Abstract
BACKGROUND:
Human epidermal growth factor receptor 2 (HER2) is an important biomarker whose status plays a pivotal role in therapeutic decision-making for breast cancer patients and in determining their clinical outcomes. Ensuring the accuracy and reproducibility of HER2 assays by immunohistochemistry (IHC) and by fluorescence in situ hybridization (FISH) requires a reliable standard for monitoring assay sensitivity and specificity, and for assessing methodologic variation. A prior NIST workshop addressed this need by reaching a consensus to create cell lines as reference materials for HER2 testing.
METHODS:
Breast carcinoma cell lines SK-BR-3 and MCF-7 were characterized quantitatively by IHC with chicken anti-HER2 IgY antibody and by FISH with biotinylated bacterial artificial chromosome DNA probes; both assays used quantum dots as detectors. Formalin-fixed and paraffin-embedded (FFPE) cell blocks were prepared and tested for suitability as candidate reference materials by IHC and FISH with commercially available reagents. IHC and FISH results were also compared with those obtained by laser-scanning cytometry and real-time PCR, respectively.
RESULTS:
MCF-7 cells had typical numbers of gene copies and very low production of HER2 protein, whereas SK-BR-3 cells contained approximately 10-fold more copies of the gene and exhibited approximately 15-fold higher amounts of HER2 protein than MCF-7 cells. FFPE SK-BR-3 cells showed results similar to those for fresh SK-BR-3 cells.
CONCLUSIONS:
SK-BR-3 and MCF-7 are suitable as candidate reference materials in QC of HER2 testing. Coupled with the associated assay platforms, they provide valuable controls for quantitative measurement of HER2 amplification and production in breast cancer samples, irrespective of the antibody/probe or detector used.
The ERBB25 gene [v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian)], commonly referred to as HER-2, is located on chromosome 17q21 and encodes human epidermal growth factor receptor 2 (HER2),6 a 185-kDa transmembrane glycoprotein belonging to the tyrosine kinase receptor family, whose members play an important role in the regulation of such fundamental processes as cell growth, survival, and differentiation (1). The tumor cells in approximately 20%–30% of women with breast cancer show amplification of ERBB2 (2). Consequently, the tumor cells in some patients produce much higher levels of the HER2 receptor on their surfaces. HER2 overproduction plays a pivotal role as a prognostic marker, by itself or in combination with other markers. HER2 positivity, alone or in association with nodal status, is generally associated with a worse prognosis, i.e., a higher rate of disease recurrence and mortality, compared with patients with a negative HER2 status (3–5).
Perhaps more importantly, the HER2 status is decisive in the selection of patients for trastuzumab therapy. Trastuzumab (Herceptin; Genentech) is a humanized monoclonal antibody cleared in 1998 by the US Food and Drug Administration (FDA) for the treatment of metastatic disease; it targets the HER2 antigen and inhibits the growth of HER2-overproducing tumor cells (6). Clinical trials of patients positive for HER2 have shown that trastuzumab is effective and well tolerated (6); however, trastuzumab is potentially harmful for patients with no HER2 production, because a similar receptor in nonpathologic heart tissue may also bind the drug, causing cardiotoxicity and, in some cases, death (7). Therefore, to avoid unnecessary risk and expense, it is vital that a reliable and reproducible assay be available for precisely determining HER2 status and identifying the right patients who will benefit from trastuzumab treatment.
There are numerous methods available for assessing HER2 status, but immunohistochemistry (IHC) measurement of protein overproduction and fluorescence in situ hybridization (FISH) measurement of ERBB2 gene amplification are the most commonly used (8). Both types of tests have the advantage of allowing evaluation of protein overproduction or gene amplification in relation to tumor morphologic features, unlike molecular techniques that require homogenization of the tumor. Unfortunately, the 2 test types do not always identify the same patient subsets as candidates for trastuzumab therapy (8), even when they are performed in the same laboratory. Moreover, there is large interlaboratory variation in the analytical sensitivity and specificity of the assays and in the different methods of evaluation frequently used to interpret the results (9–11). The results of approximately 20% of HER2 assays performed in pathology departments at the sites of primary treatment were demonstrated to be incorrect when the samples were reevaluated in a high-volume central laboratory (9, 11 ).
Ensuring the accuracy and reproducibility of HER2 results obtained by IHC and FISH testing requires precisely characterized and universally available reference control cells with known numbers of gene copies in the nucleus and known numbers of receptors on the cell surfaces. To address this need, the NIST, the Cancer Diagnosis Program of the National Cancer Institute, the FDA, and the College of American Pathologists held an NIST-sponsored workshop in 2002 ( 12). A consensus was reached at the workshop to create cell lines as reference materials for HER2 testing to facilitate the assessment of methodologic variation ( 12).
This study represents the initial effort in the production of such reference materials. The aim of the present study was to use IHC and FISH to quantitatively characterize breast carcinoma cell lines SK-BR-3 and MCF-7 in terms of HER2 production and ERBB2 amplification, respectively, and to examine the suitability of these cell lines as controls for HER2 testing.
