Abstract
A promising Cordia myxa fruit (CMF) extract targets an additional incorporation in functional foods. It retains appropriate health welfares owing to its antioxidant properties with limited incorporation in food matrices due its hydrophobicity. Therefore, CMF extract micro- and nanocapsulation was performed to protect and facilitate consistency of produced hydrophobic foods matrices. Furthermore, to determine its phytochemicals, antioxidant, and cytotoxic effects by applying analytical HPLC, FRAP and SRB assay, respectively. HPLC analysis of the tested extracts revealed the presence of, 25.59 ± 1.78 mg catechin/g, 69.68 ± 4.20 mg quercetin/g, and 112.72 ± 8.38 mg gallic acid/g extract. The CMF extract displayed a potent DPPH radicals' scavenger and FRAP high reduction capability in a dose-dependent manner. The potent pharmacological activities of CMF extract may be ascribed to high concentration of polyphenolics including flavonoids which strongly reported to possess an antitumor and antioxidant activities. To confirm the efficient CMF incorporation in micro- and nanosystems and their thermal stabilities to withstand the high temperatures applied during some food processing. DSC of the apparent melt of non-capsulated CMF and encapsulated forms (MCMF and NCMF) in sodium alginate gel and beads was studied. Results showed that melting point of CMF extract (86.17 °C) indicating its inability whereas the MCMF and NCMF melting points (226.45 and 383.87 °C, respectively) demonstrating the capability of expending alginate – packaging material to shield the vital active compounds of C. myxa fruit to be applied in different targeted delivery products especially that disclosed to high thermal treatments.
Keywords: Cordia myxa fruit, Encapsulation, Phenolic content, Flavonoids, Antioxidant, Cytotoxicity
1. Introduction
Natural sources either, phytochemicals or plants extract were reported to possess various potent biological effects as anticancer activity in comparing to chemotherapy or traditional medicines (Watkins et al., 2015). Cordia is a characteristic genus of Boraginaceae (borage) that involves many species and genera (Rupasinghe et al., 2014). Evidently four species; C. myxa, C. dichotoma, C. latifolia and C. Abyssinia were explored. Amongst, all organs of Cordia myxa have been traditionally utilized in folk medicine, including wound healing, demulcent, anthelmintic, diuretic, astringent, emollient, expectorant, hepatoprotective, analgesic, immune modulator, hypoglycemic, anti-inflammatory, laxative, antioxidative stress, hypolipidemic, aphrodisiac, and antiulcer (Oza and Kulkarni, 2017). The sticky and mucilaginous CMF pulp is the source of an eminent Unani System of medicine called “sapistan” which is prized in coughs, sore throats and chest-complaints treatments on expenses of its demulcent property (Rahman and Hussain, 2015). A Behidana (Cydonia oblonga), Unnab (Ziziphus jujuba) and Sapistan (Cordia myxa) decoction mixture was recommended for relieving Influenza (nazla-i-wabāi) in Unani medicine and other COVID-19 like epidemics. In addition, the treatment of sore throat, cough, septicemia, fever, dyspnea, pharyngitis, chest pain etc. (Ansari et al., 2020, Musa et al., 2020). C. myxa L. is rich in various secondary metabolites as, alkaloids, phenolic acids, coumarins, tannins, resins, gums, mucilage, sterols, flavonoids, saponins, terpenoids, and oil. Anti-nutritional factors (tannins and phytic acid), were detected in minute amounts, making C. myxa fruits safe for human usage (Murthy et al., 2019). Sweetness of C. myxa fruit is owed to high carbohydrate contents (fiber high total dietary, fructose, glucose, and sucrose). The fruits are rich in essential minerals, fat, ash, carbohydrates, and proteins. The CM methanolic extract has antioxidant and inhibitory activity toward α- glucosidase and α- amylase enzymes (Malik and Ahmad, 2015). Phenolic content and flavonoids of fixed seed oil of five Cordia species leaves were investigated. TLC and RP-HPLC investigations, revealed the presence of caffeic acid, chlorogenic hesperidin, dihydrorobinetin, datiscoside, rutin, and robinin (Murthy et al., 2019). Various affirmative human health effects of plant-derived phenolics credited to their antitumor properties, antioxidative, anti-inflammatory, etc. These properties intensely be contingent on their bioavailability in the organism which governed by the structure and acquainted form into the organism (purified isolates or food additives). Moreover, phenolics and macromolecules polysaccharides, dietary fibers, lipids, proteins interaction either in digestion or food, that considerably alters their bioavailability. Encapsulation can certainly influence bioavailability as it guarantees the active constituents coating and their targeted delivery to the digestive tract definite part and precise release. Nanoemulsion, a phase just before nanoencapsulation (nanostructures within the size range of 20–200 nm) with a higher kinetic stability during storage, made by emulsification of oily and aqueous phases using an emulsifier coating molecule (Ferreira and Nunes, 2019). Nanocapsules have been articulated via biodegradable polymers (wall materials), comprising whey protein, sodium caseinate and modified starch or maltodextrin (Jafari et al., 2008, Ali et al., 2015). Nanoemulsion, with particle size (20–200 nm) was made before nanoencapsulation (20–200 nm) through emulsification of the oily and aqueous phases by applying of an appropriate emulsifier agent (Ferreira and Nunes, 2019). Applications of mucilage including CMF extract is currently limited in the pharmaceutical and food industries since they may have microbial contamination caused by higher moisture content (more than 10%) and carbohydrate nature (Amiri et al., 2021). The former disadvantages make encapsulation a favorable technique that may support the application of different materials in different sectors (Deogade et al., 2012). Therefore, this study evaluated the effect of encapsulation in the nano- and microforms on the chemical constituents, antioxidant activities, and cytotoxic effects on liver cancer cell line Hep-G2 of the C. myxa fruits mucilage extract. Healthy human hepatic cells (THLE2) were used as a control to examine the assumed selectivity of the examined mucilage and its capsules. The investigation of safe materials of botanical origin in this study opens a perspective toward applying encapsulation techniques to facilitate mucilage in various food and pharmaceutical industries.
