Abstract
Aim.
To define the indications for hyperthermic isolated hepatic perfusion (IHP) in patients with unresectable liver metastases (LM) from colorectal cancer (CRC) with particular focus on IHP’s utility as a second-line option for patients whose tumors have progressed following combination systemic chemotherapy treatment.
Methods.
From June 1994 through July 2005, 120 patients with unresectable CRC LM underwent IHP with melphalan (n = 69), tumor necrosis factor (TNF) (n = 10) or both (n = 41). Hepatic arterial infusion (HAI) with floxuridine started 6–8 weeks post IHP in 46 (38%). Patients were followed for toxicity, radiographic response, and overall survival (OS). Wilcoxon rank-sum and Fisher’s exact tests were used to compare parameters by response category; survival and hepatic progression-free survival were calculated by the Kaplan–Meier method.
Results.
Of 79 males and 41 females, 96 (80%) received prior chemotherapy. There were five (4%) operative/treatment mortalities. There were 69 responses in 114 evaluable patients (61%). Total melphalan dose and combination melphalan/TNF were each associated with response; age, preoperative carcinoembryonic antigen (CEA), prior chemotherapy for established LM, tumor burden, and post-IHP HAI therapy were not. Median overall survival was 17.4 months and 2-year survival was 34%. Factors found to be independently related to survival were preoperative CEA <30 ng/mL and use of post-IHP HAI (P < 0.015).
Conclusions.
IHP results in marked tumor regression and prolonged survival in patients with CRC LM. Continued development of IHP in this clinical setting is warranted.
The development of isolated diffuse metastases to the liver from colorectal cancer (CRC) is a significant clinical problem.1 Once diagnosed, the prognosis is poor; treatment using irinotecan- or oxaliplatin-based regimens with or without bevacizumab results in median survival of 15–20 months.2 Although the initial overall response rates to treatment are high, the response duration is typically partial in character and less than 1 year. Treatment with an alternate salvage chemotherapy treatment (SCT) regimen as a second-line therapy has limited clinical benefit and patient survival is usually less than 1 year.3–5
The liver has a unique vascular anatomy that provides an opportunity to dose-intensify delivery of therapeutic agents to the cancer-burdened organ while minimizing unnecessary systemic toxicity. Experience with isolation perfusion of the liver for patients with unresectable primary or metastatic cancers confined to the liver has been reported over the past decade by several institutions. We and others have shown radiographic response rates >50% for patients with various histologies such as ocular melanoma, neuroendocrine cancer, and colorectal cancer using melphalan with or without tumor necrosis factor (TNF).6 Over the past 7 years an increasing number of patients have undergone hyperthermic isolated hepatic perfusion (IHP) after previously receiving systemic combination chemotherapy given with therapeutic intent for established metastases from CRC to the liver. We previously reported an overall radiographic response rate of 60% after IHP in 25 patients with metastatic CRC who had been treated with systemic irinotecan.7 In this report we present an analysis of factors potentially associated with outcome in all patients with metastatic CRC isolated to the liver treated with IHP at our institution in an effort to define the indications for IHP in this patient population, with particular focus on the utility of IHP as a second-line option for patients whose tumors have progressed following chemotherapy treatment.
PATIENTS AND METHODS
Between June 1994 and July 1995, 120 patients with unresectable isolated CRC liver metastases (LM) who had been treated with a 60-min hyperthermic IHP with melphalan (1–2 mg/kg, n = 69), tumor necrosis factor (0.3–2 mg, n = 10) or both (n = 41) were identified from a prospectively maintained database. All treatments were conducted in compliance with one of several related clinical research protocols approved by the Institutional Review Board and, when indicated, the Cancer Therapeutics Evaluation Program of the National Cancer Institute (NCI). All patients provided signed informed consent to participate in their clinical trial. All patients had measurable metastatic colorectal cancer confined to the liver based on standard staging studies including computed tomography scanning of the chest, abdomen, and pelvis and when indicated brain magnetic resonance imaging or bone scan. The patient’s disease in the liver was defined as unresectable based on the presence of multifocal disease in the liver or tumor abutting major vascular structures so that an adequate functional liver remnant after resection was not possible.
