Abstract
Vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV‑2) has become a major tool in the battle against the coronavirus disease 2019 (COVID-19) pandemic. Numerous products have been developed and more are to come. Vaccination success varies greatly between different countries. There are a number of different vaccine types, such as mRNA, DNA vaccines, adenovirus vector vaccines, and full-length spike protein nanoparticles with a special matrix. The different types may also cause a different spectrum of adverse events. With mass vaccination, post-marketing surveillance for product safety becomes increasingly important. In this review, we discuss possible hypersensitivity and cutaneous adverse events related to SARS-CoV‑2 vaccination—from local reactions like COVID arm to systemic and severe reactions like anaphylaxis. Vaccination may also induce or exacerbate preexisting disorders such as herpes zoster infection. This review should provide information to tailor, whenever possible, vaccination to patients’ needs. It is a contribution to patient safety as well. There is general consensus that the benefits of SARS-CoV‑2 vaccination currently outweigh the risks of possible adverse events.
Keywords: Patient safety, Post-marketing, Surveillance, Risk-benefit considerations
Abstract
Die Impfung gegen SARS-CoV‑2 (Severe Acute Respiratory Syndrome Corona Virus 2) ist zu einem wesentlichen Instrument im Kampf gegen die COVID-19(coronavirus disease 2019)-Pandemie geworden. Viele unterschiedliche Vakzine sind bisher entwickelt worden, und weitere werden folgen. Der Impffortschritt in den verschiedenen Staaten variiert erheblich. Die zur Verfügung stehenden Vakzine umfassen mRNA- und DNA-Vakzine, Adenovirus-Vektor-Vakzine und Spike-Protein-Nanopartikel in einer speziellen Matrix. Diese unterschiedlichen Produkte können auch unterschiedliche Spektren möglicher Nebenwirkungen verursachen. Im Zuge von Massenimpfungen werden Post-Marketing-Daten zur Produktsicherheit zunehmend wichtiger. In der vorliegenden Übersicht werden mögliche allergische und andere kutane Nebenwirkungen mit Bezug zur SARS-CoV-2-Vakzinierung diskutiert – von lokalen Reaktionen wie dem „COVID-Arm“ bis zu systemischen und schwerwiegenderen Reaktionen wie der Anaphylaxie. Die Impfung kann auch Dermatosen induzieren oder präexistente Hauterkrankungen exazerbieren lassen – wie den Herpes zoster. Mit dieser Übersicht werden Informationen bereitgestellt, die bei der individuellen Auswahl der Vakzine behilflich sein sollen. Sie ist auch ein Beitrag zur Patientensicherheit. Es besteht ein allgemeiner Konsens, dass derzeit der Nutzen einer Impfung gegen SARS-CoV‑2 höher eingeschätzt wird als das Risiko möglicher Nebenwirkungen.
Schlüsselwörter: Patientensicherheit, Post-Marketing, Kontrolle, Nutzen-Risiko-Abwägungen
Introduction
Introducing vaccination programs for severe acute respiratory syndrome coronavirus type 2 (SARS-CoV‑2) nourished the hope of a control of the coronavirus disease 2019 (COVID-19) pandemic [1]. A number of different vaccines have been developed and approved by various medical bodies (Table 1). With the increasing number of people who have been vaccinated, the reports on possible adverse events grow and gain public attention [2]. Adverse events following immunization (AEFI) do not necessarily have a causal relationship to vaccination. However, it is important to get an overview of possible adverse events so that vaccination can be more specifically tailored to the needs of the individual patient. This will increase patient safety and may help to further improve vaccine development.
Table 1.
