Abstract
Objective
Our aim was to investigate the impact of the sodium glucose cotransporter type 2 (SGLT2) inhibitor on the left ventricular (LV) diastolic function in type 2 diabetes mellitus (T2DM) patients with chronic heart failure (HF) complicating cardiovascular risk factors.
Methods
We analyzed data from our previous prospective multicenter study, in which we investigated the effect of dapagliflozin on the LV diastolic function of T2DM patients with stable HF at five institutions in Japan. Patients who had been taking at least 1 antidiabetic drug other than SGLT2 inhibitors started treatment with dapagliflozin. Echocardiography was performed at baseline and six months after the administration of dapagliflozin. Cardiovascular risk factors other than T2DM were age, gender, hypertension, dyslipidemia, history of cardiovascular events and overweight.
Results
The LV diastolic function, defined as the ratio of the mitral inflow E to the mitral e' annular velocities (E/e'), significantly decreased from 9.3 to 8.5 by six months after the administration of dapagliflozin (p=0.020) as previously reported. A multivariate logistic regression analysis showed that dyslipidemia was the only independent determinant of improvement in the E/e' after the administration of dapagliflozin among cardiovascular risk factors. Furthermore, the relative change in the E/e' from baseline to six months after the administration of dapagliflozin for HF patients with preserved ejection fraction (HFpEF) and dyslipidemia was significantly larger than that for HFpEF patients without dyslipidemia (-15.2% vs. 29.6%, p=0.014), but no such finding was observed in non-HFpEF patients.
Conclusion
SGLT2 inhibitors may exert a more beneficial effect on the LV diastolic function for T2DM patients with stable HF, especially those with complicating dyslipidemia, than existing treatments.
Keywords: diabetes mellitus, diastolic function, echocardiography, SGLT2 inhibitor, dyslipidemia
Introduction
Type 2 diabetes mellitus (T2DM) is a well-known risk factor for heart failure (HF), even in patients without structural heart disease or a symptom of HF, known as Stage A HF. Insufficient control of T2DM constitutes an important predictor of the new onset of HF, with every 1% increase in hemoglobin (Hb) A1c correlating with an 8-19% increase in HF incidence (1,2). Diabetes-related cardiomyopathy presents as left ventricular (LV) diastolic dysfunction, which, like cardiovascular disease, is a contributor to the development of HF in both patients with a reduced ejection fraction (HFrEF) and those with a preserved ejection fraction (HFpEF) (3,4). In addition, comorbid factors other than T2DM, such as aging, gender, hypertension, dyslipidemia, history of cardiovascular events and obesity, also have been identified as high risk factors for progression to HF (5,6).
Dapagliflozin is a sodium glucose cotransporter type 2 (SGLT2) inhibitor, and represents a new class of anti-hyperglycemic agents for T2DM, which act insulin-independently to selectively inhibit renal glucose reabsorption, thereby increasing urinary glucose excretion. A large clinical trial using dapagliflozin showed that treatment with dapagliflozin of T2DM patients at risk for atherosclerotic cardiovascular disease resulted in a lower rate of cardiovascular death or hospitalization for HF compared with the administration of a placebo (7). Furthermore, results from a recent large clinical trial showed that dapagliflozin reduced the risk of worsening HF or death from cardiovascular causes for patients with HFrEF compared to those who received a placebo, regardless of the presence of T2DM (8). However, the effect of SGLT2 inhibitors on the LV diastolic function in T2DM patients with HF who had cardiovascular risk factors other than T2DM remains uncertain.
To investigate the impact of the SGLT2 inhibitor dapagliflozin on the LV diastolic function in T2DM patients with stable HF complicating cardiovascular risk factors, we analyzed data from our previous prospective multicenter study, which investigated the effect of dapagliflozin on the LV diastolic functional parameters, including the ratio of the mitral inflow E to the mitral e' annular velocities (E/e'), LV mass index and left atrial volume index, of T2DM patients with stable HF at five institutions in Japan (9).
