root-knot nematodes because considerable damage to majority of crops including vegetables and their production depends on the correct management of these pathogens (Sikora & Fernandez, 2005). Nowadays, Root-knot nematodes are one of the top five primary plant pathogens and the first of the 10 most important plant parasite genera in the world (Mukhtar et al., 2018, Ravichandra, 2018). More efficient management methods for root-knot nematodes are currently under consideration due to environmental issues and increased restrictions on the application of chemical nematodes (Nico et al., 2004). Therefore, a range of alternative techniques are urgently needed to replace chemical control such as antagonistic bacteria are among the biological control agents tested (Hegazy et al., 2019, Kiewnick and Sikora, 2005, Tariq-Khan, 2018, Khan et al., 2021, Heflish, 2021).
Bacteria are subject to a range of suppressive activities in nematodes by various methods, which generate or interfere with recognition of toxins, antibiotics and enzymes (Khan et al., 2004). Several rhizobacteria species such as Pseudomonas and Serratia marcescens B. have been isolated from soil water, plants and insects reported to play important role as biocontrol agents against M. incognita either by promoting plant growth or by inhibiting nematode infectivity factors (Siddiqui and Akhtar, 2009a, Siddiqui and Akhtar, 2009b, Khanna et al., 2019). S. marcescens is known to be a chitinase producer, which is considered a reason for its capability to inhibit the growth of several phytopathogenic and saprophytic fungi (Okay et al., 2013).
Therefore, the main option of the present research was designed to estimate the nematicidal effect of Pseudomonas spp. and S. marcescens on egg hatching and juvenile mortality of M. incognita in vitro and observe the suppressiveness effects of rhizobacteria on M. incognita reproduction and its damages on sponge gourd under greenhouse conditions comparing with oxamyl.
2. Materials and methods
2.1. Culturing of the Root-Knot Nematode, M. incognita:
Pure culture of M. incognita was maintained in the greenhouse on the tomato susceptible cultivar Super Strain B for using as a source of egg-masses, free eggs and second stage juveniles. A single egg-mass was used to establish a nematode population. The identification of species was depending on second stage juvenile measurements and the perineal pattern method of adult females according to Eisenback et al., 1981, Jepson, 1987.
2.2. Rhizobacteria inocula source:
The tested rhizobacteria (Serratia sp. and Pseudomonas spp.) were previously isolated from collected soil samples infested with root-knot nematodes from different areas in El-Sharqia Governorate, Egypt. The obtained bacteria isolates were identified by using16S rDNA gene sequencing (Hegazy et al., 2019). The tested isolates used in the study were Pseudomonas putida (PP29 and PP22), P. fluorescens (PF131), and Serratia marcescens (A10, A15, and A20).
2.3. Preparation of egg-masses, free eggs and second juveniles stage needed for experiments:
Infected tomato roots are cut to 2-cm long pieces with 0.5% sodium hypochlorite in 200 ml of 600 ml bottle (180 ml of water + 20 ml of Clorox). Three minutes were shaken by a closely clasped pin and dissolved the gelatinous matrix then free eggs of the egg mass (Hussey and Barker, 1973). A 200-mesh sieve nestled on a 500-mesh sieve was used for the liquid suspension of eggs. Eggs collected on the 500-mesh sieve were immediately washed under a slow stream of tap water free of residual sodium hypochlorite and incubated until the hatching on Petri dishes was at around 25 ± 2 °C. Newly hatched juveniles were collected by using a micropipette. Egg-masses of equal size needed to study the effect of the tested oils on M. incognita egg hatching were hand-picked with fine forceps from small galls on the infected tomato roots obtained from previously maintained pure culture. The collected egg-masses were surface sterilized in 1:500 V/V aqueous solution of sodium hypochlorite for 5 min (Haseeb et al., 2005).
2.4. In vitro nematicidal effect of the tested bacterial isolates against M. Incognita
2.4.1. Effect of six Pseudomonas spp. and Serratia marcescens on egg hatching and juvenile mortality of M. Incognita in vitro
2.4.1.1. Effect on Egg-Masses
Five fresh and uniform size egg-masses were transferred to 10-cm diameter Petri dishes contained 10 ml of each bacterial isolates were screened in vitro for their biocontrol efficiency against M. incognita at different time periods. Each bacteria species was adjusted to about 1.8 × 108 cfu/ml, from which, 10 ml aliquots were mixed with mentioned egg-masses. The control treatment contained only nematode egg-masses in distilled water, and all treatments were replicated five times. Treatments were left under ambient temperature of 25 ± 2 °C to determine the bacterial biocontrol efficiency.
All treatments were left under laboratory temperature 25 ± 2˚C and all treatments were replicated five times. Numbers of hatched juveniles were counted using a research microscope (100X magnification). The cumulative number of hatched juveniles in each Petri dish was counted after 1, 2, 3, 5 and 7 days post treatment. Compared to control treatment, the percentage of the hatching inhibition was determined as follows:
2.4.1.2. Effect on free eggs
According to Hussey and Barker (1973) as mentioned before, M. incognita free eggs were removed from infected roots of tomatoes. Extracted eggs were suspended by distilled water and counted by using a counting slide under a research microscope (100X magnification). The number of eggs per 1 ml was adjusted to about 1000 eggs by diluting the suspension. Approximately 100 free eggs in 0.1 ml water were exposed to 1.8 × 108 cfu/ml bacterial cell free supernatant. The control treatment contained only distilled water. All treatments were replicated five times. The cumulative number of hatched juveniles was calculated in comparison with the control treatment and the percentage of egg hatching inhibition was determined by the following equation:
2.4.1.3. Effect on juvenile Mortality:
The suspension concentration of emerged juveniles was adjusted to 100 juveniles per 0.1 ml. Ten ml of the six bacterial isolates (Pseudomonas spp. and S. marcescens) were screened in vitro for their biocontrol efficiency against of M. incognita at different time periods. Bacteria species were adjusted to about 1.8 × 108 cfu/ml, from which, 10 ml aliquots were mixed with 0.1 ml of nematode suspensions (about 100 juveniles). As mentioned before, control treatment contained only distilled water and all treatments were replicated five times. Treatments were left under ambient temperature of 25 ± 2 °C to determine the bacterial biocontrol efficiency.