Eligibility criteria included Eastern Cooperative Oncology Group performance status 0 or 1, serum bilirubin ≤2.0 mg/dL, platelet count ≥150,000 per μl, and serum creatinine ≤1.5 mg/dL. Minor abnormalities in either prothrombin or partial thromboplastin times were allowed when patients otherwise appeared to have adequate hepatic synthetic reserve based on complete evaluation using radiographic and laboratory parameters. All patients had progressive disease as evidenced by increase in size or number of metastases in the liver or in increasing carcinoembryonic antigen level.
Based on previously conducted phase I trials, the maximum safe tolerated doses of recombinant human tumor necrosis factor (Knoll Pharmaceuticals, Whippany, NJ) and melphalan (GlaxoSmithKline, previously BurroughsWellcome, NC) were 1 mg and 1.5 mg/kg, respectively. In this cohort of 120 patients, the melphalan dose range was 1–2 mg/kg and the tumor necrosis factor dose range was 0.3–2 mg.
IHP
A 60-min IHP was performed via laparotomy as previously described.8,9 Briefly, via laparotomy the falciform and right and left triangular ligaments are divided, the duodenum is mobilized and reflected medially, the right lobe of the liver is reflected anteriorly and medially, and the inferior vena cava from the level of the renal veins to the diaphragm is dissected from the retroperitoneum, including ligation and division of the right adrenal vein and small direct retroperitoneal venous tributaries. The porta hepatis structures are dissected extensively to prevent perfusate leak from the liver into the systemic circulation. A 3-mm arterial inflow cannula is positioned in the gastroduodenal artery and a venous outflow cannula is positioned in the retrohepatic vena cava that is isolated above the renal veins and below the diaphragm with two vascular occluding clamps. The portal vein and common hepatic artery are occluded with vascular occluding clamps and in some cases an additional vascular occluding clamp is placed across the common bile duct. A prophylactic cholecystectomy is performed; all lymph-node-bearing tissue around the porta hepatis is resected.
A saphenous vein and left axillary vein cut-down are performed and after systemic anticoagulation with 200 units/kg heparin, a cannula is inserted into the saphenous vein and advanced into the vena cava below the renal veins. A second cannula is positioned in the axillary vein and these are connected to a veno-venous bypass circuit to actively assist the shunting of infrahepatic inferior vena cava blood flow to the systemic circulation using a centrifugal pump during treatment (Biomedicus, Medtronix, Eden Praire, NM). Early on in our experience portal venous flow was also incorporated into this shunt circuit; however, this practice has been abandoned in favor of simply occluding the portal vein.
The extracorporeal bypass circuit consists of a roller pump oxygenator, heat exchanger, and reservoir. The perfusate consists of 700 ml balanced salt solution primed for 300 ml packed erythrocytes. Arterial and venous perfusate blood gas analyses are performed at regular intervals during the perfusion, and sodium bicarbonate is added to the circuit to maintain an arterial perfusate pH between 7.2 and 7.3. Perfusate temperatures are controlled using a Hematherm cooler–heater, model 400 (Cincinnati Sub-Zero Products, Cincinnati, OH). Flow rates are adjusted upward while monitoring for stable reservoir volume, acceptable line pressures, and any evidence of perfusate leak into the systemic circulation based upon an I-131-labeled human serum albumin leak monitoring system as previously described.10 However, because of our initial experience with no perfusate leak in 50 patients using this system it is no longer routinely employed.
Stable perfusion parameters and rapid and uniform heating of the liver to target temperatures of 39.5–40°C are routinely observed. After addition of the therapeutic agents to the perfusate, treatment continues for 60 min. At the completion of the isolated hepatic perfusion the liver is flushed with 1,500 ml crystalloid and 1,500 ml colloid. Decannulation of the arterial and venous structures is performed as previously described and physiologic blood flow to the liver and through the inferior vena cava is restored without difficulty.
Forty-six patients had a catheter placed into the gastroduodenal artery for HAI therapy that was connected to a subcutaneous pump. Four to 6 weeks after IHP, patients began floxuridine (FUDR, 0.18 mg/kg/day) and leucovorin (LV) (15 mg/m2/day) given by continuous infusion over 14 days monthly (2 weeks on therapy, followed by 2 weeks off). Patients had their dose of FUDR reduced or held on the basis of toxicity from the prior dose. Treatment continued for 12 months or until there was progression of disease, toxicity, or technical problems.