2. Materials and methods
2.1. Plant material and extract preparation
During November 2018, the C. myxa fruits (CMF) were collected from El-Gharbia governorate, Egypt and characterized by Professor Abdu Mareey, plant taxonomy, Faculty of Science, Al‑Azhar University, Egypt. A voucher sample was well-preserved and stowed in the herbarium of Pharmacognosy Department, College of Pharmacy, Jouf University, Saudi Arabia. CMF were allocated into two parts; external pulp and kernel seed, which it was thrown out. The pulp was dried in an oven (40 0C), grounded, defatted with petroleum ether (40:60) and extracted with EtOH (70%). Finally, fruits mucilage extract was lyophilized and kept at −20 °C until use.
2.2. Determination of polyphenol and flavonoids of CMF extract
Assessment of flavonoids and polyphenolic contents was spectrophotometrically (Schimadzu UV/Vis-240 IPC) achieved by applying the standards aluminum chloride, and Folin Ciocalteu methods, respectively. The flavonoids assessment was estimated as μg quercetin/g extract while polyphenolics as μg gallic acid/g extract (Musa et al., 2016, Musa et al., 2020, Musa).
2.3. Phenolic acids profile by HPLC
Determination of phenolic content was accomplished by applying HPLC method (Agilent Technologies 1100 series). The method was performed as previously reported by (Kim et al., 2006, Abdelgawad et al., 2018, Ghoneim et al., 2018).
2.4. Preparation of micro- and nanocapsulated CMF
2.4.1. Microcapsulation
Microcapsulation of CMF extract was conducted using water in oil emulsion technique as previously designated (Sun et al., 2020). Inotech Encapsulator (Switzerland) was employed for achievement of the final product.
2.4.2. Nanoencapsulation
Nanoencapsulation of CMF extract was performed by using Homogenizer (PRO 400 PC, Germany) model in sodium alginate and Tween 20 (T20) matrix, through applying the adapted method (Erdmann et al., 2017).
2.5. Antioxidant assay (FRAP method)
Determination of the antioxidant potential of CMF as well as its micro- and nanocapsulation forms was conducted by applying the Ferric Reducing Antioxidant Power as stated (Benzie and Strain, 1996).
2.6. Encapsulation efficiency (EE %)
EE can be spectrophotometrically estimated through ferric reducing ability of plasma (FRAP) assay method as discussed (Benzie and Strain, 1996).
The equation was applied as:
| TCME—SCME |
| EE percentage (%) = ______________ × 100 | (1) |
| TCME |
Where TO is the total CME content and SCME is the surface CME from capsules content.
2.7. Assessment of thermal stability
The thermal stability of CMF in the form of crude extract, micro- and nanocapsulation was conducted by applying differential scanning calorimeter (DSC) according to (Hazra et al., 2004).
2.8. Cell culture and cytotoxicity assay
The targeted cell lines (human hepatocellular carcinoma (HepG2) and normal liver (THLE2) were maintained in standard conditions as reported by viability of the cells was determined by Sulforhodamine B (SRB) assay (Musa et al., 2016).
2.9. Morphology of encapsulated CMF examined by Transmission electron microscope (TEM)
The surface morphology and shape of the CMF encapsulated extracts (MCMF and NCMF) was examined by Transmission electron microscope (TEM).
2.10. Differential scanning calorimeter (DSC) of CMF
The thermal stability for CMF extract and the CMF encapsulated extracts (micro- and nano- encapsules) was determined using a differential scanning calorimeter (DSC).
3. Results
3.1. Polyphenols and flavonoids of CMF- extracts
The bioactive polyphenolic and flavonoid phytochemicals affecting the plant characteristics (morphology, growth, reproduction and environmental stress resistance) and demonstrate wide range of biological activities as powerful antioxidants and anticancer (Kim et al., 2006). Total phenolics and flavonoids contents of CMF extracts (noncapsulated, micro- and nanocapsulated) were quantified by the external standard method using Folin Ciocalteu reagent in terms of gallic acid equivalent (GAE) and quercetin equivalent (QE), respectively. The total phenolic contents of CMF extract (free, micro- and nanocapsulated) were calculated as 98.84 ± 0.50, 62.37 ± 0.18 and 68.4 ± 0.67 μg GAE/g extract, respectively. The total flavonoid contents of tested extracts were 22.4 ± 0.17, 37.2 ± 0.43 and 21.09 ± 0.12 μg quercetin/g tested extract, respectively. Ripe Indian Cordia myxa fruit pulp (CMR) had higher phenolic (2.8 mg GAE g−1) than CM green fruit pulp (1.26 mg GAE g−1). Yet, flavonoids content in green fruit pulp (28.3 mg QE g−1) in ripe fruits (24.17 mg QE g−1) (Murthy et al., 2019). Polymerization and/or areal phenolic material oxidation during drying process produces a significant variation in phenolic content of seven Kuwaiti C. myxa fruits and leaves extracts (5.50 + 0.17–11.1 + 1.47 mg/g gallic acid equivalent) (Afzal et al., 2009). Egyptian Cordia dichotoma fruits pulp extract contains 112.71 ± 8.40 mg gallic acid/g, 69.76 ± 4.18 mg quercetin/g and 25.65 ± 1.80 mg catechin/g (Ibrahim et al., 2019). Such discrepancy in the phenolic flavonoid contents and could ascribed to species/ genotypes specificity. Fifteen phenolic compounds were identified by comparing their retention indices to those of authentic standards analyzed at the same conditions (Table 1). The results pointed that CMF extracts (noncapsulated, micro- and nanocapsulted) contained significant concentrations of gentisic acid (551.75, 239.50 and 333.09 μg/ ml, respectively), gallic acid (283.36, 16.44 and 16.29 μg/ ml, respectively), rosmarinic acid (143.92 μg/ ml) and chrysin (542.40, 147.32 and 4.03 μg/ ml, respectively). Catechin (10.60, 16.81 and 7.61 μg/ ml, respectively), chlorogenic acid (3.96, 3.92 and 1.85 μg/ ml, respectively), caffeic acid (6.68, 9.94 and 1.55 μg/ ml, respectively), syringic acid (65.40 μg/ ml), vanillic acid (3.45 μg/ ml), p-coumaric acid (4.78, 10.10 and 25.88 μg/ ml, respectively), rutin (3.31 μg/ ml), apigenin-7-glucoside (9.88 μg/ ml), quercetin (46.12 μg/ ml) and kaempferol (1.93 μg/ ml) were also present. TLC chromatography and HPLC analyses of five Cordia species revealed the presence of sugars, alkaloid, flavonoids (rutin) and two phenolic derivatives as chlorgenic and caffeic acids (Tiwari et al., 1967).