Materials and Methods
Study population
The details of our prospective multicenter study have been described previously (9). In brief, the eligible patients comprised 53 T2DM patients with stable HF at the participating centers who had been taking at least 1 antidiabetic drug other than SGLT2 inhibitors for more than 1 year between December 2015 and March 2016. All patients had a history of HF but were in a clinically stable condition at the time of enrollment, defined as the absence of any exacerbation of HF symptoms for at least six months.
Patients were excluded from enrollment if they met any of the following criteria: 1) age <20 or >75 years old; 2) type I DM; 3) T2DM with HbA1c <6.5% or >10.0%; 4) insulin-dependent T2DM; 5) serious renal dysfunction defined as glomerular filtration rate <45 mL/min/1.73 m2; 6) hypotension <90/50 mmHg; 7) malignancy; 8) poor nutritional status; and 9) atrial fibrillation. According to the current guideline (10), patients were subsequently categorized as HFrEF, HFpEF or HFmrEF if their LVEF was <40%, ≥50% or 40-49%, respectively.
This study was approved by the local ethics committee of our institution.
Study protocol
Stable HF patients who had been taking at least 1 antidiabetic drug other than SGLT2 inhibitors and who had consented to their participation in this study, received the administration of 5 mg/day of dapagliflozin. Other drugs, including statins, were not changed after the start of administration of dapagliflozin. The physical examinations and blood tests were performed at baseline, three months, and six months after the administration of dapagliflozin, while echocardiography was performed at baseline and six months after the administration of the SGLT2 inhibitor. Only if a patient's HbA1c had failed to improve by 3 months after the administration of dapagliflozin, the dose was raised from 5 to 10 mg/day.
Echocardiography
All patients underwent a resting standard echocardiographic examination using commercially available echocardiography systems (Aplio Artida, Aplio 400 and Xario; Canon Medical Systems, Otawara, Japan; Vivid E9; GE-Vingmed, Horten, Norway; and iE33 and EPIQ7; Philips Medical Systems, Andover, USA). Digital routine grayscale two-dimensional cine loops from three consecutive heart beats were obtained at end-expiratory apnea from standard parasternal and apical views. Sector width was optimized to allow for complete myocardial visualization while maximizing the frame rate. Standard echocardiographic measurements were obtained in accordance with the current guidelines of the American Society of Echocardiography/European Association of Cardiovascular Imaging (11). Specifically, the early diastolic (E) and atrial wave (A) velocities and the E-wave deceleration time were measured by pulsed-wave Doppler recording from the apical four-chamber view. The spectral pulsed-wave Doppler-derived early diastolic velocity (e′) was obtained by averaging the septal and lateral mitral annulus, and the E/e′ ratio was calculated to obtain an estimate of the LV filling pressure.
Definition of cardiovascular risk factors
Cardiovascular risk factors other than T2DM were defined as age, gender, hypertension, dyslipidemia, history of cardiovascular events and overweight. Hypertension was defined as >140 mmHg systolic or >90 mmHg diastolic blood pressure or receiving hypertensive medical treatment. Dyslipidemia was defined as either fasting low-density lipoprotein cholesterol (LDL-C) ≥140 mg/dL, fasting high-density lipoprotein cholesterol (HDL-C) <40 mg/dL or triglyceride ≥150 mg/dL, or the current use of medication for dyslipidemia. Overweight was defined as a body mass index ≥25 kg/m2.
Statistical analyses
Continuous variables were expressed as the mean values and standard deviation for normally distributed data and as the median and interquartile range for non-normally distributed data. Categorical variables were expressed as frequencies and percentages. Paired t-tests or Wilcoxon's signed-rank test were used for group comparisons between baseline and six months after the start of administration of dapagliflozin. The association between relative changes in lipid profiles, such as triglycerides, LDL cholesterol and HDL cholesterol, from baseline to six months after the administration of dapagliflozin and those in the E/e' were explored by calculated Pearson's correlation coefficients.
The initial univariate logistic regression analysis to identify the independent associations of changes in the E/e' between baseline and six months after the start of administration of dapagliflozin with cardiovascular risk factors was followed by a multivariate logistic regression model using stepwise selection, with p levels for entry from the model set at <0.10. Furthermore, the Mann-Whitney U-test was used for to compare the between E/e' of patients with dyslipidemia and without dyslipidemia.
