Abstract
BACKGROUND.
Bathing intensive care unit (ICU) patients with 2% chlorhexidine gluconate (CHG)–impregnated cloths decreases the risk of healthcare-associated bacteremia and multidrug-resistant organism transmission. Hospitals employ different methods of CHG bathing, and few studies have evaluated whether those methods yield comparable results.
OBJECTIVE.
To determine whether 3 different CHG skin cleansing methods yield similar residual CHG concentrations and bacterial densities on skin.
DESIGN.
Prospective, randomized 2-center study with blinded assessment.
PARTICIPANTS AND SETTING.
Healthcare personnel in surgical ICUs at 2 tertiary-care teaching hospitals in Chicago, Illinois, and Boston, Massachusetts, from July 2015 to January 2016.
INTERVENTION.
Cleansing skin of one forearm with no-rinse 2% CHG-impregnated polyester cloth (method A) versus 4% CHG liquid cleansing with rinsing on the contralateral arm, applied with either non–antiseptic-impregnated cellulose/polyester cloth (method B) or cotton washcloth dampened with sterile water (method C).
RESULTS.
In total, 63 participants (126 forearms) received method A on 1 forearm (n =63). On the contralateral forearm, 33 participants received method B and 30 participants received method C. Immediately and 6 hours after cleansing, method A yielded the highest residual CHG concentrations (2500 μg/mL and 1250 μg/mL, respectively) and lowest bacterial densities compared to methods B or C (P<.001).
CONCLUSION.
In healthy volunteers, cleansing with 2% CHG-impregnated cloths yielded higher residual CHG concentrations and lower bacterial densities than cleansing with 4% CHG liquid applied with either of 2 different cloth types and followed by rinsing. The relevance of these differences to clinical outcomes remains to be determined.
The antiseptic properties of chlorhexidine gluconate (CHG) have been known since the 1950s.1 CHG has diverse clinical applications from oral hygiene2 to preoperative surgical site skin preparation.3 The use of no-rinse 2% CHG-impregnated cloths for routine patient bathing in the intensive care unit (ICU) has been shown to decrease the risk of healthcare-associated bloodstream infections4–6 and to reduce cross transmission of multidrug-resistant organisms (MDROs).4,7 Other ICU-based studies in which patients were bathed daily with 4% CHG formulations instead of no-rinse 2% CHG-impregnated cloths have suggested similar decreases in MDRO transmission;8,9 however, these studies have been limited by lack of randomization and by varying bathing techniques.
Few studies have directly compared outcomes between skin cleansing with no-rinse 2% CHG-impregnated cloths versus 2% or 4% CHG liquid formulations. Despite the lack of comparative data, some hospitals assume equivalent efficacy for these techniques and bathe patients with 4% CHG liquid formulations rather than 2% CHG-impregnated cloths based on relatively lower cost.10,11 The objective of the present study was to determine whether cleansing with 2% CHG-impregnated cloths versus cleansing with 4% CHG liquid delivered by 2 commonly used methods yielded comparable CHG concentrations and residual microbial densities on the skin of healthy volunteers.
METHODS
Study Population and Recruitment
Healthcare personnel from the surgical ICUs of Rush University Medical Center (RUMC) in Chicago, Illinois, and from Brigham and Women’s Hospital/Harvard Medical School (BWH) in Boston, Massachusetts, were recruited between July 2015 and January 2016. Subjects with known allergies or prior adverse reactions to CHG and those who had nonintact skin or well-defined erythema (defined as erythema grade ≥2; erythema grade scale modified from Vernon et al7) on their forearms were excluded. All other healthcare personnel who worked in the surgical ICUs were eligible for study participation. The institutional review boards of RUMC and BWH reviewed and approved the study independently, written informed consent was required at RUMC and verbal assent was required at BWH.
