Abstract
Background.
Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae (hereafter “KPC”) are an increasing threat to healthcare institutions. Long-term acute-care hospitals (LTACHs) have especially high prevalence of KPC.
Methods.
Using a stepped-wedge design, we tested whether a bundled intervention (screening patients for KPC rectal colonization upon admission and every other week; contact isolation and geographic separation of KPC-positive patients in ward cohorts or single rooms; bathing all patients daily with chlorhexidine gluconate; and healthcare-worker education and adherence monitoring) would reduce colonization and infection due to KPC in 4 LTACHs with high endemic KPC prevalence. The study was conducted between 1 February 2010 and 30 June 2013; 3894 patients were enrolled during the preintervention period (lasting from 16 to 29 months), and 2951 patients were enrolled during the intervention period (lasting from 12 to 19 months).
Results.
KPC colonization prevalence was stable during preintervention (average, 45.8%; 95% confidence interval [CI], 42.1%–49.5%), declined early during intervention, then reached a plateau (34.3%; 95% CI, 32.4%–36.2%; P < .001 for exponential decline). During intervention, KPC admission prevalence remained high (average, 20.6%, 95% CI, 19.1%–22.3%). The incidence rate of KPC colonization fell during intervention, from 4 to 2 acquisitions per 100 patient-weeks (P = .004 for linear decline). Compared to preintervention, average rates of clinical outcomes declined during intervention: KPC in any clinical culture (3.7 to 2.5/1000 patient-days; P = .001), KPC bacteremia (0.9 to 0.4/1000 patient-days; P = .008), all-cause bacteremia (11.2 to 7.6/1000 patient-days; P = .006) and blood culture contamination (4.9 to 2.3/1000 patient-days; P = .03).
Conclusions.
A bundled intervention was associated with clinically important and statistically significant reductions in KPC colonization, KPC infection, all-cause bacteremia, and blood culture contamination in a high-risk LTACH population.
Keywords: carbapenem-resistant Enterobacteriaceae, Klebsiella pneumoniae carbapenemase, long-term acute-care hospital, infection prevention, healthcare-associated infection
Healthcare-associated infections due to antibiotic-resistant bacteria often fail to respond to conventional therapy, resulting in greater risk of death and higher costs [1, 2]. Carbapenem-resistant Enterobacteriaceae (CRE) may be the most serious contemporary antibiotic resistance threat because of the number of different resistance mechanisms [3], concomitant resistance to all or nearly all alternative antibiotics [4], high attributable mortality associated with invasive infection [5, 6], and the ability of these pathogens to spread rapidly across geographic regions [7-9]. In 2013, the Centers for Disease Control and Prevention declared CRE an immediate public health threat requiring urgent and aggressive action [10].
Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae (hereafter “KPC”) are the most common CRE worldwide [3]. Colonization with KPC usually precedes infection; patients acquire colonization in healthcare settings, presumably via cross-transmission after breaches in infection prevention measures such as healthcare-worker hand hygiene [11]. Transfer of KPC-positive patients between healthcare facilities further enables dispersion of KPC throughout a geographic region [8, 12]. Thus, coordinated regional interventions have been promoted as necessary to achieve durable control [9, 13].
Long-term acute care hospitals (LTACHs) and other post-acute-care facilities, whose populations are at high risk for colonization and infection with multidrug-resistant bacteria, bear a disproportionate burden of KPC and have been shown to be a major contributor to its dissemination in multiple locales [8, 12, 14, 15]. In 2008, a cluster of KPC cases at one LTACH in metropolitan Chicago formed the epicenter of a regional outbreak that affected 42 patients and 26 healthcare facilities [8]. By 2011, the average prevalence of KPC colonization among patients in area LTACHs was 30%, more than 9-fold higher than colonization prevalence among patients in short-stay hospital intensive care units (ICUs) [14]. In response, a collaborative, LTACH-based, multifaceted regional KPC control program was developed and launched.
