Abstract
We evaluated the effectiveness of daily chlorhexidine gluconate (CHG) bathing in decreasing skin carriage of Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae (KPC) among long-term acute care hospital patients. CHG bathing reduced KPC skin colonization, particularly when CHG skin concentrations greater than or equal to 128 μg/mL were achieved.
Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae (KPC) are increasingly common in healthcare facilities, with particularly high colonization rates among long-term acute care hospital (LTACH) patients.1,2 From November 2011 to June 2013, we instituted a bundled infection control intervention at 4 LTACHs with high baseline prevalence of KPC infection; the bundle included active surveillance for rectal colonization with KPC, geographic separation of patients with KPC colonization (cohort floor or private room), a hand hygiene improvement campaign, and routine daily chlorhexidine gluconate (CHG) bathing of all patients.
In addition to gastrointestinal tract carriage, most LTACH patients who carry KPC also have skin colonization,3 which heightens the risk of healthcare worker hand contamination and patient-to-patient spread. CHG bathing is theoretically useful in reducing KPC skin burden. However, of concern is that KPC can have CHG minimum inhibitory concentrations (MICs) 10-fold or more higher than that of gram-positive organisms such as Staphylococcus aureus.4,5 To assess the effectiveness of CHG bathing as a component of a KPC control bundle in LTACHs, we evaluated patients before and after their daily CHG bath to determine rates of skin colonization with KPC as well as CHG skin concentrations achieved.
METHODS
As a part of a KPC control intervention, patients at 4 Chicago-area LTACHs received daily CHG bathing with no-rinse, 2% CHG-impregnated cloths (Sage Products), which was performed by certified nursing assistants (CNAs) employed by the hospital. Each CNA received introductory and periodic bathing instruction from study personnel.
To monitor the quality of CHG baths, we measured the concentration of CHG on KPC-positive patients’ skin and cultured skin for KPC approximately 15 minutes before and after a routine CHG bath. We identified LTACH patients who had test results that were positive for KPC rectal carriage through active surveillance. From this cohort, we randomly selected patients, approximately 4 per month, from May 2012 to June 2013. Patients admitted to the hospital within 72 hours or previously selected were excluded. Patient characteristics, including age, sex, facility length of stay, body mass index (BMI, defined as weight in kilograms divided by the square of height in meters; obesity defined as BMI greater than or equal to 30), the presence of diarrhea (2 or more liquid stools within the previous 24 hours), and the presence of a tracheostomy or gastrostomy tube were recorded.
Study personnel obtained swab samples from 5 intact skin sites (inguinal, upper back, antecubital, axilla, and neck) at risk for KPC colonization.3 Specimens for CHG concentration determination (skin surface area, 100 cm2) were tested using a semiquantitative colorimetric assay;6 from adjacent skin (100 cm2), culture specimens were obtained, with KPC testing performed as described previously.3 blaKPC was confirmed by polymerase chain reaction.3 We dichotomized CHG skin concentrations at less than 128 μg/mL versus greater than or equal to 128 μg/mL, which was the concentration of CHG, determined by agar dilution,7 that inhibited growth of 90% (MIC90) of 53 unique KPC isolates identified before bathing from the study patients’ skin.
Bivariable analysis was performed using Fisher exact or Kruskal-Wallis tests; multivariable analysis was performed using Cochran-Mentel-Haenszel statistics (SAS 9.1.3 [SAS Institute]). This project was approved by the Rush University Medical Center institutional review board.
RESULTS
Sixty-two LTACH patients participated in this study; 43% were male. The median length of stay was 29 days, with a range of 5–589 days (interquartile range [IQR], 17–51 days). The median age was 63 years (IQR, 52.5–76 years); the median BMI was 24.3 (IQR, 20.3–29.9). The majority of patients had a tracheostomy (73%) and gastrostomy (90%); 43% had diarrhea.
Although all study patients received routine CHG bathing before study enrollment, skin contamination with KPC was common. Thirty-five (56%) of 62 patients had at least 1 skin site positive for KPC immediately before bathing, versus 20 (32%) of 62 patients after bathing (P = .01). Colonization rates before bathing differed across skin sites (P < .001), with 39% of axillary and 37% of inguinal sites colonized with KPC (Table 1). Post-bath KPC colonization rates were 10% and homogeneous across skin sites, representing a 51% decrease from rates before bathing (P < .001). Notably, the neck colonization rate was unchanged (P = .40).