Table 1.
Phenolic compounds in CMF.
| Compound |
CMF extract |
Microcapsulated MCMF extract |
Nanocapsulated NCMF |
Structures |
|---|---|---|---|---|
| Concentration (mg/gm extract) | ||||
| Gallic acid | 283.36 | 16.44 | 16.29 | 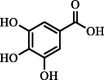 |
| Gentisic acid | 551.75 | 239.50 | 333.09 | 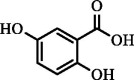 |
| Catechin | 10.60 | 16.81 | 7.61 | 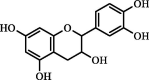 |
| Chlorogenic acid | 3.96 | 3.92 | 1.85 | 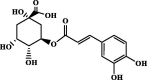 |
| Caffeic acid | 6.68 | 9.94 | 1.55 | 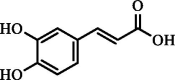 |
| Syringic acid | 65.40 | ND | 2.50 | 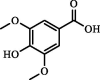 |
| Vanillic acid | 3.45 | ND | ND | 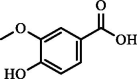 |
| p-Coumaric | 4.78 | 10.10 | 25.88 | 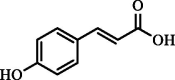 |
| Rutin | 3.31 | ND | ND | |
| Apigenin-7-glucoside | 9.88 | ND | ND | 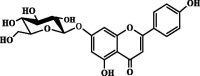 |
| Rosmarinic acid | 143.92 | ND | ND | 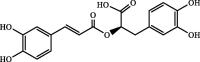 |
| Quercetin | 46.12 | ND | ND | 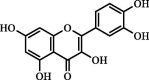 |
| Kaempferol | 1.93 | ND | ND | 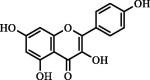 |
| Chrysin | 542.40 | 147.32 | 4.03 | 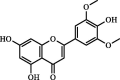 |
| Total | 1677.53 | 444.03 | 57.22 | |
3.2. CMF encapsulated particles morphology
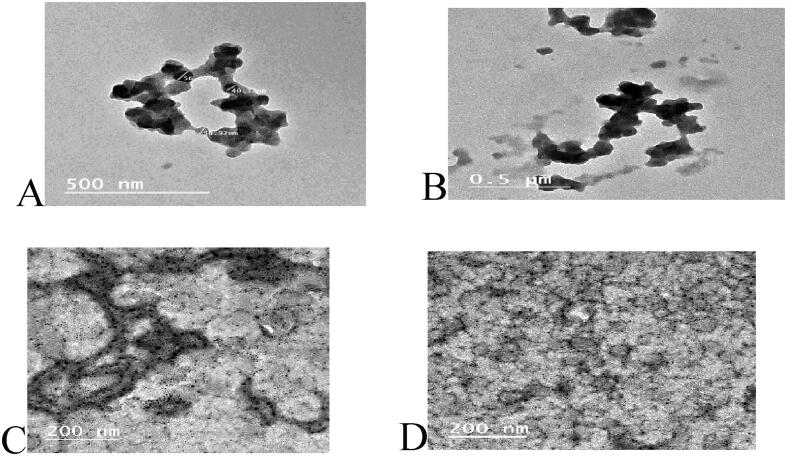
Abundant drawbacks for natural polyphenolics regarding stability, light and heat sensitivity, lower solubility, astringency, and bitter taste limiting their usage in food and oral medications. To overcome these drawbacks, encapsulation procedure is a favorable approach. The surface morphology and shape of the CMF encapsulated extracts (micro- and nanoencapsules) was examined by Transmission electron microscope (TEM). As revealed in Fig. 1, Fig. 2, Fig. 3, Fig. 4, remarkable changes in the CMF particle size and morphology were evidenced by comparing both encapsulation procedures. The nanoemulsion form gives the actual size and shape, with appearance of dark droplets. The TEM micrograph confirmed the spherical shape of the nanoparticles with average diameter 45.10 ± 0.22 nm.
Fig. 1.
The surface morphology and shape of the CMF encapsulated extracts (micro- and nanoencapsules) examined by TEM.
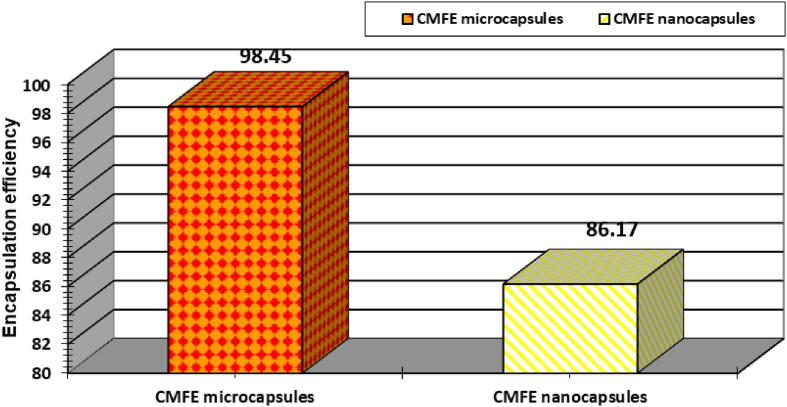
Fig. 2.
