Abstract
The present study investigated the impact of carnosic acid (CA) from rosemary on the levodopa (L-dopa)-induced dyskinesia (LID) in rats treated with 6-hydroxydopamine (6-OHDA). To establish the model of LID, 6-OHDA-lesioned rats were injected intraperitoneally with 30 mg/kg L-dopa once a day for 36 days. Rats were daily administrated with 3 or 15 mg/kg CA by oral intubation prior to L-dopa injection for 4 days. Rats pretreated with CA had reduced L-dopa-induced abnormal involuntary movements (AIMs) and ALO scores (a sum of axial, limb, and orofacial scores). Moreover, the increases of dopamine D1-receptor, p-DARPP-32, ΔFosB, p-ERK1/2, and p-c-Jun ser63, along with the decrease in p-c-Jun ser73, induced by L-dopa in 6-OHDA-treated rats were significantly reversed by pretreatment with CA. In addition, we used the model of SH-SY5Y cells to further examine the neuroprotective mechanisms of CA on L-dopa-induced cytotoxicity. SH-SY5Y cells were treated with CA for 18 h, and then co-treated with 400 μM L-dopa for the indicated time points. The results showed that pretreatment of CA attenuated the cell death and nuclear condensation induced by L-dopa. By the immunoblots, the reduction of Bcl-2, p-c-Jun ser73, and parkin and the induction of cleaved caspase 3, cleaved Poly (ADP-ribose) polymerase, p-ERK1/2, p-c-Jun ser63, and ubiquitinated protein by L-dopa were improved in cells pretreated with CA. In conclusion, CA ameliorates the development of LID via regulating the D1R signaling and prevents L-dopa-induced apoptotic cell death through modulating the ERK1/2-c-Jun and inducing the parkin. This study suggested that CA can be used to alleviate the adverse effects of LID for PD patients.
Keywords: carnosic acid, 6-hydroxydopamine, levodopa-induced dyskinesia, DARPP-32/ΔFosB, ERK1/2-c-jun
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease. Levodopa (L-dopa), the precursor of dopamine, is the primary drug used to treat PD (Cenci, 2014). However, long-term exposure to L-dopa causes motor complications, called levodopa-induced dyskinesia (LID) (Chaudhuri et al., 2019). LID develops in about 40–50% of patients in the 5 years after treatment and in up to 100% of patients after 10 years of treatment (Manson et al., 2012). The symptoms of LID include chorea, ballism, dystonia, and myoclonus (Calabresi et al., 2010). New adjunct therapies to delay or reduce these adverse effects are needed.
The mechanisms by which LID develops are complex. LID is associated with the hypersensitization of striatum dopamine D1-receptor (D1R) induced by impaired receptor trafficking (Feyder et al., 2011; Spigolon and Fisone, 2018), which results in the abnormal change of proteins of PKA, ΔFosB, and extracellular signal-regulated kinases1/2 (ERK1/2) (Fieblinger et al., 2014). An evidence has shown that pulsatile administration of L-dopa activates the proteins of DARPP-32, ERK, and ΔFosB in 6-OHDA-lesioned rats (Lebel et al., 2010). L-Dopa stimulates D1R coupled with G-protein α/olf to activate PKA, and then phosphorylates DARPP-32 protein at Thr34, leading to increase the ERK1/2 activity. The activation of ERK1/2 results in increased the activation of ΔFosB by cAMP response element-binding protein (CREB) (Fanni et al., 2018; Spigolon and Fisone, 2018). Striatal ΔFosB protein is correlated with the severity of LID in L-dopa-treated monkeys (Berton et al., 2009). ΔFosB is also found in the striatum of PD patients treated with L-dopa (Lindgren et al., 2011). Silencing of striatal ΔFosB-expression neurons improves the motor complication of LID in PD rat model (Engeln et al., 2016). Conversely, overexpression of ΔFosB in 6-hydroxydopamine (6-OHDA)-treated rats exacerbates the effects of LID (Cao et al., 2010).
Accumulating evidence indicates that cells cultured with L-dopa induced neurotoxicity via generating oxidative damage (Colamartino et al., 2015). It has been shown that the process of dopamine synthesis from L-dopa enhances the formation of reactive oxygen species (ROS) via regulating monoamine oxidase (Stansley and Yamamoto, 2013). This induction is enhanced for PD patients treated with L-dopa and leads to the apoptotic neuronal cell death (Hald and Lotharius, 2005; Dorszewska et al., 2014). Consistent with the results, the increment of cleaved-caspase 3 and -poly (ADP-ribose) polymerase (PARP) proteins is observed in PD patients treated with L-dopa (Hald and Lotharius, 2005). In addition to oxidative stress, study indicates that L-dopa-induced cell death is mediated by ERK1/2-c-Jun pathway (Park et al., 2016). The transcription factor c-Jun regulates cell survival and cell death through regulating its phosphorylation sites by ERK1/2 pathway. An observation by Park et al. suggested that the neurotoxicity caused by long-term L-dopa administration may involve the induction of c-Jun phosphorylation at ser63 and reduction of c-Jun phosphorylation at ser73, which acts as a pro- or anti-apoptotic factor, respectively (Park et al., 2016). An understanding of the possible of neurotoxicity by L-dopa in PD will help to improve the motor complication of LID.
Carnosic acid (CA), a diterpene phenolic compound from rosemary, has multiple physiological benefits, including anti-oxidant and neuroprotective (Guo et al., 2016; Lin et al., 2020). In our previous study, we reveals that the neuroprotective mechanisms of CA are related to the upregulation of anti-oxidant enzymes in PD models (Chen et al., 2012; Wu et al., 2015). CA stimulates glutathione synthesis to inhibit 6-hydroxydopamine (6-OHDA)-induced apoptosis of SH-SY5Y cells through down-regulating of c-Jun NH2-terminal kinase (JNK) and p38 (Chen et al., 2012). Furthermore, administration of CA with 6-OHDA-lesioned rats improves the antioxidant capacity, neurotoxicity, and motor impairment (Wu et al., 2015). Although CA is currently being investigated for therapeutic benefits in PD, the actions of CA on the development of LID have not yet been described. Therefore, in this study, we explored the roles of CA on LID in 6-OHDA-lesioned rats and further examined the cytotoxicity of L-dopa in SH-SY5Y cells.
