Abstract
Salinity is one of the major agricultural concern that significantly limits the crop productivity. The plant growth promoting rhizobacteria (PGPR) may contribute in sustainable crop production under salt stress. The current study was designed to isolate the Indole Acetic Acid (IAA) producing salt tolerant PGPR to promote the growth of cotton (Gossypium hirsutum, FH-142) and induce its salt stress tolerance. Ten Salt Tolerant (ST) bacterial strains were screened for their PGP trait in vitro and evaluated for their beneficial effect on cotton plants growth by plant–microbe interaction assay in lab and under natural condition. GC–MS analysis of the metabolites of the selected bacterial strains confirmed the presence of indolic compounds like indole, indole-3-butyramide, benzylmalonic acid and 4-methyl-2-pyrrolidinone. The bacterial isolates ST4, ST5, ST6, ST15, ST16, ST17, ST18, ST20, ST22 and ST25 were identified as Bacillus sp., B. sonorensis, B. cereus, B. subtilis, Brevibacillus sp. B. safensis, B. paramycoides, Bacillus sp., B. cereus and B. tequilensis respectively on the basis of 16S rDNA sequencing. Bacteria inoculated plants had a significant (P < 0.05) increase in percentage germination up to (31%), root length (17%) and shoot length (34%) in lab while in wire house pot experiments, maximum enhancement in root length (31%) and shoot length (29%) was observed. ST bacterial strains inoculation improved the chlorophyll content index (34%), relative water content (36%), leaf area (33%), absorption of K+ (28%) and decreased the uptake of Na+ (58%) from soil in plants under salt stress over control in pot experiment. These ST PGPR have the potential to act as plant defense agents by enhancing plant growth, productivity, and tolerance in saline environment.
Keywords: Salt stress, Plant growth promoting rhizobacteria, Indole acetic acid, Gossypium hirsutum, Chlorophyll content index
1. Introduction
Salinity is an edaphic stress that has affected 45 million hectares of irrigated land out of 230 million hectares, causing annual losses of approximately US$ 12 billion globally and is a major threat to global agricultural productivity (FAO, 2020). Pakistan is also dealing with extreme salinity problems with a total area of 6.30 million hectares is salt affected, out of which 1.89 million hectares is classified as saline (Abbas et al., 2019). Salt related problems have been reported in all stages of plant development and many physiological and biochemical parameters like protein synthesis, photo synthesis, water status, leaf area, lipid metabolism and membrane integrity are at risk due to high NaCl concentration (Hmaeid et al., 2019). Several biotechnological methods for improving salt resistance in plants have been tried, but these methods are expensive. The development of stress tolerance by microbes appears promising, as rhizospheric microbes both tolerate stress and confer tolerance to plants, promoting the latter's growth (Banik et al., 2018). Plant growth-promoting rhizobacteria (PGPR) promote plant growth and increase their induced systemic resistance (ISR) to a variety of environmental stresses through different process like antioxidant enzymatic activity, inorganic solutes amassing like Na+, Mg+ and K+ (Egamberdiyeva and Islam, 2008) and decline of ethylene level by ACC deaminase activity (Sarkar et al., 2018).
PGPR play a key role in combating salt stress and restoration of soil health as well as plant growth promotion. Growth of several plants under salt stress has been reported to be enhanced by PGPR like Sulla carnosa (Hmaeid et al., 2019), common ice-plant (Mesembryanthemum crystallinum L.) (Mahmood et al., 2019), rice (Oryza sativa) (Sarkar et al., 2018), wheat (Triticum aestivum) (Ansari and Ahmad, 2018), Tomato (Lycopersicon esculentum cv. Bella) (Egamberdieva et al., 2017), pea (Pisum sativum L.) (Meena et al., 2015), and soybean (Glycinemax) (Egamberdieva et al., 2015) cucumber (Cucumis sativus), sweet potato (Ipomoea batatas(L.) Lam.) (Dawwam et al., 2013), lentil (Lens esculenta) (Faisal, 2013), (Egamberdieva et al., 2011).
Among natural fibre crops, cotton is the most valuable as it is used to make biofuel and edible oil. Throughout its lifespan, it is subjected to a variety of biotic and abiotic stresses, with salinity being one of the most serious threats to global cotton production. South Punjab a semi-arid region of Punjab Pakistan, is one of the largest hub of cotton planting. Cotton and cotton-related products account for 10% of Pakistan's GDP and 55% of its foreign exchange earnings (Rehman et al., 2019). Salinization of the cultivated land is the major cause of less yield production of cotton that is a great economical loss of country. So, this research was conducted to reduce the harmful effects of salt stress in cotton by applying ST PGPR and subsequently improve the growth of cotton. The main objective of this study was to Isolate and characterize the ST PGPR and check their effects on the vegetative growth and other physiological parameters of cotton under both laboratory and field conditions.
2. Material and methods
2.1. Isolation, characterization and identification
For the exploration of salt tolerant rhizobacteria a number of rhizospheric soil samples were collected from different plants. Samples were collected in sterile plastic bags and transported to the laboratory, where they were stored at 4 °C. One gram of soil from each sample was serially diluted and spread on the LB agar plates following the method of (Iqbal and Hasnain, 2013). Isolated colonies were purified and stored at 4°C for further study. The physiological, morphological and biochemical characters of purified bacterial strains were observed following the method of Holt et al. (1994).
2.2. Screening of salt tolerant PGPR
Isolated, purified bacterial strains were checked for their salt tolerance and screened on the basis of Indole Acetic Acid (IAA) productivity. Salt tolerance capacity was determined by inoculating the spots of isolated strains on LB agar plates with varied salt concentration (0–1500 mM). Plates were incubated at 37 ± 2 °C for 48 h. Salt tolerance was determined in terms of (MIC) of salt. Salt tolerant bacterial strains were further checked for their drought tolerance and PGP traits like IAA, siderophore and HCN production as describe earlier (Batool and Iqbal, 2019).
2.3. Physiological, biochemical and molecular identification
Ten selected isolates were subjected to different physiological, morphological and biochemical tests like Gram reaction, catalase test, indole test, sucrose fermentation test, methyl red test for identification by using the standard protocol. These 10 bacterial strains were named as isolates ST4, ST5, ST6, ST15, ST16, ST17, ST18, ST20, ST22 and ST25. Furthermore, to confirm the identification of the isolates, 16S rDNA sequencing was performed by Macrogen, Seoul, South Korea. Online BLAST tool from NCBI website was used for the comparison of sequences with the already submitted sequences in NCBI nucleotide database. Sequences were submitted to the NCBI Gen-Bank database to get their accession numbers.