METHODS
Study Design and Population
The study was planned as a quality improvement project to prevent KPC colonization and infection in LTACHs in metropolitan Chicago, Illinois. Four of 7 LTACHs in the region were invited and agreed to participate in the project. LTACHs were selected for invitation based on proximity to short-stay hospitals in the city of Chicago, where regional control efforts were focused, and because they were members of a single corporation that included most LTACHs in the region. All general medical wards and high-acuity units were included in the study. A psychiatric illness and substance abuse treatment unit at one LTACH was excluded. Each LTACH employed a dedicated nurse infection preventionist. Facilities were certified by The Joint Commission; no infection-control citations were documented during the study.
A stepped-wedge design was used to introduce a bundled infection prevention intervention to LTACHs. This design was chosen because it was the most rigorous option available that allowed adoption of the intervention at all study sites [16].
Intervention Bundle and Data Collection
In the preintervention period, semiannual rectal swab culture surveys were conducted to measure prevalence of KPC colonization among patients [14]; results were reported to LTACHs. During the intervention period, patients were screened for KPC rectal colonization at the time of LTACH admission and every other week, with preemptive contact isolation of newly admitted patients pending culture results. Swabs were screened for KPC using ertapenem disks in a central laboratory [17]; blaKPC was confirmed by a polymerase chain reaction assay [18, 19]. Patients with a KPC-positive screen or clinical cultures during the intervention period were presumed to remain colonized indefinitely and were not rescreened.
In addition to every other week rectal culture surveillance, components of the KPC intervention bundle included contact isolation [20] and geographic separation of KPC-positive patients in a ward cohort or single room; universal contact isolation of all patients in high-acuity units, where geographic separation of KPC-positive and KPC-negative patients was not possible; bathing all patients daily with 2% chlorhexidine gluconate (CHG)–impregnated cloths (Sage Products, Inc, Cary, Illinois); and healthcare-worker education and adherence monitoring, with a focus on hand hygiene. Bundle components were selected based on public health recommendations and expert guidance for control of CRE [11, 13], published reports of successful KPC control programs [9, 21-23], and our preintervention assessment of frequent colonization of LTACH patients’ skin with KPC but rare contamination of the inanimate LTACH environment [24].
Demographic; admission, discharge, and transfer; and clinical culture data were obtained from corporate data warehouses. Medical device utilization was determined from review of infection-control department databases.
Study Outcomes
The primary outcome was prevalence of KPC rectal colonization. Secondary outcomes included incidence of KPC rectal colonization, KPC-positive clinical cultures, KPC bloodstream infection, bloodstream infection due to any pathogen, and blood culture contamination. Incident colonization was classified as definite (KPC-positive rectal surveillance swab on or after hospital day 4 in a patient at risk of KPC colonization, ie, no history of KPC-positive surveillance or clinical culture, and at least 1 prior KPC-negative rectal surveillance culture during hospital days 1–3) or possible (KPC-positive rectal surveillance culture on or after hospital day 4, no history of KPC-positive surveillance or clinical culture, and no prior KPC-negative rectal surveillance culture during hospital days 1–3). The National Healthcare Safety Network (NHSN) Module definition for “CRE-Klebsiella species” was used as a proxy for KPC-positive clinical culture; that is, any Klebsiella species testing intermediate or resistant to imipenem, meropenem, or doripenem by standard susceptibility testing was considered to be KPC [25]. This approach was validated by demonstrating that 87% (107/123) of KPC from a sample of surveillance swabs from LTACH patients was K. pneumoniae, and that 96% (107/112) of carbapenem-resistant Klebsiella species carried blaKPC [14]. NHSN Multidrug-Resistant Organism Module definitions for “hospital-onset, LabID event” and contaminated blood culture were used to estimate rates of clinical infection and blood culture contamination [25]. Clinical cultures were obtained at clinicians’ discretion. Sensitivity analyses included models that added the NHSN definition for “CRE-Escherichia coli” to the proxy definition of KPC [25].
Prevalence of KPC rectal colonization was chosen as the primary outcome because at the time of LTACH randomization, the only preintervention data available were results of semiannual KPC point prevalence rectal culture surveys (14 preintervention surveys at the 4 intervention LTACHs). Soon after initiation of the intervention, we determined that robust preintervention clinical culture data were available, which allowed us to take full advantage of the stepped-wedge design in the analysis. Because neither admission nor every other week surveillance was performed at participating LTACHs before the intervention, KPC incidence during the preintervention period was unknown.
