Abstract
The current research was intended to evaluate the impact of 6-shogaol in rodent model of ischemic-reperfusion induced- brain injury and also assessed 6-shogaol enhanced sevoflurane's neuroprotective effects. Ischemic-Reperfusion (I/R) injury was induced by middle cerebral artery occlusion (MCAO) method in Sprague-Dawley rats. A separate group of animal was exposed to sevoflurane (2.5%) post-conditioning for 1 h immediately after reperfusion. The 6-shogaol (25 mg or 50 mg/kg body weight) was orally administered to treatment group rats for 14 days and then subjected to I/R. The 6-shogaol treatment along with/without sevoflurane post-conditioning reduced the number of apoptotic cell counts, brain edema and cerebral infarct volume. The western blotting analysis revealed a significant stimulation of the PI3K/Akt/mTOR signal pathway. RT-PCR and western blotting studies revealed improved expressions of HIF-1α and HO-1 at both gene level and protein levels. I/R induced neurological deficits were also alleviated on sevoflurane post-conditioning with/without 6-shogaol treatment. The present findings revealed that pre-treatment with 6-shogoal enhanced the neuroprotective properties of sevoflurane post-conditioning, illustrated the efficacy of the compound against I/R injury.
Keywords: 6-shogaol, Hypoxia-inducible factor 1α, Hemeoxygenase 1, Ischemia, protein kinase B, Sevoflurane
1. Introduction
Ischemia/reperfusion (I/R) induced cerebral injury is a common pathological occurrence associated with stroke and various neurological and cardiovascular procedures as intracranial aneurysm clamping, aortic arch replacement etc (Bedirli et al., 2012). I/R induced brain damage is associated with multiple mechanisms as oxidative stress, neuroapoptosis, inflammatory responses and excitotoxicity (Liang et al., 2014). I/R-induced brain injury critically interrupts the success rates of surgical interventions, the prognosis of the disease and survival rate of patients. This necessitates the development of efficient approaches that could effectively reduce or prevent ischemic injury (Kim et al., 2009).
Investigations revealed that volatile anaesthetics like isoflurane and sevoflurane possess neuroprotective effects (Lee et al., 2008). Sevoflurane is a fast-acting extensively employed for general anaesthesia. It has been reported to possess negligible effects on cerebral metabolism and intracranial pressure. These factors contribute to the use of sevoflurane in neurosurgery (Kim et al., 2017). Further, experimental data revealed that sevoflurane post-conditioning following I/R improved neurological function and also reduced inflammation (Ye et al., 2012). Ye et al. (2012) suggested neuroprotective properties of sevoflurane post-conditioning following neonatal hypoxia–ischemia (HI) through the phosphatidylinositide 3-kinase (PI3K)/AKT signalling.
PI3K/Akt signalling is one of the major pro-survival signaling pathways that also regulate various processes, including inflammatory reactions (Zhao et al., 2006). Numerous research data has revealed that post-ischemic inflammatory reactions assist in the progression of neuronal injury and cerebral infarction (Cuartero et al., 2013). Initiation of PI3K/Akt signaling has been demonstrated to reduce brain injury in ischemic stroke models (Wang et al., 2009). Mammalian target of rapamycin (mTOR), a major effector protein of Akt, comprises the protein complexes - mTORC1 and mTORC2 (Zhang et al., 2015). mTORc1 phosphorylates ribosomal protein S6 kinases (s6K) activates Akt and eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1). The event subsequently leads to the discharge of eukaryotic translation initiation factor 4E (eIF4E) that results in cell cycle progression. mTORc2, via a positive feedback loop, stimulates Akt activation, subsequently inducing cell proliferation and survival. Activation of Akt/mTOR signal increases the survival of neurons and also induces activation of hypoxia-inducible factor-1α (HIF-1α) (Zhang et al., 2009).
HIF-1α is a significant oxygen level sensor in the cells that is involved critically in the physiological process as cell survival, cell metabolism and metabolic adaptation (Semenza, 2000). HIF-1α regulates various pivotal genes, including vascular endothelial growth factor (VEGF) and heme oxygenase-1 (HO-1) (Lee et al., 1997). Increased expressions of HIF-1α and HO-1 were reported to involve in neuroprotection (Ye et al., 2012). Compounds that could reduce inflammation, and that could potentially activate PI3K/Akt signaling and up-regulate HIF-1α and HO-1 could be of immense clinical value. 6-shogaol is a one of the major phytochemicals found in the dried rhizomes of ginger (Ali et al., 2008). Studies have explored numerous pharmacological activities of 6-shogaol including antioxidant, anticancer, neuroprotective. and anti-inflammatory effects (Han et al., 2017). The present work aimed to evaluate the neuroprotective effects of 6-shogaol in I/R injury and to analyze the effects of sevoflurane post-conditioning following I/R injury.