Study Design
With randomized laterality, each participant had 1 forearm cleansed using method A: 2% CHG-impregnated polyester cloth, ~ 500 mg CHG per cloth as per manufacturer (Sage 2% Chlorhexidine Gluconate Cloths, Sage Products [now part of Stryker], Cary, IL). Each participant’s contralateral forearm was then randomized to cleansing with 5mL of undiluted 4% CHG (Hibiclens, Mölnlycke Health Care, Norcross, GA) liquid suspension applied with either a cellulose/polyester cloth impregnated with a nonantiseptic solution (Comfort Bath, Sage Products) (Method B) or with a cotton washcloth dampened with sterile water (method C). Each cleansing method was applied to the skin for 20 seconds. For 4% CHG products, this was followed by a 2-minute dwell time,12,13 then a 20-second wipe off with a clean cloth of the same type used for application. The wipe-off technique for the 4% CHG liquid was consistent with manufacturer recommendations for general skin cleansing.14 At each participating hospital, skin cleansing protocols were standardized to be identical and were conducted by a single investigator to ensure uniformity.
Research staff, who were blinded to cleansing assignment, collected swab samples within a 5 × 5-cm2 area on the forearm at 3 time points: immediately prior to, immediately after, and 6 hours after skin cleansing. Throughout the 6 hours, participants performed their routine clinical work. To test for residual CHG on the skin of each participant, sterile swabs were moistened with sterile water (Bio-Swab, Arrowhead Forensics, Lenexa, KS), and forearms were swabbed for 10 seconds. For bacterial cultures, flocked swabs (FLOQSwabs, Copan, Murrieta, CA) were moistened in sterile water and a 5 × 5-cm2 area near the area swabbed for residual CHG was swabbed for 10 seconds. To avoid the wipe-off effect of CHG sample collection, the same area was not swabbed twice. A short survey was administered to each study participant to collect demographic data, information on use of skin products, and reports of skin reactions after cleansing. Subject-level data were deidentified before analysis.
Laboratory Methods
Chlorhexidine gluconate concentration was measured using a colorimetric method described previously.13,15 Swab samples for culture were placed immediately into 500 μL neutralizing agent16,17 without ether sulfate, as this reagent was unavailable for purchase in the United States at the time of testing. Serially diluted 100 μL volumes were aliquoted to 5% sheep’s blood agar plates in duplicate and incubated at 35 ± 2°C in ambient air for up to 48 hours. Colonies were counted and transformed to colony forming units (CFUs) after correcting for any dilution factor. Presumptive identifications were performed using standard microbiologic methods. All laboratory testing was conducted by research personnel blinded to study assignments.
Statistical Design and Analysis
Sample size estimates were based on an earlier study15 that found an effect size of d = 0.6218 for the reduction in CFUs when the CHG concentration was increased from 37.5–150 μg/mL to 300–600 μg/mL. Assuming an effect size of 0.62, a sample of 60, and a 1-tailed α of 0.05, a power of 0.96 was obtained for the current study design.
Deidentified results were recorded in an electronic database and shared between institutions. The Kruskall-Wallis test was used to compare differences in bacterial density, CHG skin concentrations, and other ordinal variables. For comparison of categorical variables, χ2 analysis or the Fischer exact test was used as appropriate for expected values. All statistical tests were 2-tailed; an α level of 0.05 was considered significant. Testing was performed using SPSS version 22 software (IBM, Armonk, NY), SAS version 9.2 software (SAS Institute, Cary, NC), or R software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Survey Results
In total, 32 participants were enrolled at RUMC and 31 participants were enrolled at BWH (63 participants and 126 forearms in total). The median age was 35 years (range, 30–45 years). Most participants were female (86%) and right-handed (87%). Nurses and patient care assistants made up the largest category of healthcare personnel (70%). Also, 2 participants reported having eczema, 1 participant reported having psoriasis, and 1 participant reported a remote history of idiopathic urticaria. When stratified by CHG cleansing intervention, no statistically significant differences were detected among groups in age, body mass index, gender, medical team role, dominant arm cleansed, presence of watch or bracelet, underlying dermatologic conditions affecting the forearms, use of topical skin products in the 24 hours prior to skin cleansing, or sleeve length prior to cleansing or throughout the 6-hour period (Table 1). Most participants reported cleansing to be relatively pleasant (≥4/5 on pleasantness scale). However, 2 participants reported minimal erythema at 6 hours after cleansing, 1 of whom had no change from baseline erythema that was caused by bracelet irritation.