SARS-CoV‑2 vaccines
| Vaccine | Company | Remarks |
|---|---|---|
| BNT162b2 | BioNTec/Pfizer, Germany & US | Nucleoside-modified mRNA encoding viral spike glycoprotein of SARS-CoV‑2 |
| mRNA-1273 | Moderna; US | Nucleoside-modified mRNA encoding viral spike glycoprotein of SARS-CoV‑2 |
| AZD1222 | AstraZeneca, Sweden & UK | Recombinant, replication-deficient chimpanzee adenovirus vector encoding SARS-CoV‑2 spike glycoprotein |
| Ad26.CoV‑2.S | Janssen-Cilag/Johnson & Johnson, US & Belgium | Human adenovirus serotype 26 carrying the spike glycoprotein of SARS-CoV‑2 |
| Gam-COVID-Vac | Gamaleya, Russia (Sputnik V) | Human adenovirus serotype 26 (first shot) and serotype 5 (second shot) carrying the spike glycoprotein of SARS-CoV‑2 |
| Ad5-nCoV | CanSino, China | Recombinant adenovirus serotype 5 carrying the spike glycoprotein of SARS-CoV‑2 |
| NVX-CoV2373 | Novavax, US | Full-length spike protein nanoparticle plus matrix M1, saponin-based adjuvant |
| BBV-152 | Bharat, India | Based on whole inactivated SARS-CoV‑2 |
| CoronaVAC | SinoVac, China | Based on whole inactivated SARS-CoV‑2 |
| Co-VLP | Medicago, Canada & Italy | Spike proteins aggregated as virus-like particles, tobacco plant-based adjuvant |
| ZF2001 | Zhifei Longcom | Recombinant tandem-repeat dimeric receptor binding domain-based protein subunit vaccine |
| INO-4800 | Inovio, US | DNA vaccine delivered intradermally with CELLECTRA® electroporation (EP) delivery system |
Allergy except anaphylaxis
Ring et al. defined allergy as follows: “Occurrence of objectively reproducible symptoms or signs initiated by exposure to a defined stimulus at a dose tolerated by normal individuals” [3]. This broad definition would include immune-mediated and non-immune reactions such as pseudo-allergies. There have been several reports on allergic/hypersensitivity reactions to SARS-CoV‑2 vaccines.
Self-reported acute allergic reactions to COVID mRNA vaccines have been observed in 2.10% among 64,900 vaccinated persons. The rate was higher in those who received Moderna mRNA-1273 compared to BioNTec/Pfizer BNT162b2: 2.20% vs. 1.95% [4].
Among 43,448 patients who received the BNT162b2 vaccine from BioNTec/Pfizer, local reactions such as soft tissue swelling or redness were observed in 5–6% of patients. The C4591001 trial, however, did not separate immediate and delayed reactions [5].
The COVE study group reported local hypersensitivity reactions known as COVID arm in 0.8% of patients after the first vaccination and in 0.2% after the second shot of mRNA-1273 (Moderna) among 15,210 patients [6]. The pathogenesis of COVID arm is thought to be a T-cell type IV immune reaction [7].
Ramos and Kelso (2021) reported 12 patients with delayed inflammatory injection site reactions after vaccination with both mRNA vaccines (11 × Moderna, 1 × BioNTec/Pfizer). The average time to onset of symptoms was 7 days after vaccination and persistence for 3–8 days. In their experience, they did not reappear after the second dose [8].
In a study from Saudi Arabia with 455 persons who received the BNT162b2 vaccine, local swellings and redness were noted in 0.8% after the first shot only. Local hypersensitivity reactions occurred in 8.0% after the first vaccination and in 14.5% after the second injection [9].