Because we had few preintervention prevalence data and no preintervention incidence data, we examined these outcomes using an a priori 1-group, longitudinal change design. This design is quasi-experimental, and is a less valid indicator of causality than the a priori stepped-wedge experimental design used with the clinical culture data. To judge the causal inference of the intervention effect, we looked for convergence of results across all quasi-experimental and experimental analyses [26].
Implementation and Adherence Monitoring
Before the intervention was introduced at each LTACH, a series of mandatory educational sessions was held for all staff, including evening, night, and weekend workers. During the intervention period, educational sessions were repeated monthly for new employees. Additional training on CHG bathing was conducted for certified nursing assistants; initial and yearly bathing competency was demonstrated by direct observation or by completion of a standard written or oral quiz. Study personnel attended monthly LTACH staff meetings and visited each LTACH 2–5 times weekly throughout the intervention to educate staff informally and assess adherence (total contact time, 10–20 person-hours per LTACH per week). Conference calls were held weekly with hospital leadership and infection preventionists at each LTACH to identify and resolve problems and to provide bidirectional feedback about the intervention, including rates of adherence with intervention bundle components.
Study Timeline
The study was conducted between 1 February 2010 (date of first available clinical culture result) and 30 June 2013 (last day of bundled intervention). The first point prevalence survey took place on 18 January 2011 [14]. LTACHs were randomized to adopt the intervention at approximately 2-month intervals beginning on 28 November 2011; after 7 months, the intervention was in effect at all LTACHs (Figure 1). Because of variability in availability of historical clinical culture data and the different dates of adoption of the intervention, the preintervention period at each LTACH ranged from 16 to 29 months and the intervention period from 12 to 19 months.
Figure 1.

Stepped-wedge design implementation at the 4 long-term acute-care hospitals (LTACHs) participating in the study. The symbol “0” in an unshaded cell indicates preintervention period. The symbol “X” in a shaded cell indicates intervention period. The start date for the preintervention period varied for each LTACH depending on availability of historical clinical culture data: February 1, 2010 (LTACH C), July 1, 2010 (LTACH B), August 1, 2010 (LTACH A), and November 1, 2010 (LTACH D).
Statistical Analysis
The anticipated effect of the intervention on KPC prevalence was calculated as follows. Prior to any LTACH adopting the intervention, average prevalence at the 4 participating facilities was 41% (standard error of the mean, 12%) [14]. The intervention was anticipated to reduce cross-transmission (KPC incidence) at the LTACHs; cross-transmission was estimated to be responsible for 75% of prevalence (estimated preintervention KPC incidence, 30%) [21]. The intervention was expected to reduce KPC incidence in each LTACH by 50% (from 30% to 15%) 12 months after all LTACHs implemented the intervention. Thus, the anticipated effect size of the bundled intervention was d = 1.25. Using this estimated effect size, an α of .05 and a sample of 4 LTACHs, a power of 0.91 was obtained, which indicated that we were likely to be able to detect a difference in prevalence due to the intervention if one occurred.
We tested for change (linear and exponential trends) in KPC colonization prevalence and incidence in separate regression models, with the null hypotheses of no change in prevalence or incidence over time during the intervention period.
Clinical culture data were analyzed using a 2-level hierarchical model and a varying time effect; the unit of analysis was the time period (month) within LTACH. The model treated differences in clinical culture incidence among LTACHs as a random effect. It also allowed us to examine the staggered initiation of the intervention and correct for site differences, and to account for the repeated measures associated with these data by allowing the use of autocorrelated error terms. The staggered initiation also corrected for seasonality effects because the staggering occurred over 7 months, with each site beginning the intervention in a different season of the year.
Compared to the preintervention period, there are 2 possible improvements that can occur for this sort of design: a simple drop in rate, and a drop in the rate over time. The combined mean effects and slope interaction term was used as the primary test of intervention effectiveness because the slopes and means were correlated and differed significantly across LTACHs. As a consequence of this interaction effect, simple mean and slope effects were uninterpretable.
Models were constructed that controlled for possible confounding effects of proportion of days patients received mechanical ventilation or urinary bladder catheterization. These factors were removed from the final model when they were found to be insignificant.