Tested materials were observed daily for juvenile mortality but tables only contain data of 1, 2, 3, 5 and 7 days as mentioned before. Juveniles showing inactive straight posture or did not show any movement after prodding were considered dead (Elizabeth et al., 2003). Mortality counts were observed using a research microscope under 100X magnification in 1 ml over the specified periods. The cumulative number of dead juveniles was calculated in comparison with the control treatment of distilled water. The mortality percentages were calculated as the following equation:
2.4.2. Efficacy of Pseudomonas spp. And S. Marcescens on galling and reproduction of M. Incognita infecting L. Aegyptiaca L. Under greenhouse conditions
Pot experiment was maintained in the greenhouse, Faculty of Agriculture, Zagazig University, Egypt on the sponge gourd (L. aegyptiaca) as a host plant. Seeds of sponge gourd (Local cultivar) were soaked in distilled water in Petri dishes and kept in an incubator at 27 ± 1 °C. After 48 h, seeds were sown in formalin sterilized 15-cm diameter plastic pots filled with about 1600 g steam sterilized soil (2:1 v/v sandy soil: clay soil) mixed with 60 g compost. Three weeks after sowing, seedlings were thinned to one plant per pot. 20 ml-mixture of Pseudomonas spp. or of S. marcescens species (at concentration of 1.8 × 108 cfu/ml) were incorporated with the upper 3 cm of soil around each plant.
Then all plants were inoculated with 1000 free eggs or 1000 freshly hatched infectious juveniles of M incognita by pipetting 2 ml of the inoculum suspension into three holes around a root system and directly covered with moist soil immediately. The chemical pesticide oxamyl (Vydate 24% EC), methyl 2- (dimethylamino) -N- [(methylcarbamoyl) oxy] −2-oxoethanimidothioate, was applied to sponge gourd seedlings at the rate of 0.3 ml/plant. Plants of the control treatment were inoculated with M. incognita alone. Five replications have been made for each treatment. The randomized complete design of all pots was arranged at 25 ± 3 °C and similar horticultural treatments have been received.
The plants were carefully removed from the cans 55 days after inoculation and plant growth data were recorded according to length and fresh weight of the shoots. An aliquot sample of 100 g of soil was treated using a mixture of sieving and Baermann trays technique for the extraction of nematodes (Hopper et al., 2005). Roots were immersed in tape water for one hour to help eliminate soil adherence and maintain egg root masses. The roots were wrapped into tissue paper during assessment phases to avoid drying and the root mass was counted for a numbers of galls and egg masses.
2.5. Statistical Analysis:
The experiments were conducted in a completely randomized design. Data were subjected to analysis of variance (ANOVA) using MSTAT VERSION 4 (1987). Means were compared by Tukey’s test: α = 0.05.
3. Results
3.1. In vitro experiments
3.1.1. Ovicidal activity of Serratia spp. And Pseudomonas spp. On egg masses hatching of M. Incognita
Different strains of Serratia spp. and Pseudomonas spp. significantly (P ≤ 0. 05) inhibited egg hatching of M. incognita (Table 1). The inhibitory effect varied according to bacteria species, strains and exposure time. Between tested bacteria, the highest values were detected with S. marcescens A10 and Pseudomonas fluorescens M. PF131 after the third day post treatment with percent inhibition reached 86.61 and 80.84%, respectively, while the lowest value was observed with S. marcescens A15 and Pseudomonas putida M. PP22 with percent reduction of 77.33 and 77.38%, respectively. On the other hand, Petri dishes treated with recommended dose of oxamyl overwhelmed those treated with bacteria strains. For instance, after one, three, five and seven-days post treatment; numbers of emerged juveniles in oxamyl treatment were 1.33, 4.66, 7.66 and 8.33 juveniles with percent inhibition of 99.41, 98.92, 98.71 and 99.16%, respectively.
Table 1.
Effect of certain bacteria species and oxamyl on number of juveniles emerged from M. incognita egg-masses at different intervals during 10 days of exposure in vitro.
| Treatments | Days after treatment |
|||||
|---|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | 7 days | 10 days | Mean hatch per egg-mass | |
| Control (distilled water) | 226.66 a | 433.33 a | 596.66 a | 993.00 a | 1193.67 a | 688.66 |
| Serratia marcescens A10 | 41.00b | 60.00c | 89.00 d | 112.33c | 265.67b | 113.60 |
| (81.91) | (86.61) | (85.08) | (88.68) | (77.74) | ||
| S. marcescens A20 | 40.00b | 58.00c | 147.33c | 269.67b | 391.67b | 181.33 |
| (82.35) | (86.15) | (75.30) | (72.84) | (67.18) | ||
| S. marcescens A15 | 60.33b | 77.33 bc | 113.33 cd | 322.33b | 360.00b | 186.66 |
| (73.38) | (82.15) | (81.00) | (67.53) | (69.84) | ||
| Pseudomonas putida PP29 | 55.66b | 87.33 bc | 155.66c | 308.00b | 343.00b | 189.93 |
| (75.44) | (79.84) | (73.91) | (68.98) | (71.26) | ||
| P. putida PP22 | 54.00b | 98.00b | 226.66b | 309.33b | 364.33b | 210.46 |
| (76.17) | (77.38) | (62.01) | (68.84) | (69.47) | ||
| P. fluorescens PF131 | 40.10b | 83.00 bc | 145.33c | 294.67b | 345.00b | 181.62 |
| (82.30) | (80.84) | (75.64) | (70.32) | (74.09) | ||
| Vydate 24% SL (Oxamyl) | 1.33c | 4.66 d | 7.66 e | 8.33 d | 9.33c | 6.26 |
| (99.41) | (98.92) | (98.71) | (99.16) | (99.21) | ||
*Reported numbers represent means of 5 replicates.
**Figures in parenthesis are percentages of egg hatching inhibition in comparison with control of distilled water.
***Treatments in the same column with the same letters are not significantly different from each other (Tukey’s test: α□= 0.05).