Materials and Methods
Experimental Animals and Treatments
Eight-week-old male Wistar rats (BioLASCO Experimental Animal Center, Taipei, Taiwan) were used in this study. The protocols for animal-related experiments were approved by the Institutional Animal Care and Use Committee of China Medical University (protocol no. 2017–189). The temperature of the animal husbandry rooms was set at 23 ± 1°C with a 12-h day and night cycle. Animals had ad libitum access to a chow diet and water. After 11 days of adaptation, 6-OHDA (Sigma, St. Louis, MO, United States) injury surgery was performed as described below to induce PD. On day 17 after lesion formation, an apomorphine (0.25 mg/kg)-induced rotation experiment (exhibited >7 full turns/min) were performed to select for next experiment (Zhang et al., 2018). The experimental groups were as follows: 1) vehicle group (6-OHDA lesion, n = 5); 2) L-dopa group: 30 mg/kg L-dopa + 15 mg/kg benserazide (n = 5); 3) L-dopa + CA3 group: 30 mg/kg L-dopa + 15 mg/kg benserazide +3 mg/kg CA (n = 5); 4) L-dopa + CA15 group: 30 mg/kg L-dopa + 15 mg/kg benserazide +15 mg/kg CA (n = 5). L-Dopa (Sigma, St. Louis, MO, United States) and benserazide (Sigma, St. Louis, MO, United States) were injected intraperitoneally with potassium phosphate buffer once per day for 36 consecutive days. In the CA-treated group, CA (Cayman, Ann Arbor, MI, Cat No.89820) was dissolved in 0.5% sodium carboxymethyl cellulose and was administered 4 days before the L-dopa injection by oral intubation once per day until the rats were sacrificed.
6-OHDA Injury Surgery
According to a previous study in our laboratory (Wu et al., 2015), rats were anesthetized with tiletamine/zolazepam (Zoletil50®; Virbac Lab., Carros, France) by intramuscular injection. The rats were fixed on a stereotaxic apparatus and 2.5 μl of 6-OHDA (5 μg/μl) was injected into the right striatum (anteroposterior: +1.5 mm; lateral: −4 mm; dorsoventral: −7.2 mm) with a flow rate of 1 μl/min. The drug delivery tube was left in place for 1 min before being removed to avoid seepage of 6-OHDA. The sham operation group was injected with 0.5% ascorbic acid-saline.
Abnormal Involuntary Movement Scores
Behavioral analysis subtypes and scoring criteria were based the study by Winkler et al. (Winkler et al., 2002). AIM scores were assessed on days 12, 26, and 33 after L-dopa administration. The rats were placed in a transparent cage for a 2-h photographic record and were scored for 1 min every 20 min. Each rat was assessed for four AIM subtypes: axial, limb, orofacial, and locomotor movements. A severity score ranging from 0 to 4 was assigned for each AIM subtype. The AIM scores from four subtypes were summed for each time point. The ALO score (sum of the axial, limb, and orofacial scores) is more responsive to human dyskinesia than are locomotive scores (Castello et al., 2020). Therefore, the ALO score was also analyzed in this study.
Preparation of Animal Tissues
After the animals were sacrificed, the striatum on the right side was separated and homogenized (10% w/v) in RIPA buffer (Biokit, Taiwan) containing 1% protease inhibitor (Sigma, St. Louis, MO, United States) and 1% phosphatase inhibitor (Sigma, St. Louis, MO, United States). The supernatant was obtained after centrifugation at 15,000 rpm for 30 min at 4°C.
Cell Culture and Sample Preparation
The SH-SY5Y cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, United States). The method of cell culture was based on our previous study (Lin et al., 2016). The cells were plated on 3.5 cm cell culture dishes and incubated with Dulbecco’s modified Eagle medium (DMEM) including 10% FBS, 1% L-glutamine, 1% non-essential amino acid, 1 mM sodium pyruvate, 1.5 g/ml sodium bicarbonate, 1% penicillin-streptomycin; pH = 7.4. Cells were pretreated with 0.1% dimethylsulfoxide (DMSO) or 0.5, 1, or 3 μM CA for 18 h followed by treatment with PBS or 20, 200, or 400 μM L-dopa for an additional 24 or 72 h. After treatment, the RIPA buffer containing 1% protease inhibitor and 1% phosphatase inhibitor was added to each plate and the cells were collected for protein analysis. Lysates were centrifuged at 14,000 rpm for 20 min at 4°C to obtain the supernatant. The protein assay dye reagent concentrate (BIO-RAD, Hercules, CA, United States) was used to measure protein concentration.
Western Blot Analysis
Samples containing the same protein concentrations were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, united States). The membranes were placed in 50 g/L skim milk at 4°C to block the nonspecific binding sites. The membranes were incubated with primary antibodies, including D1R (Santa Cruz Biotechnology (SCBT); sc-33660), p-DARPP-32 (GeneTex; GTX82714), ΔFosB (Cell Signaling Technology (CST); #14695), p-ERK1/2 (SCBT; sc-7383), ERK1/2 (SCBT; sc-93), p-c-Jun ser63 (SCBT; sc-822), p-c-Jun ser73 (CST; #3270), Bcl-2 (CST; #2876), caspase 3 (CST; #9662), cleaved caspase 3 (CST; #9661), PARP (CST; #9542), cleaved PARP (CST; #9541), parkin (SCBT; sc-32282), ubiquitin (Sigma-Aldrich; 05–944), β-tubulin (SCBT; sc-9104) and GAPDH (SCBT; sc-365062) at 4°C overnight and were then subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit (SCBT; sc-2004), goat anti-mouse IgG (SCBT; sc-2005), or mouse IgG kappa binding protein (m-IgGκ BP)-HRP (SCBT; sc-516102) secondary antibodies. The protein expression on the membrane was detected with an enhanced chemiluminescence reagent (Millipore, Burlington, MA, United States) and analyzed by a luminescent image analyzer (LAS-4000, FUJIFILM).
Cell Viability Assay
Cells were washed with phosphate-buffered saline and incubated with DMEM containing 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolim bromide (MTT) for 2 h at 37°C. After the medium was removed, the formazan crystal was dissolved with 1 ml of isopropanol and then centrifuged at 15,000 rpm for 5 min to get the supernatant. The supernatant was added to a 96-well plate, and absorbance was measured at 570 nm by ELISA (Bio-Rad, Japan). The value in the control cells was set as 100% viability.
Nuclear Staining With Hoechst 33258
Cells were washed with phosphate-buffered saline and fixed with 3.7% paraformaldehyde (pH 7.4) solution for 50 min. Then, the cells were stained with Hoechst 33258 for 1 h at 25°C in the dark and morphological changes were observed by using a fluorescence microscope. Fluorescence intensity was obtained by use of Image-Pro Plus 6.0 (Media Cybernetics, Inc., Bethesda, MD, United States).
Statistical Analysis
All in vivo data are presented as the mean ± SEM. The in vitro data are expressed as mean ± SD. Statistical analysis was performed with t-tests between two sample comparisons. The multiple comparisons were conducted with a one-way analysis of variance (ANOVA) with SAS software followed by Tukey’s post hoc test. Statistically significant differences were considered when the p-value was less than 0.05.
Results
CA Improves AIMs Induced by L-Dopa in Lesioned Rats
The axial, limb, and orofacial scores on day 26 in the L-dopa group were significantly higher than in the vehicle group (p < 0.05), whereas those in the groups pretreated with 3 or 15 mg/kg CA were significantly lower than in the L-dopa group (p < 0.05) (Figure 1A). We then divided the total recording time into 20-min intervals, and used a total of 6 intervals for score statistics. In this analysis, CA improved the ALO score (the sum of the axial, limb, and orofacial scores) during the 20–40 min and 40–60 min intervals (Figure 1B). In addition, the ALO scores in the L-dopa group were significantly higher than in the vehicle group on days 12, 26, and 33. However, the ALO scores in the group pretreated with 3 and 15 mg/kg CA were lower compared with those in the L-dopa group (p < 0.05) (Figure 1C). These results suggest that CA can improve AIMs caused by L-dopa.