2.4. Plant growth promoting traits assay
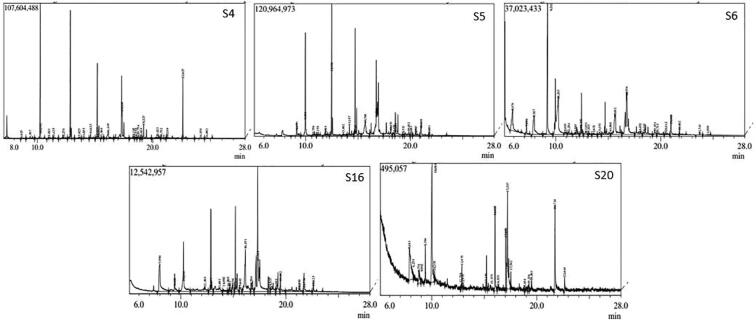
Indole acetic acid (IAA) production by ST4, ST5, ST6, ST15, ST16, ST17, ST18, ST20, ST22 and ST25 was determined by calorimetric method previously describe by Patten and Glick (1996). Bacterial strains were inoculated in LB broth supplemented with 0.1 g tryptophan in 1L and incubated at 37 °C at 100 rpm for 72hrs. After 72hrs the culture was centrifuge at 10,000 rpm for 10 min and 2 ml of supernatant was taken from each culture in a tube and allowed to react with 2 ml of Salkowski reagent for 30 min in dark. Colour change from pale yellow to pinkish red was the indication of IAA production. The IAA was quantified using a UV–vis spectrophotometer to read the color intensity at 535 nm, and the amount of IAA released was calculated using a standard graph prepared with known quantities of pure IAA. The Gas Chromatography Mass Spectrometry (GC–MS) analysis was used to identify indole compounds and their derivatives specifically (Fig. 1).
Fig. 1.
Gas Chromatogram of ST4, ST5, ST6, ST16 and ST20.
To test the ammonia production, test strains were inoculated in autoclaved 10% peptone water and incubated at 37 ± 2°C, 120 rpm in incubator shaker for 3 days. After incubation 1 ml of supernatant was reacted with 0.5 ml of Nessler’s Reagent, appearance of yellow colour designated to minimum extent and orange to brownish colour was the indication of maximum ammonia production.
For hydrogen cynide (HCN) production, selected isolates were spot inoculated on LB agar plates amended with 0.44% glycine, filter paper soaked with 2% sodium carbonate and 0.5% picric acid solution was placed on the agar plates and sealed with parafilm and incubated at 37 ± 2 °C for 72hrs. Colour change of filter paper from yellow to brown is the indication of positive result.
For siderophore production nutrient agar and Chrome Azurol S (CAS) dye was autoclaved separately and mixed before pouring in petri dishes. Test strains were spot inoculated and incubated for 72hrs. After incubation orange zone stipulated positive result.
2.5. Effect of selected isolates on plant growth promotion
2.5.1. Procurement of cotton seeds
Cotton seeds of variety FH-142 were obtained from Punjab Seeds Corporation as it is common cultivar of South Punjab. From salinized and non-salinized fields having nearby location, saline and non-saline soil was collected from District Lodhran of South Punjab. Soil samples was checked by soil analysis laboratory (Multan) for different physiochemical characters like temperature, pH, electrical conductivity, organic matter and phosphorus, potassium content. Chemical and physical properties of soil sample is given in Table 2.
Table 2.
Chemical and physical properties of soil.
| Soil type | EC mS/cm | pH | Organic matter | Available P mg/kg | Available K mg/kg | Saturation% | Texture |
|---|---|---|---|---|---|---|---|
| Saline soil | 10.51 | 8.3 | 0.47 | 4.10 | 110 | 30 | S. loamy |
| Non Saline soil | 2.87 | 7.8 | 0.49 | 4.20 | 130 | 34 | Loamy |
2.5.2. Seed inoculation
Inoculum preparation and seeds inoculation was done by following the standard guidelines (Bashan et al., 2016). Bacterial inoculum was prepared by inoculated the single colony of each selected bacterial strains in 250 ml Erlenmeyer Flasks containing 100 ml LB broth and incubated for 48 h at 37 ± 2 °C at 100 rpm. Bacterial pellet was harvested by centrifuging at 6000 rpm for 10 min at 4 °C. Bacterial pellet were washed with PBS thrice and suspended in PBS maintaining cell concentration at 106 cfu ml−1 for priming the seeds. Uniform size healthy cotton seeds (FH-142) were surface sterilized by using 2% sodium hypochlorite for 5 min, subsequently seeds were washed five times with autoclave distilled water. Sterile seeds were treated with bacterial inoculation by dipping the seeds in bacterial inoculum for 2 hrs. Seeds dipped in autoclaved distilled water without bacterial inoculation served as control.
2.6. Plant growth in gonotobiotic condition under salt stress
Pot experiment under axenic condition was performed. Each pots was filled with 600 g of autoclaved soil (Sandy, clay, loamy soil). For pots arrangement complete randomize block design was implemented. Each set has following treatment a) inoculated, non-stressed b) inoculated salt stressed c) uninoculated non stressed d) uninoculated stressed
Five cotton seeds were sown in each pot and after germination thinning was done by having three plants per pot. Soil humidity was maintained at 60%. Treatment b and d was exposed to salt stress (200 mM, NaCl) while a and c didn’t receive any salt stress with 16/8hrs, light/dark cycle, 38/30 °C day/night temperature. Seeds percentage germination was noted on 7th day of sowing. Plants were grown for four weeks. After four weeks plant shoot length and root length was measured.
2.7. Plant growth experiment in pots in natural condition
For pot experiment under natural condition saline and non-saline soil was used (Table 2). Each pot was filled by 3 kg of soil. Each treatment had two sets “stressed inoculated and non-inoculated and non-stressed inoculated and non– inoculated. Bacterial suspension was prepared and seeds were coated by bacteria by dipping them in bacterial suspension for 2hrs. Seeds soaked in autoclaved distilled water served as control. Fifteen seeds were sown in each pot and after seed germination thinning was done having six plants per pot. Plants were grown for six weeks under natural field conditions, where temperatures varies from 41 to 45°C during day while 32–37°C during night. At this stage different physiological and vegetative parameters were measured.