Encapsulation Efficiency (%) of micro- and nanocapsulated CMFE forms.
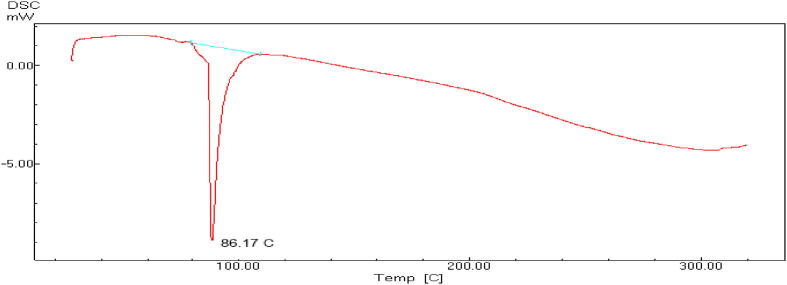
Fig. 3.
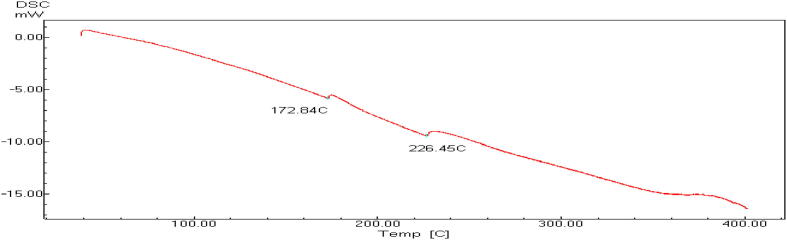
Thermogram obtained by DSC for CMF uncapsulated extract.
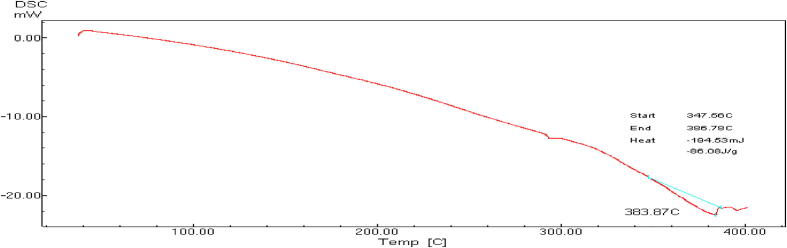
Fig. 4.
Thermogram obtained by DSC for CMF microcapsulated extract.
3.3. % of encapsulation efficiency
Fig. 2 showed that the % encapsulation efficiency value of the CMF microcapsulated extract is (98.45%) and higher than the nanocapsulated form (86.17%).
3.4. Thermal and storage stability of CMF extract and its capsulated forms
Owing to the promising medicinal value and health benefits of C. myxa fruit extract, it can be added and incorporated in functional foods as it possesses potent antioxidant properties. Hydrophobicity and limited incorporation of food matrices can be overcome by encapsulation for protection and merging in lipophilic foods matrices. The enthalpy values of the apparent melt are subtracted from the DSC of CMF non-capsulated and encapsulated forms of the extract (NCMF and MCMF) in sodium alginate gel and beads shown in Fig. 3, Fig. 4, Fig. 5, respectively. Fig. 3 shows that melting point of CMF extract is 86.17 °C indicating the inability to withstand the high temperatures applied during some food processing. In addition, CMF nanocapsules showed lower melting points; 172.84 and 226.45 °C in comparison to the microcapsules but still higher than the non-capsulated C. Myxa fruits extract. With reference to the CMF micro- and nanoparticles DSC results, the non-existence of the CMF extract melting endotherm peak at 86.17 °C produce some evidence of the CMF extract efficient incorporation in micro- and nanoparticles as melting points tends to shift or dissolve once during the bioactive C. myxa extract incorporation in a polymer-based system. On the other hand, the micro- capsulated CMF extract melting point showed in Fig. 4 was 383.87 °C demonstrating the capability of expending alginate - packaging material to shield the vital active compounds of C. Myxa fruit extract allowing it in that form to be applied in different targeted delivery products especially that disclosed to high thermal treatments. DSC analysis was used to confirm the efficient CMF extract incorporation in micro- and nanosystems indirectly by comparing the thermal stabilities of the loaded encapsulated extract forms with the non-capsulated forms (Hill et al., 2013). Furthermore, the DSC thermograms analyses permit illustrating the bioactive compound physicochemical status that articulated into microcapsules (Zhang and Gao, 2007). Alginates are a group of polysaccharides obtained from brown algae and have been carefully investigated.
Fig. 5.
Thermogram obtained by DSC for CMF nanocapsulated extract.
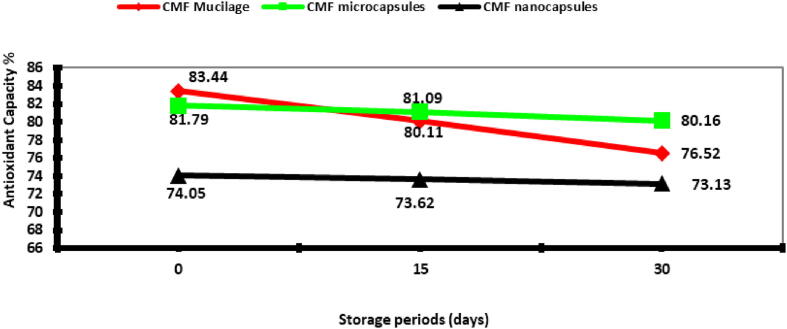
The storage stability at refrigerator temperature (4 °C) achieved by the determination of antioxidant capacity (FRAP) loss during storage for 30 days. Loss of antioxidant capacity during storage was expressed as the fraction of FRAP lost relative to CMF extract formulas before the storage. The result of the storage stability presented in the Fig. 6. It was observed that the nanocapsultion of CMF extract was the most effective for protecting the antioxidant properties of the tested extract after 30 days of storage (1.24% loss) compared to non-encapsulated CMF mucilage extract and microcapsulted CMF extract (8% and 10%, respectively) Figs. 6 and 7.