Analyses were conducted using SPSS software version 19 (IBM SPSS, Chicago, Illinois) and R version 2.13.1 (http://CRAN.R-project.org).
Ethical Review
Participating LTACHs deemed the study to be a quality improvement project and not research. The project was reviewed and determined to be a minimal-risk study by the institutional review board at Rush University Medical Center, which granted approval of the study along with a waiver of consent and Health Insurance Portability and Accountability Act waiver.
RESULTS
Study Participants and Adherence Monitoring
Patient characteristics were similar during preintervention and intervention periods, although the proportion of days that patients received mechanical ventilation or urinary bladder catheterization was smaller during the intervention period (Table 1).
Table 1.
Characteristics of the Long-term Acute-Care Hospital Population, According to Study Period
| Variable | Preintervention Perioda |
Intervention Perioda |
|---|---|---|
| Present on admission | ||
| Patients, No. | 3894 | 2951 |
| Admissions, No. | 5282 | 3738 |
| Admissions per month, mean (SD) | 231 (21) | 234 (22) |
| Age, y, mean (SD) | 63 (16) | 64 (16) |
| Female sex, % | 45.6 | 45.8 |
| Measured during hospital stay | ||
| Patient-days, No. | 178 516 | 114 070 |
| High-acuity unit patient-days, % | 8.3 | 10.1 |
| Invasive medical device utilization, %b | ||
| Mechanical ventilation | 50.5 | 43.1 |
| Central venous catheter | 50.3 | 51.9 |
| Urinary bladder catheter | 63.0 | 50.9 |
| Hospital stay, d, median (IQR) | 28 (16–43) | 26 (17–39) |
| In-hospital mortality, % | 21.5 | 17.6 |
Abbreviations: IQR, interquartile range; LTACH, long-term acute-care hospital; SD, standard deviation.
The preintervention period spanned 16–29 months and the intervention period spanned 12–19 months. Study period lengths differed at each LTACH because of variability in availability of historical clinical culture data and because of the different dates on which each LTACH was randomly assigned to adopt the bundled intervention.
Invasive medical device utilization was calculated as [(number of days a medical device was utilized by each patient/total number of patient-days) ×100.
Adherence to most components of the intervention bundle was high (Table 2). Healthcare-worker hand hygiene before room entry was low, observed in only 24% of opportunities. A total of 145 986 packages of CHG-impregnated cloths was delivered to the LTACHs during the intervention period for an estimated 116 789 baths, or approximately 1 bath per patient per day, based on baseline observations in which 1 package of 6 CHG-impregnated cloths was used for 75% of baths and 2 packages were used for 25% of baths.
Table 2.
Adherence With Components of Intervention Bundle During the Intervention Period
| Adherence Measure | No. Adherent/No. Opportunities | % Adherence | 95% CI |
|---|---|---|---|
| Collection of admission surveillance swabsa | 2872/3152 | 91.1 | 90.1–92.1 |
| Collection of every other week surveillance swabs | 5072/5316 | 95.4 | 94.8–96.0 |
| KPC-positive patient-days on a cohort floor or in a private roomb | 17 921/19 295 | 92.9 | 92.5–93.2 |
| HCW hand hygiene adherence at room entrance | 365/1499 | 24.4 | 22.2–26.6 |
| HCW hand hygiene adherence at room exit | 1304/1843 | 70.8 | 68.6–72.8 |
| Donning gloves and gown before room entryc | 387/489 | 79.1 | 75.3–82.5 |
Abbreviations: CI, confidence interval; HCW, healthcare worker; KPC, Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae; LTACH, long-term acute-care hospital.
Adherence was defined as collection of a rectal surveillance swab for KPC culture within 3 calendar days of admission. Median time from admission to availability of swab culture results was 3 days (interquartile range, 2–4 days).
Adherence was measured 3–6 days per week at each LTACH. Three LTACHs cared for KPC-positive patients on patient cohort wards. The fourth LTACH cared for KPC-positive patients in private rooms. The percentage of KPC-negative patient-days on a KPC cohort floor or in a room with a KPC-positive patient was 13% (5109/40 777).
High-acuity unit rooms only, where universal contact isolation was in effect.