2.8. Physiological parameters to detect stress effect
After 4 weeks, before harvesting plants leaf were used to determine the different physiological changes induces by salt stress and their alleviation by ST4, ST5, ST6, ST15, ST16, ST17, ST18, ST20, ST22 and ST25. Leaf chlorophyll was measured by using the chlorophyll meter (Konica Minolta, SPAD-502), three of the youngest fully expanded and sun-exposed leaves were excised in order to determine the leaf water potential using a Scholander pressure chamber (Scholander et al., 1965). An area meter (AM100, ADC, Bioscientific) was used to measure leaf area and expressed in cm2.
Plants Relative electrolyte leakage (REL) and Relative Water Content (RWC) was measured by using the method of Katam et al. (2016). For REL 1 g of fresh leaves were taken and cut into disks of 0.8 cm and incubated for 4hrs in 80 ml of ddH2O. Electrical conductivity (C1) was measured using the conductivity meter and then solution was boiled for 10 min and cooled down to room temperature to measure the C2 and REL was calculated as
| (1) |
For RWC fresh weight (FW) of leaf was measured immediately after sample collection and leaf was left to saturate in water at 4 °C for 8hrs to measure the turgid weight (TW) and dry weight (DW) was measured by drying the leaf at 80 °C for 24hrs in oven and calculated as:
| (2) |
Proline content in fresh leaves was measured using the Bates method (Bates et al., 1973). Plants were harvested carefully and washed under tap water, plants root length, shoot length was measured. For measuring the dry weight, the plants were oven dried at 65 °C for 24hrs.
Na+ and K+ content was determined by washing fresh tissues with distilled water immediately after collection, dried at 60 °C for 72hrs, and by using a mortar and pestle ground into a fine powder. Each sample's powder (almost 200–500 mg) was mixed with 12 ml of 65 percent HNO3 and 2 ml of 30 percent H2O2 and incubated at 80°C for 1hr. The concentrations of Na+ and K+ in the leaves were measured using inductively coupled plasma-optical emission spectrometry (Optima 2100 DV; Perkin-Elmer, Inc., Massachusetts, USA) according to the manufacturer’s instructions.
All data was statistical analysed by SPSS 23 software (IBM Corp, Armonk, NY, USA). Difference between mean values of vegetative and physiological parameters compared using. Duncan Multiple Range Test (DMRT) at 5% probability level. Data was expressed as means ± standard deviation.
3. Result
3.1. Isolation and screening of salt tolerant PGPR strains
Soil samples were collected from the different locations of South Punjab i-e Dunya Pur (DP), Vehari (VR), Multan (Mltn) and Lodhran (Ldr) from the rhizosphere of a number of plants including garlic, wheat, mango, coriander, black mustard, black spear grass, corn and sweet orange from fertile and barren soil (Table 1). A total of 42 halotolerant bacterial strains were isolated from 121 isolates, having the ability to withstand up to 1250 mM (NaCl) concentration. Among these isolates ST4,ST5, ST6, ST15, ST16, ST17, ST18, ST20,ST22 and ST25 showed the best growth, PGP attributes and ability to tolerate the higher salt concentration (1000 mM NaCl) and drought tolerance (10% PEG).
Table 1.
Sampling sites of rhizospheric soil collection.
| Sr# | Sample name | No of isolated bacteria | Plant Source | Soil Texture | Location |
|---|---|---|---|---|---|
| 1 | D1 | 13 | Triticum aestivum | Loamy sand | Dunya Pur |
| 2 | D2 | 7 | Medicago sativa | ||
| 3 | D3 | 7 | Brassica nigra | ||
| 4 | D4 | 14 | Allium sativum | ||
| 5 | D5 | 8 | Triticum aestivum | ||
| 6 | V1 | 13 | Heteropogon contortus | Loamy sand | Vehari |
| 7 | V2 | 10 | Triticum aestivum | ||
| 8 | V3 | 12 | Zea mays | ||
| 9 | Mlt1 | 14 | Triticum aestivum | Loamy | Multan |
| 10 | Mlt2 | 4 | Ocimum basilicum. | ||
| 11 | Mlt3 | 7 | Tagetes erecta | ||
| 12 | Ldr1 | 5 | Rosa damascena | Sandy loam | Lodhran |
| 13 | Ldr2 | 7 | Canna Indica |
3.2. Identification of isolates
On the basis of 16S rRNA gene sequencing ST4, ST5, ST6, ST15, ST16, ST17, ST18, ST20, ST22 and ST25 were identified as Bacillus sp., B. sonorensis, B. cereus, B. subtilis, Brevibacillus sp. B. safensis, B. paramycoides, Bacillus sp., B. cereus and B. tequilensis (with accession number MK511829, MK511830, MK511831, MK511833, MK511834, MK511835, MK511836, MK511837 MK511838, MK511839), respectively.
3.3. PGP traits in vitro
All selected strains showed the different behaviour regarding IAA production. ST4, ST5, ST6, ST18 and ST22 produced maximum IAA at 500 mM NaCl concentration. Maximum IAA concentration was produced by ST6 (93 µg/ml) while the lowest by ST18 that is 50 µg/ml and there was decline in it at high salinity level (850 mM NaCl). While ST15, ST16, ST20 and ST25 showed the gradual decrease in IAA production with increasing salt concentration, however ST17 showed gradual increase of IAA with increasing salt concentration. Seventy percent strains have the ability to produce ammonia and 60% showed positive result for siderophore production. ST6, ST16 and ST20 were unable to produce HCN (Table 3). GC–MS analysis of bacterial strains showed that each bacterial strain had a number of indole compounds as bacterial secondary metabolites (Table 4).
Table 3.