Materials and Methods
MATERIALS
Breast carcinoma cell lines SK-BR-3 and MCF-7 were obtained from the ATCC and cultured under the conditions recommended by the supplier. Chicken anti-HER2 IgY antibody (YXPB-IgY-hHER2/p) was prepared as described previously (13). Mouse anti-HER2 IgG monoclonal antibody (CB11) was purchased from Ventana Medical Systems. Secondary antibodies were biotinylated anti-IgY antibody (GenWay Biotech) and biotinylated anti-IgG antibody (Ventana). Each was detected by fluorescence microscopy with streptavidin–Qdot 655 (Invitrogen) as the final affinity-detection reagent. A biotinylated bacterial artificial chromosome (BAC) DNA probe for the ERBB2 gene was prepared according to a previously published procedure (14). The probe was also detected with streptavidin–Qdot 655 via fluorescence microscopy.
IHC STUDIES OF FIXED CULTURED SK-BR-3 AND MCF-7 CELLS
Cells were grown on tissue-culture chamber slides (Nunc) to a density of 30 000 cells/cm2 in medium recommended by the ATCC. Cell monolayers were fixed in 10% neutral-buffered zinc formalin (Fisher PROTOCOL* 10% Buffered Formalin; Fisher) but not embedded in paraffin. Fixed monolayers were preblocked at 20 °C for 20 min in a solution containing 50 g/L dry milk, 50 mmol/L Tris-HCl, 150 mmol/L NaCl, and 150 mmol/L Tween 20, pH 7.4. A BenchMark XT workstation (Ventana) was used to robotically prepare slides for antibody detection (reaction with primary antibodies, secondary antibodies, and fluorescence-detection reagents) (15). IgY was diluted to 1:300 (final concentration, 3.3 mg/L). Anti-IgY biotinylated antibody was used as the secondary antibody and was detected by fluorescence microscopy with streptavidin-Qdot 655. FluoSpheres carboxylate-modified microspheres (0.2 |im, crimson fluorescent; Invitrogen) were used as the calibrator. Imaging systems for 3-dimensional analysis of fluorescence signals from quantum dots, deconvolution of z-plane (depth) images, and integration of the signal with an imaging system have been described elsewhere (16).
FISH STUDIES OF FIXED CULTURED SK-BR-3 AND MCF-7 CELLS
FISH experiments were performed as previously described (14). In brief, biotinylated BAC DNA probe CTD-2019C10 was denatured by boiling for 5 min, diluted to 5 μg/L in a hybridization mixture [(0.30 mol/L NaCl and 0.030 mol/L sodium citrate) containing 500 mL/Lformamide, pH 7.0], added to the denatured cells on the slides, and sealed with a cover slip and rubber cement. After a 12-h incubation at 37 °C, the cover slips were removed, and the slides were washed 3 times in wash solution [(0.075 mol/L NaCl and 0.0075 mol/L sodium citrate) and 500 mL/L formamide] at 43 °C for 15 min each. Sites of hybridization were detected with Qdot 655. For detection, slides were mounted in VectaShield Mounting Medium with DAPI [Vector Laboratories; the product contains 1.5 mg/L 4',6-diamidino-2-phenylindole-2 (DAPI)]. Imaging and fluorescence-signal analysis have been described elsewhere (16).
LASER-SCANNING CYTOMETRY OF FIXED CULTURED SK-BR-3 AND MCF-7 CELLS
Samples were analyzed with an LSC2 laser-scanning cytometer (CompuCyte Corporation) equipped with 488-, 594- and 633-nm lasers and 4 photomultiplier tube (PMT) detectors. Qdot 655 was excited with a violet laser diode (405 nm, 15 mW) and detected through 660/20-nm bandpass filters. DAPI was excited with the violet laser diode (405 nm, 15 mW) and detected through a 461/50-nm bandpass filter. Samples were scanned in 0.5-μm steps and saved both as cytometric data and as PMT-reconstructed images. Data were acquired and analyzed with WinCyte software (version 3.7.1; CompuCyte).
REAL-TIME QUANTITATIVE PCR OF FIXED CULTURED SK-BR-3 AND MCF-7 CELLS
Genomic DNA was isolated with the QIAamp DNA-isolation kit (Qiagen) and subsequently quantified by spectrophotometric assay. The numbers of ERBB2 (i.e., HER-2) DNA copies in the 2 cell lines were evaluated with the 7500 Fast Real-Time PCR System (Applied Biosystems), a TaqMan Gene Expression Assays primer/probe set for ERBB2 (Hs01001577 g1; Applied Biosystems), and the manufacturer’s standard thermal-cycling conditions for relative quantification. Resultswere analyzed with SDS software (version 1.3.0; Applied Biosystems) on the system with the 2−–ΔΔCt method. The GAPDH gene (glyceraldehyde-3-phosphate dehydrogenase) was used as an endogenous control to correct for variation in the amount of input DNA for different samples.