Response and Follow-Up
All patients assessable for response underwent evaluation at 3, 6, 9, and 12 months after IHP and then every 4–6 months thereafter until disease progression. Responses were defined according to World Health Organization response criteria.
Statistical Methods
The primary objectives of the analysis were to determine in-liver progression-free survival probabilities (PFS) and overall survival (OS) probabilities, and to identify factors associated with these outcomes as well as with clinical response. PFS was calculated from date of treatment until date of progression within the liver or date off study as appropriate; survival was calculated from date of IHP until date of death or last follow-up. The probability of OS or PFS was calculated using Kaplan–Meier method, and the significance of the difference between actuarial survival curves was determined by Mantel–Haenszel procedure. A large number of potential prognostic factors were individually evaluated in univariate analyses with exploratory intent. Given the large number of parameters being evaluated, the resulting P-values from the log-rank test for each parameter were used primarily to screen parameters for subsequent evaluation in Cox proportional hazard models which were developed to identify a set of factors which would be jointly associated with each outcome.
As part of the initial exploration, the continuously distributed parameters were generally initially divided into four approximately equally sized quartiles in order to identify if a potential difference in survival or progression-free survival could be identified in one of the set of values versus another. For those parameters in which such a potentially useful prognostic classification could be identified, the data were regrouped and reevaluated for prognostic significance. All resulting P-values from the univariate analyses were adjusted to account for these preliminary evaluations.
For the evaluation of parameters and their association with response, continuously measured parameters were compared between response categories using the Wilcoxon rank-sum test; dichotomous parameters were compared between response categories using Fisher’s exact test or Mehta’s version of the Fisher’s exact test. All P-values are two-tailed, and except as stated above, are presented without any adjustment for multiple comparisons.
RESULTS
Patient demographics and treatment history are shown in Table 1. There was roughly a 2:1 male-to-female preponderance and a moderate to heavy burden of tumor in the liver as reflected by the median number of metastatic lesions and the percentage of liver replaced by tumor. Of note, 74 patients received systemic or regional chemotherapy with therapeutic intent for established CRC LM. The operative and treatment parameters are shown in Table 2. The maximum safe tolerated dose of melphalan defined in early phase I trials was 1.5 mg/kg. The maximum safe tolerated dose of TNF when used in combination with melphalan was 1 mg; those patients receiving lower or higher doses were treated on a TNF dose escalation trial. The bypass flow rate of almost 1.9 L/min reflects a blood flow through the shunt circuit when it incorporated both the portal venous and inferior vena cava (IVC) blood flow. Currently, portal venous blood flow is simply occluded following systemic anticoagulation which has resulted in bypass flow rates of approximately one-half of those reported in Table 2. There was prompt and uniform heating of the liver to target temperatures of between 39.5°C and 40.5°C. Operative time, length of intensive care unit (ICU) stay, and length of hospital stay reflect the major nature of the operative procedure.
TABLE 1.
Pretreatment characteristics for 120 patients with diffuse CRC liver metastases undergoing IHP
| Demographics | |
| Number of patients treated with IHP | 120 |
| Age (years, range) | 52 (22–74) |
| Female: male | 41:79 |
| Tumor characteristics | |
| No. of metastatic lesions (median/range) | 8 (1–50) |
| Percentage liver replaced by tumor (median/range) | 20% (5–75%) |
| Preoperative CEA level a | |
| Normal | 14 |
| High stable | 15 |
| High increasing | 81 |
| Unknown | 10 |
| Site of primary tumor | |
| Left colon | 17 |
| Right colon | 6 |
| Colon | 28 |
| Rectal | 25 |
| Sigmoid | 39 |
| Transverse | 5 |
| Liver metastasis | |
| Synchronous metastasis | 79 |
| Metachronous | 41 |
| Prior chemotherapy | |
| None | 26 |
| Yes | 94 |
| Chemotherapy for established liver tumors (5FU/LV and irinotecan), HAI | 74 |
| Chemotherapy as preventative treatment to dev. liver mets (5FU/LV—at least 4 cycles) | 20 |
| No chemotherapy of any kind | 26 |
5FU = 5-fluorouracil, LV = leucovorin
Upper limit of normal for CEA is 3.5 ng/mL
TABLE 2.