Plant growth promoting traits of Salt Tolerant Bacterial strains.
| IAA Production (µg/ml) | HCN Production | Ammonia Production | Siderophore production | |||
|---|---|---|---|---|---|---|
| Salt Concentration | ||||||
| Control | 500 mM | 850 mM | ||||
| ST4 | 30.09 ± 0.8a | 82.39 ± 1.1i | 62.11 ± 1.2 h | + | + | + |
| ST5 | 35.86 ± 1.4b | 69.23 ± 0.9f | 59.62 ± 1.1 g | + | + | – |
| ST6 | 40.59 ± 1.4c | 93.06 ± 1.1j | 63.00 ± 0.7 h | – | + | + |
| ST15 | 116.6 ± 1.7i | 67.63 ± 1.4e | 32 ± 1.2d | + | – | – |
| ST16 | 122.24 ± 1.1j | 48.42 ± 0.79c | 28.49 ± 0.76c | – | + | + |
| ST17 | 74.33 ± 1.1 g | 75.28 ± 0.9 h | 88.85 ± 1.2i | – | + | – |
| ST18 | 47.94 ± 0.05e | 50 ± 0.79d | 45.15 ± 1.3e | – | + | + |
| ST20 | 44.50 ± 0.50d | 24.40 ± 0.25b | 22.98 ± 0.29b | – | + | + |
| ST22 | 59.38 ± 1.2f | 71.12 ± 1.4 g | 50.43 ± 1.1f | – | – | – |
| ST25 | 87.55 ± 1.1 h | 16.75 ± 1.1a | 16.51 ± 1.4a | – | – | + |
Mean of 3 values ± Standard Deviation
Table 4.
Indole compounds and its fractions produced by ST Bacterial Strains.
| Analytes | ST4 | ST5 | ST6 | ST16 | ST20 |
|---|---|---|---|---|---|
| Indole | + | + | + | + | + |
| Benzylmalonic acid | + | + | + | + | + |
| l-Tyrosyl-l-alanyl-l-phenylalanine | – | + | + | + | – |
| 4-Methyl-2-pyrrolidinone | + | + | + | + | + |
| 3-Trifluoroacetoxypentadecane | + | + | + | + | + |
| 5-Methylhenicosane | + | – | + | + | – |
| 5-Pyrrolidino-2-pyrrolidone | + | + | + | + | + |
| Pyrrolidine | + | – | + | + | + |
| Indole-3-butyramide | + | + | + | + | + |
| Squalene | – | – | + | – | – |
| 5H-1-Pyrindine | + | + | – | + | + |
| 3-Isobutylhexahydropyrrolol | + | – | – | – | + |
3.4. Effect of bacterial inoculations on growth parameters
3.4.1. Plant vegetative attributes
Salinity adversely effects the crop productivity resulting reduction in biomass and yield of crop. The inoculation of cotton seeds with selected strains (ST4, ST5, ST6, ST15, ST16, ST17, ST18, ST20, ST22 and ST25) alleviate these adverse effects significantly by stimulating the seed germination rate as compare to control. Plants inoculated with selected strains showed better performance regarding their growth parameters in comparison to their respective control. In lab experiment, salt stress reduced the root length by 18%, shoot length by 13% as compared to unstressed seedlings (Table 5). There was significant difference in various biological parameters of ST treated cotton plants under salt stress and unstressed condition. These IAA-producing ST strains (ST4, ST5, ST6, ST15,ST9, ST12, ST13, ST16, ST19 and ST20) ameliorated the phytotoxic effect of salinity by increasing the root length by 14%, 32%, 29%, 26%, 15%, 24%, 17%, 25% 22%, 16% and shoot length by 15%, 22%, 29%, 7%, 28%, 19%, 23%, 29%, 9%, 14%, respectively over uninoculated control under natural condition (Table 6).
Table 5.
Effect of Salt Tolerant strains on plant growth parameters under both stressed and unstressed condition.
| Salt Tolerant Bacterial Strains | Lab Experiment |
|||||
|---|---|---|---|---|---|---|
| Unstressed |
Stressed |
|||||
| % age Germination | RL (cm) | SL (cm) | Germination % age | RL (cm) | SL (cm) | |
| C | 95.2 ± 1.38ab | 4.76 ± 0.25a | 8.2 ± 0.3a | 63.3 ± 1.52a | 4.03 ± 0.25a | 7.23 ± 0.25a |
| ST4 | 98.3 ± 1.52c | 5.53 ± 0.50b | 9.4 ± 0.1e | 83 ± 2.64b | 4.56 ± 0.30ab | 8.73 ± 0.25d |
| ST5 | 99.3 ± 0.57c | 5.23 ± 0.25ab | 9.26 ± 0.25e | 81.6 ± 1.52b | 4.73 ± 0.25b | 8.46 ± 0.5c |
| ST6 | 99 ± 1c | 5.4 ± 0.36ab | 8.96 ± 0.25d | 80.3 ± 1.52b | 4.46 ± 0.50ab | 8.16 ± 0.35c |
| ST15 | 95.6 ± 1.15ab | 5.2 ± 0.43ab | 8.3 ± 0.2ab | 69.6 ± 5a | 4 ± 0.11a | 7.8 ± 0.2b |
| ST16 | 98.3 ± 1.52c | 5.7 ± 0.36b | 11.73 ± 0.25 h | 81.3 ± 1.52b | 4.5 ± 0.4ab | 9.76 ± 0.25f |
| ST17 | 95.6 ± 3ab | 5.4 ± 0.35ab | 8.6 ± 0.15c | 68 ± 6a | 4.2 ± 0.25 ab | 7.7 ± 0.26b |
| ST18 | 94 ± 4a | 5.3 ± 0.61ab | 9.8 ± 0.25 g | 67.6 ± 4a | 4.3 ± 0.2 ab | 9.13 ± 0.32e |
| ST20 | 98.6 ± 1.52b | 5.53 ± 0.45b | 9.66 ± 0.15 g | 79.6 ± 2.08b | 4.73 ± 0.25b | 7.9 ± 0.4b |
| ST22 | 96 ± 2.64b | 5.5 ± 0.5ab | 9.9 ± 0.25 g | 68.6 ± 2a | 4.2 ± 0.25 ab | 8.8 ± 0.2d |
| ST25 | 95.5 ± 3.13 | 5.3 ± 0.3ab | 9.5 ± 0.3f | 70.3 ± 5.5a | 4.2 ± 0.25 ab | 8.2 ± 0.25c |
Root Length (RL), Shoot Length (SL) and Dry Weight (DW).
Values are mean of three independent replicates, ± indicates Standard Deviation. Mean values followed by different letters are significantly different within column, respectively at P ≤ 0.05 according to Duncan’s multiple range test (DMRT).
Table 6.