Outcomes
The prevalence of KPC rectal colonization was stable during the preintervention period (average, 45.8%; 95% confidence interval [CI], 42.1%–49.5% for preintervention point prevalence surveys; slope = 0.054, ie, almost zero; P = .47 for linear change; Figure 2), declined early in the intervention period, and then reached a plateau (34.3%; 95% CI, 32.4%–36.2%; P < .001 for exponential decline). Admission prevalence during the intervention period was stable (average, 20.6%; 95% CI, 19.1%–22.3%; Figure 2).
Figure 2.
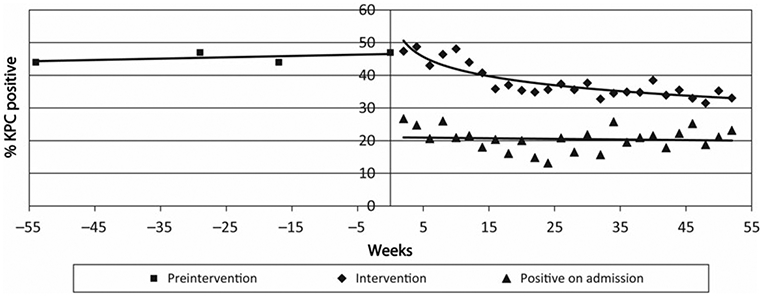
Prevalence rate of Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae (KPC) rectal colonization during the preintervention and intervention periods. Each data point in the preintervention period represents the average prevalence across the 4 long-term acute-care hospitals (LTACHs) for 1 semiannual point prevalence survey. Only 2 LTACHs (LTACHs D and C) are included in the week −17 point prevalence survey, as LTACHs A and B were already participating in the intervention at that time. During the intervention period, each data point represents the average prevalence across the 4 LTACHs for 1 every other week point prevalence survey. Data for the first 52 weeks of the intervention are shown. P < .001 for exponential decline in prevalence during the intervention period.
When only definite KPC acquisitions were considered, the incidence rate of KPC rectal colonization fell during the intervention period, from approximately 4 to 2 KPC acquisitions per 100 patient-weeks (P = .004 for linear decline; Figure 3); results were similar when possible incident cases were included.
Figure 3.

Incidence rate of Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae (KPC) rectal colonization during the intervention period. Each data point represents the number of patients who acquired KPC per 100 patient-weeks, averaged over the preceding 2 weeks. Definite incident cases and data for the first 52 weeks during which each of the 4 long-term acute-care hospitals participating in the study are shown. P = .004 for linear decline.
The intervention resulted in a 32% reduction in the rate of isolation of KPC from any clinical culture and a 56% reduction in KPC bacteremia (Table 3). Rates of bloodstream infection due to any pathogen declined by 32%; blood culture contamination declined by 53%. The magnitudes of the reductions are displayed in Figure 4A-D. There was a clear drop in rates of infection and blood culture contamination as the staggered intervention effects began, and there was some evidence that the rates continued to drop after the initiation of the intervention (based on the downward slopes of the linear trends). Neither adding the NHSN definition for “CRE Escherichia coli” to the proxy definition of KPC nor adjustment for the proportion of days that patients received mechanical ventilation or urinary bladder catheterization changed results of clinical culture analyses.
Table 3.
Effect of Intervention Bundle on Clinical Cultures and Blood Culture Contamination
| Outcome | Preinterventiona |
Interventiona |
Change in Event Rate |
P Value |
||||
|---|---|---|---|---|---|---|---|---|
| No. of Events |
Events/1000 Patient-days |
95% CI | No. of Events |
Events/1000 Patient-days |
95% CI | |||
| KPC in any clinical culture | 656 | 3.7 | 3.4–4.0 | 285 | 2.5 | 2.2–2.8 | −1.2 | .001 |
| KPC bloodstream infection | 165 | 0.9 | .8–1.1 | 48 | 0.4 | .3-.5 | −0.5 | .008 |
| Bloodstream infection due to any pathogen | 2004 | 11.2 | 10.7–11.7 | 870 | 7.6 | 7.1–8.1 | −3.6 | .006 |
| Contaminated blood culture | 865 | 4.9 | 4.5–5.2 | 261 | 2.3 | 2.0–2.6 | −2.6 | .03 |
Abbreviations: CI, confidence interval; KPC, Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae.