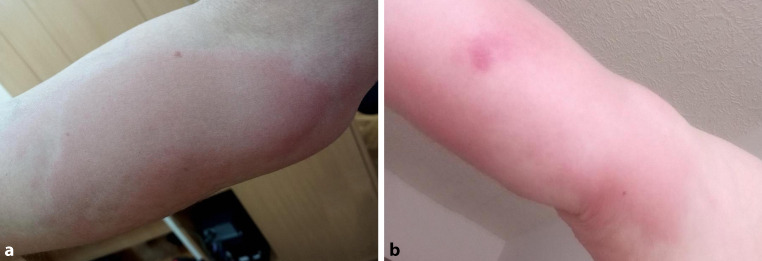
Johnston et al. (2021) reported a series of 16 patients demonstrating a delayed localized cutaneous reaction to mRNA-1273. The onset of symptoms was 2–12 days after vaccination (mean 7 days). Patients presented with a pruritic tender and erythematous or pink plaque on the arm used for vaccination [10]. This reaction should not be mistaken for the local injection site reaction with pain, swelling, and redness on days 1–3. Most patients (15/16) developed the hypersensitivity after the second dose (Fig. 1). The treatment consisted of topical corticosteroids, oral antihistamines, and cold compresses. On histology, mild perivascular and focal interstitial inflammatory infiltrate of lymphocytes and eosinophils was noted.
Fig. 1.
COVID arms of two different patients (a, b) after vaccination with CoronaVac. Erythematous, pruritic plaque persistent for 3–4 days
The international registry of cutaneous manifestations of SARS-CoV‑2 is a collaboration between the American Academy of Dermatology and the International League of Dermatological Societies. During the first 3 months of the COVID epidemic, they recorded 414 cutaneous reactions to mRNA COVID-19 vaccines from Moderna (83%) and BioNTec/Pfizer (17%). Delayed large local reactions were most common, followed by local injection site reactions. Second-dose recurrence was noted in 43% of patients [11].
In a phase II trial with the Ad5-vectored COVID-19 vaccine from China, injection site reactions such as induration, redness, swelling, and pruritus were recorded in 1–5%. Hypersensitivity reactions were not documented [12]. In phase I and II trials with ZF2001, injection site pain, swelling, and redness was observed in 4–8% for two doses and in up to 14% for three vaccination doses [13]. In a Sputnik V phase I/II trial, local itching and pain at the injection site and hives in a single patient were reported, but no delayed hypersensitivity reactions [14]. With the ChAdOx1 nCoV-19 vaccine, a single case has been observed with a delayed immune reaction [7].
A 26-year-old woman developed a fixed drug eruption after first vaccination with BNT162b2, which was challenged after the second shot. The second time the redness was darker, and she developed vesiculations with a targetoid shape. Topical corticosteroids improved pruritus and redness. A skin biopsy revealed a lichenoid interface dermatitis confirming the clinical diagnosis of a fixed drug eruption [15]. Three similar cases have been reported in middle-aged women after the second shot of BNT162b2, one with vesiculation [16].
Another differential diagnosis to painful local hypersensitivity reactions is subacromial-subdeltoid bursitis (SIRVA) due to unintentional vaccine injection into the bursa [17].
Anaphylaxis
Anaphylaxis is a typical type I immune reaction. It presents the most severe type of a hypersensitivity and is caused by activation of mast cells and basophils via binding of cell membrane receptors to immunoglobulin E (IgE) antibodies. Subsequent release of inflammatory mediators (histamine, tryptase, cytokines, and chemokines) leads to a rapid progression from mild symptoms (such as pruritus, urticaria, headache, metallic taste, and disorientation) to life-threatening symptoms such as mucosal swelling, tachycardia, bronchoconstriction, vomiting and diarrhea, seizures, vascular permeability, and shock. At least two organs should be involved to confirm the diagnosis. It warrants aggressive treatment to prevent disease-specific mortality [18].
Anaphylactic reactions to vaccines are very rare in general, with a frequency of about 1 per 1,000,000 doses [19]. The Centers for Disease Control (CDC) have reported anaphylaxis rates of 4.7 and 2.5 per 1,000,000 doses for the BioNTec/Pfizer (BNT162b2) and Moderna vaccines (mRNA-1273), respectively [20]. Data reported from the UK are much lower, with 0.027% for BioNTec/Pfizer and 0.023% for Moderna [4]. The reason for this discrepancy is not completely clear, but in the study from Blumenthal et al. (2021), only employees were considered, no pensioners [4].
About three quarters of anaphylactic reactions occur within 15 min after vaccination.