FIGURE 1.
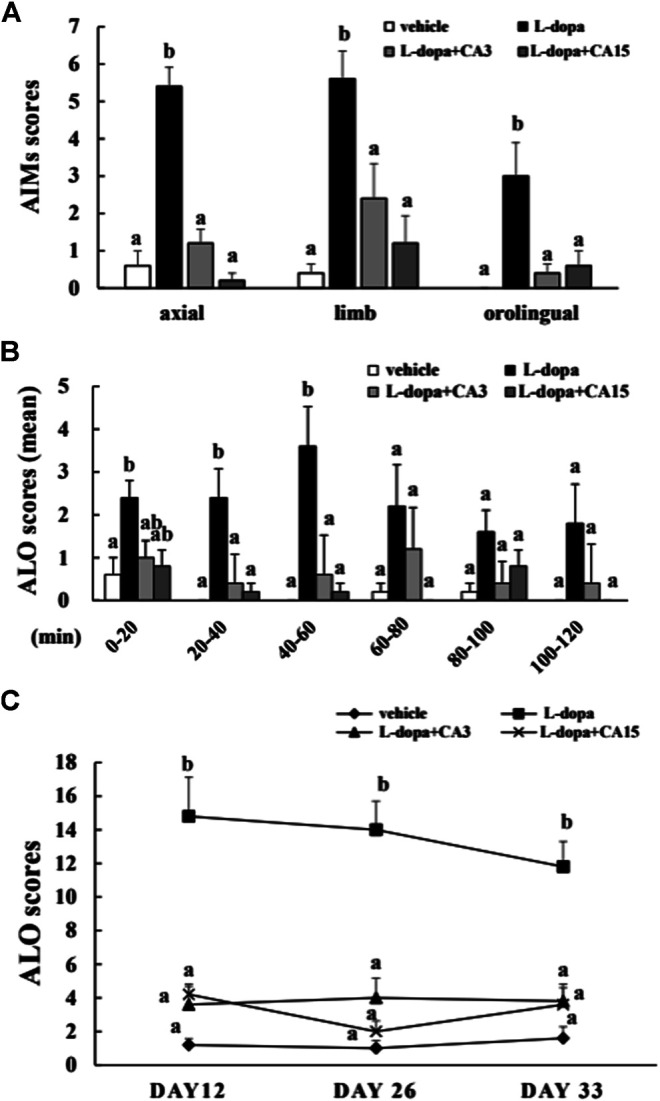
Effect of CA on L-dopa-induced abnormal involuntary movements (AIMs) in 6-OHDA lesioned rats. The axial, limb, and orofacial AIMs were calculated on days 12, 26, and 33 for 1 min every 20 min over 120 min. (A) The integrated AIM scores of axial, limb, and orofacial subtype on day 26 over 120 min. (B) The ALO score (the sum of axial, limb, and orofacial) on day 26 for 1 min every 20 min over 120 min. (C) The ALO score on days 12, 26, and 33. The results are expressed as mean ± SEM (n = 5). Means without a common letter differ, p < 0.05.
CA Reduces LID Marker Proteins in Lesioned Rats
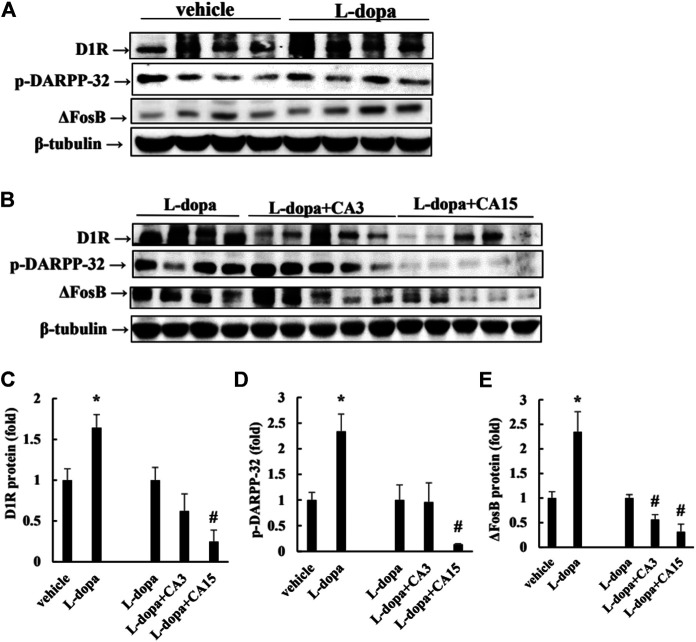
Over-activation of D1R phosphorylates DARPP-32, which ultimately leads to an increase in FosB family proteins, which causes LID (Ahn et al., 2017). Rats treated with L-dopa significantly increased D1R and p-DARPP-32, as well as ΔFosB protein (p < 0.05). Pretreatment of rats with CA at 15 mg/kg prior to 4 days of L-dopa treatment, significantly reduced D1R and p-DARPP-32 compared with that in the L-dopa group (p < 0.05). However, pretreatment with 3 mg/kg CA had no significant effect. ΔFosB proteins were reduced in the group pretreated with 3 and 15 mg/kg CA by 44 and 69%, respectively, compared with the L-dopa group (Figure 2). This result suggests that CA reduced the activation of D1R and DARPP-32, leading to improved accumulation of ΔFosB protein induced by L-dopa.
FIGURE 2.
Effect of CA on L-dopa-induced D1R, p-DARPP-32, and ΔFosB protein in 6-OHDA-lesioned rats. (A,B) D1R, p-DARPP-32, and ΔFosB protein were determined by Western blotting. β-Tubulin was used as the internal control. (C–E) The level of the L-dopa group was set at 1.0. Values are mean ± SEM (n = 3). #p < 0.05 compared with L-dopa-treated group.
