Abstract
Background
Patients with ZZ (Glu342Lys) α-1-antitrypsin deficiency (ZZ-AATD) who received augmentation therapy with α-1-antitrypsin (AAT) in randomised controlled trials over 2–3 years failed to show a significant reduction of the annual decline of forced expiratory volume in 1 s (FEV1).
Methods
To compare the trajectory of FEV1 change during 4 or more years in ZZ-AATD patients with emphysema receiving or not receiving intravenous augmentation therapy, a retrospective analysis of FEV1 values entered in the Alpha-1 International Registry (AIR) of ZZ-AATD patients from five different European countries (Germany, UK, Spain, Italy and the Netherlands) was performed. The post-bronchodilator FEV1 % predicted values for baseline and follow-up over time from patients were analysed using linear mixed effects models.
Results
Data of 374 patients were analysed: 246 untreated and 128 treated with intravenous AAT augmentation therapy. The mean±sd follow-up duration of the untreated group was 8.60±3.34 years and 8.59±2.62 years for the treated group. The mixed effects model analysis showed a mean FEV1 decline of −0.931% predicted per year (95% CI −1.144 to −0.718) in the untreated group and a decline of −1.016% predicted per year (95% CI −1.319 to −0.7145) in the treated group. The likelihood ratio test showed no difference between the two groups (p=0.71).
Conclusion
In our study population, we could not detect a significant difference in the annual decline of FEV1 by AAT augmentation treatment over a mean period of 8.6 years. Other approaches are needed to validate any benefit of augmentation therapy.
Short abstract
This analysis of European real-world follow-up data of FEV1 % predicted in ZZ α1-ATD patients showed no difference in annual FEV1 decline between the i.v. α1-AT augmentation treated and untreated patients over a period of >8 years https://bit.ly/35MglRc
Introduction
Severe α-1-antitrypsin deficiency (AATD) is a hereditary disorder that can predispose to emphysema dominant lung disease and to an accelerated decline in forced expiratory volume in 1 s (FEV1) at an early age, due to low serum α-1-antitrypsin (AAT) levels. The ZZ-AATD (Glu342Lys) phenotype is the most common deficiency with highly variable lung impact, ranging from no symptoms to the development of AATD-related emphysema [1, 2]. ZZ-AATD patients with an accelerated annual decline in FEV1 have a poorer prognosis, as a lower FEV1 is a predictor for all-cause and respiratory mortality [3–5].
More than 25 years ago, AAT augmentation therapy was introduced in the United States and some European countries. Based on the demonstration of a rise in serum AAT level above a putative “protective threshold” and the elevation of the level of AAT in bronchoalveolar lavage fluid, with weekly infusions, it was assumed that substitution of the reduced serum levels in ZZ-AATD patients with AAT purified from blood of healthy donors would protect from further progression of lung disease [6]. The American Thoracic Society (ATS)/European Respiratory Society (ERS) statement recommends weekly AAT augmentation therapy with 60 mg·kg−1 body weight for AATD patients with AAT serum levels below the putative protective threshold of 11.0 µM and an FEV1 between 30 and 65% predicted based on follow-up data of the National Heart, Lung and Blood Institute (NHLBI) registry [5, 7]. A subsequent meta-analysis suggested efficacy [8], although a systematic review [9] did not confirm a beneficial effect on annual decline in FEV1 in ZZ-AATD patients who received AAT augmentation treatment compared to those who did not.
The rate of the annual FEV1 decline is independently associated with the severity of emphysema, as quantified by lung densitometry [10]. Indeed lung densitometry can quantify pulmonary emphysema accurately and is a more sensitive and specific method to assess emphysema progression than the FEV1 [11]. Based on this validation, lung densitometry has been used as the outcome parameter in several placebo-controlled randomised clinical trials to determine the effect of intravenous (i.v.) AAT augmentation treatment on preservation of lung tissue. A consistent protective effect of i.v. AAT on lung tissue has been reported in relatively short-term studies of 2 to 4 years [12–15]. Although in these studies no effect of i.v. AAT on decline of FEV1 could be proven, it was presumed that i.v. AAT treatment effect on lung densitometry was preceding protection of change in FEV1 later in time. This implies that the follow-up time in these randomised controlled trials was too short to measure a difference between the placebo and AAT-treated group with respect to FEV1 decline. Alternatively, it suggests that the study population was too small to detect a treatment effect especially as in many patients deterioration of FEV1 can stabilise in some patients after cessation of smoking, which is a recognised driver of emphysema progression, and smokers have always been excluded from such trials [16].