All analyses were performed with a commercially available program (SPSS, version 24.0; SPSS, Chicago, USA).
Results
Patient characteristics
The baseline clinical and echocardiographic characteristics of the 53 T2DM patients are summarized in Table 1 (9). Their mean age was 68 (60-73) years old, the LV ejection fraction (LVEF) was 62.3% (49.3-68.3%), and 21 patients (38%) were woman. All clinical and echocardiographic characteristics, including the LV diastolic function, of the 53 T2DM patients at baseline and 6 months after the administration of dapagliflozin were previously reported (Table 2) (9). The E/e′ significantly decreased from 9.3 to 8.5 6 months after the administration of dapagliflozin (p=0.020), as previously described (9).
Table 1.
Baseline Characteristics of Patients.
| Clinical Characteristics | |
| Age (years) | 68 (60-73) |
| Gender (female), n (%) | 21 (38) |
| DM duration (years) | 7.0 (5.0-11.5) |
| Body weight (kg) | 66.5 (56.8-76.9) |
| BMI (kg/m2) | 25.3 (23.4-28.8) |
| Systolic blood pressure (mmHg) | 130±16 |
| Heart rate (bpm) | 71±12 |
| BNP (pg/mL) | 27.9 (9.0-58.2) |
| BUN (mg/dL) | 14.7 (12.1-19.0) |
| Creatinine (mg/dL) | 0.80±0.20 |
| eGFR (mL/min/1.73 m2) | 70.6±17.0 |
| HbA1c (%) | 7.2±0.8 |
| Hemoglobin (g/dL) | 13.7±1.7 |
| Hematocrit (%) | 41±4.7 |
| Lipid profiles (mg/dL) | |
| Triglycerides | 127 (83.7-185) |
| LDL cholesterol | 98 (86-118) |
| HDL cholesterol | 51.5 (45.0-60.2) |
| Uric acid (mg/dL) | 5.1 (4.4-5.9) |
| HF Classification, n (%) | |
| HFpEF | 37 (69) |
| HFrEF | 7 (13) |
| HFmrEF | 9 (17) |
| Comorbidities, n (%) | |
| Hypertension | 43 (81) |
| Dyslipidemia | 42 (79) |
| Cardiovascular Event | 12 (21) |
| Medications, n (%) | |
| CCB | 19 (36) |
| ACEI/ARB | 42 (79) |
| β-blocker | 27 (51) |
| Diuretics | 10 (19) |
| Statin | 37 (70) |
| Antidiabetic drugs | |
| DPP-4I | 40 (75) |
| GLP-1 RA | 1 (2) |
| SU | 11 (21) |
| α-GI | 9 (17) |
| Thiazolidinedione | 11 (21) |
| Metformin | 14 (26) |
| Echocardiographic Parameters | |
| LV end-diastolic volume (mL) | 74.2 (55.1-104.1) |
| LV end-systolic volume (mL) | 24.7 (17.0-54.5) |
| LVEF (%) | 62.3 (49.3-68.3) |
| LVMI (g/m2) | 75.0 (61.7-92.0) |
| LAVI (mL/m2) | 31 (23-45) |
| e’ (cm/s) | 6.36±1.73 |
| E/e’ | 9.3 (7.7-11.8) |
| E (cm/s) | 58.1 (46.8-70.9) |
| A (cm/s) | 76.1±17.8 |
| E/A | 0.71 (0.6-0.80) |
Data are mean±SD for normally distributed data and median and interquartile range for non-normally distributed data, or n (%).
DM: diabetes mellitus, BSA: body mass index, BNP: plasma brain natriuretic peptide, LDL: low density lipoprotein, HDL: high density lipoprotein, HFpEF: heart failure with preserved ejection fraction, HFrEF: heart failure with reduced ejection fraction, HFmrEF: heart failure with mid-range ejection fraction, CCB: calcium channel blocker, ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, DPP-4I: dipeptidyl peptidase-4 inhibitor, GLP-1 RA: glucagon-like peptide-1 receptors agonists, SU: sulfonylureas, α-GI: α-glucosidase inhibitors. LVMI: left ventricular mass index, LVEF: left ventricular ejection fraction, LAVI: left atrial volume index, E: peak early diastolic mitral flow velocity, e’: spectral pulsed-wave Doppler-derived early diastolic velocity from the septal mitral annulus
Table 2.