Effect of CA on the Phosphorylation of ERK1/2 and c-Jun in Lesioned Rats
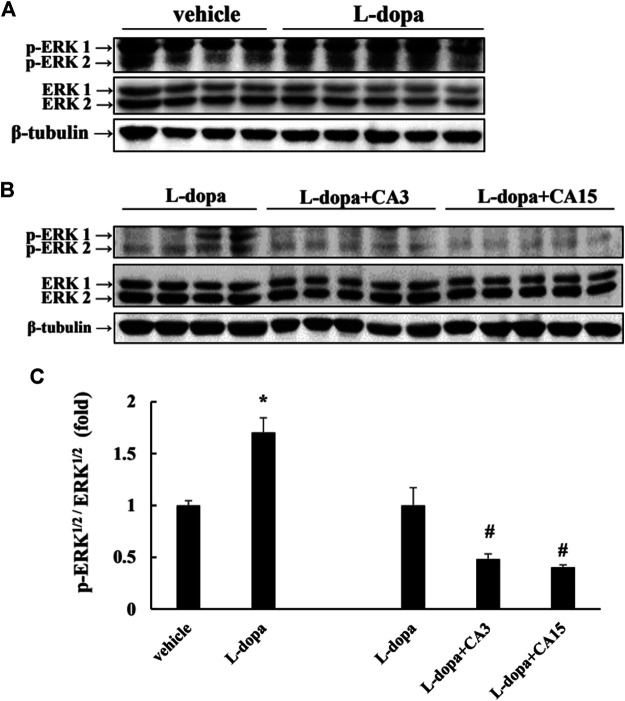
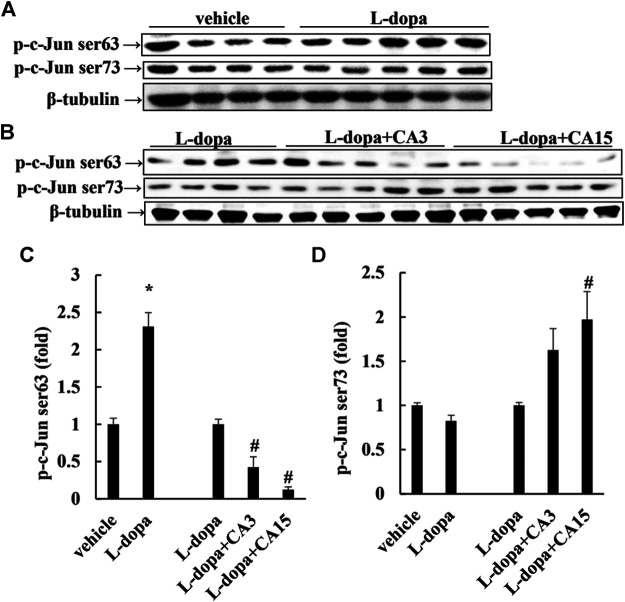
It has been reported that L-dopa induces continuous activation of ERK1/2 to promote c-Jun phosphorylation at Ser63 (as a pro-apoptotic factor) and reduces c-Jun phosphorylation at Ser73 (as an anti-apoptotic factor) (Park et al., 2016). We found that rats treated with L-dopa significantly increased the activation of ERK1/2 and c-Jun Ser63 (p < 0.05). (Figures 3A, 4A). However, phosphorylation of ERK1/2 and c-Jun Ser63 was reduced in cells pretreated with 3 and 15 mg/kg CA (Figures 3B, 4B). CA at 15 mg/kg reduced the activation of ERK1/2 and c-Jun Ser63 by 60 and 88%, respectively, compared with that in the L-dopa group (p < 0.05). However, only the group pretreated with 15 mg/kg CA showed an increase in the phosphorylation of c-Jun Ser73 of about 97% compared with the L-dopa group (p < 0.05) (Figure 4B).
FIGURE 3.
Effect of CA on L-dopa-induced activation of ERK1/2 and c-Jun in 6-OHDA lesioned rats. The phosphorylation of ERK1/2 (A,B) in striatum tissues were determined by Western blotting. β-Tubulin was used as the internal control. (C) The level of the L-dopa group was set at 1.0. Values are mean ± SEM (n = 3). #p < 0.05 compared with L-dopa-treated group.
FIGURE 4.
Effect of CA on L-dopa-induced activation of ERK1/2 and c-Jun in 6-OHDA lesioned rats. The phosphorylation of c-Jun Ser63 and c-Jun Ser73 in striatum tissues were determined by Western blotting (A,B). β-Tubulin was used as the internal control. (C,D) The level of the L-dopa group was set at 1.0. Values are mean ± SEM (n = 3). #p < 0.05 compared with L-dopa-treated group.
CA Prevents Apoptosis Induced by L-Dopa in SH-SY5Y Cells
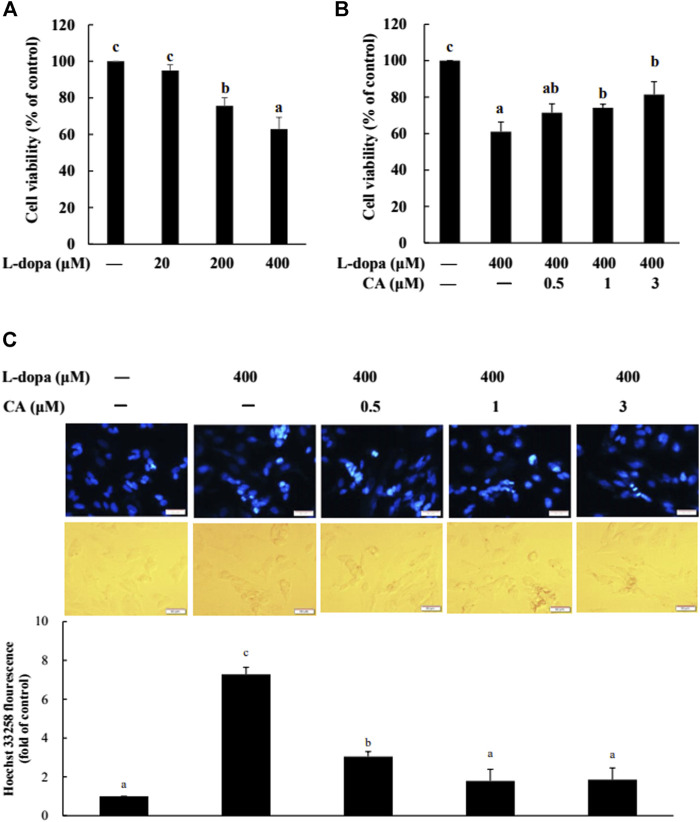
We further used SH-SY5Y cells to explore the effect of CA on cytotoxicity induced by L-dopa. The results indicated that L-dopa dose-dependently reduced cell viability. Cell viability in cells treated with 400 μM L-dopa was reduced by about 36% compared with the control group (Figure 5A) Pretreatment with 1 and 3 μM CA, however, was able to increase cell viability by 21 and 34%, respectively, in L-dopa-treated cells compared with that in cells treated with L-dopa alone (p < 0.05) (Figure 5B). The results of Hoechst 33258 staining were similar, as shown in Figure 5C. Exposure to L-dopa induced the intensity of Hoechst 33258 fluorescence, suggesting that L-dopa treatment increased nuclear condensation and then induced apoptosis. However, the effect of L-dopa on nuclear condensation was reduced in cells pretreated with CA. We then used immunoblotting to examine the effect on proteins related to apoptosis. L-Dopa dose-dependently reduced the expression of Bcl-2 protein and increased the ratio of cleaved caspase 3/caspase 3 and cleaved PARP/PARP (Figures 6A,B). CA pretreatment reversed these findings.
FIGURE 5.
Effect of CA on L-dopa-induced toxicity in SH-SY5Y cells. Cell viability was measured by MTT assay. (A) Cells were pretreated with 20, 200, and 400 μM L-dopa for 72 h. Control (−) was treated with PBS. (B) Cells were pretreated with 0.1% DMSO alone (−) or 0.5, 1, and 3 μM CA for 18 h and were then treated with 400 μM L-dopa for an additional 72 h. (C) Nuclei were visualized with Hoechst 33258 staining. Cells were pretreated with DMSO alone (−) or 0.5, 1, or 3 μM CA for 18 h and were then treated with 400 μM L-dopa for an additional 24 h. One representative image out of three independent experiments is shown. Values are mean ± SD (n = 3). Means not sharing a common letter are significantly different, p < 0.05.