Both of these vaccines are mRNA vaccines consisting of non-replicating and non-infectious RNA in a special formulation containing polyethylene glycol (PEG). The European Anaphylaxis Registry contains more than 13,000 cases of anaphylaxis from Europe and Brazil of the years between 2007 and 2020. Here, 14 cases of reactions to various PEG-containing vaccines and 7 reactions to PEG were recorded. The rate of atopic patients among those with PEG anaphylaxis reached 29% (vaccines) and 49% (PEG without vaccines) [21].
High-molecular weight PEG seems to pose a higher risk for this adverse event [22]. Usually, type I reactions are IgE mediated, but other mechanisms of anaphylaxis have been considered as well [23, 24]. PEG bound to lipid nanoparticles can activate basophil leukocytes [25]. Possible “allergic” constituents other than PEG include polysorbate, tromethamine (hydroxymethyl)aminomethane (TRIS) used as a buffer ingredient, and glycerophospholipids which are substrates for phospholipases A2 which is known for proinflammatory activity. Glycerophospholids are a constituent of mRNA-1273 [26]. Lipid nanoparticles may directly activate mast cells after phagocytosis or activate complement components [23].
The potential cause of immediate allergic reactions to recombinant ChAdOx1‑S from AstraZeneca could be polysorbate 80 [27]. Cross-reactions to PEG have occasionally been observed [7].
Pre-vaccination screening for PEG sensitization has been performed by Paoletti et al. (2021) and Banerji et al. (2021) [28, 29]. Paoletti et al. (2021) screened about 10% of their whole vaccination population and detected a single person with a positive reaction to PEG to be excluded from vaccination. Although test protocols and substances used for testing are yet not standardized nor validated, they conclude that their screening helped to reduce anaphylactic reactions to mRNA vaccines [29]. The uncertainty with unvalidated test protocols, however, has raised concerns [30].
Park et al. (2021) reported a case of anaphylaxis after first does of BNT162b2 in a 34-year-old Caucasian woman. Allergy tests and provocation confirmed cholinergic urticaria and excluded anaphylaxis to the vaccine. The second vaccination was performed without any problem [31].
Guidelines for diagnosis and treatment of anaphylactic reactions to SARS-CoV‑2 vaccines have been developed by several medical societies [32, 33].
In conclusion, most anaphylaxis cases are due to a hypersensitivity to excipients apart from the active substance.
Other cutaneous reactions
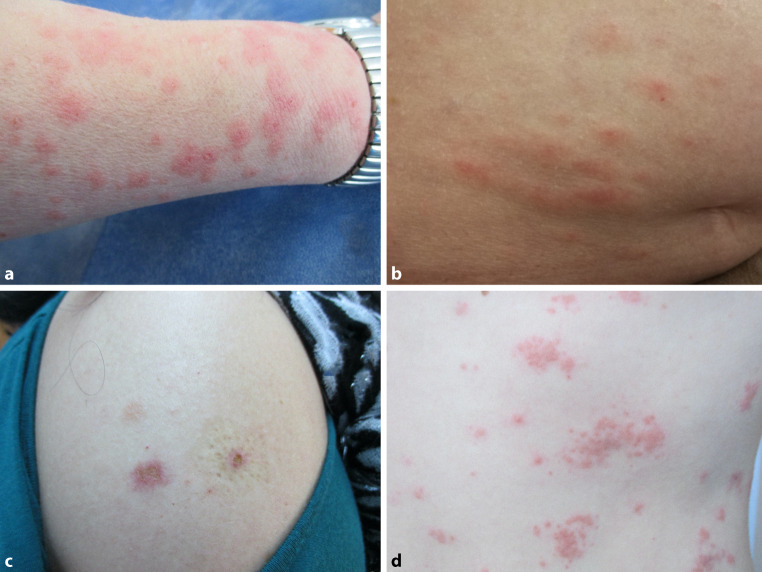
In the international registry of the American Academy of Dermatology and the International League of Dermatological Societies, urticaria (n = 16 first dose; n = 7 second dose), morbilliform exanthema (n = 11 first dose; 7 second dose), and erythromelalgia (n = 5 first dose; n = 6 second dose) were noted among 343 patients with cutaneous adverse events due to SARS-CoV‑2 vaccination (Fig. 2). Sites of soft tissue filler injection prior to vaccination may develop inflammatory reactions after vaccination. Such events were registered in up to 4.9% (Moderna) and 2.5% (BioNTec/Pfizer) of patients [11].