TABLE 1.
Demographics, Study Characteristics, Survey Feedback, and Observations by Chlorhexidine Gluconate (CHG) Skin Cleansing Intervention Groups
| Characteristic | 2% CHG Impregnated, No-Rinse Cloth (n = 63), No. (%)a | 4% CHG Applied With Non–Antiseptic-Impregnated Cloth (n = 33), No. (%)a | 4% CHG Applied With Cotton Washcloth (n = 30), No. (%)a | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR), y | 35 (30–45) | 36 (30.5–45.5) | 33 (28.8–45.3) | .73 |
| BMI, median (IQR)b | 24 (21.8–27.8) | 24.2 (22.2–27.2) | 23.3 (21.6–29.4) | .98 |
| Sex | .88 | |||
| Male | 9 (14.3) | 4 (12.1) | 5 (16.7) | |
| Female | 54 (85.7) | 29 (87.9) | 25 (83.3) | |
| Occupation | .99 | |||
| Nurse | 39 (61.9) | 21 (63.6) | 18 (60) | |
| Patient care assistant | 5 (7.9) | 2 (6.1) | 3 (10) | |
| Physician | 7 (11.1) | 4 (12.1) | 3 (10) | |
| Physical therapist | 1 (1.6) | 0 (0) | 1 (3.3) | |
| Respiratory therapist | 6 (9.5) | 4 (12.1) | 2 (6.7) | |
| Otherc | 5 (7.9) | 2 (6.1) | 3 (10) | |
| Study characteristics | ||||
| Laterality of arm cleansed | .91 | |||
| Right | 32 (50.8) | 17 (51.5) | 14 (46.7) | |
| Left | 31 (49.2) | 16 (48.5) | 16 (53.3) | |
| Dominant arm cleansed | .81 | |||
| Yes | 32 (50.8) | 15 (45.5) | 16 (53.3) | |
| No | 31 (49.2) | 18 (54.5) | 14 (46.7) | |
| Survey results as reported by study participants | ||||
| Eczema on forearm | 2 (3.2) | 2 (6.1) | 0 (0) | .56 |
| Psoriasis on forearm | 1 (1.6) | 0 (0) | 1 (3.3) | .49 |
| Other dermatologic conditions on forearmd | 1 (1.6) | 1 (3) | 0 (0) | .99 |
| ≥ 1 topical product used on forearm in the last 24 h | 55 (87.3) | 29 (87.9) | 26 (86.7) | .99 |
| Sleeve length | ||||
| Prior to cleansing | .86 | |||
| Long | 32 (50.8) | 18 (54.5) | 14 (46.7) | |
| Three-quarters | 6 (9.5) | 4 (12.1) | 2 (6.7) | |
| Short | 25 (39.7) | 11 (33.3) | 14 (46.7) | |
| Throughout most of 6 h | .30 | |||
| Long | 32 (50.8) | 19 (57.6) | 13 (43.3) | |
| Three-quarters | 10 (15.9) | 7 (21.2) | 3 (10) | |
| Short | 21 (33.3) | 7 (21.2) | 14 (46.7) | |
| Pleasantness of cleansing methode | .86 | |||
| Level 2 | 1 (1.6) | 0 (0) | 1 (3.3) | |
| Level 3 | 19 (30.2) | 9 (27.3) | 8 (26.7) | |
| Level 4 | 10 (15.9) | 5 (15.2) | 4 (13.3) | |
| Level 5 | 33 (52.4) | 19 (57.6) | 17 (56.7) | |
| Gross contamination on the forearm within 6 hours of cleansing | 3 (4.8) | 0 (0) | 0 (0) | .43 |
| Researcher observations | ||||
| Hair on forearm | 25 (39.7) | 14 (42.4) | 11 (36.7) | .89 |
| Wearing a watchf | 7 (11.1) | 1 (3) | 5 (16.7) | .18 |
| Wearing a braceletf | 4 (6.3) | 4 (12.1) | 4 (13.3) | .45 |
| Erythema on forearmg | ||||
| Prior to cleansing | .63 | |||
| Grade 0 | 62 (98.4) | 32 (97) | 30 (100) | |
| Grade 1 | 1 (1.6) | 1 (3) | 0 (0) | |
| Immediately after cleansing | .20 | |||
| Grade 0 | 63 (100) | 33 (100) | 29 (96.7) | |
| Grade 1 | 0 (0) | 0 (0) | 1 (3.3) | |
| 6 hours after cleansing | .04 | |||
| Grade 0 | 63 (100) | 33 (100) | 28 (93.3) | |
| Grade 1 | 0 (0) | 0 (0) | 2 (6.7) | |
note. IQR, interquartile range.