TABLE 1.
Klebsiella pneumoniae Carbapenemase–Producing Enterobacteriaceae (KPC) Culture Positivity and Chlorhexidine Gluconate (CHG) Concentrations, by Skin Site
| Variable | Inguinal | Back | Antecubital | Axilla | Neck | P |
|---|---|---|---|---|---|---|
| KPC positive, % | ||||||
| Before bath | 37 | 8 | 10 | 39 | 8 | <.001 |
| After bath | 15 | 5 | 5 | 11 | 15 | .16 |
| CHG concentration, median μg/mL | ||||||
| Before bath | 312.5 | 19.5 | 58.6 | 156.3 | 14.7 | <.001 |
| After bath | 1,250.0 | 234.4 | 312.5 | 625.0 | 78.1 | <.001 |
| CHG concentration ≥128 μg/mL, % | ||||||
| Before bath | 81 | 23 | 27 | 61 | 6 | <.001 |
| After bath | 97 | 66 | 77 | 84 | 47 | <.001 |
NOTE. P value tests the null hypothesis that all body sites have the same proportion or value.
The median concentration of CHG on skin was higher among patients after bathing compared with before bathing (312.5 vs 78.1 μg/mL; P < .001) but differed across body sites (P < .001); inguinal and axillary skin sites had the highest median CHG values (Table 1). The proportion of skin sites with CHG concentrations of at least 128 μg/mL was higher among patients after bathing than before bathing (74% vs 40%; P < .001), although skin site–specific differences were present for both before- and after-bathing groups (P < .001; Table 1). Controlling for skin site, a CHG concentration of 128 μg/mL or greater conferred a relative risk [RR] of 0.51 (95% confidence interval [Cl], 0.34–0.76; Figure 1) for skin colonization with KPC.
FIGURE 1.
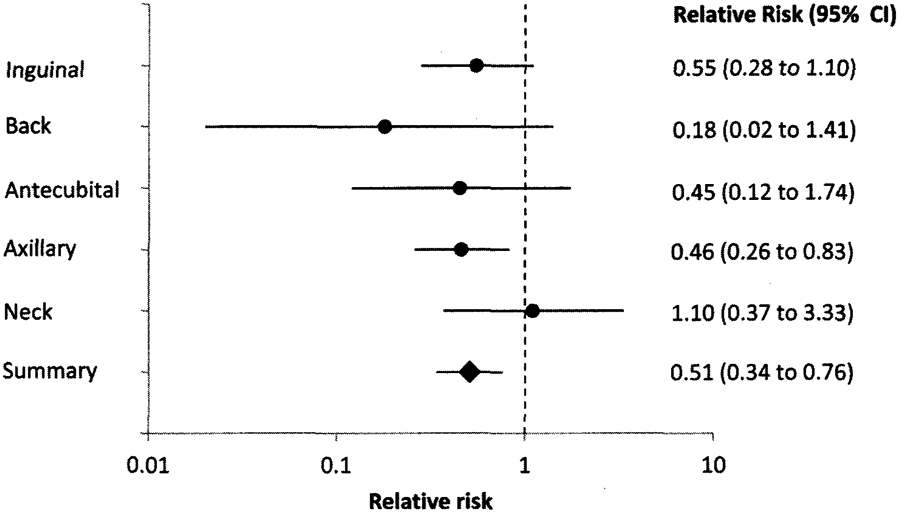
Relative risk of recovering Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae (KPC) on skin sites when achieving a chlorhexidine gluconate skin concentration of 128 μg/mL or greater, compared with less than 128 μg/mL. Relative risk of less than 1 is protective; achieving a higher chlorhexidine gluconate skin concentration led to a lower risk of recovering KPC on the skin. CI, confidence interval.
Certain patient characteristics increased the likelihood of skin colonization by KPC. The presence of diarrhea increased the risk of KPC skin colonization in the inguinal skin site (RR, 2.6 [95% CI, 1.3–5.1; P = .005), but not at other skin sites. Similarly, of the 44 patients with a tracheostomy, 9 (20%) of the patients before bathing and 5 (11%) of the patients after bathing were colonized with KPC at the neck site, whereas none of the tracheostomy-free patients had KPC neck colonization (P = .02). Age, sex, obesity, and the presence of a gastrostomy tube were not associated with increased risk of KPC skin colonization.