Comparison of Variables between Baseline and 6 Months after the Administration of Dapagliflozin.
| Baseline | 6 months | p value | ||||
|---|---|---|---|---|---|---|
| Clinical Characteristics | ||||||
| Body weight (kg) | 66.5 (56.8-76.9) | 63.9 (56.2-75.6) | <0.001 | |||
| BMI (kg/m2) | 25.3 (23.4-28.8) | 24.9 (22.8-27.8) | <0.001 | |||
| Systolic blood pressure (mmHg) | 130±16 | 128±18 | 0.218 | |||
| Heart rate (bpm) | 71±12 | 70±11 | 0.610 | |||
| BNP (pg/mL) | 27.9 (9.0-58.2) | 28.9 (9.6-62.9) | 0.132 | |||
| BUN (mg/dL) | 14.7 (12.1-19.0) | 17.2 (14.2-21.2) | <0.001 | |||
| Creatinine (mg/dL) | 0.80±0.20 | 0.86±0.23 | <0.001 | |||
| eGFR (mL/min/1.73 m2) | 70.6±17.0 | 65.6±15.3 | 0.001 | |||
| HbA1c (%) | 7.2±0.8 | 7.0±0.8 | 0.108 | |||
| Hemoglobin (g/dL) | 13.7±1.7 | 14.4±1.6 | 0.020 | |||
| Hematocrit (%) | 41.0±4.7 | 43.8±4.5 | 0.002 | |||
| Lipid profiles (mg/dL) | ||||||
| Triglycerides | 127 (84-185) | 116 (82-176) | 0.155 | |||
| LDL cholesterol | 98 (86-118) | 106 (89-126) | 0.078 | |||
| HDL cholesterol | 51.5 (45.0-60.2) | 52 (42.8-65.0) | 0.049 | |||
| Uric acid (mg/dL) | 5.1 (4.4-5.9) | 4.7 (4.2-5.7) | 0.057 | |||
| Echocardiographic Parameters | ||||||
| LV end-diastolic volume (mL) | 74.2 (55.1-104.1) | 68.5 (54.8-93.8) | 0.270 | |||
| LV end-systolic volume (mL) | 24.7 (17.0-54.5) | 20.5 (15.2-57.1) | 0.105 | |||
| LVEF (%) | 62.3 (49.3-68.3) | 63.6 (55.3-71.0) | 0.011 | |||
| LVMI (g/m2) | 75.0 (61.7-92.0) | 67.0 (55.0-81.9) | <0.001 | |||
| LAVI (mL/m2) | 31 (23-45) | 26 (21-32) | 0.001 | |||
| e’ (cm/s) | 6.36±1.73 | 6.82±1.88 | 0.031 | |||
| E/e’ | 9.3 (7.7-11.8) | 8.5(6.6-10.7) | 0.020 | |||
| E (cm/s) | 58.1 (46.8-70.9) | 55.1 (45.3-73.7) | 0.682 | |||
| A (cm/s) | 76.1±17.8 | 75.6±16.8 | 0.765 | |||
| E/A | 0.71 (0.6-0.80) | 0.70 (0.62-0.81) | 0.818 |
Data are mean±SD for normally distributed data and median and interquartile range for non-normally distributed data, or n (%). BNP: plasma brain natriuretic peptide, LDL: low density lipoprotein, HDL: high density lipoprotein, LVMI: left ventricular mass index, LVEF: left ventricular ejection fraction, LAVI: left atrial volume index, E: peak early diastolic mitral flow velocity, e’: spectral pulsed-wave Doppler-derived early diastolic velocity from the septal mitral annulus
Parameters for changes in the E/e' after the administration of dapagliflozin
Table 3 shows the results of the univariate and multivariate logistic regression analyses of the associations of the LV diastolic function assessed as the E/e' with cardiovascular risk factors after the administration of dapagliflozin. An important finding of the multiple regression analysis was that, among cardiovascular risk factors, dyslipidemia was the only independent parameter of a change in the E/e' after the administration of dapagliflozin.
Table 3.