As the period of the tested bacteria was increased, numbers of emerged juvenile were obviously increased. For instance, numbers of emerged juveniles with the highest effect bacteria, S. marcescens A10 and P. fluorescens PF131 were 89.0, 145.33; 112.33,294.67 after 5 and 7 days of exposure with percent inhibition of 85.08,75.64 and 88.68,70.64%, respectively. The same trend was found after 10 days post treatment. It was true with the six tested bacteria at the five times exposure, the distilled water treatment (control) had significantly higher numbers of eggs that hatched compared to other treatments. Ten days after treatment, numbers of emerged juveniles per egg masses was 1193.67 juveniles in distilled water treatment compared to 265.67, 345.00 and 9.33 juveniles in treatments of S. marcescens A10, P. fluorescens and oxamyl, respectively.
3.1.2. Effect of Serratia spp. And Pseudomonas spp. On free eggs hatching of M. Incognita
Six bacteria species were found to be significantly (P ≤ 0. 05) effective against free eggs of M. incognita (Table 2). The egg viability of M. incognita was influenced by the time of exposure as well as species of bacteria. Among which, S. marcescens A10 was the most effective one followed by S. marcescens A20 and P. fluorescens PF131 while S. marcescens A15 was the lowest effective one. One day after exposure, numbers of hatched juveniles and percentage of hatching inhibition in S. marcescens A10, S. marcescens A20 and P. fluorescens PF131 treatments at were 67.00 (83.22%), 122.00 (69.44%) and 124.00 (68.94%) respectively. The same arrangement was noticed after 3 and 5 days post treatment.
Table 2.
Effect of certain Serratia spp. and Pseudomonas spp. on hatching of M. incognita free eggs at five intervals during 10 days of exposure.
| Treatments | Mean number of juveniles emerged after |
||||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 10 | |
| Control (distilled water) | 399.33 a | 691.33 a | 888.67 a | 983.67 a | 993.00 a |
| Serratia marcescens A10 | 67.00 d (83.22) | 110.00 e | 148.00 d | 174.00c | 183.00 d |
| (84.08) | (83.34) | (82.31) | (81.57) | ||
| S. marcescens A20 | 122.00c | 147.33 d | 170.00c | 180.00 d | 184.67 d |
| (69.44) | (78.68) | (80.87) | (81.70) | (81.40) | |
| S. marcescens A15 | 145.67b | 183.33b | 194.67b | 195.67b | 198.67b |
| (63.52) | (73.48) | (78.09) | (80.10) | (79.99) | |
| Pseudomonas putida PP29 | 137.00 bc | 159.67c | 175.00c | 188.67c | 195.67 bc |
| (65.69) | (76.90) | (80.30) | (80.81) | (80.29) | |
| P. putida PP22 | 132.33 bc (66.86) | 157.33 cd | 173.33c | 186.67c | 193.67 bc |
| (77.24) | (80.49) | (81.02) | (80.49) | ||
| P. fluorescens PF131 | 124.00c (68.94) | 154.67 cd | 171.33c | 185.00 cd | 189.67 cd |
| (77.62) | (80.72) | (81.19) | (80.79) | ||
| Vydate 24% SL (Oxamyl) | 1.33 e (99.66) | 4.66f | 7.66 e | 8.33f | 9.33 e |
| (99.32) | (99.13) | (99.15) | (99.06) | ||
*Reported numbers represent means of 5 replicates.
**Figures in parenthesis are percentages of egg hatching inhibition in comparison with control of distilled water.
***Treatments in the same column with the same letters are not significantly different from each other (Tukey’s test: α□= 0.05).
At the seventh day of treatment, cumulative numbers of hatched juveniles and percent of hatching inhibition in Petri dishes treated with S. marcescens A10, S. marcescens A20 and P. fluorescens PF131 and P. putida PP29 were 148.00 (82.31%), 180.0 (81.70%), 185.00 (81.19%) and 188.67 (80.81%), correspondingly as compared to 983.67 and 8.33 juveniles hatched in distilled water and oxamyl treatments. It was found that as the exposure time increased, percentage of hatching inhibition was decreased with some tested bacteria. For instances, percent of reduction in Serratia marcescens A10 treatment were 84.08, 83.34, 82.31 and 81.57 after 3, 5, 7 and 10 days post treatment. Generally, the nematicidal effect of the tested bacteria showed relatively highest egg hatching inhibition on M. incognita eggs, but it was lower as compared to oxamyl.
3.1.3. Larvicidal activity of Serratia spp. And Pseudomonas spp. On infective juveniles of M. Incognita in vitro
Under laboratory conditions, effectiveness of certain Serratia and Pseudomonas species on juvenile mortality of the root-knot nematode, M. incognita were evaluated. Data in (Table 3, Table 4) and (Fig. 1) showed significant difference (P ≤ 0.05) between tested bacteria against infective juveniles of M. incognita. Among which, P. putida (PP22) was the most effective one followed by S. marcescens (A20) while S. marcescens (A10) was the lowest effective one after 7 days of exposure. The highest juvenile mortality was found with P. putida (PP22) after 7 days of exposure (47.52%) followed by S. marcescens (A20) (46.97%) at the same concentration and exposure time while, P. fluorescens (PF131) gained the least mortality after one day of exposure (3.75%). Generally, the nematicidal effect of the tested bacteria on M. incognita juveniles was directly proportion to bacteria species and exposure time. After 7 and 10 days of exposure, juvenile mortality reached to 100% in oxamyl. On contrarily, juvenile mortality ranged from 42.0 to 59.0 in the tested bacteria after 7 and 10 days of exposure, respectively.
Table 3.
Effect of certain Serratia and Pseudomonas species on juvenile mortality of M. incognita at different interval periods during 10 days of exposure compared with oxamyl.
| Treatments | Mortality (%) after exposure time |
||||
|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | 7 days | 10 days | |
| Serratia marcescens (A10) | 4.45c | 19.06 d | 30.60c | 41.71c | 51.46c |
| S. marcescens (A15) | 4.65c | 16.79 de | 34.53c | 42.88c | 55.71 bc |
| S. marcescens (A20) | 5.87b | 20.56c | 40.40b | 46.97b | 59.56b |
| Pseudomonas fluorescens (PF131) | 3.75 d | 21.61c | 33.65c | 45.08c | 56.37b |
| P. putida (PP22) | 6.84b | 25.30b | 41.36b | 47.52b | 57.66b |
| P. putida (PP29) | 4.60c | 20.98c | 35.60c | 46.40b | 58.40b |
| Vydate 24% SL (Oxamyl) | 18.80 a | 41.60 a | 95.60 a | 100.00 a | 100.00 a |
| Control (Distilled water) | 0.50 e | 1.10f | 3.40 d | 4.10 d | 4.96 d |
1Reported numbers represent means of five replicate counts.