Fig. 2.
Other cutaneous findings after SARS-CoV‑2 vaccination. a, b Morbilliform rash. c Small indurated plaque. d Herpetiform rash
In a survey from patients of 18 countries who received soft tissue filler injections prior to at least one shot of vaccination, 94.9% of patients reported no adverse reaction related to their previous soft tissue filler injection, whereas 5.1% reported pain that lasted longer than 2 days. From the current knowledge, adverse events following immunization with hyaluronic fillers don’t seem to have a causal relationship to the vaccination itself [34].
A study from Northern Italy reported cutaneous adverse reactions to BNT162b2 in 0.22% of patients. The most common reaction was urticaria followed by other rashes, i.e., morbilliform, pityriasis-form, etc. [35]. The morbilliform rash after vaccination resembles those due to COVID-19 [36, 37].
Reactivation of skin disorders
Activation of herpes zoster is not uncommon among elderly patients during any vaccination [37]. In a case series from Spain, however, all patients with herpes zoster after first or second vaccination with BTN162b2 were young and healthy [38].
McMahon et al. (2021) reported herpes zoster in 10% of patients after the second vaccination with BNT162b2 but no case after the Moderna vaccine. Exacerbation of pre-existing dermatoses was observed in 1.0 and 1.1% after the first and second dose of mRNA-1273 and in 20.0 and 7.5% after the first and second dose of BNT162b2, respectively ([11]; Fig. 3).
Fig. 3.
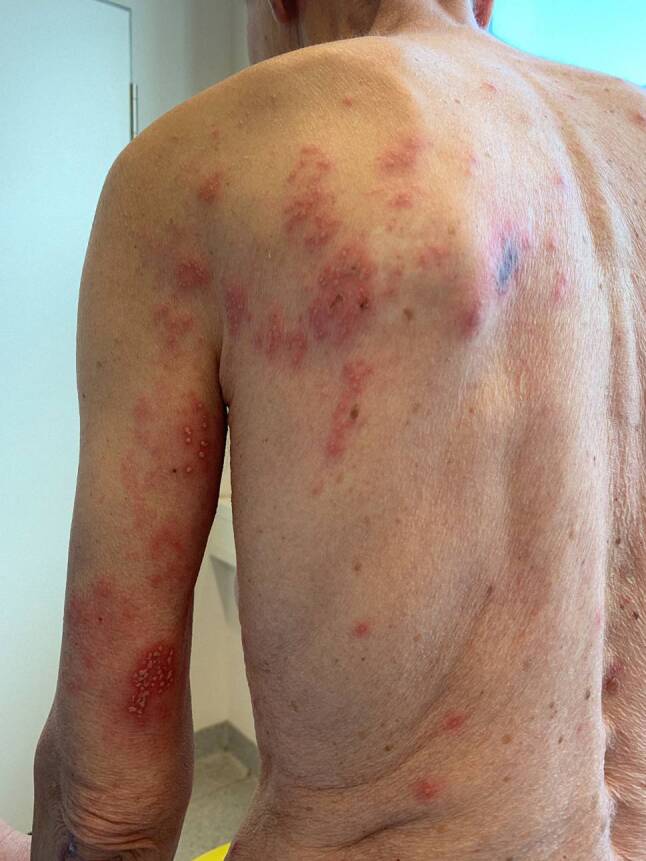
Herpes zoster
The prevalence of herpes zoster among 491 patients with autoimmune rheumatic disorders after vaccination with BNT162b2 reached 1.2% in a study from Israel [39].