Effect of Salt Tolerant strains on plant growth parameters under both stressed and unstressed condition.
| Salt Tolerant Bacterial Strains | Pot Experiment under natural condition |
|||||
|---|---|---|---|---|---|---|
| Unstressed |
Stressed |
|||||
| RL (cm) | SL (cm) | DW (g) | RL (cm) | SL (cm) | DW (g) | |
| C | 5.53 ± 0.25a | 10.16 ± 0.15a | 0.56 ± 0.01a | 4.53 ± 0.30a | 8.4 ± 0.26a | 0.45 ± 0.02a |
| ST4 | 5.8 ± 0.1a | 11.76 ± 0.25c | 0.6 ± 0.01b | 5.1 ± 0.2b | 9.66 ± 0.15c | 0.53 ± 0.01b |
| ST5 | 6.6 ± 0.2b | 12 ± 0.5b | 0.62 ± 0.01b | 5.96 ± 0.15c | 10.3 ± 0.26d | 0.54 ± 0.06b |
| ST6 | 6.43 ± 0.15b | 12.36 ± 0.60f | 0.6 ± 0.01b | 5.86 ± 0.11c | 10.8 ± 0.26f | 0.53 ± 0.04b |
| ST15 | 6.4 ± 0.3b | 11.56 ± 0.11b | 0.6 ± 0.5b | 5.73 ± 0.5c | 9 ± 0.15b | 0.5 ± 0.51b |
| ST16 | 5.53 ± 0.20a | 12.26 ± 0.25f | 0.62 ± 0.02b | 5.2 ± 0.2b | 10.76 ± 0.25e | 0.50 ± 0.02b |
| ST17 | 6.26 ± 0.25b | 11.96 ± 0.15e | 0.59 ± 0.01b | 5.6 ± 0.1c | 10 ± 0.11d | 0.51 ± 0.48b |
| ST18 | 6.73 ± 0.25c | 11.76 ± 0.25d | 0.58 ± 0.03b | 5.36 ± 0.15b | 10.4 ± 0.2d | 0.52 ± 0.5b |
| ST20 | 6.26 ± 0.25b | 12.36 ± 0.20b | 0.6 ± 0.02b | 5.66 ± 0.35c | 10.83 ± 0.15f | 0.52 ± 0.003b |
| ST22 | 6.23 ± 0.25b | 11.26 ± 0.25b | 0.59 ± 0.05b | 5.53 ± 0.47b | 9.26 ± 0.25b | 0.51 ± 0.48b |
| ST25 | 6.56 ± 0.20b | 12.03 ± 0.15e | 0.6 ± 0.02b | 5.26 ± 0.46b | 9.63 ± 0.25c | 0.50 ± 0.5b |
Root Length (RL), Shoot Length (SL) and Dry Weight (DW).
3.4.2. Plant physiological attributes
Stress alters the physiological responses of plants, so in addition to study the plant growth parameters, it is important to understand the plant resilience and adaptation to change environmental conditions. Eight physiological indicators were studied to understand the effect of salinity and its amelioration by 10 ST bacterial strains.
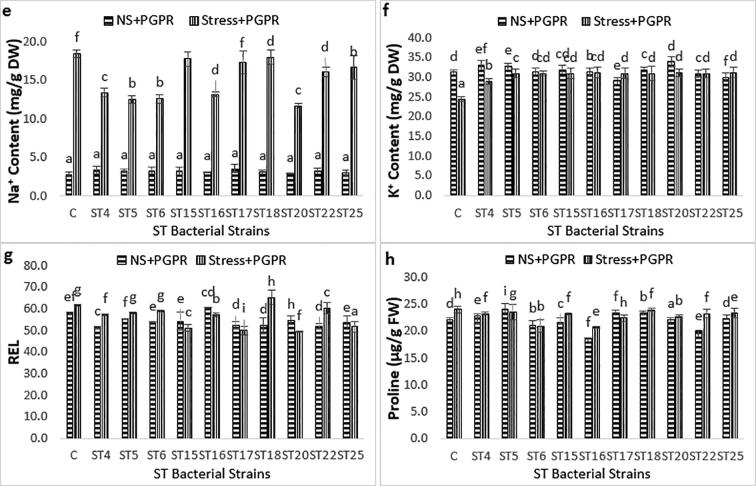
Chlorophyll concentration was highest in ST20 treated plants without stress while the best result was shown by ST16 under salt stress. The photosynthetic pigments (chlorophyll content) was significantly reduced under salinity condition (19%) while ST bacterial strains inoculation improved it significantly up to 34% (Fig. 2a). Leaf water potential (LWP) considerably decreased (became more negative) under salt stress compare to their respective control due to water loss. It is indicated that inoculated plants with ST strains enhanced osmotic potential over their respective control under stressed condition. ST16, ST20 showed maximum leaf water potential (14.6 -Mpa) with respect to stressed control (25.3 -Mpa) (Fig. 2b). The extent of salt-induced effects on relative water content (RWC) has been used as one of the critical water relation factors for determining plant salt tolerance. Maximum RWC is the indication of maximum salt tolerance of plants. Sodium chloride stress adversely affected RWC. Uninoculated control showed the RWC (55%) while maximum RWC (86%) was shown by ST5 under salt stress by combating the adverse effects of salinity (Fig. 2c). Leaf area is the good indices of stress expression as it is reported that plant respond to stress by affecting the leaf area without changing its biomass (Füzy et al., 2019). Salt stress reduced the leaf area up to 35% while up to 39% increase was induced by ST strains inoculation (Fig. 2d). Na+ content of controlled unstressed plants was 2.83 mg while stress lead it up to 18.41 mg while K+ concentration was noted 24.56 mg as compare to its corresponding control (31.24 mg). Plants treated with ST bacterial strains showed a significant decrease in Na+ content (up to 36.5%) while tremendous increase in K+ content (upto 28%) as shown in Fig (2e, f).
Fig. 2.
Effect of ST Bacterial Strains (ST4, ST5, ST6, ST15, ST16, ST17, ST18, ST20,ST22, ST25) on a) Chlorophyll Content Index (CCI), b) Leaf Water Potential (LWP), c) Relative Water Content (RWC), d) Leaf Area (LA), e) Na+ Content, f) K+ content, g) Relative Electrolyte Leakage (REL), h) Proline content under both stressed and unstressed natural condition in cotton plants. Values are mean of 03 values ± Standard Deviation. Bars with different letters show significant differences at P ≤ 0.05.
Salinity induces the production of reactive oxygen species (ROS), causing membrane injuries, protein degradation, and enzyme inactivation and thus induces oxidative stress which lead towards high value of electrolyte leakage (EL). ST bacterial strains treated plants had noticeably lower level (0.495) of EL as compared to corresponding control (0.618) which is the indicative of relative tolerance of salinity (Fig. 2g). There was no significant difference in proline content of ST4, ST5, ST6, ST15, ST17, ST18, ST20 and ST25 treated plants while ST6 and ST16 reduced it upto 14% and 15% respectively under stressed condition over respective control (Fig. 2h).