2. Materials and methods
2.1. Animals and cerebral I/R induction
The handling of animals, study design, grouping, methods and procedures for investigation were permitted by the University Ethical Committee and conducted in agreement with the guidelines of the National Institutes of Health on the care and use of animals (Garber, 2011).
From the institution’s animal breeding center, adult Sprague-Dawley rats (male; n = 96; 280–300 g) were procured. The rats (n = 3/cage) were maintained under 12 h day/12 h night cycle and controlled laboratory conditions (22–23 °C, relative humidity 55–60%). The animals were allowed freely to access diet and water. Prior initiation of the experiment, the animals were adapted for about 5 days to the laboratory environments.
The animals were assigned randomly to experimental groups (8 groups; n = 12). 6-shogaol at 25 mg or 50 mg/kg body weight was given orally to the experimental rats for 14 days prior to ischemia/reperfusion initiation. 6-shogaol was administered, 60 min before I/R treatment on day 15 (the day of induction).
On the day before treatment, the rats were subjected to 8 to 10 h fasting; however, these were permitted access to water. For I/R induction the animals were anaesthetized (25 mg/kg, zoletil) and subjected to cerebral infarction and I/R by middle cerebral artery occlusion (MCAO) method (Leonardo et al., 2010).
The left common carotid artery (CCA) was exposed via a ventral midline neck incision and the internal carotid artery (ICA) was opened, and its extra cranial branch was tied near to its origin. A 3–0 nylon monofilament suture was introduced through the external carotid artery (ECA) into the ICA to prevent blood flow into the middle cerebral artery (MCA). The nylon filament was retained in position for 120 min for induction of ischemia. For restoration of ICA-MCA blood flow, sutures were removed and for 24 h reperfusion was allowed.
Following I/R induction, the experimental animal was maintained at 37 °C heating blanket and monitored using a thermometer. It was maintained at this temperature until the animals retrieved from the surgery
2.2. Study groups
In Group I, rats were not treated to I/R and administered normal saline orally. In Group 2, rats that were administered with saline and subjected to I/R and were marked as I/R control. A separate group of rats (Group 3–4) orally administered with 6-shogaol (25 mg or 50 mg/kg body weight) were subjected to I/R. 6-shogaol (50 mg/ kg) alone treated rats not induced with I/R served as group 5. Sevoflurane post-conditioning groups (Groups 6–8) were induced with I/R and were then immediately exposed to sevoflurane (2.5% in oxygen) through a vaporizer (Sevorane Vapor 15.3, Abbott) for 1 h following reperfusion. The supply of sevoflurane was maintained at the desired concentration and monitored (Gas analyzer; Datex‐Oheda, Helsinki, Finland). Group 6- Rats were induced I/R and subjected to sevoflurane post-conditioning alone. After 24 h of reperfusion, rats from each treatment group (n = 6) were sacrificed by transcardial perfusion of saline and paraformaldehyde (4%) in 0.1 M phosphate buffer (ice-cold). Instantly, brain tissues were excised and stored at −80 °C used for analysis.
2.3. Neurobehavioral deficit evaluation
Neurobehavioral deficits were assessed in rats at 24 h post reperfusion and prior sacrifice. The extent of the neurological deficit was graded based on parameters as - dynamic behavior, the symmetry of the forelimbs, balance of movements, climbing, touch response, and response to vibrissae touch. The behaviour of animals was graded on a scale between 0 and 4 as follows: 0- normal (no deficit); 1- mild deficits; 2- moderate deficit; 3-severe deficit; and 4, very severe deficit.
2.4. Brain water content
The sectioning of excised brain tissue at 2 mm intervals in the coronal plane was carefully executed, and the wet weight of the tissue sections was performed. The dry weight of the brain slices were determined by weighing the tissue slices after drying in a hot air oven at 70 °C for 72 h.
2.5. TTC staining
Tissue viability and infarct size were measured by 2,3,5-triphenyltetrazoliumchloride (TTC) staining of the tissues, following 24 h post reperfusion. Brain tissue sections (2 mm) were treated with TTC (30 min; 37 °C) and then were plunged in para formaldehyde (4%) overnight in darkness. Tissue sections were visualised, and the infarcted region was determined using NIH ImageJ software (Version 1.42; NIH, Bethesda, MD). The infarcted region remained unstained, while normal areas appeared stained with TTC. The extent of infarction was determined as follows infarct area (%) = infarct area/total area of slice × 100.