The aim of the current study was to determine whether long-term i.v. AAT augmentation therapy preserves decline in FEV1 compared to cohorts where such therapy has been unavailable.
Methods
Study design and patients
To stimulate the availability of long-term follow-up data and appropriate research, the World Health Organization (WHO) advised the establishment of an international registry for AATD in 1997, and the Alpha-1 International Registry (AIR) was founded [17]. In this registry, patients’ data were collected by members of AIR and stored in a common database. The members were dedicated clinical scientists who entered baseline and follow-up data of AATD patients, as described in detail previously [18]. All patients included in AIR had signed written informed consent for the anonymous use of their data for research, prior to entering data into the registry [19].
In the current study, we analysed AIR data from five different countries: the Netherlands, Germany, Italy, the United Kingdom and Spain, as those countries had the most complete datasets.
Procedures
To assess the effect of AAT augmentation treatment over a longer term, data from patients with FEV1 values collected over > 4 years were extracted from the AIR database. These data were used in this retrospective cohort study of international longitudinal data of individual ZZ-AATD patients. Data registered up until January 1, 2014 were used. The individuals were selected based on the following inclusion criteria: age >25 years, phenotype ZZ-AATD, availability of ≥4 years of follow-up data, and a baseline (at the moment of entering AIR) post-bronchodilator FEV1 value between 30% and 65% predicted. Patients were excluded when there were missing data on the key variables: date of birth, sex, height, smoking status, phenotype and AAT therapy status. Also, patients who received a lung transplant, current smokers or those who stopped smoking <6 months before the start of AAT treatment were excluded. Additionally, only patients on treatment located in countries where there is augmentation therapy available and reimbursed (Spain, Germany and Italy) were included in the treatment group. Patients without treatment located in countries where there is no augmentation therapy available and reimbursed (the Netherlands and the United Kingdom) were included in the untreated group.
Outcomes
The post-bronchodilator FEV1% predicted values for baseline and each follow-up moment were calculated for each patient from the absolute FEV1 values in litres using the Global Lung Initiative (GLI) standard correction [20]. Additionally, the course of FEV1 in L of both groups were also compared. Based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD guideline, optimal bronchodilator treatment for patients was taken as the use of long-acting bronchodilators [3]. Therefore, missing follow-up values not formally stated as post-bronchodilator FEV1 were taken as the FEV1 values on prescribed bronchodilator therapy as documented in the AIR database.
Statistical analysis
Data was stratified by country, categorical variables were reported as numbers and percentages, continuous variables as mean±sd. Linear mixed effects models were used to determine the annual decline in “post-bronchodilator” FEV1 values of the two groups of ZZ-AATD patients, those with and without augmentation therapy.
For the linear mixed effects models, “post-bronchodilator” FEV1 at the different timepoints is the dependent variable, and as fixed effects we used treatment modality, follow-up duration and the interaction between follow-up duration and treatment, sex, age at follow-up moment and pack-years of previous smoking. To model the within-patient correlation we used random intercepts and slopes terms. To determine if therapy has an effect on the decline of FEV1, the likelihood ratio test was used [21]. A likelihood ratio test result with a difference of a p-value <0.05 was considered significant. Statistical analyses were performed using IBM SPSS statistics version 26 for Windows (IBM, Chicago, IL, USA). A more comprehensive description of the linear mixed effects models is documented in supplementary material, paragraph 1.1.
