Abstract
We herein report the case of a 54-year-old Japanese man with hepatitis C virus (HCV)-related membranoproliferative glomerulonephritis (MPGN), which developed at the time of relapse of immune thrombocytopenic purpura (ITP) after rituximab therapy. Antiviral therapy for HCV led to the improvement of both MPGN and ITP. Rituximab therapy may have contributed to the exacerbation of HCV infection and induced the development of HCV-related MPGN and the relapse of ITP. Our case suggested that HCV treatment should be prioritized over rituximab therapy for HCV-positive patients with ITP and that antiviral therapy for HCV may be effective for treating ITP itself.
Keywords: immune thrombocytopenia, hepatitis C virus, membranoproliferative glomerulonephritis, rituximab
Introduction
Immune thrombocytopenic purpura (ITP) is an immune-mediated acquired disease that is characterized by a transient or persistent decrease in the platelet count and an increased risk of bleeding (1,2). According to the Japanese guidelines for adult ITP (3), ITP treatment includes Helicobacter pylori eradication therapy and corticosteroid therapy as the first choice, and thrombopoietin receptor agonist, splenectomy, and rituximab therapy as the second choice. Rituximab is a chimeric monoclonal antibody against the CD20 antigen, which specifically eliminates CD20-positive B lymphocytes and reduces antibody production. In Japan, rituximab has been approved for the treatment of ITP since 2017 (4) and it is now used on a daily basis. It is widely known that the reactivation of hepatitis B virus (HBV) may occur in patients treated with rituximab-containing regimens, and there are guidelines aimed at its prevention (5). In contrast, with regard to hepatitis C virus (HCV), the onset of fulminant hepatitis is extremely rare, and there are no guidelines for its prevention (6). However, there are also some reports on the exacerbation of HCV infection by rituximab-containing regimens (7,8). We herein report a case of refractory ITP complicated by HCV-related membranoproliferative glomerulonephritis (MPGN) that developed after the initiation of rituximab therapy.
Case Report
A 54-year-old Japanese man was diagnosed with ITP at 25 years of age. He was treated with prednisolone (PSL), cyclosporin, azathioprine, high-dose intravenous immunoglobulin therapy, and eltrombopag. The effects of these treatments were all limited. Romiplostim (10 μg/kg) and PSL (10 mg) were continued for a long time. However, the dose of PSL often needed to be increased because the patient's platelet count dropped to <10.0×109/L with bleeding symptoms. The platelet-associated IgG value was in the range of 200-300 ng/107 cells. Due to long-term steroid therapy, he developed thin skin and obesity (body mass index, 30.2 kg/m2). Voglibose, linagliptin, olmesartan medoxomil/azelnidipine, enalapril maleate, and febuxostat were continued as treatments for hypertension, diabetes mellitus and hyperuricemia. Diabetic retinopathy was never noted. The estimated glomerular filtration rate remained at just over 60 mL/min/1.73 m2. Although HCV antibody positivity was pointed out, he was followed up without any treatment because there was no liver disorder. In November 2017, rituximab (375 mg/m2 per week) was administered four times. His platelet count increased and the PSL dosage could be decreased to 4 mg. In March 2018, however, his platelet count dropped in 8.0×109/L. 40 mg dexamethasone for 4 days was performed and the platelet count increased. Renal dysfunction appeared during the same period and it also worsened. Severe edema was noted on both lower legs and diuretics were required. No joint pain, swelling, Raynaud's symptoms, or numbness of the fingers or toes was observed. In April 2018, the serum creatinine concentration rose to 7.81 mg/dL and he was urgently hospitalized.