Results of the Univariate and Multivariate Logistic Regression Analysis.
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p value | OR | 95% CI | p value | |
| Age, years | 0.997 | 0.94-1.06 | 0.912 | ||||
| Gender (Female) | 1.833 | 0.52-6.46 | 0.346 | ||||
| Hypertension | 0.615 | 0.14-2.68 | 0.518 | ||||
| Dyslipidemia | 7.25 | 1.73-30.4 | 0.007 | 7.25 | 1.73-30.4 | 0.007 | |
| History of cardiovascular events | 5.625 | 0.65-49.0 | 0.118 | ||||
| Overweight | 0.379 | 0.11-1.34 | 0.131 | ||||
| HbA1c | 0.474 | 0.22-1.04 | 0.523 | ||||
| eGFR | 1.043 | 1.001-1.087 | 0.047 | ||||
OR: odds ratio, CI: confidential interval
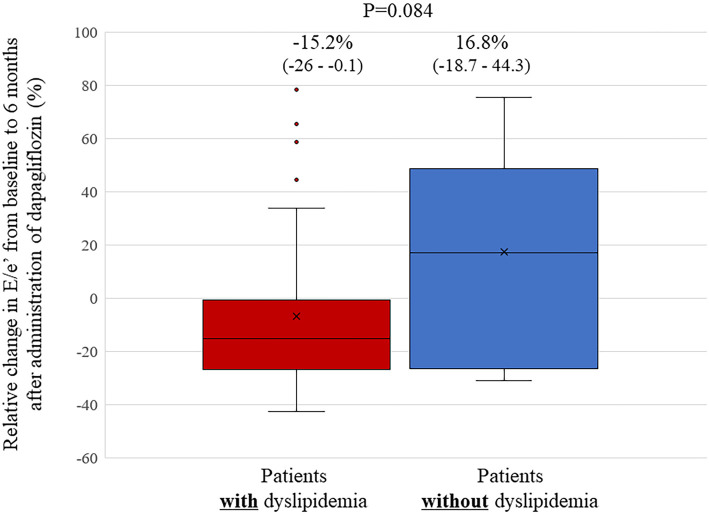
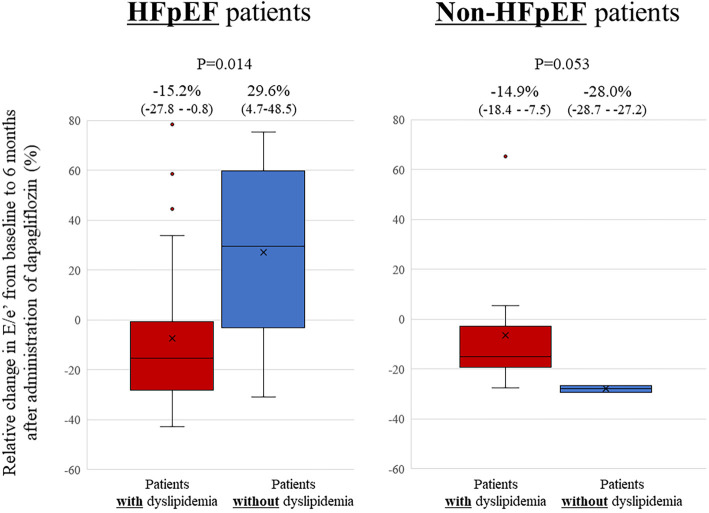
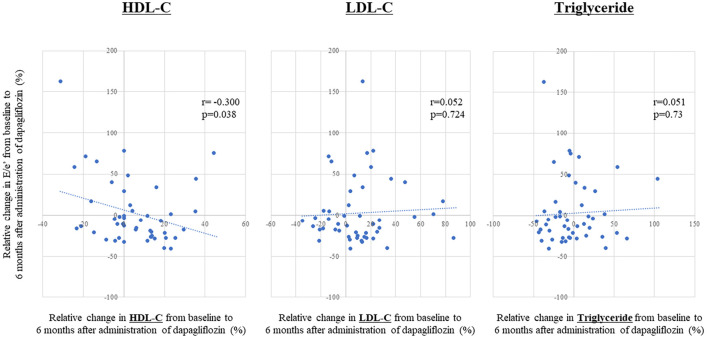
Relative changes in the E/e' from baseline to 6 months after the administration of dapagliflozin seen in patients with dyslipidemia tended to be larger than those in patients without dyslipidemia, but the difference was not statistically significant (-15.2% vs. 16.8%, p=0.084; Fig. 1). Absolute changes in the E/e' from baseline to 6 months after the administration of dapagliflozin seen in patients with dyslipidemia also tended to be larger than those in patients without dyslipidemia, but again, the difference was not statistically significant [-1.8((-2.6)-(-0.1)) vs. 1.2((-1.6)-(4.0)), p=0.06]. Furthermore, relative changes in the E/e' from baseline to 6 months after the administration of dapagliflozin seen in HFpEF patients with dyslipidemia were significantly larger than those in HFpEF patients without dyslipidemia (-15.2% vs. 29.6%, p=0.014; Fig. 2), but no such difference was observed in non-HFpEF patients. In this study, 27 patients (73%) with HFpEF had dyslipidemia. The association between relative changes in lipid profiles from baseline to 6 months after the administration of dapagliflozin and those in the E/e' are shown in Fig. 3. Relative changes in HDL-C from baseline to 6 months after the administration of dapagliflozin had a significant correlation with those in the E/e' (r=-0.300, p=0.038). However, such correlations were not observed in LDL-C or triglyceride levels (r=0.05, p=0.72 and r=0.05, p=0.73).
Figure 1.
Bar graphs of the relative changes in the E/e’ from baseline to six months after the administration of dapagliflozin, showing that these changes in the E/e’ in patients with dyslipidemia tended to be larger than in patients without dyslipidemia, although without statistically significant difference.
Figure 2.
Bar graphs of the relative changes in the E/e’ from baseline to six months after the administration of dapagliflozin, showing that the relative changes in the E/e’ in HFpEF patients with dyslipidemia were significantly larger than those in HFpEF patients without dyslipidemia, although such finding was not observed in non-HFpEF patients. HFpEF: heart failure with preserved ejection fraction
Figure 3.
A linear regression analysis comparing the relative changes in the lipid profiles from baseline to six months after the administration of dapagliflozin and those in the E/e’, showing a significant correlation between changes in the HDL-C levels and those in the E/e’. HDL-C: high-density lipoprotein cholesterol