There were 178 516 patient-days in the preintervention period and 114 070 patient-days in the intervention period.
Figure 4.
Effect of the intervention bundle on clinical culture outcomes. Shown are the moving averages of the rates of clinical infections (curved solid lines) and 95% confidence limits (curved hatched lines) for preintervention (open black circles) and intervention (closed red triangles) periods. Each data marker represents average number of clinical cultures at 1 long-term acute-care hospital (LTACH) in 1 month. Trend lines for each period are shown in black (preintervention) or red (intervention) solid bold type. Note the different ranges for y-axis in each panel. A, Klebsiella pneumoniae carbapenemase (KPC) in any clinical culture. B, KPC bloodstream infection. C, Bloodstream infection due to any pathogen. D, Contaminated blood culture.
DISCUSSION
Implementation of a bundled intervention was associated with clinically important and statistically significant reductions in KPC colonization and infection at 4 LTACHs in metropolitan Chicago: colonization incidence was reduced by 50%, colonization prevalence and all KPC infections declined by >30%, and the rate of KPC bloodstream infection fell by almost 60%. Reductions occurred despite high KPC prevalence—nearly 50% of patients were colonized with KPC at the start of the intervention—and ongoing admission of a large number of patients who already were colonized with KPC. These results demonstrate that control is possible despite high colonization pressure and repeated introduction of KPC-positive patients, and should provide support for other healthcare facilities that are working to lower the burden of KPC in their patient populations.
To our knowledge, this is the first multicenter study to show sustained decreases in cross-transmission of a multidrug-resistant pathogen and in healthcare-associated infections in an LTACH population. Patients in LTACHs are chronically critically ill [27] and at high risk of infection from multidrug-resistant organisms because of prolonged hospital stays, repeated antibiotic exposures, and elevated rates of medical device use [28, 29]. Yet, because little research has been conducted on the characterization and mitigation of risk factors for infection in this population, best practices for infection prevention in LTACHs have not been established [30]. The present study demonstrates the ability of LTACHs to participate successfully in research and adds to our understanding of how best to care for this vulnerable group of patients, which is expected to continue to grow over the next decade [31].
In addition to the targeted decreases in KPC colonization and infection, collateral benefits of the intervention were observed on relative rates of all-cause bacteremia and on blood culture contamination, which declined 32% and 53%, respectively. Because the intervention comprised a bundle of infection prevention measures, it is not possible to know with certainty which bundle component(s) were necessary and sufficient for the KPC-specific or broader improvements. Daily bathing with CHG has been shown to reduce catheter-associated bloodstream infection in both ICU and LTACH populations and to reduce blood culture contamination in ICUs [32-34]. We speculate that bathing all LTACH patients with CHG during the intervention period was largely responsible for the sharp declines observed in KPC and all-cause bloodstream infection and in blood culture contamination, reflecting rapid onset of protection of each bathed patient. In contrast, declines in KPC incidence and prevalence were more gradual, presumably reflecting the greater effort needed to control cross-colonization in a setting of high KPC admission prevalence and colonization pressure.
Although concerns have been raised about decreased susceptibility of KPC to CHG [35], we found that CHG bathing was effective in reducing KPC skin colonization in LTACH patients [36]. Thus, CHG bathing may also have helped reduce cross-transmission of KPC by lessening the risk of healthcare-worker hand contamination during direct care of KPC-positive patients. Still, active surveillance for KPC, contact isolation, and geographic separation of KPC-positive patients may have contributed to declines in KPC incidence, prevalence, and infection. Preintervention hand hygiene rates were not known, but improvements in healthcare-worker hand hygiene adherence and education about prevention of healthcare-associated infections may also have had positive effects on all outcomes.