Treatment parameters for 120 patients with diffuse CRC liver metastases undergoing IHP
| Perfusion and operative data | Median (range) |
|---|---|
|
| |
| Total melphalan (mg) dosed at 1.5 mg/kg | 105 (69–160) |
| TNF dose (mg) | Mean/median 1 (0.3–2.0) |
| Perfusion flow rate (ml/min) | 800 (550–1350) |
| Perfusion pressure (mmHg) | 145 (70–255) |
| Bypass flow rate (ml/min) | 1888 (1300–2520) |
| Central liver temperature (°C) | 40 (39–40.8) |
| Estimated blood loss (L) | 2.0 (0.5–5.0) |
| Operative time (h) | 8.5 (6–12.4) |
| Hospital stay (days) | 11 (2–78) |
| ICU stay (days) | 4 (2–71) |
Toxicities, morbidity, and mortality are shown in Table 3; there were five deaths, representing an overall mortality of 4%; three of the five deaths occurred in patients treated on phase I dose-seeking clinical trials and experienced dose-limiting toxicity. Severe hypotension was experienced in seven patients in the first 12–24 h after IHP; this was most commonly observed in patients who received TNF and is believed to be secondary to TNF-induced hepatic production of interleukin (IL)-6 and IL-8 that results in peak serum levels of the cytokines 4–6 h postoperatively.11 All other toxicities listed were reversible grade 3 or 4 (NCI common toxicity criteria, version 3.0). As previously reported in patients undergoing IHP for CRC, most individuals had transient elevations in hepatic transaminases and total serum bilirubin that peaked on postoperative day 3 or 4 and returned towards normal by postoperative day 7.12
TABLE 3.
Morbidity and toxicity associated with IHP
| Postoperative morbidity/toxicity | |
| Morbidity | |
| Bleeding (grade 4) | 4 |
| Pleural effusion | 8 |
| Atrial fibrillation/arrhythmia | 6 |
| Weight gain (grade 3) | 16 |
| Ascites | 3 |
| Wound infection | 3 |
| Infected port | 5 |
| Hypotension (grade 3/4) | 7 |
| Fever (grade 3) | 1 |
| Toxicity | Grade 3/4 |
| Bilirubin | 56 |
| Transaminases | 67 |
| Alkaline phosphatase | 5 |
| Creatinine | 2 |
| Neutropenia | 1 |
| Platelets | 12 |
| PT/PTT | 6 |
| Postoperative mortality | |
| Coagulopathy/bleeding/death | 1 |
| Hepatic failure/death | 4 |
PT/PTT = prothrombin time and partial thromboplastin time
There were 69 responses in 114 evaluable patients for an overall radiographic response rate of 61%. Median time to in-liver PFS was 7 months (Table 4). Of note, 46 patients received postoperative HAI of FUDR, leucovorin, and dexamethasone via a subcutaneously placed self-powered pump starting approximately 8 weeks after IHP. When compared with the 58 patients who received IHP without postoperative hepatic arterial infusion chemotherapy, the overall response rates were similar but the duration of response was markedly prolonged with combination treatment (Table 4). Interestingly, there was no clinically meaningful antitumor activity in a small cohort of patients treated with TNF alone. A number of continuous and categorical parameters were evaluated for their association with response (Tables 5 and 6). Age, preoperative CEA, tumor burden, and dose of TNF were not associated with response; however, patients who had a response (complete or partial) tended to have higher total doses of melphalan compared with those who had no response (minor response or stable disease). Gender, synchronous versus metachronous, and history of prior chemotherapy administered for therapeutic intent were not associated with response; in other words, patients who underwent IHP as a second-line therapy after disease progression following systemic combination chemotherapy regimens or prior hepatic arterial infusion chemotherapy had similar response rates compared with those who received IHP as first-line therapy. Also noteworthy is that HAI with FUDR did not alter overall response rates, although it was associated with a longer duration of response. Finally those patients who had TNF were more likely to have a partial or complete response compared with those treated with melphalan alone, although this association was only marginally significant.
TABLE 4.