Discussion
The findings of our study indicate that, among cardiovascular risk factors for T2DM patients with stable HF, dyslipidemia was the only independent parameter of improvement of the LV diastolic function in the E/e' after the administration of dapagliflozin. In addition, the improvement of the LV diastolic function in HFpEF patients with dyslipidemia was significantly larger than that in HFpEF patients without dyslipidemia, but such a difference was not observed in non-HFpEF patients.
Association of dyslipidemia with the LV diastolic function
T2DM is a major risk factor for cardiovascular disease and HF (12), which has been of increasing importance as a consequence of an increase in the prevalence of T2DM and in aging populations. However, the existence of a distinct diabetes-related cardiomyopathy, specifically because of hyperglycemia, is still controversial.
Dyslipidemia is a metabolic abnormality observed frequently in T2DM patients, that can lead to the development of HF. Nonetheless, the relationship of lipid profiles to HF, especially the LV diastolic function, has not been fully investigated. Horio et al. showed that HDL-C, but not total or LDL-C, had a significant association with LV diastolic dysfunction in 274 subjects with essential hypertension, and they suggested that low HDL-C levels might have an adverse effect on the LV diastolic function, regardless of the gender, blood pressure level, or LV structure (13). They also showed that triglyceride levels had a significant correlation with the LV diastolic function, and that LV diastolic dysfunction was most advanced in patients with both low HDL-C and high triglycerides levels. The serum levels of HDL-C reportedly correlate inversely with serum insulin levels (14), and some studies have reported that hyperinsulinemia or insulin resistance was related to LV diastolic dysfunction (15,16). A possible explanation for the effects of low HDL-C on cardiac structural and functional alterations may therefore involve insulin resistance and hyperinsulinemia. In addition, a study of 457 South Asians and 542 Europeans by Park et al. reported that T2DM and dyslipidemia have greater adverse effects on the LV diastolic function in the former population than in the latter population (17). Interestingly, statins reportedly improve the LV diastolic function by down-regulating the expression of genes such as collagen I, transforming growth factor-beta, matrix metalloproteinase (MMP)-2 and MMP-3, atrial natriuretic factor, interleukin (IL)-6, tumor necrosis factor (TNF)α and Rho kinase 1, inhibiting the renin-angiotensin system (RAS) and upregulating the eNOS gene expression (18-20). In addition, Warita et al. showed that pitavastatin had a beneficial effect on the LV diastolic function and LA structure and function in 220 elderly patients with hypertension (≥ 65 years old), although there were no major differences in blood pressure (21).