Results
Study population and baseline characteristics
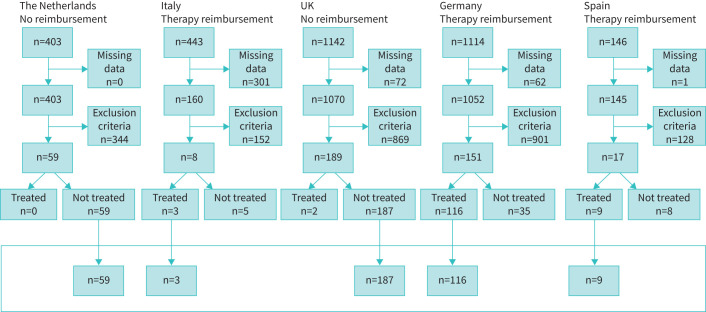
For the current study, a total of 3248 registered patients from the United Kingdom, the Netherlands, Germany, Italy and Spain were identified in the AIR database. Based on missing data or exclusion criteria, 2821 patients were excluded from subsequent analysis. Additionally, we identified 48 patients who were eligible for augmentation therapy in their country but were not treated for undocumented reasons and therefore were also excluded from the analysis. A total of 374 patients remained for the linear mixed effects model analysis; 246 patients were included in the group with no augmentation therapy (controls) and 128 patients in the group with AAT augmentation treatment. Figure 1 shows the flow-chart of the inclusion and exclusion criteria of the study individuals per country.
FIGURE 1.
When all the exclusion criteria were applied, the total number of excluded subjects is the number shown in the box. The bottom box shows the subjects who are included in the linear mixed effects model analysis. Exclusion based on missing data of key variables: date of birth, sex, length, smoking status, phenotype, α-1-antitrypsin (AAT) therapy status. Exclusion criteria: age <25 years, phenotype not ZZ (Glu342Lys) α-1-antitrypsin deficiency (ZZ-AATD), follow-up data <4 years, baseline post-bronchodilator FEV1 % predicted value <30% or >65% predicted, lung transplantation, current smokers, stopped smoking ≤6 months prior to inclusion.
A total of 2268 FEV1 values were analysed, with a mean of 6.1 annual FEV1 values per ZZ-AATD patient. All baseline FEV1 values were documented post-bronchodilator values. However, 180 (7.9%) follow-up post-bronchodilator values were not documented or formally assessed and therefore were replaced by “formal pre-bronchodilator FEV1” values from 113 ZZ-AATD patients documented in the database as on usual daily bronchodilator therapy. The mean±sd follow-up duration in the untreated group was 8.60±3.34 years and 8.59± 2.62 years in the treated group. The baseline characteristics are summarised in table 1.
TABLE 1.
Patient baseline characteristics
| The Netherlands | UK | Italy | Germany | Spain | |
| Treated/not treated n | Not treated 59 | Not treated 187 | Treated 3 | Treated 116 | Treated 9 |
| Male sex, n (%) | 33 (55.9) | 119 (63.6) | 1 (33.3) | 76 (65.5) | 6 (66.7) |
| Age at baseline years | 46.7±7.6 | 52.3±8.7 | 61.0±6.0 | 53.4±9.7 | 53.0±4.8 |
| Smoking status | |||||
| Never, n (%) | 4 (6.8) | 28 (15.0) | 1 (33.3) | 26 (22.4) | 0 (0) |
| Former, n (%) | 55 (93.2) | 159 (85.0) | 2 (66.7) | 90 (77.6) | 9(100) |
| Pack-years | 17.2±10.1 | 20.7±21.3 | 15±15 | 15.4±12.5 | 17.3±5.3 |
| Baseline FEV1 % pred | 47.3±9.1 | 46.4±9.6 | 37.3±4.9 | 46.2±10.1 | 47.2±7.7 |
Data expressed as mean±sd unless otherwise indicated. FEV1: forced expiratory volume in 1 s.
Linear mixed effects model analysis
The mean annual decline in FEV1 % predicted in the treated and untreated group was calculated by applying a linear mixed effects model analysis. The best fitted mixed model analysis showed a mean FEV1 decline of −0.931% predicted per year (95% confidence interval −1.144 to −0.718) in the untreated group, and −1.016% predicted per year (−1.319 to −0.7145) in the treated group, as summarised in figure 2. The likelihood ratio test showed no difference between the two groups (p=0.71). The mixed model analysis of the FEV1 decline in litres also showed no difference between the two groups (this analysis is documented in the supplementary material, paragraphs 1.2 and 2.2).
FIGURE 2.