4. Discussion
Salt stress alters the number of physiological process of plants by nutrient disparity, protein synthesis and photosynthesis inhibition, altered levels of growth regulator that affects the plant growth and development which leads to gradual waning in crop productivity (Saghafi et al., 2018).
In the current study, we demonstrated that the salt stress significantly affects the vegetative growth parameters of a plant like percentage germination, shoot length, root length and dry weight as compared to the non-saline condition. A reduction in seed germination and other growth parameters has been reported in number of crops under salt stress i.e., Sulla carnosa (Hmaeid et al., 2019), wheat (Triticum aestivum) (Ansari et al., 2019) and rice (Oryza sativa) (Sarkar et al., 2018). Salt stressinhibits the synthesis of phytohormones like auxin and cytokinins in plants (Figueiredo et al., 2008) so, IAA producing ST PGPR can be an effective strategy to combat salinity. As salinity agitates the hormonal balance, hormonal homeostasis can be a possible mechanism of phytohormone induced salt tolerance of plants. Salt stress in relevancy of growth parameters can be mitigated by exogenous auxin production (Egamberdieva et al., 2015). Root associated microorganisms can affect the contents of phytohormone in plants.
In this study the 10 bacterial strains, isolated from different rhizospheric samples on the basis of their salt tolerance potential (up to 1 M NaCl) and IAA production were investigated for the auxin production under different salt concentration in vitro. IAA production initially increased by ST4, ST5, ST6, ST18 and ST22 with raising salt concentration (up to 500mMNaCl) and afterwards declined at high salt concentration (850 mM NaCl), Zhang et al (2019) demonstrated a similar result in which IAA production was initially increased by under salt stress (10 mg/ml), but higher levels of NaCl (20 mg/ml) reduced IAA production. Two of the salt tolerant bacterial strains (ST16 and ST20) showed the gradual decrease in IAA production with increasing salt concentration as reported by Ansari et al (2019) where 500 mM (NaCl concentration) led to the 51.6% reduction in IAA production as compare to control while 42.9% and 30.7% reduction was reported at lower salt concentration (250 mM, 125 mM, respectively).
These bacterial strains alleviated salt stress under gonotobiotic and natural condition by increasing the seed germination, root length and shoot length up to 31%, 17%, 34% in lab, 31% and 29% in natural condition respectively. Similar results have been reported by different researchers in which PGPR improved growth of Arachis hypogaea, Triticum aestivum and Chenopodium quinoa under salt stress (Alexander et al., 2020, Orhan, 2016, Yang et al., 2016)
In addition, the effect of bacterial inoculation on a number of physiological parameters was also observed. The photosynthetic pigments (chlorophyll content) was significantly reduced under salinity conditions. It was observed that slow synthesis or fast breakdown of pigments in cells was the cause of low level of photosynthetic pigment under salt stress (Ashraf. 2003) and in this study IAA producing ST bacteria revitalized it by conferring the salt tolerant ability to plants. Similar effect of salt tolerant PGPR on photosynthetic pigment protection of host plant were also reported in common bean and peanut (Abdelmoteleb and Gonzalez-Mendoza, 2020, Alexander et al., 2020).
When plants grow under the salt stress, they also suffer from physiological drought due to difficulty in withdrawing water from soil, owing to reduced soil matric and osmotic pressure consequently leads to severe decrease in leaf water potential (becomes more negative) (El-Hendawy et al., 2017) while inoculation of ST strains help the plants to alleviate this deleterious effects of salinity. Nawaz et al (2020) reported that halotolerant PGPR improved the water related attributes of Triticum aestivum. RWC is the direct reflection of a plant water status and its reduced level is the indication of plant water deficiency. An increase in soluble solutes induced negative effect on plant water relation by slowing the uptake of water and nutrients causing osmotic effects and toxicity (Jiang et al., 2014, Yang et al., 2009). In this investigation, the ST inoculated plants were able to adjust osmotically, leading to maintenance of RWC in contrast to uninoculated plants under salt stress. Similar increase in RWC was also reported in oat seedlings by Klebsiella sp. (Sapre et al., 2018).
High salinity levels reduce leaf area due to reduced turgescence caused by salt stress, which can inhibit cell division and expansion (Manivannan et al., 2007). Plants respond to the stress effect by reducing leaf area without losing biomass. So, this parameter can be a sensitive indicator of salt stress. ST bacterial strains help to maintain the leaf area under salt stress as compared to uninoculated plants. Improved leaf area was also reported in Arabidopsis thaliana by PGPR inoculation (Fan et al., 2020).
Plants' ability to maintain ion homeostasis in saline conditions is still regarded as a reliable pointer and an effective mechanism for salt tolerance. A number of studies have found that high external NaCl concentrations cause intense competition between ions for absorption at the site of ion uptake, particularly between Na+ and K+ ions, due to their similar physiochemical properties, which does not favor metabolic functions required for salt stress adaptation (Ashraf and Ashraf, 2016, Rasheed et al., 2014). In this study ion analysis revealed that there was dramatic increase in Na+ concentration under salt stress as compare to control while the inoculation by ST bacterial strains give the salt tolerance ability by coinciding them with higher affinity of K+ over Na+ in ion uptake. In accordance to our result similar increase in K+ content and decrease in Na+ content of PGPR inoculated plants have been reported in a number of studies (Sapre et al., 2018, Ali et al., 2014).
Electrolyte leakage (EL) is a pointer to the injury occurred to plasma membrane after exposure to stresses so, EL has been considered as a quantitative, reliable, reproducible and simple test for evaluating cell sustainability after heat, salt water, or even cold stresses (Jamal et al., 2014, Ullah et al., 2014). Plasma membrane get denature or aggregate under stress according to severity of stress resulting in hyperfluidity of membrane lipids (Guo et al., 2019). The present study showed that ST PGPR inoculation decreased the electrolyte leakage by improving the membrane stability index under salt stress as compare to control. Our results are in congruence with the report of Alexander (Alexander et al., 2020) where PGPR inoculation decreased the electrolyte leakage value in peanut.