PREPARATION OF FORMALIN-FIXED AND PARAFFIN-EMBEDDED CELLS
Cells were grown in culture as recommended by the ATCC. Cells were fixed in the buffered zinc formalin solution described above, pelleted by centrifugation, and embedded in paraffin blocks as previously described (17). A Leitz microtome was used to section the blocks of formalin-fixed and paraffin-embedded (FFPE) cells into sections 4 μm thick, which were then spread on water at 41 °C and transferred to a new glass slide (Fisher). Slides were deparaffinized before analysis.
CELL LINE GENOTYPING OF FFPE CELLS
All samples were genotyped in triplicate with the PowerPlex® 16 System (Promega) on a 3130xl Genetic Analyzer with a 36-cm capillary array and POP-4 polymer (Applied Biosystems). DNA samples were diluted to 1.0 ng/μL, and 1 μL of freshly extracted cell line DNA or 2 piL of DNA extracted from FFPE cells was added to a 25-/μL final reaction volume. PCR amplification was then carried out under the conditions specified by the manufacturer. One microliter of Internal Lane Standard 600 (Promega) and 9 μd. Hi-Di™ Formamide (Applied Biosystems) were added to 1 μL of the reaction mixture [or to 1 μL of Allelic Ladder Mix (Promega), one for each run]. The mixture was then denatured at 95 °C for 3 min, placed on ice for 3 min, and then immediately sequenced. Genotyping was analyzed with GeneMapper® ID software (version 3.2; Applied Biosystems).
IHC STUDIES OF FFPE CELLS
IHC experiments on FFPE cells were identical to those described above for fixed cultured cells except for the following modifications: The primary antibody was prediluted mouse anti-HER2 IgG monoclonal antibody (CB11; Ventana), which was used without dilution (6.0 mg/L) as per the manufacturer’s instructions. Anti-IgG biotinylated antibody from the same manufacturer was used as the secondary antibody.
FISH STUDIES OF FFPE CELLS
FISH experiments on FFPE cells were identical to those described above for fixed cultured cells except that the HER2 probe from Ventana was used instead of BAC CTD-2019C10 and that fluorescein isothiocyanate was used as the detector instead of Qdot 655.
Results
IHC ANALYSIS OF FIXED CULTURED CELLS
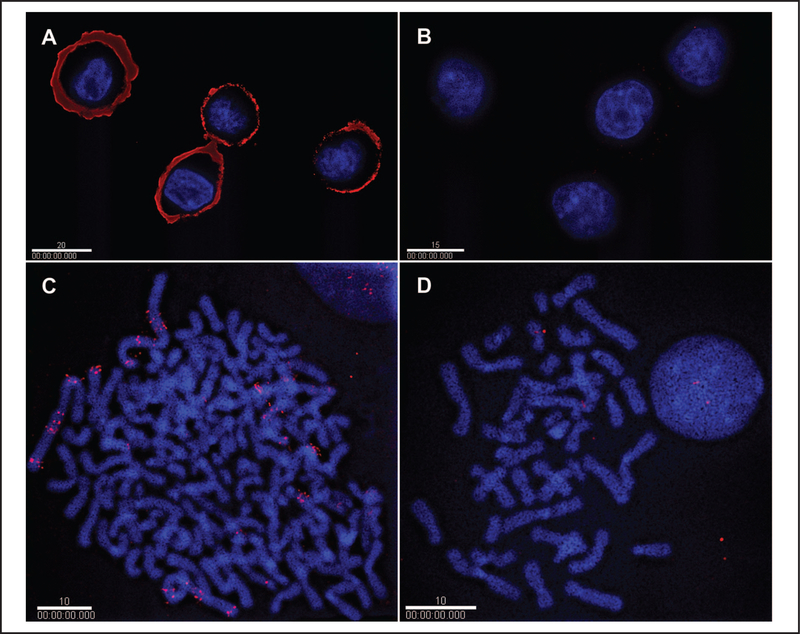
In preparations of fixed cells, IHC experiments with chick anti-HER2 antibody YXPB-IgY-hHER2/p detected high levels of HER2 protein in ERBB2-overexpressing cell line SK-BR-3 (Fig. 1A) compared with the low-expressing MCF-7 control cell line (Fig. 1B). A markedly high-intensity HER2 fluorescence signal was localized to the cell membrane in SK-BR-3 cells, as seen in optical sections from deconvolution experiments, whereas only low signal levels were found in the MCF-7 controls.
Fig. 1. IHC and FISH analyses of HER2 production and ERBB2 amplification infixed cultured cells of cell lines SK-BR-3 and MCF-7.