Results with IHP for patients with CRC liver metastases treated with IHP
| Treatment regimen | No. of patients evaluable for response | CR | PR | Median hepatic PFS (months) |
|---|---|---|---|---|
|
| ||||
| Overall | 114 | 2 | 67 | 7.0 |
| 59% | ||||
| IHP–no HAI | 58 | 0 | 33 | 5.8 |
| 57% | ||||
| IHP–HAI | 46 | 2 | 30 | 13.0a |
| 65% | ||||
| IHP (TNF alone) | 10 | 0 | 4 | 3.0 |
P < 0.001 versus IHP–no HAI and IHP (TNF alone)
TABLE 5.
Continuous parameters and their association with response in 114 evaluable patients with diffuse CRC liver metastases treated with IHP
| Variable | Response category | Mean | n | SEM | Min. | Med. | Max. | Wilcoxon P-value |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age at treatment | CR/PR | 50.8 | 69 | 1.4 | 22 | 51 | 74 | 0.45 |
| NR | 52.6 | 45 | 1.3 | 34 | 54 | 69 | ||
| Preop. CEA | CR/PR | 952.8 | 63 | 349.3 | 0.7 | 101.3 | 17000 | 0.79 |
| NR | 500.5 | 39 | 152.7 | 1.2 | 155.8 | 5140 | ||
| Number of metastatic lesions | CR/PR | 11.1 | 58 | 1.3 | 1 | 8 | 50 | 0.55 |
| NR | 9.2 | 26 | 1.6 | 2 | 7 | 38 | ||
| Percentage liver replaced by tumor | CR/PR | 28.4 | 58 | 2.7 | 5 | 20 | 75 | 0.18 |
| NR | 32.2 | 25 | 3.6 | 5 | 30 | 75 | ||
| TNF dose | CR/PR | 0.5 | 69 | 0.1 | 0 | 0 | 1.5 | 0.22 |
| NR | 0.4 | 45 | 0.1 | 0 | 0 | 2.0 | ||
| Melphalan dose | CR/PR | 103.3 | 69 | 3.9 | 0 | 105 | 160 | 0.034 |
| NR | 88.7 | 45 | 5.8 | 0 | 95 | 160 | ||
CR = complete response, PR = partial response, NR = no response
TABLE 6.
Categorical parameters and their association with response in 114 evaluable patients with diffuse CRC liver metastases treated with IHP
| Response |
P-value (Fisher’s) | ||
|---|---|---|---|
| CR/PR | NR | ||
|
| |||
| Synchronous | 47 | 27 | |
| Metachronous | 22 | 18 | 0.42 |
| Male | 49 | 25 | |
| Female | 20 | 20 | 0.11 |
| No prior chemotherapy | 18 | 7 | |
| Preventative | 13 | 6 | 0.26a |
| Tx for established tumor | 38 | 32 | |
| Any HAI treatment | |||
| Yes | 32 | 14 | 0.12 |
| No | 37 | 31 | |
| TNF alone | |||
| Yes | 4 | 6 | 0.19 |
| No | 65 | 39 | |
| Melphalan alone, no HAI | |||
| Yes | 7 | 15 | |
| No | 62 | 30 | 0.0032 |
| Melphalan alone (±HAI) | |||
| Yes | 36 | 29 | 0.25 |
| No | 33 | 16 | |
