Abstract
Selenium is essential for human health; its deficiency leads to cardiac dysfunction. We herein report a 79-year-old man on peritoneal dialysis who presented with refractory hypotension caused by selenium deficiency. He was admitted to our hospital with bacterial pneumonia and hypotension and abnormal electrocardiogram (ECG) findings. Despite improvement of pneumonia, his hypotension continued, and intravenous noradrenalin could not be discontinued. His serum selenium level was extremely low, and he was started on intravenous selenium. His hypotension and ECG findings gradually improved, and noradrenalin was discontinued. Physicians should consider selenium deficiency when patients on peritoneal dialysis show refractory hypotension.
Keywords: peritoneal dialysis, hypotension, selenium deficiency
Introduction
Selenium is a trace element that is necessary for many bodily processes, including thyroid hormone metabolism, DNA synthesis, reproduction, and protection from oxidative damage and infection (1). Therefore, selenium is essential for human health, and its deficiency is related to diseases such as hypothyroidism, deforming arthritis, and cardiovascular diseases (2).
We herein report a peritoneal dialysis patient who presented with refractory hypotension and abnormal electrocardiogram (ECG) findings caused by selenium deficiency. His refractory hypotension was successfully treated with intravenous selenium administration.
Case Report
A 79-year-old Japanese man on peritoneal dialysis (PD) was hospitalized because of bacterial pneumonia and hypotension. His medical history included hypertension, ischemic heart disease, aortic dissection, gout, cerebral infarction, chronic heart failure, and chronic kidney disease caused by nephrosclerosis. His blood pressure had been controlled at 110-130 mmHg. He had taken esomeprazole, pitavastatin, nicorandil, cilostazol, clopidogrel, carvedilol, and amiodarone. He had been on peritoneal dialysis for eight years and he had been repeatedly hospitalized for heart failure caused by left ventricular (LV) dysfunction due to ischemic heart disease. He had been treated with ultrafiltration by peritoneal dialysis every time. With the decline in physical strength, his food intake had gradually decreased over the past year.
On admission, a physical examination showed blood pressure of 66/42 mmHg, heart rate of 89 beats/min, temperature of 37.3 °C, and SpO2 of 91% (room air). His body weight could not be measured because of deterioration of his general condition. He was conscious and had no edema. Right chest coarse crackles were identified. His laboratory findings were as follows: white blood cell (WBC) 5,900/μL, red blood cell (RBC) 2.84×104/μL, hemoglobin (Hb) 8.4 g/dL, hematocrit (Ht) 26.1%, platelet (Plt) 16.3×104/μL, albumin 1.9 g/dL, blood urea nitrogen 39.4 mg/dL, creatinine 4.86 mg/dL, brain natriuretic peptide (BNP) 1967.5 pg/mL, and C-reactive protein (CRP) 10.90 mg/dL. Chest X-ray showed infiltration on the right side. Computed tomography showed patchy shadows in the bilateral lungs, and pleural effusion and atelectasis were observed in the right lung. Echocardiography showed diffuse hypokinesis of the left ventricle and a left ventricular ejection fraction of 40%, as before (2014: 68.1%, 2015: 75.9%, 2017: 75%, 2018: 68%, 2019: 48%). Bronchial pneumonia and heart failure were diagnosed, and he was treated with intravenous tazobactam/piperacillin and intravenous noradrenalin. His sputum culture was positive for Klebsiella pneumoniae, and two sets of blood culture were negative. His PD menu was daily extraneal and APD (1.5% reguneal 3 times) at night. Three months before admission, his peritoneal equivalent test (PET) category had shown a low average. After admission, his APD menu was changed to 2.5% fluid intended to increase ultrafiltration to improve overhydration.