IHC with IgY antibody and quantum dot fluorophores for detection of HER2 production is shown for high-producing SK-BR-3 cells (A) and for very low-producing MCF-7 cells (B). FISH with the BAC CTD-2019C10 probe and quantum dot fluorophores for detecting ERBB2 amplification is shown in high copy-number SK-BR-3 cells (C) and in low copy-number MCF-7 cells (D). The white bars represent 20 μm (A), 15 μm (B), and 10 μm (C, D).
FISH ANALYSIS OF FIXED CULTURED CELLS
FISH experiments were performed with fixed cultured cells as the hybridization substrate. BAC CTD-2019C10, a 168-kb genomic probe encompassing human ERBB2, was detected in ERBB2-amplified SK-BR-3 cells (Fig. 1C) and in low-copy MCF-7 cells (Fig. 1D) with streptavidin-conjugated Qdot 655 as the detector. High levels of fluorescence due to multiple copies of the ERBB2 gene were localized to the chromosomes in SK-BR-3 cells (Fig. 1), whereas only low fluorescence signals were found in the MCF-7 controls because of the small number of ERBB2 copies (i.e., 2) in these cells.
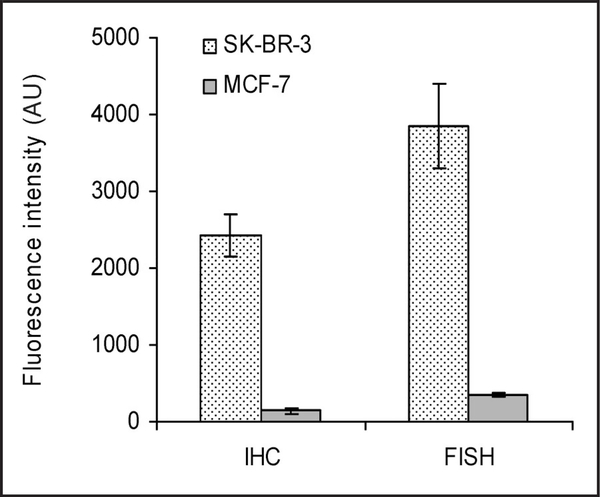
Fig. 2 shows the mean intensity of Qdot 655–detected fluorescence in 10 randomly selected fixed cultured cells from the I HC and FISH experiments de scribed above. SK-BR-3 cells produced appr oximately 15-fold more protein than MCF-7 cells and contained approximately 10-fold more copies of the ERBB2 gene.
Fig. 2. Quantification of IHC and FISH images with quantum dot fluorophores.
Qdot 655–detected fluorescence was calculated for 10 randomly selected cells in the IHC and FISH experiments depicted in Fig. 1. Fluorescence intensities are expressed as the mean (SD). AU, absorbance units.
QUANTIFICATION OF IHC RESULTS BY LASER-SCANNING CYTOMETRY
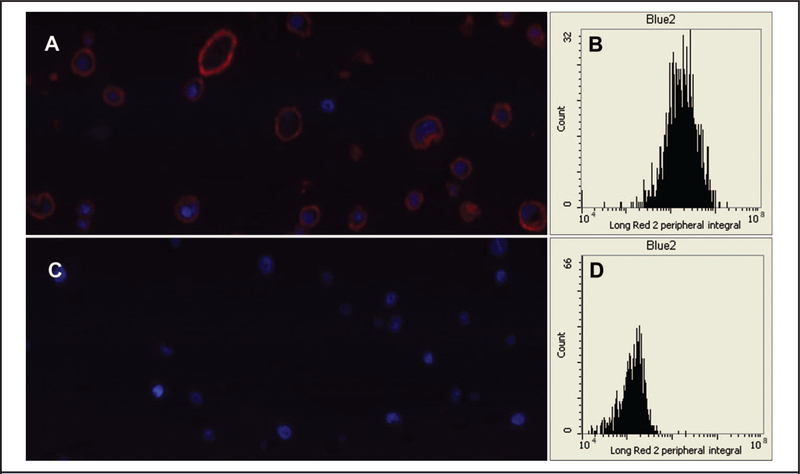
To confirm the results of the IHC experiment, we performed identical experiments and quantified the results by laser-scanning cytometry. Representative PMT-reconstructed images are shown in Fig. 3A (SK-BR-3) and Fig. 3C (MCF-7). Similarly to Fig. 1, high levels of HER2 fluorescence were detected in the cell membrane of SK-BR-3 cells, whereas the corresponding signals in the control MCF-7 cells were very low. The cytometric data for peripheral Qdot 655 fluorescence intensity (with spatial exclusion of the nucleus by DAPI contouring) are shown in Fig. 3B (SK-BR-3) and Fig. 3D (MCF-7). The ratio of the integral of the fluorescence intensity for SK-BR-3 (2 298 722) to that for MCF-7 (140 600) was 16.3, a result concordant with the results in Fig. 2.
Fig. 3. Laser-scanning cytometry experiments confirming HER2 production in fixed SK-BR-3 and MCF-7 cells.