Effect of SGLT2 inhibitor on lipid profiles
SGLT2 inhibitors are well known to affect lipid profiles as well as reduce blood pressure, induce weight loss and reduce HbA1c levels. Hayashi et al. reported the effect of the SGLT2 inhibitor dapagliflozin on the lipid profiles of 80 T2DM patients assigned to receive dapagliflozin or sitagliptin, a dipeptidyl peptidase-4 inhibitor (22). They showed that the LDL-C and apolipoprotein B levels were not significantly changed by dapagliflozin, whereas the HDL-C and apolipoprotein AI levels increased. Furthermore, dapagliflozin did not alter the concentrations of LDL-C, but small dense LDL-C levels decreased by 20%, and large buoyant LDL-C levels increased by 18%. Dapagliflozin also increased HDL2-C by 18% without affecting HDL3-C. In contrast, sitagliptin did not alter the plasma lipids or lipoprotein subspecies. In addition, Roes et al. showed that HDL-C was independently associated with the LV diastolic function in patients with metabolic syndrome, suggesting adverse cardiovascular changes in the presence of low HDL-C levels (23).
In our study, triglyceride levels tended to be decreased, while LDL-C levels tended to be increased, and HDL-C levels were significantly increased six months after the administration of dapagliflozin, as previously reported. While a small change, only the relative changes in HDL-C from baseline to six months after the administration of dapagliflozin had a significant correlation with those in the LV diastolic function.
SGLT2 inhibitors have a multifaceted effect on the LV diastolic function, and dyslipidemia may be strongly involved, based on our findings. These findings suggest potential new insight into the positive impact of dapagliflozin on the LV diastolic function in T2DM patients. In addition, improvement of the LV diastolic function, assessed as the E/e' after the administration of dapagliflozin was prominent in T2DM patients with complicating dyslipidemia in the present study. Interestingly, this phenomenon was more prominent in T2DM patients with HFpEF than in those with non-HFpEF.
Clinical implications
Diabetes-related cardiomyopathy presents as an LV diastolic dysfunction, which plays an important role in the development of cardiovascular events and outcomes both for patients with HFrEF and those with HFpEF. The present problem is that there is no effective treatment for LV diastolic dysfunction to improve the outcomes for HF patients.
SGLT2 inhibitors are well known to have pleiotropic effects other than diabetes, so these multifaceted effects of SGLT2 inhibitors on such various risk factors may well lead to improvement in the LV diastolic function for T2DM patients. Our recent prospective multicenter trial using T2DM patients with stable HF showed that use of the SGLT2 inhibitor dapagliflozin was associated with improvements in LV diastolic functional parameters including the E/e', LV mass index and left atrial volume index. Furthermore, a recent and important large clinical trial, DAPA-HF, which used the SGLT2 inhibitor dapagliflozin and included 4,744 patients with HFrEF showed that the risk of the primary composite outcome of worsening HF or death from cardiovascular causes was significantly lower in the dapagliflozin group than in the placebo group, regardless of the presence of T2DM (8). Our findings indicate that SGLT2 inhibitors have the potential to improve the LV diastolic function in T2DM patients with stable HF, with a more beneficial effect for patients with complicating dyslipidemia than for those without such complications. The indication of SGLT2 inhibitors for T2DM patients may thus be expanding.