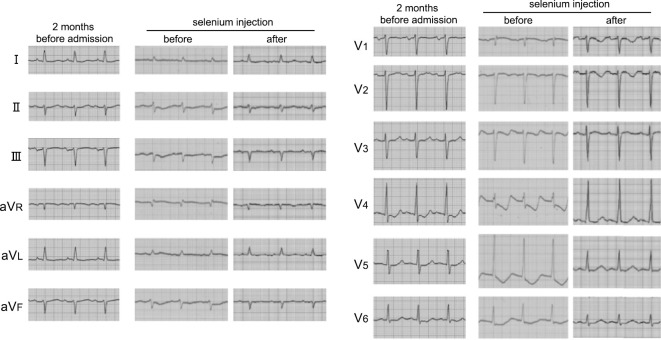
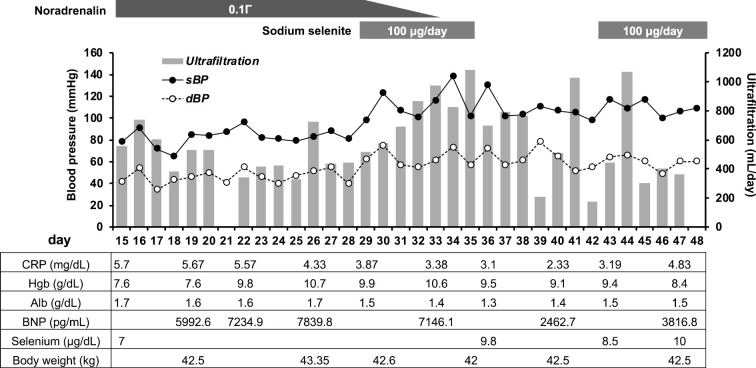
Although his general condition improved gradually, his volume overload and hypotension persisted. To determine why his hypotension persisted, his serum vitamin B1 level was measured, and vitamin B1 was administered, but it did not improve his condition. Several days later, his serum vitamin B1 level was reported as being in the normal range (4.9 μg/dL). Based on these findings and his abnormal ECG findings (Fig. 1), we investigated the possibility of selenium deficiency. His serum selenium level (reference range: 10.6-17.4 μg/dL) was then checked and was found to be extremely low (7.0 μg/dL) (Fig. 2). Sodium selenite injection (Fujimoto Pharmaceutical Corporation, Matsubara, Japan) 100 μg/day was then given intravenously; his blood pressure gradually increased, and intravenous noradrenalin was able to be discontinued five days after selenium administration (Fig. 2). Interestingly, his fluid removal by PD ultrafiltration increased after selenium administration, and his pulmonary congestion and serum BNP level also improved (Fig. 2). Since we were unable to estimate the serum selenium level after selenium administration, and there was concern about overcorrection (3), selenium administration was stopped on day 35. Discontinuation for 1 week resulted in a significant decrease in the serum selenium concentration (9.8 to 8.5 μg/dL). Intravenous selenium was then re-started, and an elevated serum selenium level was confirmed 4 days after administration (8.5 to 10.0 μg/dL).
Figure 1.
Electrocardiogram findings two months before admission and before and after treatment with intravenous selenium.
Figure 2.
Clinical course of the present case. BNP: brain natriuretic peptide, dBP: diastolic blood pressure, sBP: systolic blood pressure
Selenium deficiency often causes ECG changes (4), and this case also showed abnormal ECG findings that improved after selenium administration (Fig. 1). The patient's general condition improved, and he was transferred to another hospital for rehabilitation on day 48.
Discussion
A case of refractory hypotension with abnormal ECG findings that was diagnosed as selenium deficiency was described. The patient's hypotension and pulmonary congestion were successfully treated with intravenous selenium administration. Hypotension can be caused by vitamin B1 deficiency, anemia, hypoalbuminemia, infectious state, and myocarditis. However, the patient's hemoglobin, albumin, and CRP levels did not change markedly after the improvement of hypotension (Fig. 1), and two sets of blood culture were negative. His serum vitamin B1 levels were also normal. As we did not perform a myocardial biopsy, we were unable to exclude the possibility of acute or chronic myocarditis. However, hypotension and his ECG finding improved just after selenium supplementation without intervention of myocarditis. Based on these findings, we diagnosed the cause of his hypotension as selenium deficiency.
Historically, selenium deficiency has been famous for causing Keshan disease, which can lead to cardiac dysfunction (4,5). Keshan disease is an endemic cardiomyopathy occurring in low-selenium areas of China (5). The main clinical features are cardiac dysfunction and electrocardiographic changes. The present patient showed refractory hypotension and abnormal electrocardiogram findings that improved after intravenous selenium administration. Based on this clinical course and the diagnostic criteria for selenium deficiency (6), his hemodynamic instability was attributed to selenium deficiency.