2Tested nematodes were observed daily for mortality up to 10 days.
3Different bacterial isolates in the same column with the same letters are not significantly different from each other (Tukey’s test: α□= 0.05).
Table 4.
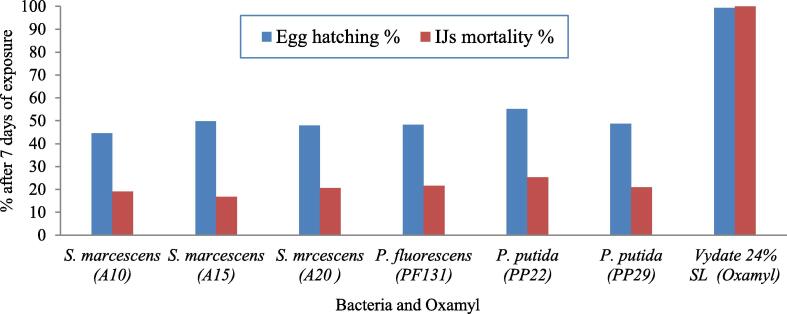
Effect of Serratia spp., Pseudomonas spp. and oxamyl on hatchability and mortality of M. incognita after 96 hrs.
| Treatments | Hatching % | Mortality % |
|---|---|---|
| Control (Distilled water) | 691.33 a | 1.10f |
| Serratia marcescens (A10) | 383.33b (44.55) | 19.06 d |
| Serratia marcescens (A15) | 347.33 d (49.75) | 16.79 de |
| Serratia marcescens (A20) | 359.67c (47.97) | 20.56c |
| Pseudomonas fluorescens (PF131) | 357.33 cd (48.31) | 21.61c |
| Pseudomonas putida (PP22) | 310.00 e (55.15) | 25.30b |
| Pseudomonas putida (PP29) | 354.67 cd (48.69) | 20.98c |
| Vydate 24% SL (Oxamyl) | 4.66f (99.32) | 100.00 a |
1Reported numbers represent means of five replicate counts.
2Tested nematodes were observed daily for hatching and mortality but only table contains data of 96 hrs.
3Different bacterial isolates in the same column with the same letters are not significantly different from each other (Tukey’s test: α□= 0.05).
Fig. 1.
Comparison between percentages of egg hatching inhibition in free eggs and dead infective juveniles of M. incognita after immersion by Serratia spp. and Pseudomonas spp. compared with oxamyl at the seventh day post treatment.
3.2. Greenhouse experiments
3.2.1. Greenhouse evaluation of Serratia spp. And Pseudomonas spp
The effect of Serratia spp. and Pseudomonas spp. compared to oxamyl on M. incognita infected sponge gourd (L. aegyptiaca) under greenhouse conditions is presented in (Table 5). The obtained results revealed that all treatments significantly (P ≤ 0. 05) reduced galling (as indicated by number of galls) and reproduction (as indicated by number of egg-masses on roots and number of juveniles in soil) and enhanced growth of sponge gourd (as indicated by root length, fresh roots weight and fresh shoots weight) compared to plants inoculated with M. incognita alone. Pots treated with oxamyl overwhelmed those treated with Serratia spp. and Pseudomonas spp. Among the tested plants, Serratia spp. and Pseudomonas spp. gave the best results in shoot weight (5.41 and 5.16 g) in pots infected by eggs of M. incognita as compared with positive control (4.30). While, Serratia spp. and Pseudomonas spp. were less effective in pots infected by infective juveniles of M. incognita. While, oxamyl gave the best results in pots infected by eggs and infective juveniles but, Serratia spp. and Pseudomonas spp. showed best results with plants infected with eggs only.
Table 5.
Effect of Serratia spp. and Pseudomonas spp. in comparison with oxamyl on galling and reproduction of M. incognita in relation to growth of Luffa aegyptiaca under greenhouse conditions.
| Treatments | Shoot weight (%increase) | Root weight (g) (%increase) | Root length (cm) (%increase) | Number of galls (%reduction) | Root Gall Index (GI) | Number of egg-masses (%reduction) | Egg mass Index (EI) | infective juveniles /100 g soil (% reduction) |
|---|---|---|---|---|---|---|---|---|
| Control- Luffa (without PPN or bacteria) | 6.98 a | 5.22 a | 16.34 a | 0.00 d | (0) | 0.00c | (0) | 0.00 g |
| Control −2 Luffa Treated with Infective juveniles of M. incognita | 3.21 d | 3.89 d | 11.91 d | 67.00 a | (4.0) | 108.00 a | (5.0) | 78.0 a |
| Control-3 Luffa Treated with eggs of M. incognita | 4.30c | 3.75 d | 12.17 d | 62.66 a | (4.0) | 98.33 a | (4.3) | 67.00b |
| Luffa + infective juveniles and bacteria (Serratia spp.) | 5.39b (67.91) | 4.55c (16.96) | 14.01 bc (17.63) | 53.66b (19.91) | (4.0) | 92.66 ab (14.20) | (4.0) | 51.33c (34.19) |
| Luffa + eggs and bacteria (Serratia spp.) | 5.41b (25.18) | 4.76 bc (22.36) | 14.10 bc (15.85) | 42.33 bc (32.44) | (4.0) | 59.33c (39.66) | (4.0) | 32.00 d (52.23) |
| Luffa + infective juveniles and bacteria (Pseudomonas spp.) | 5.99 ab (86.60) | 4.57c (17.48) | 12.16 d (2.09) | 52.33b (21.89) | (4.0) | 83.66 ab (45.06) | (4.6) | 49.33c (36.75) |
| Luffa + eggs and bacteria (Pseudomonas spp.) | 5.16b (20.00 | 4.78 bc (22.87) | 12.33 d (1.31) | 47.00b (24.99) | (4.0) | 51.67c (47.45) | (4.0) | 36.00 d (46.26) |
| Vydate 24% SL (Oxamyl) + of M. incognita | 6.07 ab (89.09) | 4.95b (27.24) | 14.73 ab (23.67) | 27.66 d (58.71) | (3.3) | 31.66 d (70.68) | (4.0) | 17.33 e (77.78) |
| Vydate 24% SL (Oxamyl) + eggs of M. incognita | 6.48 a (50.69) | 4.87b (29.86) | 14.60 ab (19.96) | 31.00 d (50.52) | (3.3) | 32.66 d (66.78) | (4.0) | 9.33 ef (86.07) |
*Each value is a mean of five replicates.