Study limitations
Several limitations associated with the present study warrant mention. This study comprised a small number of patients and did not use a placebo-controlled group, so future prospective studies with larger patient populations, including placebo-controlled groups, will be needed to validate our findings. In addition, the E/e' at baseline was relatively low in this study, as all patients were in a stable HF condition. The changes in the E/e' in this study were also small as that in estimated LV end-diastolic pressure (24). Since a significant correlation was reportedly observed between the E/e' and pulmonary capillary wedge pressure in HF patients (25,26), we believed that a small decrease in the E/e', even in patients with a normal range of E/e', could be beneficial.
Conclusion
Dapagliflozin exerts a more beneficial effect on the LV diastolic function of T2DM patients with stable HF, especially those with complicating dyslipidemia, than existing treatments. Our findings may thus offer new insight into the management of T2DM patients with HF.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful for the support of the entire staff of the echocardiography laboratory of Kobe University Hospital.
References
- 1.Vaur L, Gueret P, Lievre M, Chabaud S, Passa P; study DSG. Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: observations from the DIABHYCAR (type 2 DIABetes, Hypertension, CArdiovascular Events and Ramipril) study. Diabetes Care 26: 855-860, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation 103: 2668-2673, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez-Benitez G, Desai JR, Xu S, et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care 38: 905-912, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 241: 2035-2038, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Writing Committee Members, Yancy CW, Jessup M, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 134: e282-e293, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H. Utility of strain imaging in conjunction with heart failure stage classification for heart failure patient management. J Echocardiogr 17: 17-24, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347-357, 2019. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995-2008, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Soga F, Tanaka H, Tatsumi K, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol 17: 132, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28: 1-39. e14, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation 100: 1132-1133, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Horio T, Miyazato J, Kamide K, Takiuchi S, Kawano Y. Influence of low high-density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am J Hypertens 16: 938-944, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension 25: 30-36, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Lind L, Andersson PE, Andren B, Hanni A, Lithell HO. Left ventricular hypertrophy in hypertension is associated with the insulin resistance metabolic syndrome. J Hypertens 13: 433-438, 1995. [PubMed] [Google Scholar]
- 16.Watanabe K, Sekiya M, Tsuruoka T, Funada J, Kameoka H. Effect of insulin resistance on left ventricular hypertrophy and dysfunction in essential hypertension. J Hypertens 17: 1153-1160, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Park CM, Tillin T, March K, et al. Hyperglycemia has a greater impact on left ventricle function in South Asians than in Europeans. Diabetes Care 37: 1124-1131, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indolfi C, Di Lorenzo E, Perrino C, et al. Hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin prevents cardiac hypertrophy induced by pressure overload and inhibits p21ras activation. Circulation 106: 2118-2124, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Tousoulis D, Oikonomou E, Siasos G, Stefanadis C. Statins in heart failure--With preserved and reduced ejection fraction. An update. Pharmacol Ther 141: 79-91, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Xu Z, Okamoto H, Akino M, Onozuka H, Matsui Y, Tsutsui H. Pravastatin attenuates left ventricular remodeling and diastolic dysfunction in angiotensin II-induced hypertensive mice. J Cardiovasc Pharmacol 51: 62-70, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Warita S, Kawasaki M, Tanaka R, et al. Effects of pitavastatin on cardiac structure and function and on prevention of atrial fibrillation in elderly hypertensive patients: a prospective study of 2-years' follow-up. Circ J 76: 2755-2762, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol 16: 8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roes SD, Alizadeh Dehnavi R, Westenberg JJ, et al. Assessment of aortic pulse wave velocity and cardiac diastolic function in subjects with and without the metabolic syndrome: HDL cholesterol is independently associated with cardiovascular function. Diabetes Care 31: 1442-1444, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzl M, Ojeda F, Zeller T, et al. Risk factors for heart failure are associated with alterations of the LV end-diastolic pressure-volume relationship in non-heart failure individuals: data from a large-scale, population-based cohort. Eur Heart J 37: 1807-1814, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53: 1119-1126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita K, Minamishima T, Goda A, et al. Comparison of the reliability of E/E' to estimate pulmonary capillary wedge pressure in heart failure patients with preserved ejection fraction versus those with reduced ejection fraction. Int J Cardiovasc Imaging 31: 1497-1502, 2015. [DOI] [PubMed] [Google Scholar]