FIGURE 6.
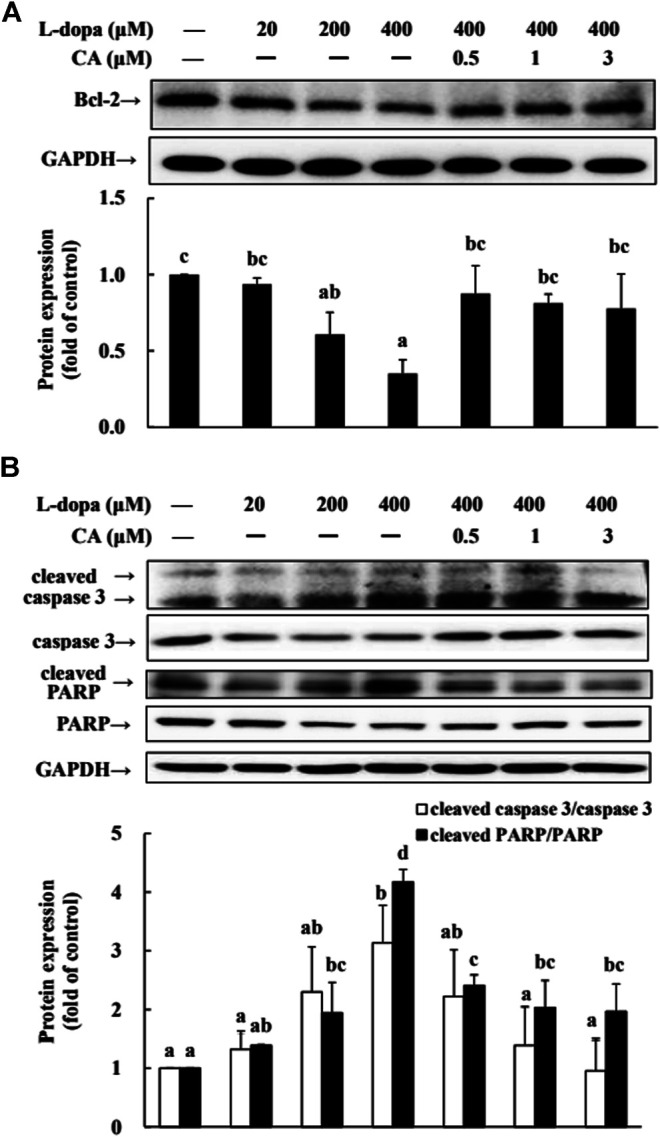
Involvement of reduction in apoptosis-related proteins in the neuroprotective effects of CA. (A) Cells were pretreated with DMSO alone (−) or 0.5, 1, or 3 μM CA for 18 h and were then treated with 20, 200, and 400 μM L-dopa for an additional 24 h. One representative immunoblot out of three independent experiments is shown. (B) The control group was regarded as 1. Values are mean ± SD (n = 3). Means not sharing a common letter are significantly different, p < 0.05.
CA Regulates ERK1/2/c-Jun Signaling Induced by L-Dopa in SH-SY5Y Cells
The phosphorylation of ERK1/2 and c-Jun Ser63 was increased in cells treated with L-dopa, and the activation of c-Jun Ser73 was reduced (Figure 7). However, pretreatment with CA improved the effects of L-dopa on these proteins (p < 0.05).
FIGURE 7.
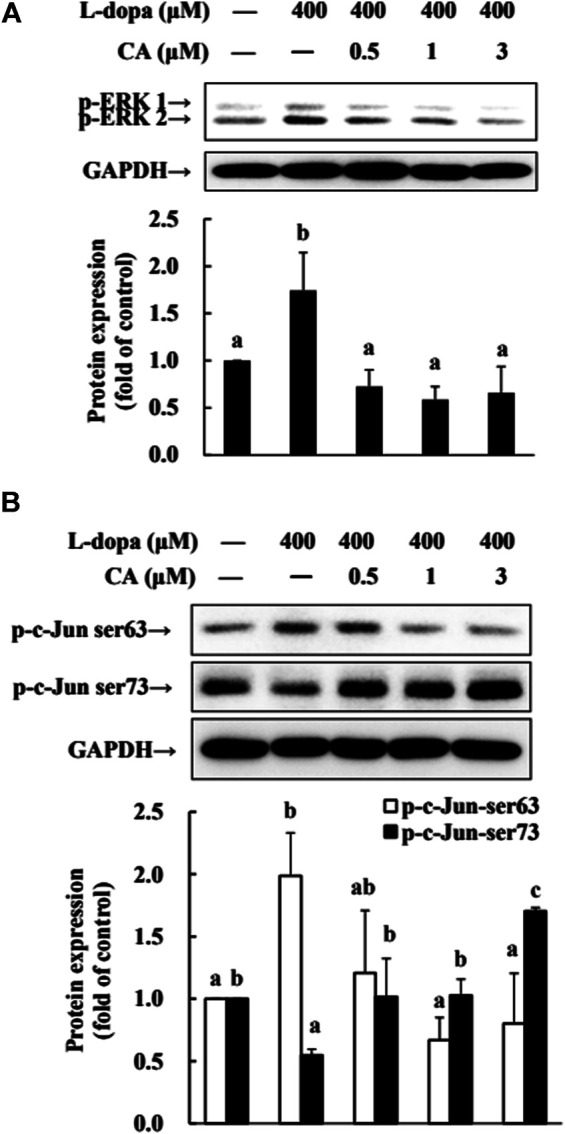
Effect of CA on the activation of ERK1/2 and c-Jun in L-dopa-treated SH-SY5Y cells. (A) Cells were pretreated with DMSO alone (−) or 0.5, 1, or 3 μM CA for 18 h and were then treated with 400 μM L-dopa for an additional 0.5 h. One representative immunoblot out of three independent experiments is shown. (B) The control group was regarded as 1. Values are mean ± SD (n = 3). Means not sharing a common letter are significantly different, p < 0.05.
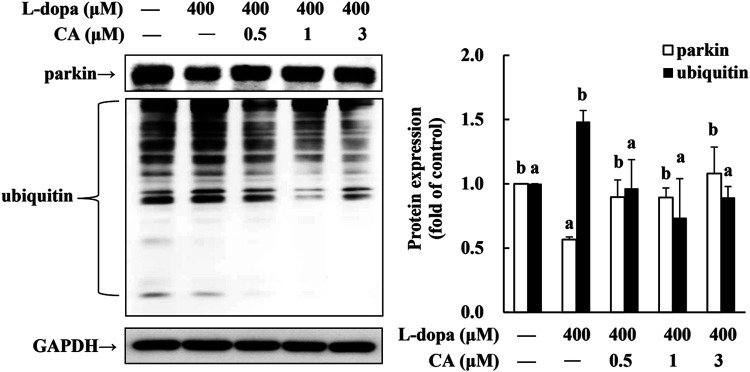
CA Improves the Expression of Parkin and Ubiquitinated Protein Induced by L-Dopa in SH-SY5Y Cells
Parkin is a ubiquitin protein ligase E3 and plays a critical role in the ubiquitin-proteasome system (UPS) (Higashi et al., 2004). The activation of D1R by L-dopa is associated with the dysregulation of the (Berthet et al., 2012). Therefore, in this study, we also examined ubiquitinated-related proteins. We found that parkin protein was reduced and ubiquitinated protein was increased in cells treated with L-dopa (Figure 8). CA pretreatment improved both proteins in cells treated with L-dopa (p < 0.05).