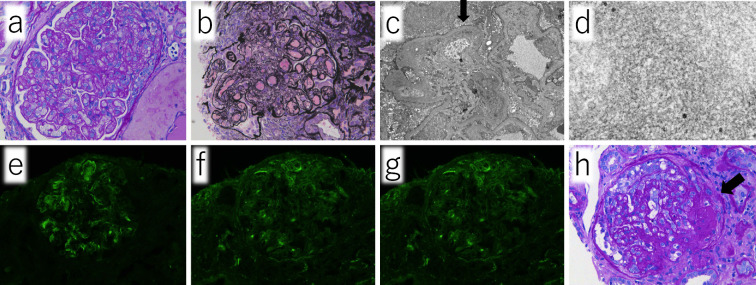
A physical examination on admission revealed the following: body temperature, 35.8℃; heart rate, 84 bpm; blood pressure, 147/80 mmHg; and respiratory rate, 19 breaths/min. His oxygen saturation was 99% while breathing room air. A physical examination revealed purpura on his hand and breast. No edema was found in either of the lower limbs on admission. The laboratory findings are shown in Table. The patient's platelet count had increased due to the administration of high-dose dexamethasone therapy just before admission. He presented with mild liver dysfunction, severe renal dysfunction, and hyperkalemia due to renal dysfunction. The levels of complement proteins were low. A cryoglobulin test was positive, and IgG and IgM cryoglobulins were detected. Because M-protein was not found by immunoelectrophoresis, Type III cryoglobulinemia was thus diagnosed. A urinalysis revealed protein, occult blood, and various urinary casts. Abdominal echography showed normally sized kidneys, increased echogenicity of the renal parenchyma, and renal cysts. There were moderate to large amount of ascites without findings suggestive of cirrhosis. A renal biopsy revealed an enlargement of the glomeruli with mesangial proliferation, lobulation of the glomeruli (Fig. 1a, b), and double contours in the basement membranes (Fig. 1c, arrow). Microtubule-like deposits were diffusely observed in the subendothelial basement membrane and paramesagiual area on electron microscopy (Fig. 1c, d). Immunofluorescence staining showed IgM positivity in the basement membrane in the form of small granules (Fig. 1e), while IgG and C3 were negative (Fig. 1f, g). There was no marked difference between kappa and lambda staining. Exudative lesions in the capillaries were observed in some glomeruli (Fig. 1h, arrow).
Table.
Laboratory Examination at the Admission.
| Complete blood cell count | Blood chemistry | Serological test | |||||||
| White blood cell | 9.0 | ×109/L | Total protein | 5.3 | g/dL | C-reactive protein | 0.77 | mg/dL | |
| Neutrophil | 87.4 | % | Albumin | 3.5 | g/dL | IgG/IgA/IgM | 990/89/49 | mg/dL | |
| Lymphocyte | 10.2 | % | Aspartate transaminase | 69 | U/L | M-protein | negative | ||
| Eosinophil | 0.1 | % | Alanine aminotransferase | 64 | U/L | C3 | 61 | mg/dL (73-138) | |
| Hemoglobin | 11.4 | g/dL | Lactate dehydrogenase | 364 | U/L | C4 | 14 | mg/dL (86-138) | |
| Hematocrit | 35.1 | % | Alkaline phosphatase | 400 | U/L | CH50 | 28.0 | U/mL (31.6-57.6) | |
| Platelet count | 136.0 | ×109/L | Total bilirubin | 0.5 | mg/dL | Cryoglobulin | positive | ||
| Urea nitrogen | 75 | mg/dL | Antinuclear antibody | ×80 | |||||
| Coagulation test | Creatinin | 7.81 | mg/mL | Rheumatoid factor | <5.0 | IU/mL | |||
| PT-INR | 1.04 | Sodium | 137 | mEq/L | ds-DNA antibody | <0.5 | U/mL | ||
| APTT | 45.3 | sec | Potassium | 5.8 | mEq/L | MPO/PR3-ANCA | <1.0/<1.0 | U/mL | |
| Fibrinogen | 273 | mg/dL | Corrected calcium | 8.4 | mg/dL | Anti-SS-A/B antibody | <1.0/<1.0 | U/mL | |
| D-dimer | 0.7 | μg/mL | eGFR | 6.7 | mL/min/1.73m2 | Anti-GBM antibody | <2.0 | U/mL | |
| Urine test | |||||||||
| Virological examination | Protein | 3+ | White blood cell | <0-1 | /HPF | ||||
| Hepatitis B surface antigen | (-) | Glucose | 1+ | Red blood cell | 1-4 | / HPF | |||
| HCV-antibody | (+) | Occult blood | 1+ | Oval fat body | + | Granular cast | + | ||
| HCV-genotype | 2a | Protein | 6.20 | g/gCre | Hyalin cast | + | Fatty cast | + | |
| HCV-RNA | 6.5 | log IU/mL | Waxy cast | + | Epithelial cast | + | |||
PT-INR: international normalized ratio, APTT: activated partial thromboplastin, HCV: hepatitis C virus, Cre: creatinine, Ig: immunoglobulin, MPO: myeloperoxidase, PR3: proteinase 3, ANCA: anti-neutrophil cytoplasmic antibody, SS: Sjogren’s syndrome, GBM: glomerular basement membrane, HPF: high power field. The numbers in parentheses after the C3, C4, and CH50 values are the normal range at the time of measurement
Figure 1.
Renal biopsy revealed enlargement of the glomeruli with mesangial proliferation, lobulation of the glomeruli (a, b), and double contours in the basement membranes (c, arrow). Microtubule-like deposits are diffusely observed in the subendothelial basement membrane and paramesagiual area on electron microscopy. (c, d) Immunofluorescence staining revealed IgM positivity in the basement membrane in the form of small granules (e), while IgG and C3 were negative (f, g). Exudative lesions in capillaries were observed in some glomeruli (h, arrow).