Fig. 6.
Storage stability of CMF extract forms during storage at four °C.
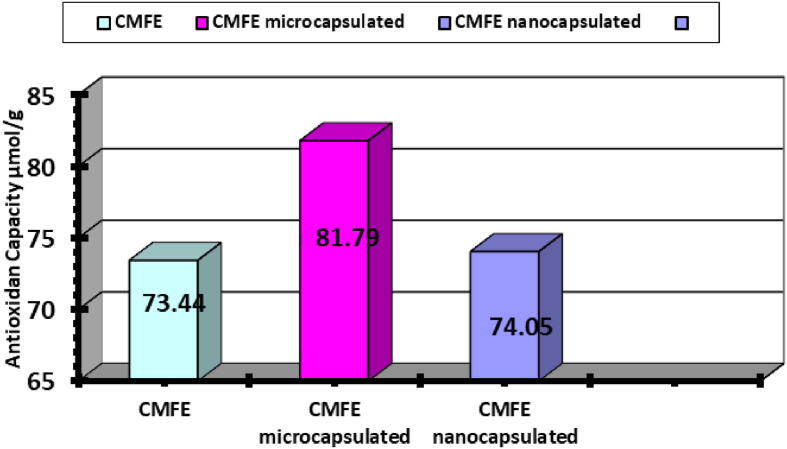
Fig. 7.
FRAP of CMF (non-, micro- and nanocapsulated) extracts.
3.5. Antioxidant activity
In aerobic environment, antioxidants are crucial for the living being by inhibiting or delaying oxidative progression chain reactions induced by free radicals. They are affecting the lipid cell membranes and some enzymes containing SH, so they are eventually toxic and associated to some neuro- generative disorders (Sairam et al., 2000). However, two assays should be employed to evaluate antioxidant activity as no single one could reflect the action mechanism of all antioxidants in a complex system, due to various oxidative processes. A significant correlation found between CMF phytonutrient and biological potencies of tested extracts. DPPH assay method was used to evaluate the total antioxidant potential. CMF extracts antioxidant activities found to increase in a dose dependent manner. DPPH radical scavenging inhibition values found to be 90.93, 78.23 and 82.13% for non-capsulated, MCMF and NCMF extracts at 300 μL, respectively. The IC50 of FRAP was 81.79 µmol/g in microcapsulated CMF extract followed by nano- and noncapsulated extracts (74.05 and 73.44 µmol/g, respectively) (Sairam et al., 2000).
3.6. Cytotoxicity effect of CMF extracts
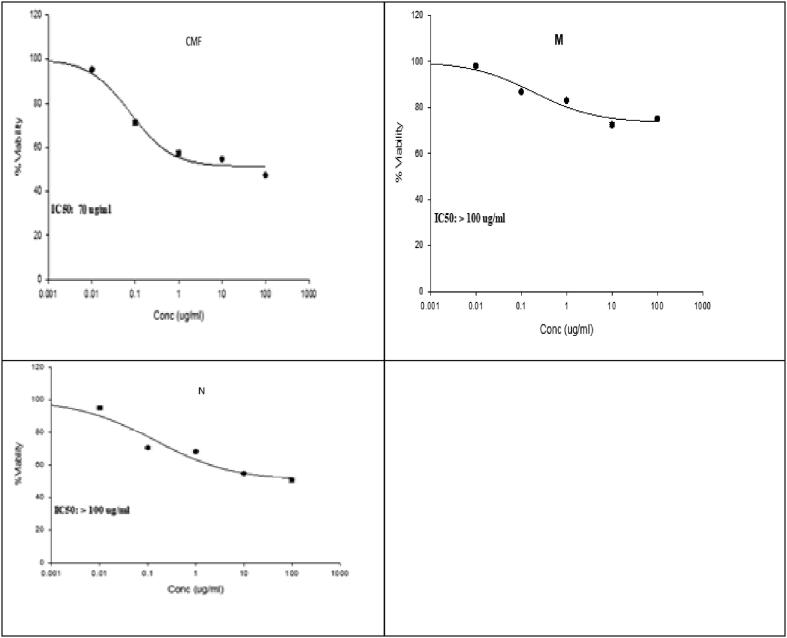
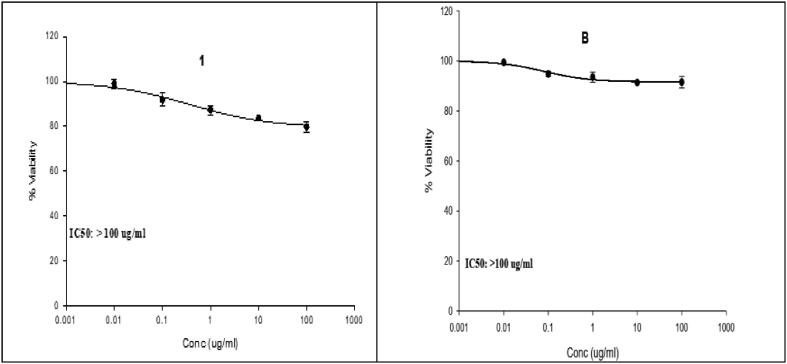
SRB assay was employed to evaluate in vitro cytotoxic activity of CMF extract and its micro- and nanocapsulated forms on THLE2 and HepG-2 cell lines in comparison to Cisplatin (CIS) as a reference drug (Fig. 8, Fig. 9). The CMF extract showed cytotoxicity effect (IC50: 70 μg/mL) in comparison to its micro- and nanocapsules (IC50 ˃ 100 μg/mL). The reduction in viability of hepatocellular carcinoma cells (HepG-2) with CMF extract in comparison with the treated normal cells (THLE2) indicated the selectivity of CM fruit extract against only cancerous cells. In general, the CMF extract exhibited a lower growth inhibitory activity against the Hep G-2 cell line than the reference drug, CIS (IC50: 20.71 μg/mL). Meanwhile, micro- and nanocapsules showed a very low cytotoxicity in comparison to the reference drug. According to the established cytotoxicity scale for plant metabolites, the investigated extract showed a low cytotoxicity (20 μg/mL < IC50 < 100 μg/mL) while micro- and nanocapsules has low or no cytotoxicity against cancer cell lines (IC50 ˃ 100 μg/mL) (Kuete and Efferth, 2015). No cytotoxicity was recorded in normal cell lines (IC50 greater than 100 μg/mL) for CMF extract and micro- and nanocapsulated according to the same scale. In several cancer cell lines studies, chrysin and apigenin revealed anti-inflammatory and radical scavenging properties and tumor cell invasion inhibition, metastasis, and mitogen- activated protein kinases (MAPK) and their downstream oncogenes (Rupasinghe et al., 2014). No mortality up to a dose level of 2500 mg/Kg of C. myxa L. indicating the safety of plant and this was in accordance with published data which reported that both the leaves and the fruits are safe and edible in India and other countries (Abdel-Aleem et al., 2019, Gupta and Gupta, 2017). HPLC analysis explored the differences between phenolic and flavonoid compounds in the CMF extract and its capsulated forms. However, the recorded quantitative differences could be regarded as the main factor affecting the variation in the cytotoxic activity among all CMF extract forms. Most of the studies that concerned with extracts and their forms of encapsulation has focused only on their stability and biological activities, neglecting changes in the other aspects such as ingredients of the encapsulated forms. Based on energy-intensive techniques, scarce studies have reported little variations in biological activities and physical stability of the produced formulations as a result of Ostwald ripening or flocculation of the emulsions (Baldan, 2002). Although high shear homogenization may lead to disintegration and/or accumulations of the essential oil ingredients, nothing has been reported regarding the influence of encapsulation techniques on bioactive compounds.