DISCUSSION
We assessed the effectiveness of daily CHG bathing in reducing skin colonization with KPC, in the context of a multifaceted KPC control intervention among LTACH patients. We found that CHG bathing reduced the likelihood of skin colonization by KPC, particularly if a CHG skin concentration of 128 μg/mL or more was achieved. However, we still commonly found KPC on patients’ skin, particularly at the inguinal and axillary sites, and more often before the daily bath, when CHG skin concentrations were lowest.
blaKPC-positive K. pneumoniae multilocus sequence type (ST) 258, which is the most common lineage of KPC-producing Enterobacteriaceae worldwide, may have reduced susceptibility to CHG compared with other multidrug-resistant K. pneumoniae isolates.4 Although the CHG MICs of intensive care unit gram-positive organisms such as S. aureus, coagulase-negative staphylococci, and enterococcus are typically 4 μg/mL or less,5,8 ST258 isolates have CHG MICs ranging from 32 to 256 μg/mL.4 Meticulous attention to proper bathing technique (ie, gently but firmly scrubbing each area of skin for 20 seconds with CHG-impregnated cloths) may be necessary for CHG bathing to be an effective component of a KPC control program. When 2 patients from the eligible study population with skin colonized with KPC were bathed by study personnel using proper technique, the 5 skin sites had a median after-bath CHG concentration of 1,250 μg/mL and none grew KPC (data not shown).
Bathing quality differed across skin sites, with the back, neck, and antecubital skin sites having lower CHG concentrations. The presence of diarrhea (presumably increasing fecal skin contamination) appeared to particularly affect inguinal KPC carriage. Additionally, we speculate that the axillary and inguinal skin sites, which are moist and rich in apocrine glands, may represent microbial niches that are particularly favorable for long-term colonization with KPC.9 The presence of tracheostomy appears to be a strong risk factor for neck colonization by KPC.
Some limitations exist. This evaluation was performed in LTACHs, and results may not be generalizable to short-stay acute care hospitals. Furthermore, we did not score the quality of CHG bathing on a routine basis, nor did we directly observe the quality and timing of the proximal bath before study enrollment. Aside from initial and periodic training, bathing personnel performed CHG bathing in a routine manner.
In conclusion, CHG bathing reduces the skin burden of KPC, although its effectiveness varies by skin site. Our findings stress the importance of monitoring adequacy of CHG bathing and of assessing whether, at some body sites, KPC has advanced from transient to resident flora.
ACKNOWLEDGMENTS
We thank Monica K. Sikka, MD, and Caroline J. Thurlow, MD, for coordinating and collecting a subset of patient samples. We thank the patients and staff of participating hospitals for their assistance and cooperation.
Financial support.
This study was supported in part by the Centers for Disease Control and Prevention (CDC) Epicenters program ( U54CK000161 to R.A.W.) and CDC contract number 200–2011-M-41103 (to M.K.H.). Sage Products provided 2% chlorhexidine gluconate-impregnated cloths to participating hospitals at no charge.
Footnotes
Potential conflicts of interest. M.K.H. and R.A.W. report having received previous research funding from Sage Products. All other authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
REFERENCES
- 1.Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 2.Lin MY, Lyles RD, Lolans K, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2013;57:1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurlow CJ, Prabaker K, Lin MY, Lolans K, Weinstein RA, Hayden MK. Anatomic sites of patient colonization and environmental contamination with Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae at long-term acute care hospitals. Infect Control Hosp Epidemiol;2013;34(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naparstek L, Carmeli Y, Chmelnitsky I, Banin E, Navon-Venezia S. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect 2012;81(1):15–19. [DOI] [PubMed] [Google Scholar]
- 5.McDanel JS, Murphy CR, Diekema DJ, et al. Chlorhexidine and mupirocin susceptibilities of methicillin-resistant staphylococcus aureus from colonized nursing home residents. Antimicrob Agents Chemother 2013;57(1):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg 2008;207(2):233–239. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, Eighth Edition. Wayne, PA: CLSI, 2009. [Google Scholar]
- 8.Popovich KJ, Lyles R, Hayes R, et al. Relationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically ill patients bathed daily with chlorhexidine gluconate. Infect Control Hosp Epidemiol 2012;33(9):889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011;9(4):244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]