Unless otherwise specified.
For BMI, data were missing for 2 participants for 2% CHG intervention group and 1 participant for each 4% CHG intervention group.
Other occupations included clerk, occupational therapist, and pharmacist.
Other dermatologic conditions includes one patient with remote idiopathic urticaria.
Level corresponds to degree of pleasantness and ranges from 1 to 5, with 1 indicating very unpleasant and 5 indicating very pleasant.
Subjects who wore a watch or bracelet at the wrist or distal forearm prior to cleansing were allowed to keep the jewelry on throughout the 6 hours.
Grade of erythema corresponds to severity and ranges from 0 to 4 with a grade of 0 indicating no erythema, grade 1 indicating very slight erythema (barely perceptible), grade 2 indicating well-defined erythema, grade 3 indicating moderate to severe erythema, and grade 4 indicating severe erythema (beet redness).
Chlorhexidine Gluconate Concentration and Bacterial Density on Skin
Chlorhexidine gluconate was not detected on any participant’s forearm immediately prior to cleansing (Figure 1). Cleansing with no-rinse 2% CHG yielded the highest residual CHG skin concentrations immediately and 6 hours after cleansing; this difference remained statistically significant when compared to each of the two 4% CHG cleansing methods independently (P< .001 for each comparison) or combined (P <.001). Cleansing with 4% CHG liquid and non–antiseptic-impregnated cloths resulted in higher residual CHG concentrations than cleansing with 4% CHG with cotton washcloth immediately and 6 hours after cleansing (P <.001 and P =.002, respectively).
FIGURE 1.
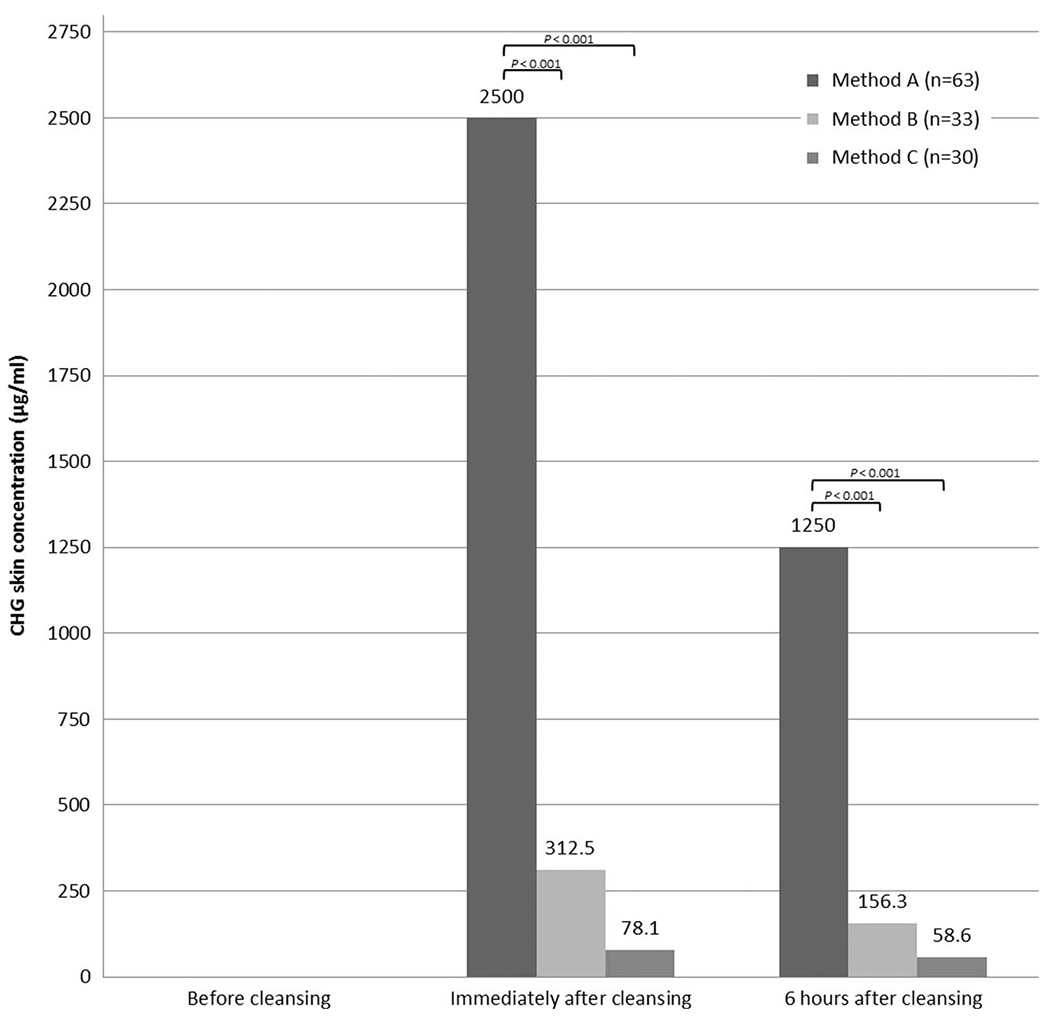
Chlorhexidine gluconate (CHG) concentrations measured on the skin of participants randomized to 3 different CHG cleansing methods. NOTE. Median values are reported. Data are also significant at P< .001 between all 3 groups immediately and 6 hours after cleansing. Method A: 2% CHG-impregnated cloth; method B: 4% CHG liquid with non–antiseptic-impregnated cloth; method C: 4% CHG liquid with cotton washcloth. No participants had CHG detected on skin at the baseline measurement.