Representative PMT-reconstructed images are shown for SK-BR-3 cells (A) and MCF-7 cells (C). The cytometric data for peripheral fluorescence intensity (with spatial exclusion of the nucleus by DAPI contouring) collected from a channel optimized for Qdot 655 (designated as Long Red 2) are shown for SK-BR-3 (B) and MCF-7 (D).
QUANTIFICATION OF ERBB2 GENE COPY NUMBER BY REAL-TIME PCR
The results of the FISH experiment were confirmed by studies of gene copy number with real-time quantitative PCR. The numbers of copies of the ERBB2 gene in SK-BR-3 cells were approximately 11- to 12-fold higher than those in MCF-7 cells (see Fig. 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol55/issue7), a finding in accordance with the FISH results in Fig. 2.
CELL LINE GENOTYPING
To confirm the identities of the cell lines in the FFPE cell preparations, we genotyped 16 loci in samples of DNA prepared from FFPE cells. A comparison of the genotypes for DNA recovered from FFPE cells and from freshly prepared cultured cells showed exact matches for allele calls for all 16 loci for both cell lines (Table 1). Table 1 also lists the DNA profile from ATCC for 9 of these 16 loci, and the allele calls in the fresh and FFPE samples for these 9 loci matched exactly those of the ATCC profile, for both cell types.
Table 1.
Genotyping of DNA extracted from fresh cultures and FFPE preparations of MCF-7 and SK-BR-3 cells.
| SK-BR-3 genotype |
MCF-7 genotype |
|||||
|---|---|---|---|---|---|---|
| Locusa | Fresh | FFPE | ATCC | Fresh | FFPE | ATCC |
| D3S1358 | 17 | 17 | 16 | 16 | ||
| TH01 | 8, 9 | 8, 9 | 8, 9 | 6 | 6 | 6 |
| D21S11 | 30, 30.2 | 30, 30.2 | 30 | 30 | ||
| D18S51 | 10, 13 | 10, 13 | 14 | 14 | ||
| Penta_E | 10, 11 | 10, 11 | 7, 12 | 7, 12 | ||
| D5S818 | 9, 12 | 9, 12 | 9, 12 | 11, 12 | 11, 12 | 11, 12 |
| D13S317 | 11, 12 | 11, 12 | 11, 12 | 11 | 11 | 11 |
| D7S820 | 9, 12 | 9, 12 | 9, 12 | 8, 9 | 8, 9 | 8, 9 |
| D16S539 | 9 | 9 | 9 | 11, 12 | 11, 12 | 11, 12 |
| CSF1PO | 12 | 12 | 12 | 10 | 10 | 10 |
| Penta_D | 9, 12 | 9, 12 | 12 | 12 | ||
| Amel | X, X | X, X | X | X, X | X, X | X |
| vWA | 17 | 17 | 17 | 14, 15 | 14, 15 | 14, 15 |
| D8S1179 | 11, 12 | 11, 12 | 10, 14 | 10, 14 | ||
| TPOX | 8, 11 | 8, 11 | 8, 11 | 9, 12 | 9, 12 | 9, 12 |
| FGA | 20 | 20 | 23, 24, 25 | 23, 24, 25 | ||
STR loci listed using UniSTS nomenclature (http://www.ncbi/nlm.nih.gov/sites/entrez?db=unists). Detailed information on the exact chromosomal locations of the loci can also be found at: http://www.cstl.nist.gov/strbase/pub_pres/Butler2006JFS_coreSTRreview.pdf.
IHC ANALYSIS OF FFPE CELLS
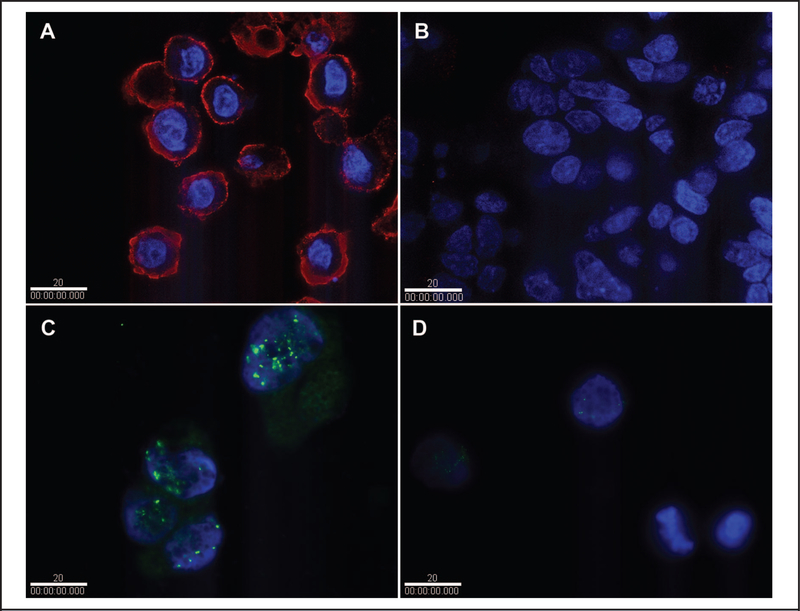
IHC experiments performed with the commercial mouse IgG anti-HER2 antibody on FFPE cells yielded results very similar to those obtained with fixed cultured cells. We detected substantially higher levels of HER2 protein in ERBB2-overexpressing cell line SK-BR-3 (Fig. 4A) than in the low-expressing MCF-7 controls (Fig. 4B). The fluorescence signal was predominantly localized to the cell membrane in SK-BR-3 cells, as seen in optical sections from deconvolution experiments. Fluorescence signals in the MCF-7 controls were very low.