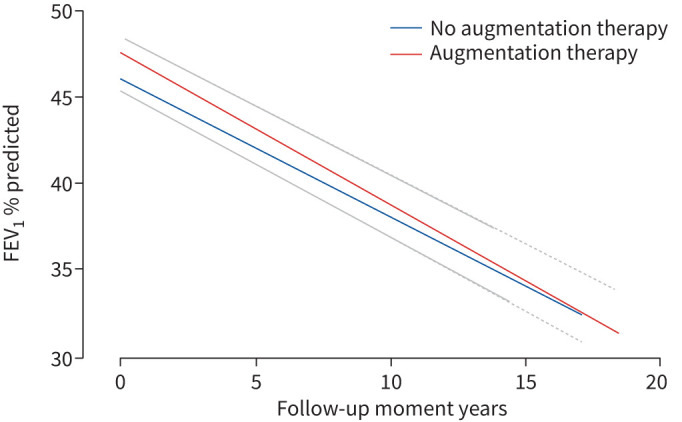
Decline in forced expiratory volume in 1 s (FEV1) as % predicted over the years calculated by the mixed model without confounders, for both groups: untreated and treated. The confidence intervals of both graphs are overlapping, thereby only the upper confidence interval of the treated graph and the lower confidence interval of the untreated graph is shown. There was no significant difference in decline in FEV1.
Discussion
This retrospective analysis of European real-world follow-up data of the decline in FEV1 % predicted in ZZ-AATD patients showed no difference in annual FEV1 decline between the AAT augmentation treated and untreated patients over a period of >8 years. The mean decline of FEV1 in ZZ-AATD patients was best expressed as the % predicted to account for the natural ageing decline and normalise data between subjects for sex and height.
The annual FEV1 decline reported in our long-term study is less than that reported in the most recently performed short-term randomised controlled trial for AAT augmentation therapy, the RAPID study [13]. That study also showed no difference in FEV1 decline (p=0.21) between groups. Indeed, mean±sd FEV1 decline was −2.3±13.1% predicted over 2 years in the placebo group and −3.1±10.7% predicted in the treated group [13]. Whereas the shorter period of follow-up in the RAPID trial may have affected the detection of any difference in decline rate of FEV1, our longer-term follow-up study has not provided support of the statement in the RAPID-OLE study [14], that lung density change in favour of i.v. AAT augmentation treatment will eventually be reflected in a reduced FEV1 decline in the longer term.
The results of our study also support the findings of previous limited analyses of ZZ-AATD patients. The national German registry analysis with patients who had not received augmentation therapy (n=15) and patients who did receive therapy (n=85) over a mean follow-up of 4.89 years [22] showed no difference in FEV1 decline between groups. Neither did the analysis of the Spanish national database of patients who did not receive augmentation therapy (n=45) and patients who did receive augmentation therapy (n=77) over an average of >8 years [23].
Our database study has some important strengths. First, in this study data from five different countries are combined to form the two groups of interest. The use of data from an international registry of a rare disease makes it possible to analyse data of a higher number of ZZ-AATD patients and creates the opportunity to combine data from countries where there is no augmentation therapy available with data from countries where AAT augmentation therapy is both available and reimbursed. Second, most included patients had multiple follow-up FEV1 values over a mean follow-up time of >8 years with a mean of 6.1 FEV1 values for slope analysis. These two factors increase the accuracy of the presented mean of both groups in our study [24]. Third, selection bias for inclusion of patients in our study was limited using the ATS/ERS statement for diagnosis and treatment of individuals with AATD [7]. Another strength of our analysis is that in our study FEV1 decline is primarily expressed in % predicted instead of litres per year, to account for a corrected value for the age-related decline of FEV1 [16].