**Treatments in the same column with the same letters are not significantly different from each other (Tukey’s test: α□= 0.05)
On the other hand, number of galls, egg-masses and juveniles in soil in treatments of Luffa infected by eggs and treated with Serratia spp. and Pseudomonas spp. were 42.33(47.00), 59.33 (51.67), and 32.00 (36.00), respectively. However, these values in treatment of Luffa infected by infective juveniles were 53.66 (52.33), 92.66 (83.66) and 51.33 (49.33), respectively. The parallel values in control treatment were 67.00, 108.00, and 78.0 with number of galls, egg-masses and juveniles in soil, respectively. Percent reduction in galls, egg- masses and soil juvenile number in treatments of Serratia spp. and Pseudomonas spp. were 32.44 (24.99), 39.66 (47.45) and 52.23(46.26) % in pots infected by eggs, respectively. For plant growth parameters, it was clear that all tested treatments ameliorated growth of the sponge gourd to certain extend as compared to plants inoculated with M. incognita alone.
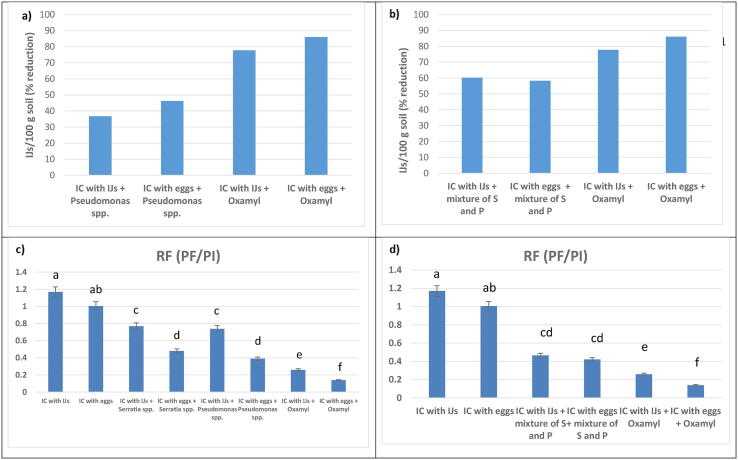
Percent increase in fresh shoot weight and root length in treatments of Serratia spp. and Pseudomonas spp. were 67.91(86.60) and 17.63 (2.09) %, respectively in pots infected by infective juveniles. The parallel values in oxamyl treatment were 89.09 and 23.67%, respectively. Values of reproduction factor (RF) clearly displayed effect of Serratia spp. and Pseudomonas spp. in suppressing number of eggs and infective juveniles in soil. For example, in treatments of Serratia spp. and Pseudomonas spp., RF values were 0.769 (0.480) and 0.739 (0.390) in plants infected by eggs and infective juveniles of M. incognita. The parallel values in positive control were 1.170 and 1.005, respectively. Generally, oxamyl treatment surpassed Serratia spp. in improving plant growth parameters and soil parameters (Fig. 2).
Fig. 2.
Percent reduction in infective juveniles of Meloidogyne incognita as influenced by tested Rhizobacter (S. marcescens and Pseudomonas spp.) applied alone or mixture compared with oxamyl (a & b) as well as, effect of different bacteria isolates (alone of mixture) on RF ratio in pot soil of Luffa aegyptiaca infected with eggs and infective juveniles (c & d). Bars with same letters are not differ significantly from each other (Tukey’s test: α□= 0.05).
3.2.2. Greenhouse evaluation mixture of Serratia spp. And Pseudomonas spp
The obtained results in (Table 6) revealed that, mixture of Serratia spp. and Pseudomonas spp. treatments significantly (P ≤ 0.05) reduced galling and reproduction and number of juveniles in soil and enhanced growth of sponge gourd more than application each of them alone. As well as, plant growth parameters were increased to reach nearby those in pots treated with oxamyl. As well, soil parameters such as number of galls, egg-masses and infective juveniles were decreased significantly (P ≤ 0. 05) to close those in oxamyl treatments.
Table 6.
Effect of mixture of Serratia spp. and Pseudomonas spp. in comparison with oxamyl on galling and reproduction of M. incognita in relation to growth parameters of Luffa aegyptiaca under greenhouse conditions.
| Treatments | Shoot weight (%increase) | Root weight (g) (%increase) | Root length (cm) (%increase) | Number of galls (% reduction) | Root Gall Index (GI) | Number of egg-masses (%reduction) | Egg mass Index (EI) | infective juveniles /100 g soil (%reduction) |
|---|---|---|---|---|---|---|---|---|
| Control- Luffa (without PPN or bacteria) | 6.98 a | 5.22 a | 16.34 a | 0.00 d | (0) | 0.00c | (0) | 0.00 g |
| Control −2 Luffa Treated with Infective juveniles of M. incognita | 3.21 d | 3.89c | 11.91c | 67.00 a | (4.0) | 108.00 a | (5.0) | 78.0 a |
| Control-3 Luffa Treated with eggs of M. incognita | 4.30c | 3.75c | 12.17c | 62.66 a | (4.0) | 98.33 a | (4.3) | 67.00b |
| Luffa + infective juveniles of root-knot nematode and mixture of Serratia spp. and Pseudomonas spp. | 6.19 ab (91.90) | 4.70b (20.82) | 14.00b (22.85) | 29.00b (56.71) | 3.00b | 65.33b (39.50) | 3.66b | 31.66c (60.25) |
| Luffa + eggs of root-knot nematode and mixture of Serratia spp. and Pseudomonas spp. | 6.23 ab (44.88) | 4.89b (30.40) | 14.60b (19.96) | 21.66 d (65.43) | 3.00b | 54.33c (44.74) | 3.66b | 28.00 d (58.20) |
| Vydate 24% SL (Oxamyl) + infective juveniles of M. incognita | 6.07 ab (89.09) | 4.95b (27.24) | 14.73 ab (23.67) | 27.66 d (58.71) | (3.3) | 31.66 d (70.68) | (4.0) | 17.33 e (77.78) |
| Vydate 24% SL (Oxamyl) + eggs of M. incognita | 6.48 a (50.69) | 4.87b (29.86) | 14.60 ab (19.96) | 31.00 d (50.52) | (3.3) | 32.66 d (66.78) | (4.0) | 9.33 ef (86.07) |
*Each value is a mean of five replicates.