Accumulation of compatible solutes under salt stress is one of the common physiological phenomena and proline is the common attuned solute that accumulates in response to changes in external osmotic potential. So, proline can be considered as one of the biochemical marker of salt stress. ST bacterial strains helped to maintain the proline content under stressed condition to the level of unstressed condition. To strengthen our finding that ST PGPR helped to lower the proline content similar results were also reported by number of studies (Adhikari et al., 2020, Sapre et al., 2018) in which the halotolerant PGPR inoculation lower the proline content in oat and soybean, respectively. In our study out of ten finally selected ST PGPR nine were Bacillus strains, our study coincided with previous findings where Bacillus species considered as predominant PGP bacteria (Akinrinlola et al., 2018, Radhakrishnan et al., 2017) and ST5, ST16 and ST20 isolated from rhizosphere of Solanum lycopersicum, Triticum aestivum and Allium sativum, respectively showed the most promising results. Due to the positive effects demonstrated by these bacterial strains under salinity conditions, must be evaluated in trial field experiments for further manipulation in crop production.
5. Conclusion
Result showed that ST inoculated plants are able to maintain their physio-mopholigical characters under salt stress not only in lab but also in field condition (EC: 10.51mS/cm). So, IAA producing ST bacterial strains which colonize the roots and helpful in improving the seed germination and growth parameters by improving the number of physiological functions like mitigating the osmotic stress, increasing the absorbability of K+ and decreasing the absorption of Na+ and maintaining the proline content, CCI, RWC and EL in inoculated plants may be a good source of cotton growth promotion under salt stress in field condition. By keeping the data analysis of cotton loss in recent years in Southern Punjab, our results are of great importance to improve cotton yield qualitatively as well as quantitatively.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.05.056.
Contributor Information
Sarwat Saleem, Email: Sarwat.1645@wum.edu.pk.
Atia Iqbal, Email: atiaiqbal01@gmail.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abbas R., Rasul S., Aslam K., Baber M., Shahid M., Mubeen F., Naqqash T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King. Saud. Univ. Sci. 2019;4:1195–1201. [Google Scholar]
- Abdelmoteleb A., Gonzalez-Mendoza D. Isolation and identification of phosphate solubilizing Bacillus spp. from Tamarix ramosissima rhizosphere and their effect on growth of Phaseolus vulgaris under salinity stress. Geomicrobiol. J. 2020;23:1–8. [Google Scholar]
- Adhikari A., Khan M.A., Lee K.E., Kang S.M., Dhungana S.K., Bhusal N., Lee I.J. The halotolerant rhizobacterium—Pseudomonas koreensis MU2 enhances inorganic silicon and phosphorus use efficiency and augments salt stress tolerance in Soybean (Glycine max L.) Microorganisms. 2020;8:1256–1263. doi: 10.3390/microorganisms8091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinrinlola R.J., Yuen G.Y., Drijber R.A., Adesemoye A.O. Evaluation of Bacillus strains for plant growth promotion and predictability of efficacy by in vitro physiological traits. Int. J. Microbiol. 2018;18:1–11. doi: 10.1155/2018/5686874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A., Singh V.K., Mishra A. Halotolerant PGPR Stenotrophomonas maltophilia induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front. Microbiol. 2020;11:2525–2532. doi: 10.3389/fmicb.2020.568289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Charles T.C., Glick B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant. Physiol. Biochem. 2014;80:160–167. doi: 10.1016/j.plaphy.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Ansari F.A., Ahmad I., Pichtel J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil. Ecol. 2019;143:45–54. [Google Scholar]
- Ashraf M. Relationships between leaf gas exchange characteristics and growth of differently adapted populations of Blue panicgrass (Panicum antidotale Retz.) under salinity or waterlogging. Plant. Sci. 2003;165:69–75. [Google Scholar]
- Ashraf M.A., Ashraf M. Growth stage-based modulation in physiological and biochemical attributes of two genetically diverse wheat (Triticum aestivum L.) cultivars grown in salinized hydroponic culture. Environ. Sci. Pollut. Res. 2016;23:6227–6243. doi: 10.1007/s11356-015-5840-5. [DOI] [PubMed] [Google Scholar]
- Banik A., Pandya P., Patel B., Rathod C., Dangar M. Characterization of halotolerant, pigmented, plant growth promoting bacteria of groundnut rhizosphere and its in-vitro evaluation of plant-microbe protocooperation to withstand salinity and metal stress. Sci. Total. Environ. 2018;630:231–242. doi: 10.1016/j.scitotenv.2018.02.227. [DOI] [PubMed] [Google Scholar]
- Bashan, Y., Kloepper, J.W., de-Bashan, L.E. and Nannipieri, P., 2016. A need for disclosure of the identity of microorganisms, constituents, and application methods when reporting tests with microbe-based or pesticide-based products. Biol. Fert. Soils. 52, 283–284.
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant. Soil. 1973;39:205–207. [Google Scholar]
- Batool S., Iqbal A. Phosphate solubilizing rhizobacteria as alternative of chemical fertilizer for growth and yield of Triticum aestivum (Var. Galaxy 2013) Saudi. J. Biol. Sci. 2019;26:1400–1410. doi: 10.1016/j.sjbs.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawwam G.E., Elbeltagy A., Emara H.M., Abbas I.H., Hassan M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci. 2013;58:195–201. [Google Scholar]
- Egamberdieva, D., Davranov, K., Wirth, S., Hashem, A., Abd Allah, E.F., 2017. Impact of soil salinity on the plant-growth–promoting and biological control abilities of root associated bacteria. Saudi. J. Biol. Sci. 24, 1601–1608. [DOI] [PMC free article] [PubMed]
- Egamberdieva D., Jabborova D., Hashem A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi. J. Biol. Sci. 2015;22:773–779. doi: 10.1016/j.sjbs.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D., Kucharova Z., Davranov K., Berg G., Makarova N., Azarova T., Chebotar V., Tikhonovich I., Kamilova F., Validov S.Z., Lugtenberg B. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fert. Soils. 2011;47:197–205. [Google Scholar]
- Egamberdiyeva, D., Islam, K.R., 2008. Salt-tolerant rhizobacteria: plant growth promoting traits and physiological characterization within ecologically stressed environments. In: Plant-Bacteria Interactions: Strategies and techniques to promote plant growth, pp. 257–281.