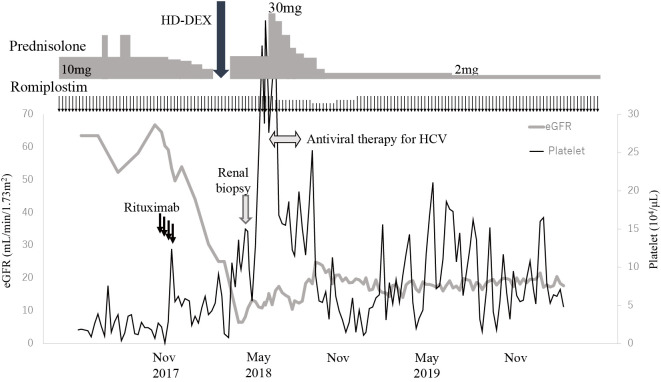
The main cause of renal failure was diagnosed as HCV-related MPGN, which is classified as immune complex-mediated MPGN, and diabetic nephropathy was also considered to be present. The administration of glecaprevir hydrate/pibrentasvir for 12 weeks was initiated and the dose of PSL was increased to 30 mg for 2 weeks. At two weeks after the start of the antiviral therapy, HCV-RNA was no longer detected. The serum creatinine levels tended to improve, and the urinary occult blood disappeared, although mild proteinuria remained. The dose of PSL was gradually reduced to 3 mg, and the dose of romiplostim was reduced to 5 μg/kg. Because the platelet count decreased again, the dose of romiplostim was returned to 10 μg/kg. More than one year has passed since the dose of PSL was reduced to 2 mg. Although the patient's platelet count has fluctuated, no clear bleeding tendency has been observed (Fig. 2). With the reduction of the PSL dose, the patient's body mass index improved to 23.1 kg/m2, and voglibose and linagliptin could be discontinued. The levels of complement protein improved to 13 (normal range, 11-31) mg/dL for C3, 56 (73-138) mg/dL for C4, and 36.6 (25.0-48.0) U/mL for CH50. The elevated serum creatinine level and mild proteinuria persisted, a finding that was attributed to the diabetic nephropathy, and the management of chronic kidney disease was continued with a focus on antihypertensive therapy.
Figure 2.
The clinical course. HD-DEX: high dose dexamethasone, eGFR: estimated glomerular filtration rate, HCV: hepatitis C virus
Discussion
MPGN is a type of glomerulonephritis that is characterized by mesangial proliferation and basement membrane duplication. The clinical presentation and course are extremely variable (9). MPGN occurs as a primary or secondary condition. Secondary MPGN is most often due to hepatitis C and other infections (10). Patients with HCV-related MPGN have a higher incidence of liver dysfunction, cryoglobulinemia, rheumatoid factor, and hypocomplementemia (11,12). In our case, after rituximab therapy HCV-related MPGN developed with a relapse of ITP and both improved with antiviral therapy for HCV. HCV-RNA elevation due to rituximab therapy may have contributed to the development of MPGN and the relapse of ITP. Unfortunately, however, the patient's HCV-RNA levels were not measured until the onset of MPGN, as there was no liver disorder nor any indication for antiviral therapy. This clinical course may have been due to an iatrogenic cause. At the very least, the HCV-RNA levels should have been monitored before the initiation of rituximab therapy.
Several reports have described the successful treatment of MPGN with rituximab therapy. The addition of rituximab therapy to antiviral therapy for HCV-related MPGN is well tolerated and it has been reported to be more effective than antiviral therapy alone (13,14). Mak et al. described the successful treatment of thrombotic thrombocytopenic purpura and MPGN with rituximab therapy in a case associated with HCV infection (15). In that case, the MPGN improved, but liver dysfunction and HCV-RNA elevation were transiently observed. In contrast to the present case, neither a blood examination nor a renal biopsy showed cryoglobulin. There is no doubt that rituximab is effective as a treatment for MPGN itself. However, it may not be possible to prevent the onset of MPGN, and in our case, it was considered that rituximab indirectly induced MPGN.