While the intervention conferred clear benefit on patients who were cared for in participating LTACHs, it is likely to also have had a favorable effect on prevalence of KPC in other healthcare facilities in the region. In Chicago, as in much of the United States, LTACHs serve as a reservoir for KPC [14]. Nationally in 2012, 3.9% of short-stay hospitals that submitted data to NHSN reported at least 1 healthcare-associated infection due to CRE, compared with 17.8% of LTACHs [37]. Patients in LTACHs typically have contact with multiple different healthcare facilities over time as their clinical needs change [8]. Transfer from an LTACH is a risk factor for KPC colonization at the time of short-stay hospital admission [12, 38]. Reducing the number of LTACH patients who are colonized and infected with KPC should result in fewer KPC-positive patients transferred from an LTACH to another healthcare facility, thus slowing regional dissemination. Formal testing of this hypothesis using simulation modeling and highly discriminating molecular epidemiologic methods such as whole-genome sequencing is needed.
Our study has limitations. The study was conducted in hospitals with high KPC prevalence, and results may not be generalizable to settings with lower prevalence of KPC. Few preintervention colonization data were available for analysis, but convergent responses to the intervention of all colonization and infection outcomes strengthen our confidence in the validity of the observed declines. Sequential rollout of the intervention and contact between personnel at different LTACHs may have resulted in some intervention effect on LTACHs before they officially implemented the intervention; this effect would have reduced the difference between preintervention and intervention outcomes. Infections were identified using NHSN standardized laboratory surveillance definitions without clinical assessment, which may have resulted in misclassification of colonization as infection, or of infection acquired prior to LTACH admission as LTACH onset. Data on severity of patient illness and antibiotic use were not available; these variables remain potential unmeasured confounders. Our bundle did not include antimicrobial stewardship and so the effect of inclusion of stewardship in the bundle is unknown. Whether daily CHG bathing will select for CHG resistance in KPC or other skin microbes is unknown and should be monitored. Finally, the bundled intervention preluded assessment of the impact of individual bundle components.
In conclusion, a bundled intervention was associated with reduced colonization and infection due to KPC, declines in bloodstream infection due to all pathogens, and decreased blood culture contamination in a high-risk LTACH population. Evaluation of long-term and regional effects of the intervention is warranted.
Acknowledgments.
We thank the patients and staff of the 4 study Long-term acute-care hospitals (LTACHs) for their participation; Huiyuan Zhang, MS (John H. Stroger Hospital, Chicago, Illinois) for data aggregation; and David Schwartz, MD (John H. Stroger Hospital), William Trick, MD (John H. Stroger Hospital), and Gordon Trenholme, MD (Rush University Medical Center) for critical review of the manuscript.
Financial support.
This work was supported by the Centers for Disease Control and Prevention (grant numbers U54CK000161 to R. A. W. and 200-2011-M-41103 to M. K. H.) and by an unrestricted gift from the Foglia Family Foundation to R. A. W. Sage Products, Inc, provided CHG-impregnated cloths to participating LTACHs at no cost.
Footnotes
Potential conflicts of interest. M. K. H. has conducted unpaid research for Cepheid Corporation. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek, San Francisco, California, 2–6 October, 2013. Abstract 1209.
References
- 1.Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther 2008; 6:751–63. [DOI] [PubMed] [Google Scholar]
- 2.Huttner A, Harbarth S, Carlet J, et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011; 17:1791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordmann P, Cornaglia G. Carbapenemase-producing Enterobacteriaceae: a call for action! Clin Microbiol Infect 2011; 18:411–2. [DOI] [PubMed] [Google Scholar]
- 5.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–106. [DOI] [PubMed] [Google Scholar]
- 6.Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–6. [DOI] [PubMed] [Google Scholar]
- 7.Canton R, Akova M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 2012; 18:413–31. [DOI] [PubMed] [Google Scholar]
- 8.Won SY, Munoz-Price LS, Lolans K, Hota B, Weinstein RA, Hayden MK. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae. Clin Infect Dis 2011; 53:532–40. [DOI] [PubMed] [Google Scholar]
- 9.Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011; 52:848–55. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed 6 May 2014.
- 11.Tacconelli E, Cataldo MA, Dancer SJ, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant gram-negative bacteria in hospitalized patients. Clin Microbiol Infect 2014; 20(suppl 1):1–55. [DOI] [PubMed] [Google Scholar]
- 12.Marchaim D, Chopra T, Bogan C, et al. The burden of multidrug-resistant organisms on tertiary hospitals posed by patients with recent stays in long-term acute care facilities. Am J Infect Control 2012; 40:760–5. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). 2012. toolkit. Available at: http://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed 6 May 2014.