**Treatments in the same column with the same letters are not significantly different from each other (Tukey’s test: α□= 0.05).
Percent reduction in galls, egg-masses and soil juvenile number in treatments of mixture of Serratia spp. and Pseudomonas spp. were 65.43 (56.71), 44.74(39.50) and 58.20 (60.25) % in pots infected by eggs and IJs, respectively. For plant growth parameters, mixture of Serratia spp. and Pseudomonas spp. ameliorated growth of the sponge gourd to certain extend as compared to control treatments. Percent increase in fresh shoot weight and root length in treatments of mixture Serratia spp. and Pseudomonas spp. and oxamyl were 91.90 (22.85) %, and 89.09 (23.67) %, respectively in pots infected by IJs with insignificant differences (P ≤ 0. 05). Values of reproduction factor (RF) were 0.465 (0.420) and 0.259 (0.139) in treatments of tested bacteria and oxamyl, respectively (Fig. 2). Generally, mixture of Serratia spp. and Pseudomonas spp. surpassed Serratia spp. or Pseudomonas spp. alone in improving plant growth parameters and suppression of galls, egg-masses and infective juveniles in soil of pots.
4. Discussion
The root-knot nematode has an important effect on crop growth and productivity. The protection of plants from root-knot nematode, particularly for increasing crop yields, has thus become an important task. Control of root-knot nematodes, Meloidogyne spp. with synthetic nematicides is expensive and cause many problems to environment and human health. On the other hand, as organic agriculture increased, new alternative control methods need to be developed because chemical nematicides are not acceptable in organic farms. Nowadays, bioagents such as rhizosphere bacteria are important to the use for the management of nematodes, since they are eco-friendly, ease to apply, not expensive and are available to farmers (Chitwood, 2002, Oka et al., 2002, Prasad et al., 2002). The potential of applying rhizosphere bacteria in management of root – knot nematodes has been reported by many authors (Ketabchi et al., 2016, Zavaleta-Mejia and Van Cundy, 1982). In addition to Pseudomonas, the bacteria belonging to the genus Serratia may make polyketides and strains of P. fluorescens are dominant plant rhizobacteria that promote growth in several cultivars (Beneduzi et al., 2012, Ho¨keberg M., Cerhardxon B., Johnxxon L. , 1997), Bacterial nematodes which are attracted to feed on P. fluorescens in the rhizosphere may indirectly contribute to crop defense from abiotic or biotic stressors (Blanc et al., 2006). Paecilomyces lilacinus L. is known to be a colonizer of the rhizosphere and thus only infested by sedentary stage (eggs and females) from the cyst and the root-knot nematode. Therefore, this fungus will therefore not prevent initial damage to the root (Siddiqui and Mahmood, 1999, Siddiqui, 2000).
Microbial proteases have been suggested as nematode virulence factors (Siddiqui and Akhtar, 2009c, Tian et al., 2007). nematophagous fungal extracellular serine protectase prevents nematode infection through the degrading cuticle defense (Meyer et al., 2004, Morton et al., 2004). Sikora (1992) reported that the rhizobacteria have great ability to control plant parasite nematodes. Also, plant growth-promoting rhizobacteria are beneficial bacteria colonizing the rhizosphere and the vegetable roots which enhance plant growth or plant pathogens protection (Kloepper and Ryu, 2006, Marleny et al., 2008, Sharma and Sharma, 2017). Our results indicate that the nematicidal properties of Serratia spp. and Pseudomonas spp. in vitro bioassay using M. incognita juveniles caused mortality and reduced egg hatching and suppressed number of galls, egg-masses and infective juveniles in soil to a lower degree and had positive effect on plant growth parameters under glasshouse conditions. As well as, rhizosphere bacteria were more effective against eggs of Meloidogyne spp. than infective juveniles in vitro and under greenhouse conditions. The obtained results might agreement with those obtained by Mohamed et al., (2009) in a greenhouse experiment, where they showed that P. fluorescens had higher effect than S. marcescens in reduction of M. incognita population. Also, these findings corroborate the results obtained by Zhao et al., 2018 who showed that P. putida (Sneb 821), P. fluorescens (Sneb 825) and Serratia proteamaculans (Sneb 851) had high potential as a biocontrol agent against M. incognita, causing 99.17% juvenile mortality and 61.11% egg mortality. As well as, in the pot experiment displayed significantly higher levels of growth in root and shoot compared to control plants and reduced the number of galls and juveniles in the soil.
Moreover, application of Serratia sp., Pseudomonas spp. caused the higher mortality of second stage juvenile and had positive effect on plant growth parameters, besides decreased the nematode-related parameters, such as number of gall, egg and egg mass, as well as the reproduction factor (Mahgoob and El-Tayeb, 2010, Ketabchi et al., 2016; and Khan et al., 2012). Khan et al., 2012 showed that the suppressive effects of Serratia sp., Pseudomonas spp. on the nematode were less than that of fenamiphos. Although chemical nematicides such as carbofurane can have a high degree of effectiveness against root-knot nematodes, inducing resistance from microorganisms such as S. marcescens and Pseudomonas spp. may be considered a more natural and environmentally acceptable alternative to such chemicals. Viljoen et al., 2019 mentioned that plant growth-promoting rhizobacteria plant growth-promoting rhizobacteria strains caused second-stage juvenile paralyses and have potential as biological control agents of M. incognita on carrots and tomatoes.