There are also some limitations of our study. First, most data were added retrospectively from paper files into the registry. Unfortunately, this resulted in some missing data, even following two requests for data from contributors checking their source data, and thereby led to a high number of excluded patients which might have led to inadvertent selection bias and a marked reduction in patient number fulfilling all the inclusion criteria. Second, over the years the i.v. AAT dosing regimen in the three countries has been variable and occasionally interrupted for some months due to drug supply problems. This break in therapy was not carefully documented in the registry. Third, in this study the choice for analysing the post-bronchodilator FEV1 instead of the pre-bronchodilator FEV1 values was based on the ATS/ERS guideline for the analysis of follow-up FEV1 in COPD [25]. This guideline does not state about the formality or use of the type or dose of bronchodilator, i.e. short-acting β2-agonist or short-acting muscarinic antagonist. In the AIR database no data were collected about the type of bronchodilator used for the measurement of the post-bronchodilator FEV1 values. Also, the registry does not include data about the use and timing of long-acting bronchodilators on the day of the spirometry. Fourth, several other factors are known to influence FEV1 decline in AATD: baseline FEV1, smoking history, age, body mass index (BMI) and exacerbation rate [22, 26]. In our database, no exacerbation rate or respiratory symptoms are registered, apart from the St. George's Respiratory Questionnaire score at baseline. Values of BMI and exacerbation rates were often missing in the follow-up database. However, the other known influencing factors on FEV1 decline, i.e. previous smoking history and patient age, were applied as confounders in the mixed models. Although smoking has a major effect on FEV1 decline, it should be noted that all patients on augmentation had to have stopped smoking, which in its own right would likely reduce subsequent decline [16]. The time from quitting smoking to the date of starting augmentation treatment (the period of being an ex-smoker) could not be used as a confounder in the mixed effects model. By doing so, we were unable to model the never-smokers without creating another and smaller subgroup analysis. Therefore, we only applied the number of pack-years in our mixed models.
Even though the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) guidance for new drug studies in COPD have determined that the FEV1 should be the primary outcome in drug development [27], the findings in our study suggest that FEV1 decline would be inappropriate to evaluate the effect of AAT augmentation therapy in ZZ-AATD patients. Historically, AAT augmentation therapy with a dose of 60 mg·kg−1 has been introduced based on the hypothetical effect on reducing FEV1 decline by restoring the protease–antiprotease balance through AAT substitution. Indeed limited studies of airway secretions indicate both a rise in local AAT levels and a reduction in protease activity consistent with this concept [28]. More recent studies also indicate that excess protease activity is present in the lung [29], albeit not specific for the lung [30, 31]. These biomarkers or footprints of excess protease activity might be useful to perform dose-ranging studies of AAT augmentation therapy to evaluate the effect on the protease balance and whether the current dose of 60 mg·kg−1 is sufficient for an individual patient as intimated by Campos et al. [32].
However, other molecular mechanisms affected by the ZZ-AATD phenotype may also be responsible for emphysema development such as quantitative and qualitative properties of circulating Z-AAT polymers, dysregulation of immune cell responses and endothelial cell function or generation of unknown pro-inflammatory substances [33].
Finally, it should be re-emphasised that the FEV1 is a poor surrogate for the emphysema process and far less sensitive to change than lung densitometry. In addition, decline in FEV1 is both variable in AATD and even stabilises (other than normal age-related decline) in a proportion of ex-smokers. Studies of patients not documented for rapid decline post smoking will include a variable portion of patients who stabilise and reduce the power for using FEV1 as an outcome parameter thus requiring large numbers of subjects to identify statistical differences as in the NHLBI study [5] and in the meta-analysis by Chapman et al. [8]. To support development of new outcome parameters for research in AATD, the EARCO initiative was established together with the European Respiratory Society [34]. A protocol was developed to assess complete phenotyping of AATD patients (www.earco.org).
We conclude that our “real-world data” could not show a difference in long-term annual FEV1 decline in ZZ-AATD patients who received intravenous AAT augmentation treatment for emphysema compared to those who did not. Other approaches are needed to validate any benefits of i.v. augmentation therapy or determine reasons other than number, current status and length of study required to be confident of the benefits of such therapy.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00194-2021.SUPPLEMENT (449.6KB, pdf)
Acknowledgements
We thank S. Janciauskiene, Dept of Pulmonology, Hannover Medical School, for critical reading of the manuscript.
Submitted article, peer reviewed.
This article has supplementary material available from openres.ersjournals.com.
Conflict of interest: I.G.M. Schouten has nothing to disclose.
Conflict of interest: M.J. Kasteleyn has nothing to disclose.
Conflict of interest: R. Tsonaka has nothing to disclose.
Conflict of interest: R. Bals reports a grant paid to his institution by Grifols Spain in the last 36 months.
Conflict of interest: A.C. Turner reports grants paid to her institution by Grifols Spain and CSL Behring in the last 36 months.
Conflict of interest: I. Ferrarotti reports grants paid to her institution by Grifols Spain and CSL Behring in the last 36 months.