- 14.Lin MY, Lyles-Banks RD, Lolans K, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2013; 57:1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2011; 65:1807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol 2006; 6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lolans K, Calvert K, Won S, Clark J, Hayden MK. Direct ertapenem disk screening method for identification of KPC-producing Klebsiella pneumoniae and Escherichia coli in surveillance swab specimens. J Clin Microbiol 2010; 48:836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole JM, Schuetz AN, Hill CE, Nolte FS. Development and evaluation of a real-time PCR assay for detection of Klebsiella pneumoniae carbapenemase genes. J Clin Microbiol 2009; 47:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangold KA, Santiano K, Broekman R, et al. Real-time detection of blaKPC in clinical samples and surveillance specimens. J Clin Microbiol 2011; 49:3338–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control 2007; 35:S165–93. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Price LS, Hayden MK, Lolans K, et al. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect Control Hosp Epidemiol 2010; 31:341–7. [DOI] [PubMed] [Google Scholar]
- 22.Chitnis AS, Caruthers PS, Rao AK, et al. Outbreak of carbapenem-resistant enterobacteriaceae at a long-term acute care hospital: sustained reductions in transmission through active surveillance and targeted interventions. Infect Control Hosp Epidemiol 2012; 33:984–92. [DOI] [PubMed] [Google Scholar]
- 23.Bilavsky E, Schwaber MJ, Carmeli Y. How to stem the tide of carbapenemase-producing Enterobacteriaceae?: proactive versus reactive strategies. Curr Opin Infect Dis 2010; 23:327–31. [DOI] [PubMed] [Google Scholar]
- 24.Thurlow CJ, Prabaker K, Lin MY, Lolans K, Weinstein RA, Hayden MK. Anatomic sites of patient colonization and environmental contamination with Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae at long-term acute care hospitals. Infect Control Hosp Epidemiol 2013; 34:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Multidrug-Resistant Organism and Clostridium difficile Infection (MDRO/CDI) Module. Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf. Accessed 9 June 2014.
- 26.Smoking and health: report of the advisory committee to the Surgeon General of the Public Health Service United States Surgeon General’s Advisory Committee on Smoking. 1964. Available at: http://profiles.nlm.nih.gov/ps/access/NNBBMQ.pdf. Accessed 6 January 2015.
- 27.Macintyre NR. Chronic critical illness: the growing challenge to health care. Respir Care 2012; 57:1021–7. [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Price LS. Long-term acute care hospitals. Clin Infect Dis 2009; 49:438–43. [DOI] [PubMed] [Google Scholar]
- 29.Kalb TH, Lorin S. Infection in the chronically critically ill: unique risk profile in a newly defined population. Crit Care Clin 2002; 18:529–52. [DOI] [PubMed] [Google Scholar]
- 30.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA 2010; 303:2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zilberberg MD, Shorr AF. Prolonged acute mechanical ventilation and hospital bed utilization in 2020 in the United States: implications for budgets, plant and personnel planning. BMC Health Serv Res 2008; 8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007; 167:2073–9. [DOI] [PubMed] [Google Scholar]
- 33.Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol 2009; 30:959–63. [DOI] [PubMed] [Google Scholar]
- 34.Munoz-Price LS, Hota B, Stemer A, Weinstein RA. Prevention of bloodstream infections by use of daily chlorhexidine baths for patients at a long-term acute care hospital. Infect Control Hosp Epidemiol 2009; 30:1031–5. [DOI] [PubMed] [Google Scholar]
- 35.Naparstek L, Carmeli Y, Chmelnitsky I, Banin E, Navon-Venezia S. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect 2012; 81:15–9. [DOI] [PubMed] [Google Scholar]
- 36.Lin MY, Lolans K, Blom DW, et al. The effectiveness of routine daily chlorhexidine gluconate bathing in reducing Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae skin burden among long-term acute care hospital patients. Infect Control Hosp Epidemiol 2014; 35:440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 2013; 62:165–70. [PMC free article] [PubMed] [Google Scholar]
- 38.Prabaker K, Lin MY, McNally M, et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol 2012; 33:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]