| Melphalan + TNF (±HAI) | |||
| Yes | 29 | 10 | 0.043 |
| No | 40 | 35 | |
By Mehta’s version of Fisher’s exact test
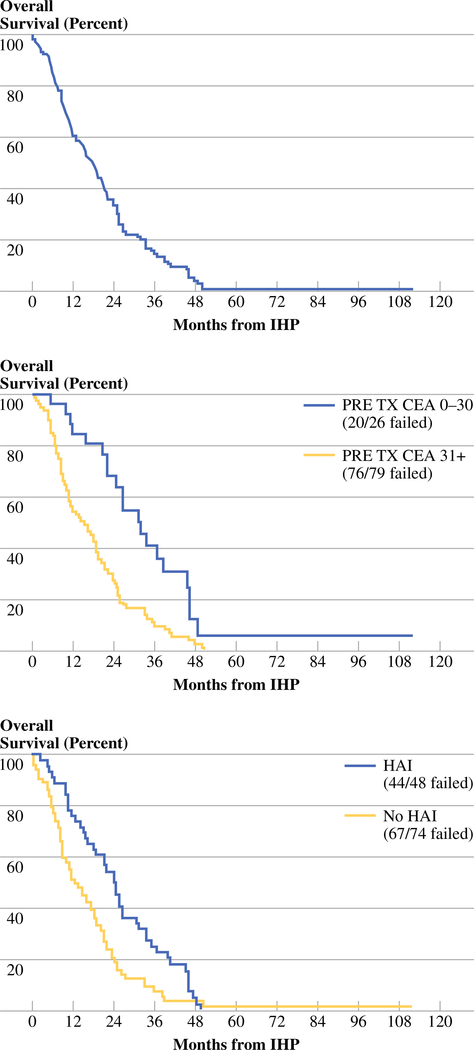
The actuarial OS in all 120 treated patients is shown in Fig. 1; the median OS was 17.4 months. Factors found to be potentially associated with longer hepatic PFS in univariate analyses included use of HAI (P < 0.0001), lack of use of TNF (P = 0.0015), metachronous metastases (P = 0.024), pretreatment CEA <30 ng/mL (adjusted P = 0.0084), 5–20% hepatic replacement (adjusted P = 0.0021), and melphalan dose 91 mg + (adjusted P = 0.066). With respect to OS, use of any melphalan (P = 0.032), HAI (P = 0.0035), pretreatment CEA <30 ng/ml (adjusted P = 0.0012), and 5–10% hepatic replacement (adjusted P = 0.054) were identified as being of importance in univariate analyses. When these factors were evaluated for their joint significance by a Cox model with backward elimination, the use of HAI following IHP and preoperative CEA value of ≤30 ng/mL were independently and significantly associated with both improved hepatic PFS and OS (Table 7, Fig. 1). A typical response to IHP in a male with bulky CRC LM is shown in Fig. 2.
FIG. 1.
Actuarial OS in all 120 patients with diffuse CRC LM who underwent hyperthermic IHP (top panel) and in patients based on baseline CEA level (middle panel) or with or without HAI therapy (bottom panel) following IHP
TABLE 7.
Cox proportional hazards models (following backward elimination) showing the relationship between OS and liver PFS in 120 patients with diffuse CRC LM treated with IHP and in 105 patients for whom preoperative CEA was known
| Parameter estimate | P-value | Hazard ratio (HR) | 95% CI for HR | |
|---|---|---|---|---|
|
| ||||
| Survival (n = 120) | ||||
| HAI | 0.58 | 0.0039 | 1.78 | 1.20, 2.64 |
| Survival (n = 105) | ||||
| HAI | 0.54 | 0.013 | 1.72 | 1.12, 2.63 |
| Preoperative CEA | 0.83 | 0.0012 | 2.29 | 1.39, 3.78 |
| Liver PFS (n = 120) | ||||
| HAI | 1.02 | <0.0001 | 2.79 | 1.87, 4.16 |
| Liver PFS (n = 105) | ||||
| HAI | 1.17 | <0.0001 | 3.22 | 2.06, 5.03 |
| Preoperative CEA | 0.85 | 0.0006 | 2.35 | 1.44, 3.82 |
CI confidence interval
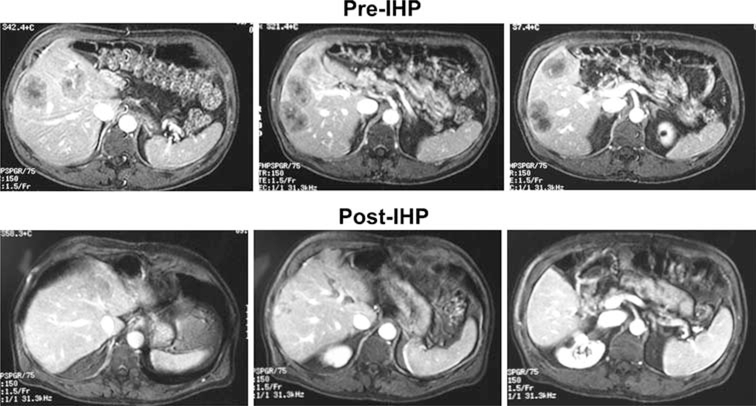
FIG. 2.