2.6. Assessment of neuronal apoptosis
Terminal transferase-mediated dUTP nick end-labelling (TUNEL) staining was done for evaluation of neuronal apoptosis following I/R. Tissue sections of a 5-μm thickness (n = 6/ group) were treated as per the instructions specified (DeadEnd TM fluorometric TUNEL system kit, Promega, Madison, WI, USA) and were analysed for apoptotic cells by NIS-Elements BR imaging processing and analysis software (Nikon Corporation, Japan).
2.7. Semi-quantitative RT-PCR
Brain samples excised 24 h after perfusion was used for analysing the expression using RT-PCR and western blotting. For RT-PCR process, Total tissue RNA isolated from ischemic cortical samples from the different treatment groups and were used for the assay. NucleoSpin RNA II kit and Titanium One-step RT-PCR kit from BD Biosciences were employed to isolate RNA and for RT-PCR respectively. Following primers were used: HIF-1α – Forward: 5′-AAG TCT AGG GAT GCA GCA C-3′, Reverse: 5′-CAA GATCAC CAG CAT CTA G-3′ HO-1- Forward: 5′- GAG ATT GAG CGC AAC AAG GA-3′, Reverse: 5′-AGC GGT AGA GCT GCT TGA ACT‐3′. The amplified PCR products of the test genes were normalised with the expressions of β-actin as an internal control. Forward: 5′–CCC TCA AGA TTG TCA GCA ATG C-3′, Reverse: 5′-GTC CTC AGT GTA GCC CAG GAT‐3′. PCR products were visualised by Molecular Imager FX (Bio-Rad, USA) and analysed using Quantity One software (Bio-Rad).
2.8. Western blotting
The excised ischemic cortical samples (n = 6/ group) were homogenized in ice-cold cell lysis buffer containing cocktail of protease inhibitors (Cell Signaling Technology). The total protein content of the tissues was quantified using protein assay kit from Thermo Fischer Scientific. Protein samples (n = 6/group) of equal concentrations (60 µg) from the different treatment groups were loaded on SDS-PAGE (to 10–12%) gels and were separated. The size-fractioned proteins were electrotransfered onto a nitrocellulose membrane (ThermoFischer, USA). The blotted membranes were treated with non-fat dry milk (5%) followed by overnight incubation at 4 °C with appropriate concentration of specific primary antibodies and further incubated at room temperature (1 h) with secondary antibodies (peroxidase-conjugated; Santa Cruz Biotechnology). Primary antibodies against the following were used: Bcl-2,Bax, Bad, β-actin, cleaved-caspase-3, HIF-1α, HO-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Akt , PI3K, p-Akt, p-PI3K (Cell Signaling Technology, Beverly, MA, USA), mTOR, p-mTOR, s6K and p-s6K (Abcam, Cambridge, MA, USA).The immunoreactive bands were detected and analysed in an electro-chemiluminescence system (ECL, Millipore, USA). The intensity of the bands were standardised to expressions of β-actin that was run in parallel as the internal control.
2.9. Statistical analysis
The data obtained are presented as mean ± SD (n = 6) and analysed by one-way analysis of variance (ANOVA) and Duncan's Multiple Range Test (DMRT) using SPSS version 21.0 (IBM Corporation, USA); p-values < 0.05 were marked as statistically significant.
3. Results
3.1. 6-shogaol and sevoflurane attenuated I/R-induced neurobehavioral deficits
The animals of all treatment groups exhibited normal behavioural responses and neuromuscular coordination an hour before I/R induction. However, 24 h following I/R substantial (p < 0.05) neurological deficits were observed (Fig. 1). The flexibility of the left limbs was found to be reduced. The animals were noticed to struggle to respond to the test stimulus. The neurological deficits were observed to substantially (p < 0.05) reduce treatment with 6-shogaol given at 25 and 50 mg doses. Animals administered 6-shogaol and exposed to sevoflurane exhibited better neuroprotective effects.
Fig. 1.
Effects of 6-shogaol and sevoflurane on I/R induced neurobehavioural deficits The data are represented as mean ± SD, n = 6. p < 0.05 as obtained by one-way ANOVA and DMRT analysis. * represents p < 0.05 vs. control; # represents p < 0.05 vs. I/R control; @ represents 6-shogaol (50 mg/Kg) + I/R vs. 6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%) at p < 0.05 a-f represents means from various study groups that differ at p < 0.05.