Conflict of interest: A.G. Corsico reports grants paid to his institution by Grifols Spain and CSL Behring in the last 36 months.
Conflict of interest: B. Lara reports a grant paid to her institution by Grifols Spain in the last 36 months.
Conflict of interest: M. Miravitlles reports personal fees from CSL Behring, AstraZeneca, Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Spin Therapeutics, pH Pharma, Novartis, Sanofi, Grifols, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Sandoz, Zambon and Novartis, during the conduct of the study.
Conflict of interest: R.A. Stockley reports grants paid to his institution by Grifols Spain and CSL Behring in the last 36 months.
Conflict of interest: J. Stolk reports personal fees from CSL Behring and Kamada Ltd during the conduct of the study.
Support statement: This study was supported by Stichting AIR. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Talamo RC, Allen JD, Kahan MG, et al. . Hereditary alpha-1-antitrypsin deficiency. N Engl J Med 1968; 278: 345–351. doi: 10.1056/NEJM196802152780701 [DOI] [PubMed] [Google Scholar]
- 2.Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2012; 185: 246–259. doi: 10.1164/rccm.201108-1428CI [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2018. Available from: http://goldcopd.org [Google Scholar]
- 4.Hiller AM, Piitulainen E, Jehpsson L, et al. . Decline in FEV1 and hospitalized exacerbations in individuals with severe alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis 2019; 14: 1075–1083. doi: 10.2147/COPD.S195847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med 1998; 158: 49–59. doi: 10.1164/ajrccm.158.1.9712017 [DOI] [PubMed] [Google Scholar]
- 6.Wewers MD, Casolaro MA, Sellers SE, et al. . Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med 1987; 316: 1055–1062. doi: 10.1056/NEJM198704233161704 [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society/European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168: 818-900. doi: 10.1164/rccm.168.7.818 [DOI] [PubMed] [Google Scholar]
- 8.Chapman KR, Stockley RA, Dawkins C, et al. . Augmentation therapy for alpha1 antitrypsin deficiency: a meta-analysis. COPD 2009; 6: 177–184. doi: 10.1080/15412550902905961 [DOI] [PubMed] [Google Scholar]
- 9.Gøtzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy: systematic review. Dan Med Bull 2010; 57: A4175. [PubMed] [Google Scholar]
- 10.Nishimura M, Makita H, Nagai K, et al. . Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 44–52. doi: 10.1164/rccm.201106-0992OC [DOI] [PubMed] [Google Scholar]
- 11.Dirksen A, Friis M, Olesen KP, et al. . Progress of emphysema in severe alpha 1-antitrypsin deficiency as assessed by annual CT. Acta Radiol 1997; 38: 826–832. [DOI] [PubMed] [Google Scholar]
- 12.Dirksen A, Dijkman JH, Madsen F, et al. . A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999; 160: 1468–1472. doi: 10.1164/ajrccm.160.5.9901055 [DOI] [PubMed] [Google Scholar]
- 13.Chapman KR, Burdon JG, Piitulainen E, et al. . Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386: 360–368. doi: 10.1016/S0140-6736(15)60860-1 [DOI] [PubMed] [Google Scholar]
- 14.McElvaney NG, Burdon J, Holmes M, et al. . Long-term efficacy and safety of alpha1 proteinase inhibitor treatment for emphysema caused by severe alpha1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med 2017; 5: 51–60. doi: 10.1016/S2213-2600(16)30430-1 [DOI] [PubMed] [Google Scholar]
- 15.Dirksen A, Piitulainen E, Parr DG, et al. . Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J 2009; 33: 1345–1353. doi: 10.1183/09031936.00159408 [DOI] [PubMed] [Google Scholar]
- 16.Stockley RA, Edgar RG, Pillai A, et al. . Individualized lung function trends in alpha-1-antitrypsin deficiency: a need for patience in order to provide patient centered management? Int J Chron Obstruct Pulmon Dis 2016; 11: 1745–1756. doi: 10.2147/COPD.S111508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alpha 1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Organ 1997; 75: 397–415. [PMC free article] [PubMed] [Google Scholar]