Fig. 4. IHC and FISH analyses of HER2 production and ERBB2 amplification in FFPE SK-BR-3 and MCF-7 cells.
IHC analysis with mouse IgG antibody and quantum dot fluorophores for detecting HER2 production in SK-BR-3 cells (A) and MCF-7 cells (B). FISH analysis with the Ventana probe and fluorescein isothiocyanate detector for detection of ERBB2 gene amplification in SK-BR-3 cells (C) and MCF-7 cells (D). White bars indicate 20 μm.
FISH ANALYSIS OF FFPE CELLS
For FISH experiments on FFPE cells, we used the HER2 probe from Ventana (FDA cleared for clinical diagnosis) instead of the BAC CTD-2019C10 probe used for fixed cultured cells, and we used the organic dye fluorescein isothiocyanate as the label instead of Qdot 655. Despite these changes, we obtained results very similar to those obtained with fixed cultured cells. High levels of fluorescence due to multiple copies of the ERBB2 gene were localized to the chromosomes in SK-BR-3 cells (Fig. 4C), whereas only a few fluorescence signals of low intensity were found in the MCF-7 control cells because of the small number of ERBB2 copies (i.e., 2; Fig. 4D).
DEMONSTRATION OF HER2 IHC WITH AN INTERNAL CALIBRATOR
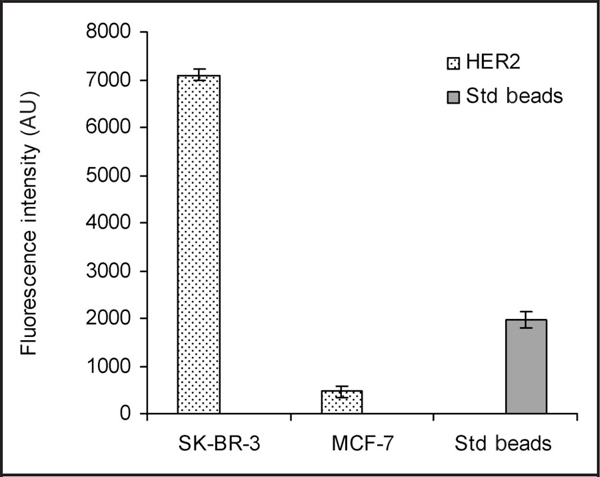
To demonstrate the feasibility of the use of these cell lines as possible reference materials for quantitative analysis of HER2, we performed an IHC experiment with fluorescent microspheres used as the calibrator for image capture and analysis (Fig. 5). Values obtained from similarly treated unknown samples can be evaluated from the values obtained for SK-BR-3 and MCF-7.
Fig. 5. IHC analysis with fluorescent microspheres as a calibrator for image capture and analysis, demonstrating the feasibility of quantitative analysis of HER2 production with the FFPE cell lines as controls.
AU, absorbance units; Std beads, fluroescent microspheres.
Discussion
In the emerging clinical paradigm of individualized medicine (18), accurate quantitative data are important to reach the correct treatment decision, such as in the case of HER2 testing for selecting patients for trastuzumab therapy. The potential for substantial benefits contrasted with the high cost and potential cardiotoxicity of trastuzumab demands accurate HER2 testing. Despite attempts within the international pathology community to improve the status of HER2 testing in routine practice, testing inaccuracy remains a major issue with both IHC and FISH (19). Among the various factors that can account for the large variation observed in clinical practice and in clinical trials, the lack of validated reference standards and calibrators for QC of assay sensitivity is the most notable. The present study sought to provide a solution to this problem by testing the suitability of 2 breast carcinoma cell lines, SK-BR-3 and MCF-7, as candidate reference materials for HER2 testing.
The current practice of the use of paraffin blocks of tumor tissues with varying levels of ERBB2 gene expression and HER2 protein content is suboptimal for 2 reasons. First, tumor material is often difficult to acquire; therefore, the quantity available is likely to be limited, making it unfeasible to use as QC material on a large scale. Second, each patient’s biopsy material differs from biopsy samples from other patients in that each patient sample has individual differences in tumor expression and fixation. Obviously, this lack of reproducibility makes such material less ideal as a standard by which to stringently gauge assay sensitivity for ERBB2 gene expression or HER2 protein content.