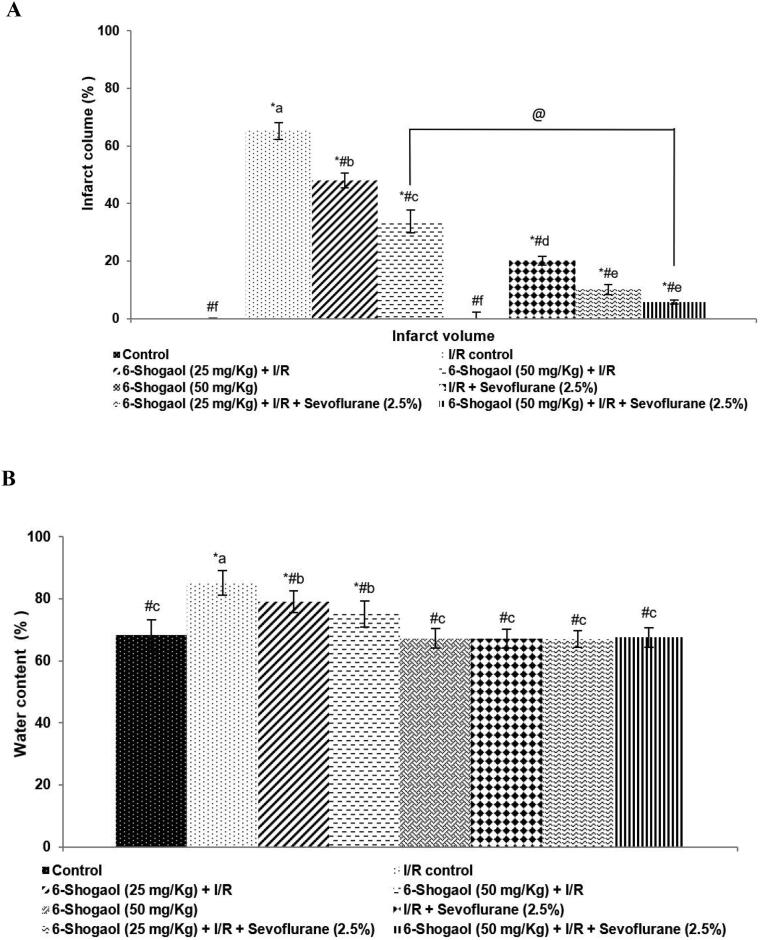
3.2. 6-shogaol and sevoflurane reduced brain edema and infarct area
Infarcted area following I/R was determined by TTC staining. Infarcted regions appeared as unstained regions vs. normal regions stained with TTC. The ischemic areas were observed predominantly in the striatum and the frontoparietal cortex regions (Fig. 2). Pre- treatment with 6-shogaol at 25 and 50 mg doses resulted in a substantially (p < 0.05) reduced brain edema and infarct volume following I/R (Fig. 2 a and b). Interestingly, post-conditioning with sevoflurane though exerted protective effects pre-treatment with 6-shogaol irrespective of the dosage given, was noticed to exert greater protective effects compared to sevoflurane post-conditioning alone. The infarct volume was observed to decrease from 65.15% to 5.80% on 50 mg 6-shogaol treatment and post-conditioning with sevoflurane. Further, group 5 rats that were treated with 6-shogaol at 50 mg dose alone did not exhibit any neuronal injury.
Fig. 2.
Effect of 6-shogaol and sevoflurane on brain edema and infarct area. 6-shogaol and sevoflurane reduced cerebral infarct area (a) 6-shogaol and sevoflurane reduced brain edema following I/R injury (b). The data are represented as mean ± SD, n = 6. p < 0.05 as obtained by one-way ANOVA and DMRT analysis. * represents p < 0.05 vs. control; # represents p < 0.05 vs. I/R control; @ represents 6-shogaol (50 mg/Kg) + I/R vs. 6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%) at p < 0.05. a-f represents means from various study groups differing at p < 0.05.