FIGURE 8.
Effect of CA on the expression of parkin and ubiquitinated protein in L-dopa-treated SH-SY5Y cells. Cells were pretreated with DMSO alone (−) or 0.5, 1, or 3 μM CA for 18 h and were then treated with 400 μM L-dopa for an additional 24 h. One representative immunoblot out of three independent experiments is shown. The control group was regarded as 1. Values are mean ± SD (n = 3). Means not sharing a common letter are significantly different, p < 0.05.
Discussion
LID is the adverse events after long-term use of L-dopa in PD patients (Chaudhuri et al., 2019). Several strategies for overcoming LID have been developed, but some of these have limitations (Ryu et al., 2018). In the current study, we revealed that CA could improve the development of LID in a 6-OHDA-lesioned rat model. CA inhibited D1R-induced signaling, including p-DARPP-32, p-ERK1/2, and ΔFosB. Moreover, we revealed that CA attenuated the levels of cleaved-caspase 3 and -PARP by L-dopa is associated with the regulation of the ERK1/2-c-Jun pathway. In addition, we found that CA increased parkin protein to reduce the ubiquitinated protein by L-dopa. It is the first study to show the favorable effect of CA against the side effects inducing by L-dopa treatment for PD.
LID is a serious motor complication that develops after long-term L-dopa therapy for PD (Sun et al., 2020). Study indicated that the time-course of L-dopa during 0–60 min exhibits higher total AIM and ALO scores (Ryu et al., 2018). Consistent with these results, our results revealed that the ALO scores are higher after L-dopa administration during 0–60 min. CA group decreased the ALO scores during 20–60 min. Moreover, CA treatment exhibited lower the ALO scores up to 33 days. These results suggested that CA is beneficial for long-term treatment of LID for PD. Recent studies have reported that the behavioral expression of LID is associated with D1R signaling. However, the signaling was unchanged by continuous administration of L-dopa through a subcutaneous mini-pump, suggesting prevention the fluctuation of L-dopa concentrations relieved the LID (Lebel et al., 2010). In the present study, CA attenuated the activation of D1R/DARPP32/ERK1/2 cascade and subsequently led to counteract the effect of LID in 6-OHDA-lesioned rats. Because D1R signaling is triggered by the activation of PKA, study reported that treatment with PKA inhibitor Rp-cAMPS alleviated the LID in 6-OHDA-lesioned rats (Lebel et al., 2010). Similarly, researchers showed that blocking ERK1/2 phosphorylation by SL327 reduces LID in 6-OHDA-lesioned mice treated with L-dopa (Santini et al., 2007). Study also indicated that ΔFosB is accumulated after abusing some drugs and increased the sensitivity to the behavioral effects (Nestler et al., 2001). The present study found that striatal ΔFosB protein induction is related with the AIM and ALO scores by L-dopa. In parallel with behavior reversal, CA treatment reduced D1R/DARPP32/ERK1/2 cascade and decreased ΔFosB protein caused by L-dopa treatment.
Research shows that ERK1/2 activation is involved in the neuronal cell viability induced by L-dopa treatment (Park et al., 2016). Treatment of PC12 cells with L-dopa induces the activation of ERK1/2 and caspase 3 (Jin et al., 2010; Park et al., 2014). Similarly, in 6-OHDA-lesioned rats, administration of L-dopa upregulated the ERK1/2 activation, leading to increase c-Jun phosphorylation at ser63, but decrease c-Jun phosphorylation at ser73 (Park et al., 2016). It is because L-dopa at high concentrations was cytotoxic and stimulated the activities of ERK1/2 and caspase 3. These results could explain that PD patients after long-term exposure to L-dopa increased neurotoxic events (Blandini et al., 2004) and stimulated abnormal involuntary movements (Chaudhuri et al., 2019). Our results in the present study supported the report that L-dopa increased ERK1/2/c-Jun activation and enhanced caspase 3 activation (Figures 3, 4, 6, 7). CA treatment could increase the cell survival through reducing the ERK1/2 activation and alleviating the alternation of c-Jun pathway to prevent apoptotic neuronal cell death.
Recently, evidence reported that dysregulation membrane localization of dopamine D1R impairs the striatal ubiquitin-proteasome system and increases the accumulation of ubiquitinated protein to exaggerate the D1R transmission (Barroso-Chinea et al., 2015). Parkin, an ubiquitin E3 ligase, facilitates multiple misfolded proteins degradation by 26S proteasome, and plays an important role in neuroprotection (Wilhelmus et al., 2012). Study reported that knockdown of parkin displays the higher AIMs scores than in wild-type mice. Moreover, compare to control mice, parkin mutant mice shows an earlier onset of AIMs (Berthet et al., 2012). Our previous study indicated that CA acts to attenuate 6-OHDA-induced neurotoxicity associated with the induction of parkin through enhancing UPS and preventing apoptosis (Lin et al., 2016). In this study, we have shown that SH-SY5Y cells treated with L-dopa decreased the parkin and increased the ubiquitinated protein; however, CA pretreatment reversed parkin and ubiquitinated proteins by L-dopa. Therefore, we speculated that CA up-regulated the parkin protein and reduced the D1R abnormal trafficking, leading to alleviation of D1R signaling and LID.
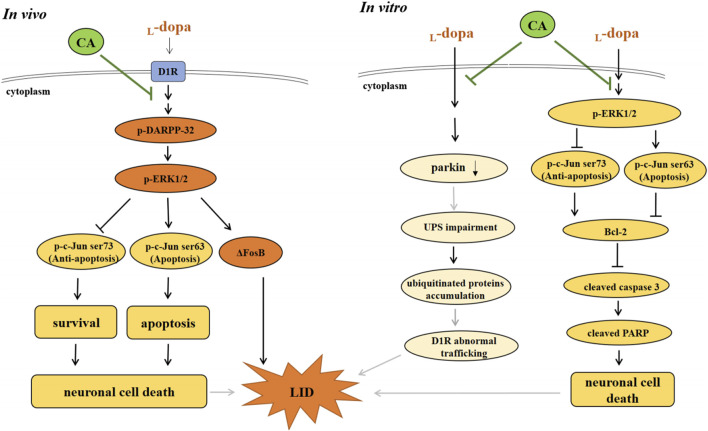
In conclusion, the results of the current indicate that CA can alleviate LID-induced behavior changes in 6-OHDA-treated rats by regulating the D1R-mediated activation of DARPP-32 and ΔFosB. The protective mechanisms of CA are involved with the inactivation of ERK1/2/c-Jun pathway and the induction of parkin protein, leading to reduction of apoptotic neuronal cell death and LID (Figure 9). CA could be recommended as a beneficial therapy for delaying the development of LID in PD patients.