To create reproducible standards that are available in unlimited supply, Sompuram et al. (20) used short synthetic peptides as a control in IHC analysis. The peptides, which mimic the 3-dimensional conformation of the analyte epitope for a particular antibody, are attached to the same slides as the patient sample. Although this method does provide a highly available, reproducible, stable, and inexpensive source of analytical-control material, it has several major drawbacks. First and foremost, because the peptides are not susceptible to preanalytical treatments such as fixation and antigen retrieval, the method is sensitive only to analytical errors, such as those associated with reagents, instrumentation, and technique. This issue is particularly important, because preanalytical errors contribute substantially to the error in IHC testing. Second, the method is specific to a particular monoclonal antibody used in the analysis. Lastly, the method is applicable only to IHC, and not to FISH.
The ideal reference material for standards and calibrators in HER2 testing (as well as other biomarkers) would appear to be cell lines. Cell lines can be grown in vitro; thus, such cells are potentially available in large quantities. Cell lines can be fixed and processed in ways similar to those of histopathologic samples. Although analyte levels may differ among different batches and with different treatments, a single harvest of a large-scale production of cells and their subsequent fixation and processing at the same time could yield a long-lasting bank of control material, thereby providing reproducibility and consistency among different assays. In addition, such cell-based reference materials would be useful for both IHC and FISH testing.
In fact, the use of cell lines mounted on slides as controls was proposed long ago for IHC analysis of hormone receptors in breast cancer (21, 22 ). In the case of HER2 IHC testing, the Dako HercepTest kit includes cell line–based control slides as an essential component. Aside from being relatively expensive, the controls are confined to use only with the antibody included in the kit. More recently, Rhodes et al. (23) reported the use of 4 cell lines with differing levels of ERBB2 overexpression as graded calibrators for HER2 IHC and FISH testing. The cell lines were FFPE in a manner similar to that of tissue samples and were stable for at least 8 weeks. Although this method seemed to provide a solution to the problem in question, the use of 4 cell lines makes it tedious for routine use in a histopathology laboratory. More importantly, although the authors claimed that the use of 4 cell lines was an advantage of the method, the use of a graded control system subjects the evaluation of a patient sample to potential bias by the individual interpreting the results, rendering test results not totally objective.
In the system presented in this study, our use of only 2 cell lines makes the system more feasible for routine use in histopathology laboratories. The 2 cell lines were characterized in terms of HER2 protein production and ERBB2 gene amplification. MCF-7 cells have a typical number of gene copies (i.e., 2) and very low HER2 protein content, whereas SK-BR-3 cells contain approximately 10-fold more copies of the ERBB2 gene and approximately 15-fold higher HER2 protein content (Figs. 1 and 2). Laser-scanning cytometry and real-time PCR confirmed these results (Fig. 3; see Fig. 1 in the online Data Supplement). The fixation and embedding procedures for preparing FFPE cells did not cause changes in analyte levels (Fig. 4).
By virtue of the high specificity of the IgY antibody (13) and the BAC probe (14), as well as the high intensity and stability of the quantum dot detectors (14), both the IHC method and the FISH method used in this study were quantitative (Fig. 1). When an internal calibrator is included in the assay systems, as exemplified in the IHC experiment shown in Fig. 5, values obtained for the 2 control cell lines may provide reference points for absolute quantification of unknown samples, thus making it possible for the 2 cell lines to serve as controls and to provide reproducibility and consistency among different assays.
From a practical point of view, the system of 2 cell lines described in this report has a major perceived drawback compared with multiple-cell, graded controls, such as those used in the Dako HercepTest kit. Current guidelines (19) recommend that an equivocal result in an IHC test be further evaluated by FISH to determine the HER2 status for therapeutic decision-making, and vice versa. In either case, control materials in the equivocal range are needed in current clinical practice to guide pathologists in evaluating the test results. Our system of 2 cell lines does not provide control material in this intermediate range; however, provided that absolutely quantitative and highly reproducible IHC and FISH results can be obtained from this system, it is not inconceivable that the development of future guidelines will be based on clinical data collected by this system in correlation with outcomes from clinical trastuzumab trials.
In addition, in the current configuration of our 2–cell line system, internal calibrators such as the fluorescent microspheres used in this study have the drawback of not being susceptible to preanalytical treatments, thus requiring that samples and control cell lines be treated in exactly the same way (e.g., on the same slide) to obtain a meaningful absolute value. It is beyond the scope of this study, however, to design the optimal assay platform for the use of standard cell lines as controls or to provide an adequate evaluation scale for predicting clinical outcomes. Nevertheless, the value of these cell lines as controls in HER2 testing remains. As we have demonstrated, we rigorously evaluated the 2 cell lines as candidate reference materials for HER2 testing. SK-BR-3 and MCF-7 appear to be suitable for these purposes. When their use is coupled with the associated assay platforms, they provide valuable calibrators for quantitative measurement of ERBB2 amplification and expression in breast cancer samples, irrespective of the antibody/probe or detector used.