Fig. 8.
Cell viability percentage (posttreatment) of Hep G2: (A) CMF extract, (M) microcapsulated CMF extract, and (N) nanocapsulated CMF extract Cisplatin using SRB assay.
Fig. 9.
Cell viability percentage (posttreatment) of THLE2: (A) CMF extract, (M) microcapsulated CMF extract, and (B) nanocapsulated CMF extract Cisplatin using SRB assay.
4. Discussion
4.1. Polyphenols and flavonoids of CMF-extracts
Flavonoids play a prime role in antioxidant activity depending on their molecular structure and hydroxylation pattern. Obtained results revealed a considerable variation and higher phenolic concentration in all CMF extracts. This is due to variation in the solvent strength associated with their polarities. The presence of chrysin and apigenin supported the medicinal value of the CMF extract as they are potent antioxidants and can protect the cells against oxidative damage (Siddiqui et al., 2018, German-Ponciano et al., 2018). Additionally, both chrysin and apigenin possess anxiolytic effect on the benzodiazepine (BDZ) receptor with no sedative effect or muscle relaxation (Wolfman et al., 1994). Traditionally, Cordia myxa L. used for various neuro disorders treatment. The methanol extract exhibited considerable anti-anxiety activity (200 mg/kg) with respect to standard drug (Diazepam) (Mortazavi).
4.2. CMF encapsulated particles morphology
Obviously, the detected nanoparticles average diameter was higher than that informed for comparable other tested extracts under the same conditions and this could be related to the Ostwald ripening phenomenon where a mild solubility of the tested CMF extracts phase exhibited in the surrounding aqueous phase of the emulsion system transferring from small to large droplets (Tadros et al., 2004).
4.3. % of encapsulation efficiency
Depending upon the findings, microcapsulation form is more reliable to maintain the inner CMF contents more than nanocapsulation packaging method. However, the nanocapsulated CMF extract is more widespread and distributed inside the sodium alginate capsule. Countless issues can affect the encapsulation efficiency as technique type, properties of coating materials and active compounds, interactions among these compounds, etc. (Jyothi et al., 2010).
4.4. Thermal and storage stability of CMF extract and its capsulated forms
Regrettably, bioactive compounds as polyphenols have an inadequate long-term stability, as they are altered numerous factors (oxygen, temperature, light, pH variation, metals exitance, and enzymes). Additionally, low water solubility leads to deficient bioavailability. The promising encapsulation allow to enhance the products stability and bioavailability managing the level of active agent release extending the shelf-life of encapsulated compounds can be considerably extended (Bakowska-Barczak and Kolodziejczyk, 2011). Yet, the CMF thermal properties were hardly ever analyzed using thermal analysis techniques in which the relationships between the food sample properties and sample heating or cooling temperature. Obtained DSC curves can show endo- and exo-thermic peaks where higher temperature of peaks can be indicating the higher thermal stability of tested materials. As reported for the ascorbic acid thermo-oxidative stability by gum Arabic and sodium alginate polymers up to 188 °C that is higher than the traditional processing temperature used in the fish feed industry (Barra et al., 2019). The nano capsule has improved the rosemary essential oil thermal stability and can also be preserve the antioxidant capacity especially in the industry of thermal treatment various applications (Dehghan et al., 2020). Bovine serum albumin enhanced by gum Arabic- alginate globules were efficient to transport to the small intestine and could be good potential protein drugs delivery vehicle (Mohamed et al., 2016, Ezekiel et al., 2020). Gum cordia as a polymer was effectively utilized for the jelly formulation for improving techno-functional properties of Apple jelly (Hasani and Yazdanpanah, 2020). Obviously, that thermal and storage stability were increased by encapsulation as compared with our reported results.