Herpes zoster has also been observed among children with COVID-19. We have seen a number of cases between 1 and 11 years of age [40].
The exact mechanisms of herpes zoster activation by COVID-19 vaccination are not yet fully understood. There must be a failure of T lymphocytes responsible for control of the VZV virus. It has been suggested that there might be similarities with a paradoxical reaction to immune reconstitution inflammatory syndrome [41].
Inflammatory reactions within Bacillus Calmette-Guérin (BCG) vaccination scars have been observed 24–36 h after the second vaccination with both mRNA vaccines. They disappeared after 4 days [42].
Conclusion
Adverse cutaneous and hypersensitivity reactions to SARS-CoV‑2 vaccines are not uncommon. A more frequent example of these benign conditions that can resolve without treatment is COVID arm—a delayed hypersensitivity reaction. While most of these adverse events are self-limited and temporary, anaphylaxis has been observed in rare cases, some with a fatal outcome. There is general consensus that an absolute contraindication to COVID-19 vaccination is a known hypersensitivity to ingredients of the vaccine. Patients with lower and medium risk for SARS-CoV-19 vaccination should be monitored for at least 15 or 30 min after injection [43]. For a number of other vaccines, public safety data are sparse or missing [44].
Acknowledgments
Funding
No funding was provided for this article.
Conflict of interest
U. Wollina, A. Chiriac, H. Kocic, A. Koch, and P. Brzezinski declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perez Perez GI, Talebi Bezmin Abadi A. Ongoing challenges faced in the global control of COVID-19 pandemic. Arch Med Res. 2020;51(6):574–576. doi: 10.1016/j.arcmed.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumental S, Debré P. Challenges and issues of anti-SARS-CoV-2 vaccines. Front Med (Lausanne) 2021;8:664179. doi: 10.3389/fmed.2021.664179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ring J, Jutel M, Papadopoulos N, et al. Provocative proposal for a revised nomenclature for allergy and other hypersensitivity diseases. Allergy. 2018;73(10):1939–1940. doi: 10.1111/all.13561. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal KG, Robinson LB, Camargo CA, Jr, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdanov G, Bogdanov D, Kazandjieva J, Tsankov N. Cutaneous adverse effects of the available COVID-19 vaccines. Clin Dermatol. 2021 doi: 10.1016/j.clindermatol.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos CL, Kelso JM. “COVID arm”: very delayed large injection site reactions to mRNA COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021 doi: 10.1016/j.jaip.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021 doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mintoff D, Pisani D, Betts A, Scerri L. SARS-CoV-2 mRNA vaccine-associated fixed drug eruption. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17390. [DOI] [PubMed] [Google Scholar]
- 16.Tammaro A, Adebanjo GAR, Parisella FR, et al. Local reactions to the second dose of the BNT162 COVID-19 vaccine. Dermatol Ther. 2021 doi: 10.1111/dth.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantarelli Rodrigues T, Hidalgo PF, Skaf AY, Serfaty A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA) Skelet Radiol. 2021 doi: 10.1007/s00256-021-03803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer D, Vander Leek TK, Ellis AK, Kim H. Anaphylaxis. Allergy Asthma Clin Immunol. 2018;14(2):54. doi: 10.1186/s13223-018-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—december 14, 2020–january 18, 2021. JAMA. 2021;325(11):1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraft M, Renaudin JM, Ensina LF, et al. Anaphylaxis to vaccination and polyethylene glycol (PEG): a perspective from the European anaphylaxis registry. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdeljic Turk V. Anaphylaxis associated with the mRNA COVID-19 vaccines: approach to allergy investigation. Clin Immunol. 2021;227:108748. doi: 10.1016/j.clim.2021.108748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risma KA, Edwards KM, Hummell DS, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021 doi: 10.1016/j.jaci.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radice A, Fassio F, Meucci E, et al. Potential culprits for immediate hypersensitivity reactions to BNT162b2 mRNA COVID-19 vaccine: not just PEG. Eur Ann Allergy Clin Immunol. 2021 doi: 10.23822/EurAnnACI.1764-1489.214. [DOI] [PubMed] [Google Scholar]