Images of a representative response to IHP in a male with CRC liver metastases. Panels show pretreatment T1-weighted gadolinium-enhanced MRI images (top row) and corresponding images taken 8 months after IHP
DISCUSSION
The treatment approach for patients with diffuse unresectable liver metastases from colorectal cancer is changing rapidly.13 Because of the improved efficacy with new combination chemotherapy regimens using irinotecan or oxaliplatin with or without bevacizumab, systemic chemotherapy as a first-line approach is commonly used.14,15 Up to 30% of patients may have sufficient tumor regression that their metastases are rendered resectable.16,17 However, despite overall response rates greater than 50%, responses are usually partial in character and their duration is usually less than 1 year.2,14 Even in patients whose lesions are rendered undetectable on contrast-enhanced computed tomography (CT) scan after treatment, active disease persists in over 80% of all sites of previously established metastases.18 Unfortunately, treatment with second-line systemic chemotherapy regimens after disease recrudescence has limited clinical benefit.4,5,19
Regional therapy to the liver as a second-line treatment approach for patients with CRC liver metastases has several potential advantages. Previous work has demonstrated that metastases in the liver derive blood supply more from arterial than from portal blood flow and that intra-arterial administration will selectively deliver chemotherapeutics primarily to the tumors.20,21 Regional therapy eliminates unnecessary systemic toxicity and when used as second-line therapy can be used selectively for those who may benefit most, that is, those with disease that has remained confined to liver by serial imaging over time. IHP with hyperthermia and melphalan (with or without TNF) has been shown to have the ability to cause regression of advanced cancers in the liver.6,22 The data reported in this study are derived from a large cohort of patients treated on sequentially performed prospective clinical trials evaluating the utility of IHP in patients with diffuse isolated CRC LM. The data demonstrate several important observations regarding the use of IHP specifically in patients with CRC liver metastases. The likelihood of response after IHP was not adversely influenced by burden of disease in the liver as reflected by level of CEA, number of metastatic tumors, or percentage of liver replaced by tumor. Response rates after IHP were not different in patients who had or had not been previously treated with chemotherapy for established LM, suggesting that IHP may have utility as a second-line treatment option for those with diffuse CRC LM. Interestingly, the data show an association between total melphalan dose and response. Our data with IHP confirm what has been shown by Posner et al. in isolated limb perfusion: that the use of TNF as a single agent in isolation perfusion is associated with no meaningful antitumor activity.23 There are accumulating data that TNF acts principally on tumor neovasculature by increasing permeability and thereby delivery of chemotherapeutics to the tumor interstitium, which is followed by intravascular coagulation resulting in tumor ischemia.24,25 Although these effects may be important in some clinical settings, our data confirm that these effects are not sufficient for clinically meaningful tumor regression. The administration of FUDR-based HAI chemotherapy after IHP was associated with a markedly prolonged duration of response and survival compared with IHP alone. This observation suggests that post-IHP systemic combination chemotherapy using currently available agents may provide a similar benefit and would have the advantage of also treating systemic micrometastases.
The morbidity and toxicities associated with IHP are not inconsequential. The operative or treatment-related mortality of 4% represent five patients, of whom three were treated on phase I dose-seeking clinical trails and experienced dose-limiting toxicity. The maximum safe tolerated dose of melphalan is 1.5 mg/kg body weight; higher doses are associated with severe veno-occlusive disease. The maximum safe tolerated dose of TNF is 1 mg, with higher doses resulting in severe coagulopathy. Most patients do experience transient grade 3 or 4 hepatic toxicity that requires no specific intervention.
The median survival for patients with metastatic CRC who have disease progression after first-line systemic chemotherapy is 1 year or less.3,26,27 The median overall survival in our patient cohort was 17.4 months and was not adversely affected by a history of previous treatment with systemic chemotherapy, suggesting that IHP may be an effective option in appropriately selected patients with diffuse CRC LM.
ACKNOWLEDGMENT
This work was supported by the Center for Cancer Research, NCI, Bethesda, and the University of Maryland Marlene and Stewart Greenebaum Cancer Center, Baltimore, Maryland.