Bacterial density on forearms varied by participant prior to cleansing, but the differences were not statistically significant (P= .29) (Table 2). Immediately and 6 hours after cleansing, bacterial densities decreased after all 3 cleansing methods. The no-rinse 2% CHG cloth cleansing yielded the lowest bacterial densities at both time points when compared to both 4% CHG cleansing methods combined (P< .001). Differences in bacterial densities between 4% CHG cleansing methods were not statistically significant at either time point after cleansing. Residual bacteria isolated after cleansing were commensal skin microbiota such as coagulase-negative staphylococci, micrococcus, and Bacillus species.
TABLE 2.
Bacterial Density Measured on the Skin of Participants Randomized to 3 Different Chlorhexidine Gluconate (CHG) Cleansing Methods
| Bacterial Density (CFU/25 m2)a |
|||
|---|---|---|---|
| CHG Cleansing Method | Before Cleansing | Immediately After Cleansing | 6 Hours After Cleansing |
| Method A: 2% CHG-impregnated cloth (n = 63) | 465 | 0 | 0 |
| Method B: 4% CHG liquid with non–antiseptic-impregnated cloth (n = 33) | 442.5 | 2.5 | 15 |
| Method C: 4% CHG liquid with cotton washcloth (n = 30) | 685 | 5 | 16.3 |
| P valueb | .29 | <.001 | <.001 |
note. CFU, colony-forming units.
Median values are reported.
P values shown test the null hypothesis that all 3 groups are equal immediately and 6 hours after cleansing. Data also significant at P=.002 for method A vs method B immediately after cleansing, and P< .001 for method A vs method B 6 hours after cleansing. P<.001 for method A vs method C immediately after and 6 hours after cleansing.
DISCUSSION
We found that cleansing the skin of healthy volunteers with no-rinse 2% CHG-impregnated cloths yielded the highest residual CHG concentrations and lowest bacterial densities compared to cleansing with 4% CHG liquid applied with non–antiseptic-impregnated cellulose/polyester cloths or with cotton washcloths dampened with sterile water, followed by rinsing. The strengths of our study design include the use of highly standardized cleansing methods and incorporation of blinded sample collection and outcome assessment. More importantly, we compared residual CHG concentrations and microbial densities on the skin directly between participants. Our findings thus extend those of 2 other separate investigations in which the application of 2% CHG-impregnated cloths yielded higher residual CHG concentrations13 or greater microbial reductions at skin sites19 in healthy volunteers compared to cleansing with 4% CHG liquid.