3.3. 6-shogaol and sevoflurane inhibited I/R-induced neuronal cell death
The data obtained illustrated that post-conditioning with sevoflurane following 6-shogaol pre-treatment produced a significant (p < 0.05) reduction in I/R-induced nerve cell death (Fig. 3). 6-shogaol administered alone at both the doses (25 mg and 50 mg) before I/R caused a signficant (p < 0.05) reduction in TUNEL positive cell counts. Further, significant neuroprotection following I/R brain injury was observed in rats that were treatment with 6-shogaol followed by sevoflurane post-conditioning vs. rats exposed to sevoflurane or 6-shogaol. The apoptotic cell counts decreased from 141 cells/mm2 in I/R control to 58 cells/mm2 on 6-shogaol treatment at 50 mg/kg dose. While 6-shogaol and sevoflurane post conditioning decreased apoptotic cell counts to 19 cells/ mm2.
Fig. 3.
Effects of 6-shogaol and sevoflurane post-conditioning on neuronal apoptosis. The data are represented as mean ± SD, n = 6. p < 0.05 as obtained by one-way ANOVA and DMRT analysis.* represents p < 0.05 vs. control; # represents p < 0.05 vs. I/R control; @ represents 6-shogaol (50 mg/Kg) + I/R vs. 6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%) at p < 0.05. a-f represents means of the various study groups that differ at p < 0.05.
Significant (p < 0.05) higher expressions of Bad, Bax and Cleaved caspase-3, were noticed in the I/R control group vs. normal control. The expressions of Bcl-2, the anti-apoptotic protein were reduced in I/R control in comparison with the control. These enhanced expressions of pro-apoptotic proteins possibly could have contributed to the increased neuroapoptosis as noticed in TUNEL assay. 6-shogaol treatment prior to I/R induction resulted in decreased expressions of Bax, Bad and cleaved caspase-3 vs. I/R control (Fig. 4 a-d). However, the data revealed that 6-shogaol pre-treatment, along with sevoflurane post-conditioning more effectively down-regulated the expressions of pro-apoptotic proteins. Bcl-2 expression increased to 85% on exposure to 2.5% sevoflurane following reperfusion vs. 48% in I/R control. Interestingly, shogaol pre-treatment at doses 25 mg/kg and 50 mg/kg along with sevoflurane post-conditioning, enhanced the expressions of Bcl-2 to 98% and 100% respectively, demonstrating the anti-apoptotic efficacy of 6-shogaol. The results indicated that 6-shogaol have potential neuroprotective effects.
Fig. 4.
Effects of 6-shogaol and sevoflurane post-conditioning on the expressions of apoptotic protein following ischemia/reperfusion injury. Representative immunoblot (A) Expressions of test proteins relative to control expressions set at 100% (B and C). The data are represented as mean ± SD, n = 6. p < 0.05 as obtained by one-way ANOVA and DMRT analysis. * represents p < 0.05 vs. control; # represents p < 0.05 vs. I/R control; @ represents 6-shogaol (50 mg/Kg) + I/R vs. 6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%) at p < 0.05. a-f represents means from various study groups differing at p < 0.05 [L1-Control; L2-I/R control; L3-6-shogaol (25 mg/Kg) + I/R; L4-6-shogaol (50 mg/Kg) + I/R; L5-6-shogaol (50 mg/Kg); L6-I/R + sevoflurane (2.5%); L7-6-shogaol (25 mg/Kg) + I/R + sevoflurane (2.5%); L8-6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%)]
3.4. 6-shogaol and sevoflurane post-conditioning improved HO-1 and HIF-α
6-shogaol treatment was found to enhance expressions levels of HO-1 and HIF-α both at mRNA and protein levels following I/R (Fig. 5 a-d). The substantially increased (p < 0.05) mRNA expressions of HO-1 and HIF-α in I/R control was further enhanced on 6-shogaol treatment at 25 mg and 50 mg doses. The mRNA levels of HIF- α and HO-1 increased to 1.9 and 2.23 folds respectively in rats treated with 6-shogaol and sevoflurane vs. 1.35 and 1.23 folds in IR control. Post-conditioning with sevoflurane following 6-shogaol pre-treatment was noticed to significantly (p < 0.05) elevate protein expressions of HO-1 and HIF-α vs. I/R control. Post-conditioning with sevoflurane alone enhanced the translation of HIF-α and HO-1, as seen in the raise of the protein levels from 114% and 109% in IR control to 147.76% and 141.82%. While 6-shogaol treatment along with sevoflurane post-conditioning up-regulated the expressions of HO-1 and HIF-α to 171.2% and 169% respectively vs. 136% and 139.8% in 6-shogaol alone treated group. The results suggest 6-shogaol, and sevoflurane post-conditioning was more effective in regulating the expressions HIF-α and HO-1 in comparison with sevoflurane or 6-shogaol administered alone.
Fig. 5.