Supplementary Material
Acknowledgments:
We thank Drs. Laurie E. Locascio and Steven J. Choquette for critically reading the manuscript.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: NIST Standard Reference Material Development Project for HER2, and an Interagency Agreement of NIST and the NIH Office of Women’s Health.
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played a direct role in the final approval of the manuscript.
Footnotes
Human genes: ERBB2 (alias, HER-2), v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian); GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Nonstandard abbreviations: HER2, human epidermal growth factor receptor 2; FDA, Food and Drug Administration; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; BAC, bacterial artificial chromosome; DAPI, 4’,6-diamidino-2-phenylindole-2; PMT, photomultiplier tube; FFPE, formalin-fixed and paraffin-embedded.
Disclaimer: Certain commercial equipment or materials are identified in this report to specify adequately the experimental procedures. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- 1.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644–6. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. [DOI] [PubMed] [Google Scholar]
- 3.Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, et al. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res 1993;53:4960–70. [PubMed] [Google Scholar]
- 4.Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 1997;15:2894–904. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi H, Stearns V, Hayes DF. When is a tumor marker ready for prime time? A case study of c-erbB-2 as a predictive factor in breast cancer. J Clin Oncol 2001;19:2334–56. [DOI] [PubMed] [Google Scholar]
- 6.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17: 2639–48. [DOI] [PubMed] [Google Scholar]
- 7.Hayes DF, Picard MH. Heart of darkness: the downside of trastuzumab. J Clin Oncol 2006;24: 4056–8. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez RE, Wallis T, Tabasczka P, Visscher DW. Determination of Her-2/Neu status in breast carcinoma: comparative analysis of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol 2000;13:37–45. [DOI] [PubMed] [Google Scholar]
- 9.Paik S, Bryant J, Tan-Chiu E, Romond E, Hiller W, Park K, et al. Real-world performance of HER2 testing—National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst 2002;94:852–4. [DOI] [PubMed] [Google Scholar]
- 10.Perez EA, Suman VJ, Davidson NE, Martino S, Kaufman PA, Lingle WL, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol 2006;24:3032–8. [DOI] [PubMed] [Google Scholar]
- 11.Roche PC, Suman VJ, Jenkins RB, Davidson NE, Martino S, Kaufman PA, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst 2002;94:855–7. [DOI] [PubMed] [Google Scholar]
- 12.Hammond ME, Barker P, Taube S, Gutman S. Standard reference material for Her2 testing: report of a National Institute of Standards and Technology-sponsored Consensus Workshop. Appl Immunohistochem Mol Morphol 2003;11: 103–6. [PubMed] [Google Scholar]
- 13.Xiao Y, Gao X, Gannot G, Emmert-Buck MR, Srivastava S, Wagner PD, et al. Quantitation of HER2 and telomerase biomarkers in solid tumors with IgY antibodies and nanocrystal detection. Int J Cancer 2008;122:2178–86. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Barker PE. Semiconductor nanocrystal probes for human metaphase chromosomes. Nucleic Acids Res 2004;32:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bankfalvi A, Boecker W, Reiner A. Comparison of automated and manual determination of HER2 status in breast cancer for diagnostic use: a comparative methodological study using the Ventana BenchMark automated staining system and manual tests. Int J Oncol 2004;25:929–35. [PubMed] [Google Scholar]
- 16.Xiao Y, Telford WG, Ball JC, Locascio LE, Barker PE. Semiconductor nanocrystal conjugates, FISH and pH. Nat Methods 2005;2:723. [DOI] [PubMed] [Google Scholar]
- 17.Morgan JM. A protocol for preparing cell suspensions with formalin fixation and paraffin embedding which minimizes the formation of cell aggregates. J Cell Pathol 2001;5:171–80. [Google Scholar]
- 18.Elledge RM, Osborne CK. Oestrogen receptors and breast cancer. BMJ 1997;314:1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18–43. [DOI] [PubMed] [Google Scholar]
- 20.Sompuram SR, Kodela V, Zhang K, Ramanathan H, Radcliffe G, Falb P, et al. A novel quality control slide for quantitative immunohistochemistry testing. J Histochem Cytochem 2002;50: 1425–34. [DOI] [PubMed] [Google Scholar]
- 21.Allred DC. Should immunohistochemical examination replace biochemical hormone receptor assays in breast cancer? Am J Clin Pathol 1993;99: 1–3. [DOI] [PubMed] [Google Scholar]
- 22.Riera J, Simpson JF, Tamayo R, Battifora H. Use of cultured cells as a control for quantitative immunocytochemical analysis of estrogen receptor in breast cancer. The Quicgel method. Am J Clin Pathol 1999;111:329–35. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes A, Jasani B, Couturier J, McKinley MJ, Morgan JM, Dodson AR, et al. A formalin-fixed, paraffin-processed cell line standard for quality control of immunohistochemical assay of HER-2/neu expression in breast cancer. Am J Clin Pathol 2002;117:81–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.