4.5. Antioxidant activity
The present study discloses that the correlation of DPPH results with level of flavonoid contents and total phenolics. The FRAP method has a reputation for reducing the ferric-triridyl triazine to its colored ferrous reduced form in the presence of antioxidants indicating that antioxidants are electron donors that reduce the oxidative moieties of the lipid peroxidation process. CMF extracts were found to be effective in scavenging the FRAP radical.
4.6. Cytotoxicity effect of CMF extracts
Plant polyphenols are positively affecting the human health properties due to their anti-inflammatory, antioxidant and antitumor properties, which intensely depend on their structure bioaccessibility and subsequently bioavailable form that internally reach the organism, either incorporated with food matrix or as purified isolates. Both bioaccessibility and bioavailability are positively affected by encapsulation that ensures the active ingredient coating and consequently reaching the targeted delivery at its specific site with a controlled release.
5. Conclusion
Resembling utmost natural compounds, applying Cordia myxa fruit (CMF) in food or pharmaceutical industries could have some restrictions due to its aroma, flavor, poor solubility in hydrophilic media, and oxidation liability. Encapsulation looks to be an attractive unique approach avoiding these limitations. The micro- and nanoemulsion of CMF extract effect on the chemical characteristics, antioxidant activity and anticancer potential was inspected as a delivering system incorporating plant bioactive compounds in foods and drugs for cancer treatment. Encapsulation using high-pressure homogenization techniques intensely damage the chemical constituents and thermal stabilities of delivery systems in association with the encapsulated CMF extracts and therefore the biological activities. Supporting studies are necessary to explicate the chemical constituent's behavior through encapsulation procedures and thereby assess the diverse encapsulation techniques compatibility for regular naturally occurring flavoring additives.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Appreciation from authors to the Deanship of Scientific Research at Jouf University, Sakaka, Saudi Arabia for finance this work through research grant No. 772/39, and to, and to Taif University Researchers Supporting Project number (TURSP-2020/56), Taif University, Taif, Saudi Arabia. The authors also would like to thank AlMaarefa University for support.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.05.064.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abdel-Aleem E.R., Attia E.Z., Farag F.F., Samy M.N., Desoukey S.Y. Total phenolic and flavonoid contents and antioxidant, anti-inflammatory, analgesic, antipyretic and antidiabetic activities of Cordia myxa L. leaves. Clin. Phytosci. 2019;5:1–9. [Google Scholar]
- Abdelgawad M.A., Mohamed A.M., Musa A., Mostafa E.M., Awad H.M. Synthesis, chromatographic separation and antimicrobial evolution of new azoquinoline-8-ol. J. Pharm. Sci. Res. 2018;10:1314–1318. [Google Scholar]
- Afzal M., Obuekwe C., Khan A., Barakat H. Influence of Cordia myxa on chemically induced oxidative stress. Nutrit. Food Sci. 2009 [Google Scholar]
- Ali W.R., Al-Asady Z., Ibrahim A. Immunomodulatory of Cordia myxa (L.) aqueous extract fruit in immunized mice with hydatid cyst fluid. J. Nat. Sci. Res. 2015;5:75–83. [Google Scholar]
- Amiri S., Saray F.R., Rezazad-Bari L., Pirsa S. Optimization of extraction and characterization of physicochemical, structural, thermal, and antioxidant properties of mucilage from Hollyhock’s root: a functional heteropolysaccharide. J. Food Meas. Charact. 2021:1–15. [Google Scholar]
- Ansari, A.P., Zaheer Ahmed, N., Rather, S.A., Rafeeqi, T.A., Beigh, B.S., 2020. Immune boosting and anti-influenza effects of an Unani decoction in influenza like illness and COVID-19 like epidemics: a rationale approach. Int. J. Res. Med. Sci. 8, 4544.
- Bakowska-Barczak A.M., Kolodziejczyk P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011;34:1301–1309. [Google Scholar]
- Baldan, A., 2002. Review progress in Ostwald ripening theories and their applications to nickel-base superalloys Part I: Ostwald ripening theories. J. Mater. Sci. 37, 2171-2202.
- Barra P.A., Márquez K., Gil-Castell O., Mujica J., Ribes-Greus A., Faccini M. Spray-drying performance and thermal stability of L-ascorbic acid microencapsulated with sodium alginate and gum Arabic. Molecules. 2019;24:2872. doi: 10.3390/molecules24162872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Dehghan, B., Esmaeilzadeh Kenari, R., Raftani Amiri, Z., 2020. Nano‐encapsulation of orange peel essential oil in native gums (Lepidium sativum and Lepidium perfoliatum): Improving oxidative stability of soybean oil. J. Food Process. Preservat. 44, e14889.
- Deogade U.M., Deshmukh V.N., Sakarkar D.M. Natural gums and mucilage's in NDDS: applications and recent approaches. Int. J. PharmTech Res. 2012;4:799–814. [Google Scholar]
- Erdmann M.E., Lautenschlaeger R., Schmidt H., Zeeb B., Gibis M., Brüggemann D.A., Weiss J. Influence of droplet size on the antioxidant efficacy of oil-in-water emulsions loaded with rosemary in raw fermented sausages. Eur. Food Res. Technol. 2017;243:1415–1427. [Google Scholar]
- Ezekiel O.O., Ojuola O.F., Adedeji O.E. Stability of encapsulated Lactobacillus rhamnosus GG in cocoa (Theobroma cacao L.) juice. Acta Periodica Technol. 2020:61–75. [Google Scholar]
- Ferreira C.D., Nunes I.L. Oil nanoencapsulation: development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019;14:1–13. doi: 10.1186/s11671-018-2829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German-Ponciano, L. J., Rosas-Sánchez, G. U., Rivadeneyra-Domínguez, E., Rodríguez-Landa, J. F., 2018. Advances in the preclinical study of some flavonoids as potential antidepressant agents. Scientifica, 2018. [DOI] [PMC free article] [PubMed]
- Ghoneim M.M., Musa A., El-Hela A.A., Elokely K.M. Evaluation and understanding the molecular basis of the antimethicillin-resistant Staphylococcus aureus activity of secondary metabolites isolated from Lamium amplexicaule. Pharm. Magazine. 2018;14:3. [Google Scholar]
- Gupta R., Gupta G.D. Toxicity assessment and evaluation of analgesic, antipyretic and anti-inflammatory activities on Cordia obliqua leaf methanol extract. Pharm. J. 2017;9 [Google Scholar]
- Hasani, M., Yazdanpanah, S., 2020. The Effects of Gum Cordia on the Physicochemical, Textural, Rheological, Microstructural, and Sensorial Properties of Apple Jelly. J. Food Quality, 2020.