6-shogaol and sevoflurane regulated HIF-α and HO-1 expressions following ischemia/reperfusion injury. Representative gel of RT-PCR analysis (A) Relative expressions of HIF-α and HO-1mRNA (B) Representative immunoblot (C) Protein expressions relative to control set at 100% (D). The data are represented as mean ± SD, n = 6. p < 0.05 as obtained by one-way ANOVA and DMRT analysis. * represents p < 0.05 vs. control; # represents p < 0.05 vs. I/R control; @ represents 6-shogaol (50 mg/Kg) + I/R vs. 6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%) at p < 0.05. a-f represents means from various study groups differing at p < 0.05. [L1-Control; L2-I/R control; L3-6-shogaol (25 mg/Kg) + I/R; L4-6-shogaol (50 mg/Kg) + I/R; L5-6-shogaol (50 mg/Kg); L6-I/R + sevoflurane (2.5%); L7-6-shogaol (25 mg/Kg) + I/R + sevoflurane (2.5%); L8-6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%)]
3.5. 6-shogaol and sevoflurane post-conditioning activated the PI3K/Akt/ mTOR/s6K signaling
Analysis of the protein expression by western blotting showed up-regulation in the phosphorylated forms of PI3K, Akt, mTOR, s6K phosphorylation in I/R and normal control. 6-shogaol treatment was found to enhance the phosphorylation levels of PI3K, Akt, mTOR and s6K proteins (Fig. 6 a-e) and I/R control. Interestingly, 6-shogaol treatment and sevoflurane post conditioning were found to be more effective in the stimulation of PI3K/Akt/ mTOR/s6K signaling pathway in comparison to sevoflurane post-conditioning alone. p-PI3K expressions were noticed to increase to 2.1 fold from 1.4 fold in rats treated with 6-shogaol and exposed to sevoflurane vs. sevoflurane alone exposed rats (Fig. 6 a and b). p-mTOR and p-Akt expression enhanced to 2.5 and 2.7 folds (Fig. 6 a, c-d). p-s6K levels increased from 1.58 fold in I/R control to 3.6 fold on treatment with 6-shogaol and sevoflurane post-conditioning (Fig. 6 a and e). Further, 6-shogaol (50 mg/kg) administered to rats that were not induced with I/R did not affect the expression of proteins in comparison to normal control.
Fig. 6.
Effects of 6-shogaol and sevoflurane post-conditioning on the PI3K/Akt/ mTOR/s6K signaling following ischemic/reperfusion injury. Representative immunoblot of protein expressions (A) Phosphorylated protein levels to respective total protein levels (B-D). The data are represented as mean ± SD, n = 6. p < 0.05 as obtained by one-way ANOVA and DMRT analysis. * represents p < 0.05 vs. control; # represents p < 0.05 vs. I/R control; @ represents 6-shogaol (50 mg/Kg) + I/R vs. 6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%) at p < 0.05. a-f represents means from various study groups differing at p < 0.05. [L1-Control; L2-I/R control; L3-6-shogaol (25 mg/Kg) + I/R; L4-6-shogaol (50 mg/Kg) + I/R; L5-6-shogaol (50 mg/Kg); L6-I/R + sevoflurane (2.5%); L7-6-shogaol (25 mg/Kg) + I/R + sevoflurane (2.5%); L8-6-shogaol (50 mg/Kg) + I/R + sevoflurane (2.5%)]
4. Discussion
I/R-induced brain injury, a pathological complication resulting from various neurological and cardiovascular procedures may source many clinical concerns. Consequently, the identification of more effective strategies to prevent IR-induced brain injury is inevitable for cerebral protection (Tapuria et al., 2008). Studies have revealed that sevoflurane post-conditioning alleviated I/R induced brain injury (Ye et al., 2012). Preconditioning with sevoflurane has been shown to enhance spatial learning and also improve memory in rodents following I/R (Hu et al., 2013).
Apoptosis is well documented as a major mechanism resulting in neuronal cell death in I/R (Guo et al., 2013). Significantly (p < 0.05) lesser neuroapoptotic cell counts were noticed in rats administered with 6-shogaol and/or post-conditioned with sevoflurane. Previous investigations have illustrated that the neuroprotective effects of volatile anaesthetics following cerebral IR injury involve anti-apoptotic mechanisms (Konia et al., 2009). Bedirli et al. (2012) showed that pre-conditioning with sevoflurane caused inhibition of apoptosis via downregulation of pro-apoptotic protein as Tp53 and through elevated anti-apoptotic proteins as Bcl-2. The PI3K/Akt signal is critically regulated neuronal survival. The pathway is articulated extensively in the CNS and is stimulated by several growth factors as the nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) (Brunet et al., 2001).