The present patient had several risks for selenium deficiency, including receiving total parenteral nutrition (TPN) and chronic kidney disease (CKD). In addition, the PD also likely contributed to selenium deficiency. A previous study evaluated the nutritional status in end-stage kidney disease (ESKD) patients before and six months after renal replacement therapy (hemodialysis or peritoneal dialysis) or renal transplantation (7). Interestingly, the level of serum selenium was significantly increased in hemodialysis and renal transplant patients but not in PD patients. Furthermore, one cross-sectional study showed that the serum selenium level was significantly lower in PD patients than in hemodialysis patients (8). Although these papers did not mention the precise mechanism responsible for such a difference, it may have been due to selenium bonding to serum albumin that was being removed by peritoneal dialysate. Indeed, peritoneal dialysate from some patients contained selenium (8). Thus, PD should be considered a risk factor for selenium deficiency. Although one study reported that PD did not lead to a loss of selenium, peritoneal dialysate from some patients showed the loss of selenium (9). However, the factor responsible for the loss of selenium was not confirmed. Furthermore, this study did not focus on the total amount of selenium but rather its concentration (9), so it was unable to conclude that PD did or did not lead to the loss of selenium.
As described above, the present patient had several risk factors for a low selenium level. Of these, we considered PD to have contributed the most for two reasons. First, in most reported cases of selenium deficiency in patients on TPN in Japan, the durations of treatment were much longer than in the present patient (6). Second, the serum selenium level in the present case decreased just four days after the discontinuation of intravenous selenium administration, although selenium could not have been excreted in the urine of the present patient, as he had anuria. Physician should evaluate the serum selenium level when patients on PD receiving TPN show refractory hypotension.
While our patient had several risk factors associated with low selenium levels, patients with low selenium levels do not always have symptoms of selenium deficiency, and many of them are actually asymptomatic according to previous reports (10,11). Selenium deficiency may be crucial to maintaining stable hemodynamics in patients with a history of chronic heart failure and ischemic heart disease. Indeed, there have been several reports suggesting that selenium levels affect chronic heart failure or coronary heart disease (12-14). Another study showed the effects of selenium supplementation on reducing NT-proBNP levels and cardiovascular mortality (15).
Conclusion
In conclusion, patients on PD receiving parenteral nutrition are at risk of selenium deficiency. Physicians should consider selenium deficiency as a cause of refractory hypotension in patients undergoing PD, especially those with a history of heart disease.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Sunde RA. Selenium. In: Modern Nutrition in Health and Disease. 11th ed. Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, Eds. Lippincott Williams & Wilkins, Philadelphia, 2012: 225-237. [Google Scholar]
- 2.Rayman MP. The importance of selenium to human health. Lancet 356: 233-241, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Levander OA, Burk RF. Report on the 1986 A.S.P.E.N. research workshop on selenium in clinical nutrition. J Parenter Enteral Nutr 10: 545-549, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Chen J. An original discovery: selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 21: 320-326, 2012. [PubMed] [Google Scholar]
- 5.Liu Y, Chiba M, Inaba Y, Kondo M. Keshan disease-a review from the aspect of history and etiology. Nihon Eiseigaku Zasshi 56: 641-648, 2002(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 6.Kodama H, Asagiri K, Etani Y, et al. Diagnosis and treatment of selenium deficiency. J Jpn Soc Clin Nutr 40: 238-283, 2018(in Japanese). [Google Scholar]
- 7.Dizdar OS, Yıldız A, Gul CB, Gunal AI, Ersoy A, Gundogan K. The effect of hemodialysis, peritoneal dialysis and renal transplantation on nutritional status and serum micronutrient levels in patients with end-stage renal disease; multicenter, 6-month period, longitudinal study. J Trace Elem Med Biol 60: 126498, 2020. [DOI] [PubMed] [Google Scholar]
- 8.Pakfetrat M, Malekmakan L, Hasheminasab M. Diminished selenium levels in hemodialysis and continuous ambulatory peritoneal dialysis patients. Biol Trace Elem Res 137: 335-339, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Sriram K, Abraham G. Loss of zinc and selenium does not occur through peritoneal dialysis. Nutrition 16: 1047-1051, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Lipkin E, Schumann L, Young JH, Ivey M. Prediction of whole blood selenium levels in patients on long term parenteral nutrition. J Parenter Enteral Nutr 10: 40-44, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Fleming CR, McCall JT, O'Brien JF, Forsman RW, Ilstrup DM, Petz J. Selenium status in patients receiving home parenteral nutrition. J Parenter Enteral Nutr 8: 258-262, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol 37: 1765-1774, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 84: 762-773, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirdamadi A, Rafiei R, Kahazaipour G, Fouladi L. Selenium level in patients with heart failure versus normal individuals. Int J Prev Med 10: 210, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson P, Dahlström Ö, Dahlström U, Alehagen U. Effect of selenium and Q10 on the cardiac biomarker NT-proBNP. Scand Cardiovasc J 47: 281-288, 2013. [DOI] [PubMed] [Google Scholar]