- Hazra A., Alexander K., Dollimore D., Riga A. Characterization of some essential oils and their key components: thermoanalytical techniques. J. Therm. Anal. Calorim. 2004;75:317–330. [Google Scholar]
- Hill L.E., Gomes C., Taylor T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT-Food Sci. Technol. 2013;51:86–93. [Google Scholar]
- Ibrahim A.Y., El-Newary S.A., Ibrahim G.E. Antioxidant, cytotoxicity and anti-tumor activity of Cordia dichotoma fruits accompanied with its volatile and sugar composition. Ann. Agric. Sci. 2019;64:29–37. [Google Scholar]
- Jafari S.M., Assadpoor E., Bhandari B., He Y. Nano-particle encapsulation of fish oil by spray drying. Food Res. Int. 2008;41:172–183. [Google Scholar]
- Jyothi N.V.N., Prasanna P.M., Sakarkar S.N., Prabha K.S., Ramaiah P.S., Srawan G. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010;27:187–197. doi: 10.3109/02652040903131301. [DOI] [PubMed] [Google Scholar]
- Kim K.-H., Tsao R., Yang R., Cui S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–473. [Google Scholar]
- Kuete, V., Efferth, T., 2015. African flora has the potential to fight multidrug resistance of cancer. BioMed. Res. Int., 2015. [DOI] [PMC free article] [PubMed]
- Malik A., Ahmad A.R. Determination of phenolic and flavonoid contents of ethanolic extract of Kanunang leaves (Cordia myxa L.) Int. J. PharmTech Res. 2015;7:243–246. [Google Scholar]
- Mohamed H., Mustafa S., Fitrianto A., Manap Y.A. Development of alginate–gum arabic beads for targeted delivery of protein. SMU Med. J. 2016;3:486–508. [Google Scholar]
- Mortazavi, S.A., Antidepressant Effects of a Reformulated Traditional Tablet. Iranian Red Crescent Med. J., 22.
- Murthy H.N., Joseph K.S., Gaonkar A.A., Payamalle S. Evaluation of chemical composition and antioxidant activity of Cordia myxa fruit pulp. J. Herbs Spices Med Plants. 2019;25:192–201. [Google Scholar]
- Musa, A. Phytochemistry, Pharmacological Potency, and Potential Toxicity of Myoporum spp.
- Musa, A., Al-Muaikel, N., Abdel-Bakky, M., 2016. Phytochemical and pharmacological evaluations of ethanolic extract of. Bassia eriophora, Der Pharma. Chem., 8, 169-178.
- Musa A., Mostafa E.M., Al-Sanea M.M., Ahmed S.R., Mostafa-Hedeab G., Abdelgawad M.A. Insights studies for certain natural fda approved polyphenolics and repurposing for COVID-19. International Journal of Research. Pharm. Sci. 2020;11 [Google Scholar]
- Oza M.J., Kulkarni Y.A. Traditional uses, phytochemistry and pharmacology of the medicinal species of the genus Cordia (Boraginaceae) J. Pharm. Pharmacol. 2017;69:755–789. doi: 10.1111/jphp.12715. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Hussain A. Phytochemical and analytical evaluation of Cordia dichotoma Linn. leaves. Pharm. J. 2015;7 [Google Scholar]
- Rupasinghe H.V., Nair S.V., Robinson R.A. Chemopreventive properties of fruit phenolic compounds and their possible mode of actions. Stud. Nat. Prod. Chem. 2014;42:229–266. [Google Scholar]
- Sairam R., Srivastava G., Saxena D. Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000;43:245–251. [Google Scholar]
- Siddiqui, A., Badruddeen, Akhtar, J., Uddin Ms, S., Khan, M. I., Khalid, M., Ahmad, M., 2018. A naturally occurring flavone (Chrysin): chemistry, occurrence, pharmacokinetic, toxicity, molecular targets and medicinal properties. J. Biol. Active Prod. Nature 8, 208-227.
- Sun X., Cameron R.G., Manthey J.A., Hunter W.B., Bai J. Microencapsulation of Tangeretin in a Citrus Pectin Mixture Matrix. Foods. 2020;9:1200. doi: 10.3390/foods9091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros T., Izquierdo P., Esquena J., Solans C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004;108:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Tiwari R., Srivastava K., Shukla S., Bajpai R. Chemical examination of the fixed oil from the seeds of Cordia myxa. Planta Med. 1967;15:240–244. doi: 10.1055/s-0028-1099978. [DOI] [PubMed] [Google Scholar]
- Watkins R., Wu L., Zhang C., Davis R.M., Xu B. Natural product-based nanomedicine: recent advances and issues. Int. J. Nanomed. 2015;10:6055. doi: 10.2147/IJN.S92162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman C., Viola H., Paladini A., Dajas F., Medina J.H. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol. Biochem. Behav. 1994;47:1–4. doi: 10.1016/0091-3057(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Zhang H., Gao S. Temozolomide/PLGA microparticles and antitumor activity against glioma C6 cancer cells in vitro. Int. J. Pharm. 2007;329:122–128. doi: 10.1016/j.ijpharm.2006.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.