The CHG concentration needed to reduce microbial bioburden on skin appears to be dependent at least in part on microbial susceptibility to CHG.20 In a study of ICU patients, Popovich et al15 found that a CHG skin concentration ≥18.75 μg/mL was inversely associated with gram-positive bacterial colony counts, including Staphylococcus aureus and Enterococcus species. Lin et al21 calculated that the relative risk of skin contamination with Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae was decreased by approximately half in long-term acute-care hospital patients who had CHG skin concentrations ≥128 μg/mL. In both studies, the optimal concentration of CHG on skin exceeded the minimum inhibitory concentrations (MICs) of the targeted bacteria. Notably, some strains of bacteria have been reported to have CHG MICs as high as 2500 μg/mL.22 In our study, cleansing with no-rinse 2% CHG-impregnated cloth was the only method that achieved CHG concentrations as high as 2500 μg/mL.
Several factors may account for the observed differences in effects of 2% CHG-impregnated cloth and 4% CHG liquid skin cleansing methods. First, 2% CHG-impregnated cloth bathing is “no-rinse,” whereas manufacturers of 4% liquid CHG solutions instruct users to rinse off the solution after skin cleansing, an instruction that we followed.14,23 Few data have been published on the safety of bathing patients with undiluted CHG liquid without rinsing. In a study of patients admitted to a long-term acute-care hospital, daily bathing with 2% CHG liquid solution without rinsing was discontinued in 3 of 405 patients due to mild and reversible generalized erythema or pruritus.24 Swan et al25 published a study of 350 surgical ICU patients who were bathed every other day with CHG liquid without rinsing, alternating with soap and water. Their results showed the same incidence of adverse skin reactions compared to bathing patients daily with soap and water only. More recently, Alserehi et al26 demonstrated that bathing patients in a trauma ICU with diluted 4% CHG solution without rinsing resulted in near equivalent proportions of skin samples that had adequate CHG skin concentrations (defined as ≥18.75 μg/mL) compared to bathing with CHG-impregnated cloths. However, only 10 patients were studied, and adverse skin reactions related to the no-rinse solution were not evaluated.
The cloth that was used to apply 4% CHG solution to the skin may have also affected residual CHG concentrations and bacterial densities. In our study, the application of 4% CHG with cotton washcloths yielded consistently lower CHG concentrations than application with cellulose/polyester cloths that were impregnated with a nonantiseptic solution. Cotton fibers are known to bind CHG27 and may release less CHG to patients’ skin during bathing, in comparison to noncotton cloths. Finally, the differential effects of the 3 bathing methods may have been due to the different quantities of CHG that were applied to skin: 200 mg CHG per 5 mL 4% CHG liquid versus 500 mg CHG per 2% CHG-impregnated cloth. Our skin cleansing protocols were developed to simulate actual patient bathing practices at our institutions.7 Other studies of patient bathing with 4% CHG liquid have used various approaches, including different dwell times9,12 and CHG dilutions.8,25,28 The optimal method of 4% liquid CHG bathing remains to be determined.
Our study has limitations. First, we measured CHG concentrations and bacterial skin densities up to 6 hours from time of CHG cleansing (partly due to availability of healthcare professionals in an average shift), yet patients in most hospitals are bathed only once daily. We do not know whether the differences in CHG skin concentration and microbial densities observed for the different bathing methods persisted longer than 6 hours. In an earlier study, we found that reductions in microbial bioburden on the skin of ICU patients after cleansing with no-rinse 2% CHG-impregnated cloths persisted for up to 24 hours.15 Second, we elected to study healthy healthcare personnel because they comprise a more homogenous population than do hospital patients. Skin microbial communities of healthy volunteers are likely different from skin microbial communities of hospital patients; the latter group may be more prone to harboring MDROs with high CHG minimum inhibitory concentrations (MICs).21,29 Although residual CHG concentrations in our study were greater than CHG MICs reported for many MDROs,20 additional studies of hospital patients are needed to determine whether the relative differences in CHG concentration and bacterial densities that we observed are present over a longer time. Finally, the relation between the outcomes we studied—CHG concentration and microbial density on skin—and clinically relevant outcomes such as bacteremia is unknown.