- 25.Troelnikov A, Perkins G, Yuson C, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in PEG allergic patients. J Allergy Clin Immunol. 2021 doi: 10.1016/j.jaci.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Hatziantoniou S, Maltezou HC, Tsakris A, et al. Anaphylactic reactions to mRNA COVID-19 vaccines: a call for further study. Vaccine. 2021;39(19):2605–2607. doi: 10.1016/j.vaccine.2021.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caballero ML, Quirce S. Excipients as potential agents of anaphylaxis in vaccines: analyzing the formulations of currently authorized COVID-19 vaccines. J Investig Allergol Clin Immunol. 2021;31(1):92–93. doi: 10.18176/jiaci.0667. [DOI] [PubMed] [Google Scholar]
- 28.Paoletti G, Racca F, Piona A, et al. Successful SARS-coV-2 vaccine allergy risk-management: the experience of a large Italian university hospital. World Allergy Organ J. 2021;14(5):100541. doi: 10.1016/j.waojou.2021.100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone BD. PEG skin testing for COVID-19 vaccine allergy. J Allergy Clin Immunol Pract. 2021;9(4):1765. doi: 10.1016/j.jaip.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park HJ, Montgomery JR, Boggs NA. Anaphylaxis after the Covid-19 vaccine in a patient with cholinergic urticaria. Mil Med. 2021 doi: 10.1093/milmed/usab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ring J, Worm M, Wollenberg A, et al. Risk of severe allergic reactions to COVID-19 vaccines among patients with allergic skin diseases—practical recommendations. A position statement of ETFAD with external experts. J Eur Acad Dermatol Venereol. 2021;35(6):e362–e365. doi: 10.1111/jdv.17237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimek L, Bergmann KC, Brehler R, et al. Practical handling of allergic reactions to COVID-19 vaccines: a position paper from German and Austrian allergy societies AeDA, DGAKI, GPA and ÖGAI. Allergo J Int. 2021 doi: 10.1007/s40629-021-00165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotkin RH, Gout U, Sattler S, et al. Global recommendations on COVID-19 vaccines and soft tissue filler reactions: a survey-based investigation in cooperation with the international society for Dermatologic and aesthetic surgery (ISDS) J Drugs Dermatol. 2021;20(4):374–378. doi: 10.36849/JDD.6041. [DOI] [PubMed] [Google Scholar]
- 35.Farinazzo E, Ponis G, Zelin E, et al. Cutaneous adverse reactions after m-RNA COVID-19 vaccine: early reports from North-East Italy. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jedlowski PM, Jedlowski MF. Morbilliform rash after administration of Pfizer-BioNTech COVID-19 mRNA vaccine. Dermatol Online J. 2021;27(1):13030/qt4xs486zg. doi: 10.5070/D3271052044. [DOI] [PubMed] [Google Scholar]
- 37.Wollina U, Karadağ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33(5):e13549. doi: 10.1111/dth.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021 doi: 10.1002/jmv.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford) 2021 doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines (Basel) 2021;9(6):572. doi: 10.3390/vaccines9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brzezinski P, Wollina U. HSV infections during the COVID-19 pandemic. Our Dermatol Online. 2020;11(2):19–20. doi: 10.7241/ourd.2020S2.7. [DOI] [Google Scholar]
- 43.Lopatynsky-Reyes EZ, Acosta-Lazo H, Ulloa-Gutierrez R, et al. BCG Scar local skin inflammation as a novel reaction following mRNA COVID-19 vaccines in two international healthcare workers. Cureus. 2021;13(4):e14453. doi: 10.7759/cureus.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forni G, Mantovani A, COVID-19 Commission of Accademia Nazionale dei Lincei, Rome COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]