FIGURE 9.
The actions of neuroprotective mechanism of CA on LID in in vivo and in vitro studies. (A) In in vivo study, L-dopa stimulates DIR protein, and then activates the phosphorylation of DARPP-32 and ERK1/2, which elevates ΔFosB protein, leading to develop the LID in 6-OHDA-lesioned rats. Moreover, L-dopa administration induced neuronal cell death through regulating ERK1/2-c-Jun pathway. However, CA alleviates these effects induced by L-dopa. (B) In in vitro study, pretreatment of CA with SH-SY5Y cells attenuates L-dopa-triggered apoptotic cell death mediated via increasing c-Jun ser73 activation and decreasing c-Jun ser63 activation by ERK1/2. Additionally, CA could be improved the development of LID is related to the induction of parkin protein, leading to prevent the ubiquitinated protein accumulation and D1R abnormal trafficking (gray line).
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
All animal manipulations were carried out according to the Institutional Animal Care and Use Committee of China Medical University (protocol no. 2017–189).
Author Contributions
C-WT designed research; C-YLa and C-YLi performed research; C-RW and C-HT contributed analytic tools and ideas; C-YLa and C-WT wrote the paper. C-YLi and C-WT revised the paper.
Funding
This study was supported by the Ministry of Science and Technology (MOST 106-2320-B-039-055-MY3) and CMU109-MF-79.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ahn S., Song T. J., Park S. U., Jeon S., Kim J., Joo-Young O., et al. (2017). Effects of a Combination Treatment of KD5040 and L-Dopa in a Mouse Model of Parkinson's Disease. BMC Compl. Altern. Med. 17, 220. 10.1186/s12906-017-1731-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Chinea P., Thiolat M.-L., Bido S., Martinez A., Doudnikoff E., Baufreton J., et al. (2015). D1 Dopamine Receptor Stimulation Impairs Striatal Proteasome Activity in Parkinsonism through 26S Proteasome Disassembly. Neurobiol. Dis. 78, 77–87. 10.1016/j.nbd.2015.02.024 [DOI] [PubMed] [Google Scholar]
- Berthet A., Bezard E., Porras G., Fasano S., Barroso-Chinea P., Dehay B., et al. (2012). L-DOPA Impairs Proteasome Activity in Parkinsonism through D1 Dopamine Receptor. J. Neurosci. 32, 681–691. 10.1523/jneurosci.1541-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O., Guigoni C., Li Q., Bioulac B. H., Aubert I., Gross C. E., et al. (2009). Striatal Overexpression of ΔJunD Resets L-DOPA-Induced Dyskinesia in a Primate Model of Parkinson Disease. Biol. Psychiatry 66, 554–561. 10.1016/j.biopsych.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F., Cosentino M., Mangiagalli A., Marino F., Samuele A., Rasini A., et al. (2004). Modifications of Apoptosis-Related Protein Levels in Lymphocytes of Patients with Parkinson's Disease. The Effect of Dopaminergic Treatment. J. Neural Transm. 111, 1017–1030. 10.1007/s00702-004-0123-1 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Filippo M. D., Ghiglieri V., Tambasco N., Picconi B. (2010). Levodopa-induced Dyskinesias in Patients with Parkinson's Disease: Filling the Bench-To-Bedside gap. Lancet Neurol. 9, 1106–1117. 10.1016/s1474-4422(10)70218-0 [DOI] [PubMed] [Google Scholar]
- Cao X., Yasuda T., Uthayathas S., Watts R. L., Mouradian M. M., Mochizuki H., et al. (2010). Striatal Overexpression of FosB Reproduces Chronic Levodopa-Induced Involuntary Movements. J. Neurosci. 30, 7335–7343. 10.1523/jneurosci.0252-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello J., Cortés M., Malave L., Kottmann A., Sibley D. R., Friedman E., et al. (2020). The Dopamine D5 Receptor Contributes to Activation of Cholinergic Interneurons during L-DOPA Induced Dyskinesia. Sci. Rep. 10, 2542. 10.1038/s41598-020-59011-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci M. A. (2014). Presynaptic Mechanisms of L-DOPA-Induced Dyskinesia: The Findings, the Debate, and the Therapeutic Implications. Front. Neurol. 5, 242. 10.3389/fneur.2014.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri K. R., Jenner P., Antonini A. (2019). Should There Be Less Emphasis on Levodopa-Induced Dyskinesia in Parkinson's Disease? Move. Disord. 34, 816. 10.1002/mds.27691 [DOI] [PubMed] [Google Scholar]
- Chen J.-H., Ou H.-P., Lin C.-Y., Lin F.-J., Wu C.-R., Chang S.-W., et al. (2012). Carnosic Acid Prevents 6-Hydroxydopamine-Induced Cell Death in SH-Sy5y Cells via Mediation of Glutathione Synthesis. Chem. Res. Toxicol. 25, 1893–1901. 10.1021/tx300171u [DOI] [PubMed] [Google Scholar]
- Colamartino M., Santoro M., Duranti G., Sabatini S., Ceci R., Testa A., et al. (2015). Evaluation of Levodopa and Carbidopa Antioxidant Activity in normal Human Lymphocytes In Vitro: Implication for Oxidative Stress in Parkinson's Disease. Neurotox. Res. 27, 106–117. 10.1007/s12640-014-9495-7 [DOI] [PubMed] [Google Scholar]
- Dorszewska J., Prendecki M., Lianeri M., Kozubski W. (2014). Molecular Effects of L-Dopa Therapy in Parkinson's Disease. Curr. Genomics 15, 11–17. 10.2174/1389202914666131210213042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeln M., Bastide M. F., Toulmé E., Dehay B., Bourdenx M., Doudnikoff E., et al. (2016). Selective Inactivation of Striatal FosB/ΔFosB-Expressing Neurons Alleviates L-DOPA-Induced Dyskinesia. Biol. Psychiatry 79, 354–361. 10.1016/j.biopsych.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Fanni S., Scheggi S., Rossi F., Tronci E., Traccis F., Stancampiano R., et al. (2018). 5alpha-reductase Inhibitors Dampen L-DOPA-Induced Dyskinesia via Normalization of Dopamine D1-Receptor Signaling Pathway and D1-D3 Receptor Interaction. Neurobiol. Dis. 121, 120–130. 10.1016/j.nbd.2018.09.018 [DOI] [PubMed] [Google Scholar]