- 18.Luisetti M, Miravitlles M, Stockley, et al. Alpha1-antitrypsin deficiency: a report from the 2nd meeting of the Alpha One International Registry, Rapallo (Genoa, Italy), 2001. Eur Respir J 2002; 20: 1050-1056. [DOI] [PubMed] [Google Scholar]
- 19.Stockley RA, Luisetti M, Miravitlles M, et al. . Ongoing research in Europe: Alpha One International Registry (AIR) objectives and development. Eur Respir J 2007; 29: 582–586. doi: 10.1183/09031936.00053606 [DOI] [PubMed] [Google Scholar]
- 20.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzmaurice GM, Nan LM, Ware JH. Applied Longitudinal Analysis. 2nd Edn.Hoboken/New Jersey, John Wiley & Sons, Inc, 2011; 97. [Google Scholar]
- 22.Fahndrich S, Bernhard N, Lepper PM, et al. . Exacerbations and duration of smoking abstinence are associated with the annual loss of FEV1 in individuals with PiZZ alpha-1-antitrypsin deficiency. Respir Med 2017; 129: 8–15. doi: 10.1016/j.rmed.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 23.Esquinas C, Serreri S, Barrecheguren M, et al. . Long-term evolution of lung function in individuals with alpha-1 antitrypsin deficiency from the Spanish registry (REDAAT). Int J Chron Obstruct Pulmon Dis 2018; 13: 1001–1007. doi: 10.2147/COPD.S155226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ML, Gunel E, Petsonk EL. Design strategies for longitudinal spirometry studies: study duration and measurement frequency. Am J Respir Crit Care Med 2000; 162: 2134–2138. doi: 10.1164/ajrccm.162.6.2003171 [DOI] [PubMed] [Google Scholar]
- 25.Graham BL, Steenbruggen I, Miller MR, et al. . Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200: e70-e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawkins PA, Dawkins CL, Wood AM, et al. . Rate of progression of lung function impairment in alpha1-antitrypsin deficiency. Eur Respir J 2009; 33: 1338–1344. doi: 10.1183/09031936.00061208 [DOI] [PubMed] [Google Scholar]
- 27.van Haarst A, McGarvey L, Paglialunga S. Review of drug development guidance to treat chronic obstructive pulmonary disease: US and EU perspectives. Clin Pharmacol Ther 2019; 106: 1222–1235. doi: 10.1002/cpt.1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockley RA, Bayley DL, Unsal I, et al. . The effect of augmentation therapy on bronchial inflammation in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2002; 165: 1494–1498. doi: 10.1164/rccm.2109013 [DOI] [PubMed] [Google Scholar]
- 29.Sinden NJ, Stockley RA. Proteinase 3 activity in sputum from subjects with alpha-1-antitrypsin deficiency and COPD. Eur Respir J 2013; 41: 1042–1050. doi: 10.1183/09031936.00089712 [DOI] [PubMed] [Google Scholar]
- 30.Carter RI, Mumford RA, Treonze KM, et al. . The fibrinogen cleavage product Aalpha-Val360, a specific marker of neutrophil elastase activity in vivo. Thorax 2011; 66: 686–691. doi: 10.1136/thx.2010.154690 [DOI] [PubMed] [Google Scholar]
- 31.Newby PR, Crossley D, Crisford H, et al. . A specific proteinase 3 activity footprint in α1-antitrypsin deficiency. ERJ Open Res 2019; 5: 00095-2019. doi: 10.1183/23120541.00095-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campos MA, Geraghty P, Holt G, et al. . The biological effects of double-dose alpha-1 antitrypsin augmentation therapy. A pilot clinical trial. Am J Respir Crit Care Med 2019; 200: 318–326. doi: 10.1164/rccm.201901-0010OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siebers K, Fink B, Zakrzewicz A, et al. . Alpha-1 antitrypsin inhibits ATP-mediated release of interleukin-1β via CD36 and nicotinic acetylcholine receptors. Front Immunol 2018; 9: 877. doi: 10.3389/fimmu.2018.00877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greulich T, Altraja A, Barrecheguren M, et al. . Protocol for the EARCO registry: a pan-European observational study in patients with α(1)-antitrypsin deficiency. ERJ Open Res 2020; 6: 00181-2019. doi: 10.1183/23120541.00181-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00194-2021.SUPPLEMENT (449.6KB, pdf)