REFERENCES
- 1.Stangl R, tendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–10. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347–53. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, et al. Superiority of oxaliplatin and fluourouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–69. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg ML. Current status of second-line therapy for metastatic colorectal cancer. Clin Colorectal Cancer. 2004;4(1):S16–21. [PubMed] [Google Scholar]
- 6.Grover A, Alexander HR Jr. The past decade of experience with isolated hepatic perfusion. Oncologist. 2004;9:653–64. [DOI] [PubMed] [Google Scholar]
- 7.Alexander HR Jr, Libutti SK, Pingpank JF, Bartlett DL, Helsabeck C, Beresneva T. Isolated hepatic perfusion for the treatment of patients with colorectal cancer liver metastases after irinotecan-based therapy. Ann Surg Oncol. 2005;12:138–44. [DOI] [PubMed] [Google Scholar]
- 8.Libutti SK, Bartlett DL, Fraker DL, Alexander HR. Technique and results of hyperthermic isolated hepatic perfusion with tumor necrosis factor and melphalan for the treatment of unresectable hepatic malignancies. J Am Coll Surg. 2000;191:519–30. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;129:176–87. [DOI] [PubMed] [Google Scholar]
- 10.Barker WC, Andrich MP, Alexander HR, Fraker DL. Continuous intraoperative external monitoring of perfusate leak using I–131 human serum albumin during isolated perfusion of the liver and limbs. Eur J Nucl Med. 1995;22:1242–8. [DOI] [PubMed] [Google Scholar]
- 11.Lans TE, Bartlett DL, Libutti SK, Gnant MFX, Liewehr DJ, Venzon DJ, et al. Role of tumor necrosis factor (TNF) on toxicity and cytokine production following isolated hepatic perfusion (IHP). Clin Cancer Res. 2001;7:784–90. [PubMed] [Google Scholar]
- 12.Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;129:176–87. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM, Rothenberg ML, van CE, Benson AB, Ill, Blanke CD, Diasio RB, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist. 2007;12:38–50. [DOI] [PubMed] [Google Scholar]
- 14.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- 15.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–14. [DOI] [PubMed] [Google Scholar]
- 16.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhii SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9. [DOI] [PubMed] [Google Scholar]
- 17.Masi G, Cupini S, Marcucci L, Cerri E, Loupakis F, Allegrini G, et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol. 2006;13:58–65. [DOI] [PubMed] [Google Scholar]
- 18.Benoist S, Brouquet A, Penna C, Julie C, El HM, Chagnon S, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–45. [DOI] [PubMed] [Google Scholar]
- 19.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. [DOI] [PubMed] [Google Scholar]
- 20.Breedis C, Young G. Blood supply of neoplasms of the liver. Am J Pathol. 1954;30:969–85. [PMC free article] [PubMed] [Google Scholar]
- 21.Ridge JA, Bading JR, Gelbard AS, Benua RS, Daly M. Perfusion of colorectal hepatic metastases. Relative distribution of flow from the hepatic artery and portal vein. Cancer. 1987;59:1547–53. [DOI] [PubMed] [Google Scholar]
- 22.Alexander HR, Bartlett DL, Libutti SK. Current status of isolated hepatic perfusion with or without tumor necrosis factor for the treatment of unresectable cancers confined to liver. Oncologist. 2000;5:416–24. [DOI] [PubMed] [Google Scholar]
- 23.Posner MC, Lienard D, Lejeune FJ, Rosenfelder D, Kirkwood J. Hyperthermic isolated limb perfusion with tumor necrosis factor alone for melanoma. Cancer J Sci Am. 1995;1:274–80. [PubMed] [Google Scholar]
- 24.Friedl J, Puhlmann M, Bartlett DL, Libutti SK, Turner EN, Gnant MF, et al. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood. 2002;100:1334–9. [PubMed] [Google Scholar]
- 25.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haller DG, Rothenberg ML, Wong AO, Koralewski PM, Miller WH Jr, Bodoky G, et al. Oxaliplatin plus irinotecan compared with irinotecan alone as second-line treatment after single-agent fluoropyrimidine therapy for metastatic colorectal carcinoma. J Clin Oncol. 2008;26:4544–50. [DOI] [PubMed] [Google Scholar]
- 27.Kemeny N, Garay CA, Gurtler J, Hochster H, Kennedy P, Benson A, et al. Randomized multicenter phase II trial of bolus plus infusional fluorouracil/leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J Clin Oncol. 2004;22:4701–9. [DOI] [PubMed] [Google Scholar]