Sevoflurane post-conditioning following reperfusion resulted in marked activation of PI3K, Akt, mTOR and s6K proteins. Similar observations were presented by Wang et al. (2010). The PI3K/Akt pathway upon activation induces the activity of the anti-apoptotic proteins and deactivates the pro-apoptotic factors (Liu et al., 2009). Activation of the signal by sevoflurane and 6-shogaol could have contributed to the increased expressions of Bcl-2 in line with down-regulated Bad and Bax levels. Activated Akt exerts anti-apoptotic effects by phosphorylating and inactivating Bad and inducing the release of Bcl-xL, an anti-apoptotic protein, that inhibits pro-apoptotic- Bax (Koh, 2011).
Sevoflurane-induced up-regulation of p-Akt as observed in the present suggests inhibition of pro-apoptotic proteins. Isoflurane pre-conditioning was shown to exert neuroprotective effects via up-regulation of the Akt/mTOR/s6K signaling pathway (Yan et al., 2016). The findings our study also revealed a significantly (p < 0.05) increased mRNA and HIF-1α and HO-1 protein levels on treatment with sevoflurane and/or 6-shogaol. Further, prior treatment with 6-shogaol followed by sevoflurane post-conditioning more effectively enhanced HIF-1α and HO-1 expressions at transcriptional and translational levels as well. HIF-1α, activated under hypoxic conditions, plays a critical role in ischemia (Harms et al., 2010). HIF-1 regulates many genes associated with vital events of the cells including cell survival, proliferation, and iron metabolism (Otterbein et al., 2003). HO-1 is one of the major proteins that are regulated by HIF-1α that is induced under ischemic condition (Lee et al., 1997). Protective effects of HO-1 in ischemia are well documented (Shah et al., 2011). Neuroprotective effects of HO-1 could be attributed to the effective regulation of intracellular heme levels and conversion of heme to antioxidant bilirubin by HO-1 (Stocker et al., 1987). Thus, markedly improved HIF-1α and HO-1 noticed on 6-shogaol and/or sevoflurane post-conditioning in part could have contributed to the decrease in neuronal apoptosis as observed.
5. Conclusions
The results clearly revealed that 6-shogaol supplementation prior to induction of I/R exhibited neuroprotective effects. 6-shogaol treatment was found to enhance expression levels of HO-1 and HIF-α at mRNA levels following I/R. The present investigation revealed that 6-shogaol is a novel therapeutic candidate in the treatment of I/R brain injury.
6. Declarations
Contributions of Authors statement
The authors have equally contributed in the study design, layout, and conduction of the experiments and in the presentation of this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ali B.H., Blunden G., Tanira M.O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem. Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Bedirli N., Bagriacik E.U., Emmez H., Yilmaz G., Unal Y., Ozkose Z. Sevoflurane and isoflurane preconditioning provides neuroprotection by inhibition of apoptosis-related mRNA expression in a rat model of focal cerebral ischemia. J. Neurosurg. Anesthesiol. 2012;24:336–344. doi: 10.1097/ANA.0b013e318266791e. [DOI] [PubMed] [Google Scholar]
- Brunet A., Datta S.R., Greenberg M.E. Transcription-dependent and - independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cuartero M.I., Ballesteros I., Moraga A., Nombela F., Vivancos J., Hamilton J.A., Corbí A.L., Lizasoain I., Moro M.A. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- Garber J.C. Guide for the care and use of laboratory animals. 8th ed. National Academy of Sciences; USA: 2011. Committee for the update of the guide for the care and use of laboratory animals. [Google Scholar]
- Guo, F., Jin, W.L., Li, L.Y., Song, W.Y., Wang, H.W., Gou, X.C, Mi., Y.J., Wang, Q., Xiong, L., 2013. M9, a novel region of amino-Nogo-A, attenuates cerebral ischemic injury by inhibiting NADPH oxidase-derived superoxide production in mice. CNS Neurosci. Ther. 19, 319-328. [DOI] [PMC free article] [PubMed]