- El-Hendawy S.E., Hassan W.M., Al-Suhaibani N.A., Refay Y., Abdella K.A. Comparative performance of multivariable agro-physiological parameters for detecting salt tolerance of wheat cultivars under simulated saline field growing conditions. Front. Plant. Sci. 2017;8:435–450. doi: 10.3389/fpls.2017.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal M. Inoculation of plant growth promoting bacteria Ochrobactrum intermedium, Brevibacterium sp. and Bacillus cereus induce plant growth parameters. J. Appl. Biotech. 2013;1:45–53. [Google Scholar]
- Fan D., Subramanian S., Smith D.L. Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci. Rep. 2020;10:1–18. doi: 10.1038/s41598-020-69713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . FAO; Rome: 2020. Salt-Affected Soils [Online] [Google Scholar]
- Figueiredo M.V., Burity H.A., Martínez C.R., Chanway C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil. Ecol. 2008;40:182–188. [Google Scholar]
- Füzy A., Kovács R., Cseresnyés I., Parádi I., Szili-Kovács T., Kelemen B., Rajkai K., Takács T. Selection of plant physiological parameters to detect stress effects in pot experiments using principal component analysis. Acta. Physiol. Plant. 2019;41:56–64. [Google Scholar]
- Guo Q., Liu L., Barkla B.J. Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 2019;17:4264–4295. doi: 10.3390/ijms20174264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmaeid N., Wali M., Mahmoud O.M.B., Pueyo J.J., Ghnaya T., Abdelly C. Efficient rhizobacteria promote growth and alleviate NaCl-induced stress in the plant species Sulla carnosa. Appl. Soil. Ecol. 2019;133:104–113. [Google Scholar]
- Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T., S.T.W., 1994. Bergey's Manual of Determinative Bacteriology, ninth ed.
- Iqbal A., Hasnain S. Auxin producing Pseudomonas strains: biological candidates to modulate the growth of Triticum aestivum beneficially. Am. J. Plant. Sci. 2013;4:1693–1700. [Google Scholar]
- Jamal A., Shahid M.N., Aftab B., Rashid B., Sarwar M.B., Mohamed B.B., Hassan S., Husnain T. Water stress mediated changes in morphology and physiology of Gossypium arboreum (var FDH-786) J. Plant. Sci. 2014;2:179–186. [Google Scholar]
- Jiang X., Qi W., Xu X., Li Y., Liao Y., Wang B. Higher soil salinity causes more physiological stress in female of Populus cathayana cuttings. Sheng Tai Xue Bao. 2014;34:225–231. [Google Scholar]
- Katam R., Sakata K., Suravajhala P., Pechan T., Kambiranda D.M., Naik K.S., Guo B., Basha S.M. Comparative leaf proteomics of drought-tolerant and-susceptible peanut in response to water stress. J. Proteom. 2016;143:209–226. doi: 10.1016/j.jprot.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Mahmood A., Amaya R., Turgay O.C., Yaprak A.E., Taniguchi T., Kataoka R. High salt tolerant plant growth promoting rhizobacteria from the common ice-plant Mesembryanthemum crystallinum L. Rhizosphere. 2019;9:10–17. [Google Scholar]
- Manivannan P., Jaleel C.A., Sankar B., Somasundaram R., Murali P.V., Sridharan R., Panneerselvam R. Salt stress mitigation by calcium chloride in Vigna radiata (L.) Wilczek. Acta. Biol. Cracov. Bot. 2007;49:105–109. [Google Scholar]
- Meena V.S., Maurya B.R., Verma J.P., Aeron A., Kumar A., Kim K., Bajpai V.K. Potassium solubilizing rhizobacteria (KSR): isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015;81:340–347. [Google Scholar]
- Nawaz A., Shahbaz M., Imran A., Marghoob U., Imtiaz M., Mubeen F., Khan A.U. Potential of salt tolerant PGPR in growth and yield augmentation of wheat (Triticum aestivum L.) under saline conditions. Front. Microbiol. 2020;11:1–12. doi: 10.3389/fmicb.2020.02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan F. Alleviation of salt stress by halotolerant and halophilic plant growth promoting bacteria in wheat (Triticum aestivum) Braz. J. Microbiol. 2016;47:621–627. doi: 10.1016/j.bjm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten C.L., Glick B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996;42:207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan, R., Hashem, A., Abd Allah, E. F., 2017. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 8, 667–681. [DOI] [PMC free article] [PubMed]
- Rasheed R., Ashraf M.A., Parveen S., Iqbal M., Hussain I. Effect of salt stress on different growth and biochemical attributes in two canola (Brassica napus L.) cultivars. Commun. Soil. Sci. Plan. 2014;45:669–679. [Google Scholar]
- Rehman A., Jingdong L., Chandio A.A., Hussain I., Wagan S.A., Memon Q.U.A. Economic perspectives of cotton crop in Pakistan: A time series analysis (1970–2015)(Part 1) J. Saudi. Soc. 2019;18:49–54. [Google Scholar]
- Saghafi D., Ghorbanpour M., Lajayer B.A. Efficiency of Rhizobium strains as plant growth promoting rhizobacteria on morpho-physiological properties of Brassica napus L. under salinity stress. J. Soil Sci. Plant. Nutr. 2018;18:253–268. [Google Scholar]
- Sapre S., Gontia-Mishra I., Tiwari S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa) Microbiol. Res. 2018;206:25–32. doi: 10.1016/j.micres.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Sarkar A., Ghosh P.K., Pramanik K., Mitra S., Soren T., Pandey S., Mondal M.H., Maiti T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018;169:20–32. doi: 10.1016/j.resmic.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Scholander P.F., Bradstreet E.D., Hemmingsen E.A., Hammel H.T. Sap pressure in vascular plants: negative hydrostatic pressure can be measured in plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Ullah K., Khan N.U., Khan S.J., Khan M.I., Khan I.U., Gul S., Khan R.U. Cell membrane thermo-stability studies through joint segregation analysis in various wheat populations. Pak. J. Bot. 2014;46:1243–1252. [Google Scholar]
- Yang A., Akhtar S.S., Iqbal S., Amjad M., Naveed M., Zahir Z.A., Jacobsen S.E. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant. Biol. 2016;43:632–642. doi: 10.1071/FP15265. [DOI] [PubMed] [Google Scholar]
- Yang F., Xiao X., Zhang S., Korpelainen H., Li C. Salt stress responses in Populus cathayana Rehder. Plant. Sci. 2009;176:669–677. [Google Scholar]
- Zhang S., Gan Y., Xu B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant. Biol. 2019;1:22–39. doi: 10.1186/s12870-018-1618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.