5. Conclusion
Inhibition of egg hatching and increasing juvenile mortality of M. incognita in vitro as well as reduction of galling and reproduction of such nematode in vivo strongly suggest the presence of compounds that possess ovicidal and larvicidal properties. On the other hand, the mechanisms of development, including nematicidal compounds and/or enhanced bacterial-induced defense mechanisms in plants can operate simultaneously or sequentially and can be important in the abolition of root-knot nematode. Moreover, it is recommended the use of organic modifications with productive strains of plant growth-promoting rhizobacteria because organic materials promote growth of pathogens competing with or destruction of species.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This study was financed by Taif University Researchers Supporting Project number (TURSP -2020/92), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Elasyed M. Abd El-Aal, Email: ahmedaioub1991@gmail.com.
Mohamed Shahen, Email: mshahen@science.tanta.edu.eg.
Mohammad Javed Ansari, Email: mjavedansari@gmail.com.
References
- Beneduzi A., Ambrosini A., Passaglia L.M. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genetics and Molecular Biology. 2012;35:1044–1051. doi: 10.1590/s1415-47572012000600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc C., Sy M., Djigal D., Brauman A., Normand P., Villenave C. Nutrition on bacteria by bacterial-feeding nematodes and consequences on the structure of soil bacterial community. European Journal of Soil Biology. 2006;42:570–578. [Google Scholar]
- Chitwood D.J. Phytochemicals based strategies for nematode control. Annual Review of Phytopathology. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- Eisenback J.D., Hirschmann H., Sasser J.N., Triantaphyllou A.S. North Carolina State University and U.S. Agency for International Development; Raleigh: 1981. A guide to the four most common species of root-knot nematodes (Meloidogyne species), with a pictorial key; p. 48. [Google Scholar]
- Elizabeth A., De-Nardo B., Grewal P.S. Compatibility of Steinernema feltiae (Nematoda: Steinernematidae) with pesticides and plant growth regulators used in glasshouse plant production. Biocontrol Science and Technology. 2003;13(4):441–448. [Google Scholar]
- Haseeb A., Sharma A., Shukla P.K. Studies on the management of root-knot nematode, Meloidogyne incognita-wilt fungus, Fusarium oxysporum disease complex of green gram, Vigna radiata cv ML-1108. J Zhejiang Univ. Sci. B. 2005;6(8):736–742. doi: 10.1631/jzus.2005.B0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflish A.A., Hanfy A.E., Ansari M.J., Dessoky E.S., Attia A.O., Elshaer M.M., Gaber M.K., Kordy A., Doma A.S., Abdelkhalek A., Behiry S.I. Green Biosynthesized Silver Nanoparticles Mediated by Acalypha wilkesiana Extract control root-knot nematode. Journal of King Saud University-Science. 2021;33(6):101516. doi: 10.1016/j.jksus.2021.101516. [DOI] [Google Scholar]
- Hegazy M.I., Salama A.S.A., El-Ashry R.M., Othman A.I. Serratia marcescens and Pseudomonas aeruginosa are promising candidates as biocontrol agents against root-knot nematodes (Meloidogyne spp.) Middle East Journal of Agriculture Research. 2019;8(2):828–838. [Google Scholar]
- Ho¨keberg M., Cerhardxon B., Johnxxon L. (1997). Biological control of sereal seed-borne diseases by bacterization with greenhouse-selected bacteria. European Journal of Plant Pathology 103, 23–33.
- Hopper, D.J.; Hallmann, J. and Subbotin, S.A. (2005).Methods of extraction, processing and detection of plant and soil nematodes.Pp 53-84. In: Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, Eds: M. Luc; A.R. Sikora and J. Bridge, 2 Edition, CAB International, Wallingford, UK, 871pp.
- Hussey R.S., Barker K.R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973;57:1025–1028. [Google Scholar]
- Jepson S.B. 1sted. CAB International; Wallingford, UK: 1987. Identification of root-knot nematodes (Meloidogyne species) [Google Scholar]
- Ketabchi S., Charehgani H., Majzoob S. Impact of rhizosphere antagonistic bacteria and urea fertilizer on root knot nematode (Meloidogyne incognita) under greenhouse condition. JAPS, Journal of Animal and Plant Sciences. 2016;26(6):1780–1786. [Google Scholar]
- Khan A., Williams K.L., Nevalainen H.K.M. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and Root –Knot nematode. Microorganisms. 2004;8,401. doi: 10.3390/microrganisms:8030401. doi: 10.3390/microorganisms8030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.R., Khan M.M., Anwer M.A., Haque Z. Laboratory and field performance of some soil bacteria used as seed treatments on Meloidogyne incognita in chickpea. Nematologia Mediterranea. 2012;40(2):143–151. [Google Scholar]
- Khan M.A., Riaz H., Raheel M., Shakeel Q., Waheed U., Ahmed N., Bashair M., Ashraf W., Abbas H.T., Siddique M., Khan M. In-vitro and In-vivo management of Meloidogyne incognita (Kofoid and White) Chitwood and Rhizoctonia bataticola (Taub.) Butler in cotton using organic’s. Saudi Journal of Biological Sciences. 2021;28:1–9. doi: 10.1016/j.sjbs.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna K., Jamwal V.L., Kohli S.K. Role of plant growth promoting bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant and Soil. 2019;436(2):325–345. [Google Scholar]
- Kiewnick S., Sikora R. Biological control of the root knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol Control. 2005;38:179–187. [Google Scholar]
- Kloepper J.W., Ryu C.M. Bacterial endophytes as elicitors of induced systemic resistance. In: Schulz B., editor. Microbial Root Endophytes. Springer-Verlag; Heildelberg: 2006. pp. 33–51. [Google Scholar]
- Mahgoob A.E.A., El-Tayeb T.S. Biological control of the root-knot nematode, Meloidogyne incognita on tomato using plant growth promoting bacteria. Egyptian Journal of Biological Pest Control. 2010;20(2):95–103. [Google Scholar]
- Marleny B.C., Nancy K.B., Kathy S.L., Edzard V.S., Joseph W.K. Suppressiveness of root- knot nematodes mediated by rhizobacteria. Biol. Cont. 2008;47:55–59. [Google Scholar]
- Meyer S.L.F., Huettel R.N., Liu X.Z., Humber R.A., Juba J., Nitao J.K. Activity of fungal culture filtrates against soybean cyst nematode and Root-knot nematode egg hatch and juvenile motility. Nematology. 2004;6:23–32. [Google Scholar]
- Moens M., Perry R. N., Starr J. L. (2009). Meloidogyne species: a diverse group of novel and important plant parasites. Pp. 1–17, In: Root-Knot Nematodes, Eds: R. N. Perry,M. Moens, and J. L. Starr, Wallingford, UK: CABI Publishing.