In individuals who are infected with HCV, the incidence of ITP is increased in comparison individuals without HCV infection (16). Several reports have suggested that HCV infection is involved in the development of ITP through various mechanisms (16-18). Johia et al. described the case of an HCV-positive patient with refractory ITP in whom the eradication of HCV was effective for ITP (18). In this case, although rituximab therapy seemed to improve the responsiveness of ITP to steroid therapy, the reduced dose of PSL (4 mg) caused a relapse of ITP. Although the combined use of romiplostim was still necessary after antiviral therapy for HCV, the dosage of PSL could be reduced to 2 mg, suggesting that antiviral therapy for HCV was also effective against ITP. Thrombocytopenia is no longer a major problem in the treatment of chronic HCV infection because of the recent change in direct antiviral agents without interferon (19). The US guidelines for ITP recommend that testing for HCV be considered in all patients with acute ITP and that antiviral therapy be considered in HCV-positive patients with ITP in the absence of contraindications (20). Although whether or not all HCV-positive patients with ITP should be treated remains controversial in Japan, antiviral therapy for HCV should be actively performed as a treatment for refractory ITP. At least, it is certain that antiviral therapy for HCV should be given before rituximab therapy.
In conclusion, we experienced a case in which HCV-related MPGN that developed with a relapse of ITP after rituximab therapy, in which both conditions were improved by antiviral therapy for HCV. Rituximab therapy may have contributed to the exacerbation of HCV infection and induced the development of HCV-related MPGN and relapse of ITP. Our case suggested that HCV treatment should be prioritized over rituximab therapy for HCV-positive patients with ITP and that antiviral therapy for HCV may be effective for ITP itself.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Yoshitaka Miyakawa of Department of General Internal Medicine, Saitama Medical University for his advice on the treatment of ITP in the present case.
References
- 1.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med 346: 995-1008, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Cooper N, Bussel J. The pathogenesis of immune thrombocytopaenic purpura. Br J Haematol 133: 364-374, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Kashiwagi H. Reference guide for management of adult idiopathic thrombocytopenic purpura (ITP): 2019 version. Rinsho Ketsueki 60: 877-896, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Miyakawa Y, Katsutani S, Yano T, et al. Efficacy and safety of rituximab in Japanese patients with relapsed chronic immune thrombocytopenia refractory to conventional therapy. Int J Hematol 102: 654-661, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsumi Y, Yamamoto Y, Ito S, et al. Hepatitis B virus reactivation with a rituximab-containing regimen. World J Hepatol 7: 2344-2351, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firpi RJ, Nelson DR. Management of viral hepatitis in hematologic malignancies. Blood Rev 22: 117-126, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Sagnelli E, Pisaturo M, Sagnelli C, Coppola N. Rituximab-based treatment, HCV replication, and hepatic flares. Clin Dev Immunol 2012: 945950, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennishi D, Terui Y, Yokoyama M, et al. Monitoring serum hepatitis C virus (HCV) RNA in patients with HCV-infected CD20-positive B-cell lymphoma undergoing rituximab combination chemotherapy. Am J Hematol 83: 59-62, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis―a new look at an old entity. N Engl J Med 366: 1119-1131, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int 47: 643-656, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Yamabe H, Johnson RJ, Gretch DR, et al. Hepatitis C virus infection and membranoproliferative glomerulonephritis in Japan. J Am Soc Nephrol 6: 220-223, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Gretch DR, Yamabe H, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 328: 465-470, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Saadoun D, Resche Rigon M, Sene D, et al. Rituximab plus Peg-interferon-alpha/ribavirin compared with Peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood 116: 326-334, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Dammacco F, Tucci FA, Lauletta G, et al. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term study. Blood 116: 343-353, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Mak SK, Lo KY, Lo MW, et al. Refractory thrombotic thrombocytopenic purpura and membranoproliferative glomerulonephritis successfully treated with rituximab: a case associated with hepatitis C virus infection. Hong Kong Med J 15: 201-208, 2009. [PubMed] [Google Scholar]
- 16.Chiao EY, Engels EA, Kramer JR, et al. Risk of immune thrombocytopenic purpura and autoimmune hemolytic anemia among 120 908 US veterans with hepatitis C virus infection. Arch Intern Med 169: 357-363, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Almeida AJ, Campos-de-Magalhaes M, de Melo Marcal OP, et al. Hepatitis C virus-associated thrombocytopenia: a controlled prospective, virological study. Ann Hematol 83: 434-440, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Johira Y, Hatooka H, Mori N, et al. Regression of idiopathic thrombocytopenic purpura in a patient with eradication of hepatitis C virus by direct-acting antivirals. Hepatology 71: 389-391, 2020. [DOI] [PubMed] [Google Scholar]
- 19.Dahal S, Upadhyay S, Banjade R, Dhakal P, Khanal N, Bhatt VR. Thrombocytopenia in patients with chronic hepatitis C virus infection. Mediterr J Hematol Infect Dis 9: e2017019, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 117: 4190-4207, 2011. [DOI] [PubMed] [Google Scholar]