- Han Q., Yuan Q., Meng X., Huo J., Bao Y., Xie G. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget. 2017;8:42001–42006. doi: 10.18632/oncotarget.16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms K.M., Li L., Cunningham L.A. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Zhang Y., Li W., Liu J., Li Y. Preconditioning with sevoflurane ameliorates spatial learning and memory deficit after focal cerebral ischemia-reperfusion in rats. Int. J. Dev. Neurosci. 2013;31:328–333. doi: 10.1016/j.ijdevneu.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Kim S., Jung W.Y., Park S.J., Park D.H., Kim J.M., Cheong J.H., Ryu J.H. he neuroprotective effects of the seeds of Cassia obtusifolia on transient cerebral global ischemia in mice. Food Chem. Toxicol. 2009;47:1473–1479. doi: 10.1016/j.fct.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Kim H.C., Kim E., Bae J.I., Lee K.H., Jeon Y.T., Hwang J.W., Lim Y.J., Min S.W., Park H.P. Sevoflurane postconditioning reduces apoptosis by activating the JAK-STAT pathway after transient global cerebral ischemia in rats. J. Neurosurg. Anesthesiol. 2017;29:37–45. doi: 10.1097/ANA.0000000000000331. [DOI] [PubMed] [Google Scholar]
- Koh P.O. Nicotinamide attenuates the ischemic brain injury-induced decrease of Akt activation and Bad phosphorylation. Neurosci. Lett. 2011;498:105–109. doi: 10.1016/j.neulet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Konia M.R., Schaefer S., Liu H. Nuclear factor-[kappa]B inhibition provides additional protection against ischaemia/reperfusion injury in delayed sevoflurane preconditioning. Eur. J. Anaesthesiol. 2009;26:496–503. doi: 10.1097/eja.0b013e328324ed2e. [DOI] [PubMed] [Google Scholar]
- Lee J.J., Li L., Jung H.H., Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.J., Jiang B.H., Chin B.Y., Iyer N.V., Alam J., Semenza G.L. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase 1 gene in response to hypoxia. J. Biol. Chem. 1997;272:5371–5381. [PubMed] [Google Scholar]
- Leonardo C.C., Hall A.A., Collier L.A., Green S.M., Willing A.E., Pennypacker K.R. Administration of a sigma receptor agonist delays MCAO-induced neurodegeneration and white matter injury. Transl. Stroke Res. 2010;1:135–145. doi: 10.1007/s12975-009-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Li Z., Mo N., Li M., Zhuang Z., Wang J., Wang Y., Guo X. Isoflurane preconditioning ameliorates renal ischemia-reperfusion injury through anti-inflammatory and antiapoptotic actions in rats. Biol. Pharm. Bull. 2014;37:1599–1605. doi: 10.1248/bpb.b14-00211. [DOI] [PubMed] [Google Scholar]
- Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3- kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein L.E., Soares M.P., Yamashita K., Bach F.H. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Shah Z.A., Nada S.E., Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Tapuria N., Kumar Y., Habib M.M., Abu Amara M., Seifalian A.M., Davidson B.R. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury - A review. J. Surg. Res. 2008;150:304–330. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Wang G.L., Yu Y.H., Wang Y. The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res. 2009;1297:177–184. doi: 10.1016/j.brainres.2009.08.054. [DOI] [PubMed] [Google Scholar]
- Wang J.K., Yu L.N., Zhang F.J., Yang M.J., Yu J., Yan M., Chen G. Post-conditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res. 2010;1357:142–151. doi: 10.1016/j.brainres.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Yan W., Chen Z., Chen J., Chen H. Isoflurane preconditioning protects rat brain from ischemia reperfusion injury via up-regulating the HIF-1α expression through Akt/mTOR/s6K activation. Cell. Mol. Biol. 2016;Noisy-le-grand), 62:38–44. [PubMed] [Google Scholar]
- Ye Z., Guo Q., Xia P., Wang N., Wang E., Yuan Y. Sevoflurane postconditioning involves an up-regulation of HIF-1alpha and HO-1 expression via PI3K/Akt pathway in a rat model of focal cerebral ischemia. Brain Res. 2012;1463:63–74. doi: 10.1016/j.brainres.2012.04.050. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yu X.H., Yan Y.G., Wang C., Wang W.J. PI3K/Akt signaling in osteosarcoma. Clin. Chim. Acta. 2015;444:182–192. doi: 10.1016/j.cca.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Zhang L., Qu Y., Yang C., Tang J., Zhang X., Mao M., Mu D., Ferriero D. Signaling pathway involved in hypoxia-inducible factor-1alpha regulation in hypoxic-ischemic cortical neurons in vitro. Neurosci. Lett. 2009;461:1–6. doi: 10.1016/j.neulet.2009.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Sapolsky R.M., Steinberg G.K. Phosphoinositide- 3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol. Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]