In conclusion, our findings using a randomized study design with blinded assessment demonstrated that cleansing with no-rinse 2% CHG-impregnated cloths yielded significantly higher residual skin concentrations and lower bacterial density in healthy volunteers for at least 6 hours after application compared to cleansing with 4% CHG liquid using 2 alternative cloth delivery vehicles, followed by rinsing. Reasons for poorer performance of 4% CHG liquid bathing methods in our study are likely multifactorial, including rinsing after cleansing, cloth material, and lower absolute quantity of CHG applied. Clinical studies that compare standardized CHG formulations and bathing techniques are needed to evaluate the effect of different CHG bathing methods on patient outcomes.
ACKNOWLEDGMENTS
We would like to thank Ann Lough, Timothy Rog, Nicole Murphy, Jalpa Sarup Patel, Pamela Bell, the SICU staff, nurses, and the clinical microbiology laboratory at RUMC. We would also like to thank the BWH Surgical Critical Care Translational Research (STAR) Center, clinical microbiology laboratory (Dr Lynn Bry), Dr Michael Klompas of the BWH Department of Infectious Diseases, and the BWH surgical, burn, trauma, and thoracic ICUs.
Financial support:
This study was supported by the Centers for Disease Control and Prevention (Epicenter Grant Cooperative Agreements U54-CK00016 and U54 CK000481) and the Surgical Critical Care Translational Research (STAR) Center (G. Frendl) at the Brigham and Women’s Hospital, Boston, Massachusetts.
Potential conflicts of interest
M.K.H. has an investigator-initiated research grant from Clorox, which manufactures CHG products. Sage Products, LLC (now part of Stryker Corporation) provides CHG-containing products to institutions participating in research projects in which R.A.W., M.K.H., and M.Y.L. are investigators. Mölnlycke Health Care provides CHG-containing products to institutions participating in research projects in which R.A.W. and M.K.H. are investigators. OpGen Company provides laboratory testing at no cost for a research project in which M.Y.L., M.K.H., and R.A.W. are investigators. M.Y.L. receives an investigator-initiated grant from CareFusion Foundation (now part of Becton Dickinson).
Footnotes
PREVIOUS PRESENTATION. This study was presented in part at The Society for Healthcare Epidemiology of America Spring 2016 Conference, May 20, 2016, Atlanta, GA, USA, in a poster titled “Comparison of 2% Chlorhexidine Gluconate (CHG)— impregnated Cloth vs. 4% CHG Cleansing.”
REFERENCES
- 1.Davies GE, Francis J, Martin AR, Rose FL, Swain G. 1:6-Di-4’-chlorophenyldiguanidohexane (hibitane); laboratory investigation of a new antibacterial agent of high potency. Brit J Pharmacol Chemother 1954;9:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 2012;39:1042–1055. [DOI] [PubMed] [Google Scholar]
- 3.Edmiston CE Jr, Bruden B, Rucinski MC, Henen C, Graham MB, Lewis BL. Reducing the risk of surgical site infections: does chlorhexidine gluconate provide a risk reduction benefit? Am J Infect Control 2013;41(5 Suppl):S49–S55. [DOI] [PubMed] [Google Scholar]
- 4.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. New England J Med 2013;368:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol 2009;30:959–963. [DOI] [PubMed] [Google Scholar]
- 6.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–2079. [DOI] [PubMed] [Google Scholar]
- 7.Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–312. [DOI] [PubMed] [Google Scholar]
- 8.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–1865. [DOI] [PubMed] [Google Scholar]
- 9.Borer A, Gilad J, Porat N, et al. Impact of 4% chlorhexidine whole-body washing on multidrug-resistant Acinetobacter baumannii skin colonisation among patients in a medical intensive care unit. J Hosp Infect 2007;67:149–155. [DOI] [PubMed] [Google Scholar]