- Feyder M., Bonito-Oliva A., Fisone G. (2011). L-DOPA-Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission. Front. Behav. Neurosci. 5, 71. 10.3389/fnbeh.2011.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieblinger T., Graves S. M., Sebel L. E., Alcacer C., Plotkin J. L., et al. (2014). Cell Type-specific Plasticity of Striatal Projection Neurons in Parkinsonism and L-DOPA-Induced Dyskinesia. Nat. Commun. 5, 5316. 10.1038/ncomms6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Shen Z., Yu H., Lu G., Yu Y., Liu X., et al. (2016). Carnosic Acid Protects against Acetaminophen-Induced Hepatotoxicity by Potentiating Nrf2-Mediated Antioxidant Capacity in Mice. Korean J. Physiol. Pharmacol. 20, 15–23. 10.4196/kjpp.2016.20.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A., Lotharius J. (2005). Oxidative Stress and Inflammation in Parkinson's Disease: Is There a Causal Link? Exp. Neurol. 193, 279–290. 10.1016/j.expneurol.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Higashi Y., Asanuma M., Miyazaki I., Hattori N., Mizuno Y., Ogawa N. (2004). Parkin Attenuates Manganese‐induced Dopaminergic Cell Death. J. Neurochem. 89, 1490–1497. 10.1111/j.1471-4159.2004.02445.x [DOI] [PubMed] [Google Scholar]
- Jin C. M., Yang Y. J., Huang H. S., Kai M., Lee M. K. (2010). Mechanisms of L-DOPA-Induced Cytotoxicity in Rat Adrenal Pheochromocytoma Cells: Implication of Oxidative Stress-Related Kinases and Cyclic AMP. Neuroscience 170, 390–398. 10.1016/j.neuroscience.2010.07.039 [DOI] [PubMed] [Google Scholar]
- Lebel M., Chagniel L., Bureau G., Cyr M. (2010). Striatal Inhibition of PKA Prevents Levodopa-Induced Behavioural and Molecular Changes in the Hemiparkinsonian Rat. Neurobiol. Dis. 38, 59–67. 10.1016/j.nbd.2009.12.027 [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Chen W.-J., Fu R.-H., Tsai C.-W. (2020). Upregulation of OPA1 by Carnosic Acid Is Mediated through Induction of IKKγ Ubiquitination by Parkin and Protects against Neurotoxicity. Food Chem. Toxicol. 136, 110942. 10.1016/j.fct.2019.110942 [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Tsai C.-W., Tsai C.-W. (2016). Carnosic Acid Protects SH-Sy5y Cells against 6-Hydroxydopamine-Induced Cell Death through Upregulation of Parkin Pathway. Neuropharmacology 110, 109–117. 10.1016/j.neuropharm.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Lindgren H. S., Rylander D., Iderberg H., Andersson M., O'Sullivan S. S., Williams D. R., et al. (2011). Putaminal Upregulation of FosB/ΔFosB-like Immunoreactivity in Parkinson's Disease Patients with Dyskinesia. J. Parkinsons Dis. 1, 347–357. 10.3233/jpd-2011-11068 [DOI] [PubMed] [Google Scholar]
- Manson A., Stirpe P., Schrag A. (2012). Levodopa-induced-dyskinesias Clinical Features, Incidence, Risk Factors, Management and Impact on Quality of Life. J. Parkinsons Dis. 2, 189–198. 10.3233/jpd-2012-120103 [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Barrot M., Self D. W. (2001). FosB: A Sustained Molecular Switch for Addiction. Proc. Natl. Acad. Sci. 98, 11042–11046. 10.1073/pnas.191352698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. H., Park H. J., Shin K. S., Lee M. K. (2014). Multiple Treatments with L-3,4-Dihydroxyphenylalanine Modulate Dopamine Biosynthesis and Neurotoxicity through the Protein Kinase A-Transient Extracellular Signal-Regulated Kinase and Exchange Protein Activation by Cyclic AMP-Sustained Extracellular Signa. J. Neurosci. Res. 92, 1746–1756. 10.1002/jnr.23450 [DOI] [PubMed] [Google Scholar]
- Park K. H., Shin K. S., Zhao T. T., Park H. J., Lee K. E., Lee M. K. (2016). L-DOPA Modulates Cell Viability through the ERK-C-Jun System in PC12 and Dopaminergic Neuronal Cells. Neuropharmacology 101, 87–97. 10.1016/j.neuropharm.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Ryu Y.-K., Park H.-Y., Go J., Choi D.-H., Kim Y.-H., Hwang J. H., et al. (2018). Metformin Inhibits the Development of L-DOPA-Induced Dyskinesia in a Murine Model of Parkinson's Disease. Mol. Neurobiol. 55, 5715–5726. 10.1007/s12035-017-0752-7 [DOI] [PubMed] [Google Scholar]
- Santini E., Valjent E., Usiello A., Carta M., Borgkvist A., Girault J.-A., et al. (2007). Critical Involvement of cAMP/DARPP-32 and Extracellular Signal-Regulated Protein Kinase Signaling in L-DOPA-Induced Dyskinesia. J. Neurosci. 27, 6995–7005. 10.1523/jneurosci.0852-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigolon G., Fisone G. (2018). Signal Transduction in L-DOPA-Induced Dyskinesia: from Receptor Sensitization to Abnormal Gene Expression. J. Neural Transm. 125, 1171–1186. 10.1007/s00702-018-1847-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley B. J., Yamamoto B. K. (2013). L-dopa-induced Dopamine Synthesis and Oxidative Stress in Serotonergic Cells. Neuropharmacology 67, 243–251. 10.1016/j.neuropharm.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Wang T., Li N., Qiao J. (2020). Analysis of Motor Complication and Relative Factors in a Cohort of Chinese Patients with Parkinson's Disease. Parkinsons Dis. 2020, 8692509. 10.1155/2020/8692509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus M. M. M., Nijland P. G., Drukarch B., de Vries H. E., van Horssen J. (2012). Involvement and Interplay of Parkin, PINK1, and DJ1 in Neurodegenerative and Neuroinflammatory Disorders. Free Radic. Biol. Med. 53, 983–992. 10.1016/j.freeradbiomed.2012.05.040 [DOI] [PubMed] [Google Scholar]
- Winkler C., Kirik D., Björklund A., Cenci M. A. (2002). L-DOPA-induced Dyskinesia in the Intrastriatal 6-hydroxydopamine Model of Parkinson's Disease: Relation to Motor and Cellular Parameters of Nigrostriatal Function. Neurobiol. Dis. 10, 165–186. 10.1006/nbdi.2002.0499 [DOI] [PubMed] [Google Scholar]
- Wu C.-R., Tsai C.-W., Chang S.-W., Lin C.-Y., Huang L.-C., Tsai C.-W. (2015). Carnosic Acid Protects against 6-Hydroxydopamine-Induced Neurotoxicity in In Vivo and In Vitro Model of Parkinson's Disease: Involvement of Antioxidative Enzymes Induction. Chemico-Biological Interactions 225, 40–46. 10.1016/j.cbi.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Zhang S. F., Xie C. L., Lin J. Y., Wang M. H., Wang X. J., Liu Z. G. (2018). Lipoic Acid Alleviates LDOPA induced Dyskinesia in 6OHDA Parkinsonian Rats via Anti-oxidative S-tress. Mol. Med. Rep. 17, 1118–1124. 10.3892/mmr.2017.7974 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.