- Mohamed K., Zeinat S.A., El-Sayed T.E., Radwan Gh.ada.S., El-Wahab Abd. Potency evaluation of Serratia marcescens and Pseudomonas fluorescens as biocontrol agents for root-knot nematodes in Egypt. J. Appl. Sci. Res. 2009;4(1):93–102. [Google Scholar]
- Morton C.O., Hirsch P.R., Kerry B.R. Infection of plant-parasitic nematodes by nematophagous fungi: a review of the application of molecular biology to understand infection processes and to improve biological control. Nematology. 2004;6:161–170. [Google Scholar]
- Mukhtar T., Jabbar A., Raja M.U., Javed H. (2018). Pakistan J. Zool., 50: 1195-1198. https://doi. org/10.17582/journal.pjz/2018.50.3.sc4
- Nico A.I., Rafael R.M., Jiménez-daza M., Castillo P. Control of root-knot nematodes by composted agroindustrial wastes in potting mixtures. Crop Prot. 2004;23:581–587. [Google Scholar]
- Oka Y., Keltai H., Bar-Eyal M., Mor M., Sharon E., Chet I., Spiegal Y. New strategies for the control of plant parasitic nematodes. Pest Management Science. 2002;56:983–988. [Google Scholar]
- Okay S., Ozdal M., Kurbanoğlu E.B. Characterization, antifungal activity, and cell immobilization of achitinase from Serratia marcescens MO-1. Turk. J Biol. 2013;37:639–644. [Google Scholar]
- Prasad D., Ram D., Imtiyaz A. Management of plant parasitic nematodes by the use of botanicals. Annals of Plant Protection Sciences. 2002;10(2):360–364. [Google Scholar]
- Ravichandra N.G. Heidelberg, New York, Dordrecht, Springer Publishing, London, UK; New Delhi: 2018. Horticultural nematology; p. 412. [Google Scholar]
- Sharma I.P., Sharma A.K. Effective control of root-knot nematode disease with Pseudomonad rhizobacteria filtrate. Rhizosphere. 2017;3:123–125. [Google Scholar]
- Siddiqui I.A. Effects of sell suspension and sell-free culture filtrate of Pseudomonas aeruginosa in the control of root rot–root knot disease complex of tomato (Lycopersicon esculentum Mill.) Acta Agro- botanica. 2000;33:47–55. [Google Scholar]
- Siddiqui Z.A., Akhtar M.S. Effects of antagonistic fungi and plant growth promoting rhizobacteria on growth of tomato and reproduction of root-knot nematode Meloidogyne incognita. Austral Plant Pathol. 2009;38:22–28. [Google Scholar]
- Siddiqui Z.A., Akhtar M.S. Effect of plant growth promoting rhizobacteria, nematode parasitic fungi and root-nodule bacterium on root-knot nematodes Meloidogyne javanica and growth of chickpea. Biocont Sci Technol. 2009;19:511–521. [Google Scholar]
- Siddiqui Z.A., Akhtar M.S. Effect of antagonistic fungi, plant growth- promoting rhizobacteria, and arbuscular mycorrhizal fungi alone and in combination on the reproduction of Meloidogyne incognita and growth of tomato. J. Gen. Plant Pathol. 2009;75:144–153. doi: 10.1007/s10327-009-0154-4. [DOI] [Google Scholar]
- Siddiqui Z.A., Mahmood I. Role of bacteria in the management of plant parasitic nematodes. A review. Bioresource Technol. 1999;69:167–179. [Google Scholar]
- Sikora R.A. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Ann. Rev. Phytopathol. 1992;30:245–270. [Google Scholar]
- Sikora R.A., Fernandez E. Nematode parasites of vegetables. In: Luc M., Sikora R.A., Bridge J., editors. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. CABI Publishing, Wallingford; UK: 2005. pp. 319–392. [Google Scholar]
- Tariq-Khan M. management of root-knot nematode, Meloidogyne incognita, in tomato with two Trichoderma species. Pakistan J. Zool. 2018;50(4):1589–1592. [Google Scholar]
- Tian B., Yang J., Zhang K.Q. Bacteria used in the biological control of plantparasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol. Ecol. 2007;61:197–213. doi: 10.1111/j.1574-6941.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Trinh Q., Le T., Nguyen T., Nguyen H., Liebanas G., Nguyen T. Meloidogyne daklakensis n. sp. (Nematoda: Meloidogynidae), a new root-knot nematode associated with Robusta coffee (Coffea canephora Pierre ex A. Froehner) in the Western Highlands. Vietnam. J. Helminthol. 2019;93(2):242–254. doi: 10.1017/S0022149X18000202. [DOI] [PubMed] [Google Scholar]
- Viljoen J.J.F., Labuschagne N., Fourie H., Sikora R.A. Biological control of the root-knot nematode Meloidogyne incognita on tomatoes and carrots by plant growth-promoting rhizobacteria. Tropical Plant Pathology. 2019;44(3):284–291. [Google Scholar]
- Zavaleta-Mejia E., Van Cundy S.D. Effects of rhizobacteria on Meloidogyne infection. Journal of Nematology. 1982;14:473–476. [Google Scholar]
- Zhao Zhao Dan, Di Hui Zhao, XiaoFeng Zh.u., Yuan Wang, Duan Yuan, Xuan YuXi, YuanHu Chen LiJie. Isolation and identification of bacteria from rhizosphere soil and their effect on plant growth promotion and root-knot nematode disease. Biological Control. 2018;119:12–19. [Google Scholar]
Further Reading
- Akhtar M.S., Siddiqui Z.A. Use of plant growth-promoting rhizobacteria for the biocontrol of root-rot disease complex of chickpea. Australas. Plant. Path. 2009;38(1):44–50. [Google Scholar]