- 10.Petlin A, Schallom M, Prentice D, et al. Chlorhexidine gluconate bathing to reduce methicillin-resistant Staphylococcus aureus acquisition. Crit Care Nurse 2014;34:17–25; quiz 26. [DOI] [PubMed] [Google Scholar]
- 11.Ritz J, Pashnik B, Padula C, Simmons K. Effectiveness of 2 methods of chlorhexidine bathing. J Nurs Care Qual 2012;27:171–175. [DOI] [PubMed] [Google Scholar]
- 12.Edmiston CE Jr, Lee CJ, Krepel CJ, et al. Evidence for a standardized preadmission showering regimen to achieve maximal antiseptic skin surface concentrations of chlorhexidine gluconate, 4%, in surgical patients. JAMA Surg 2015;150:1027–1033. [DOI] [PubMed] [Google Scholar]
- 13.Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: Can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg 2008;207:233–239. [DOI] [PubMed] [Google Scholar]
- 14.Hibiclens Drug Facts. Mölnlycke website. http://www.molnlycke.us/antiseptics/general-skin-cleansing/hibiclens-information/hibiclens-drug-facts/.Accessed December 3, 2017.
- 15.Popovich KJ, Lyles R, Hayes R, et al. Relationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically ill patients bathed daily with chlorhexidine gluconate. Infect Control Hosp Epidemiol 2012;33:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampf G What is left to justify the use of chlorhexidine in hand hygiene? J Hosp Infect 2008;70(Suppl 1):27–34. [DOI] [PubMed] [Google Scholar]
- 17.Reichel M, Heisig P, Kampf G. Pitfalls in efficacy testing—How important is the validation of neutralization of chlorhexidine digluconate? Ann Clin Microbiol Antimicrob 2008;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 19.Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate-impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control 2007;35:89–96. [DOI] [PubMed] [Google Scholar]
- 20.Kampf G Acquired resistance to chlorhexidine—is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect 2016;94:213–227. [DOI] [PubMed] [Google Scholar]
- 21.Lin MY, Lolans K, Blom DW, et al. The effectiveness of routine daily chlorhexidine gluconate bathing in reducing Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae skin burden among long-term acute care hospital patients. Infect Control Hosp Epidemiol 2014;35:440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenzuela AS, Benomar N, Abriouel H, Canamero MM, Lopez RL, Galvez A. Biocide and copper tolerance in enterococci from different sources. J Food Protect 2013;76:1806–1809. [DOI] [PubMed] [Google Scholar]
- 23.Gluconate Chlorhexidine. Lexi-Comp Lexi-Drugs Online database website, http://online.lexi.com. Published 2007. Updated June1, 2017. Accessed July 9, 2017. [Subscription required to view.].
- 24.Munoz-Price LS, Hota B, Stemer A, Weinstein RA. Prevention of bloodstream infections by use of daily chlorhexidine baths for patients at a long-term acute care hospital. Infect Control Hosp Epidemiol 2009;30:1031–1035. [DOI] [PubMed] [Google Scholar]
- 25.Swan JT, Ashton CM, Bui LN, et al. Effect of chlorhexidine bathing every other day on prevention of hospital-acquired infections in the surgical ICU: a single-center, randomized controlled trial. Critical Care Med 2016;44:1822–1832. [DOI] [PubMed] [Google Scholar]
- 26.Alserehi H, Filippell M, Emerick M, et al. Chlorhexidine gluconate bathing practices and skin concentrations in intensive care unit patients. Am J Infect Control 2017. pii S0196-6553 (17):31002–7; doi: 10.1016/j.ajic.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Denton GW. Chlorhexidine. In Block SS, ed. Disinfection, Sterilization, and Preservation. 5th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:321–336. [Google Scholar]
- 28.Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol 2012;33:1094–1100. [DOI] [PubMed] [Google Scholar]
- 29.Naparstek L, Carmeli Y, Chmelnitsky I, Banin E, Navon-Venezia S. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect 2012;81:15–19. [DOI] [PubMed] [Google Scholar]


