Abstract
Background:
There is paucity of data focusing on females’ outcomes after the use of impeller pumps percutaneous ventricular assist devices (IPVADs).
Methods:
Patients who received IPVADs during the period of October 1st, 2015-December 31, 2017, were identified from the United States National Readmission Database. A 1:1 propensity score matching was used to compare the outcomes between females and males.
Results:
A total of 19,278 (Female = 5,456; Male = 13,822) patients were included in the current analysis. After propensity score matching and among all-comers who were treated with IPVADs, females had higher in-hospital major adverse events (MAEs) (38 vs. 32.6%, p < .01), mortality (31 vs. 28%, p < .01), vascular complications (3.3 vs. 2.1%, p < .01), major bleeding (7.8 vs. 4.8%, p < .01), nonhome discharges (21.6 vs. 16.3%; p < .01), and longer length of stay (7 days [IQR 2–12] vs. 6 days [IQR 2–12], p = .02) with higher 30-day readmission rate compared to males (20.5 vs.16.4%, p < .01). Furthermore, among patients who received the IPVADs for high-risk percutaneous coronary intervention (HRPCI), females continued to have worse MAEs, which was driven by high rates of major bleeding. However, among patients who received IPVADs for cardiogenic shock (CS) the outcomes of females and males were comparable.
Conclusions:
Among all-comers who received IPVADs, females suffered higher morbidity and mortality compared to males. Higher morbidity driven mainly by higher rates of major bleeding was seen among females who received IPVADs for the hemodynamic support during HRPCI and comparable outcomes were observed when the IPVADs were used for CS.
Keywords: 30-day readmission, cardiogenic shock, gender disparity, high-risk percutaneous coronary intervention, in-hospital outcomes, percutaneous ventricular assist devices
1 |. INTRODUCTION
Impellor pumps percutaneous ventricular assist devices (IPVADs) have gained increasing popularity in the last decade and became an essential component of the cardiovascular therapeutic armamentarium.1 The American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) Guideline for Percutaneous Coronary Intervention (PCI) recommends consideration of the use of percutaneous left ventricular assist devices as an adjunct to high-risk-PCI (HRPCI) (Class IIb) and for the hemodynamic support during cardiogenic shock (CS) in the context of ST-elevation myocardial infarction (Class Ib).1,2
Among the currently available IPVADs, the Impella (Abiomed, Danvers, Massachusetts) family of devices are transvalvular microaxial pumps that can be rapidly deployed in the catheterization laboratory and provide direct left ventricular unloading with forward blood flow of up to 5 L/min.1 The safety and feasibility of a strategy of early upfront use of IPVADs before PCI in patients with CS secondary to acute myocardial infarction (AMI) has recently been demonstrated.3,4 Moreover, earlier studies have supported an improvement in the hemodynamic profile, reduced rates of acute kidney injury and trend toward better 90-day clinical endpoints with the use of IPVADs compared to the intra-aortic balloon pump when the IPVADs are used as an adjunct to HRPCI.5,6 Although there is a wealth of registry data on the outcomes of IPVADs in the contemporary practice, there is paucity of data focusing on females outcomes.7 Females are under-presented in all the trials which assessed the safety and efficacy of IPVADs.8,9 Hence, in the current study, we used the National Readmission Database (NRD) to compare the outcomes between females and males in patients who were treated with IPVADs.
2 |. METHODS
2.1 |. Study data
The study was derived from the NRD, from October 1, 2015 to December 31, 2017. The NRD is part of Healthcare Cost and Utilization Project (HCUP) databases and is sponsored by the Agency for Healthcare Research and Quality (AHRQ).10 In its most recent release, the NRD reported data collected from 28 states, accounting for 60% of the total U.S. population and 58.2%t of all U.S. hospitalizations.10 Unweighted, each year of the NRD provide data on about 17 million discharges and weighted it estimates more than 36 million discharges. The Institutional Review Board exempted the study because it utilizes publicly de-identified data.
2.2 |. Study population
Adult patients (≥18 years) who received IPVADs between October 1, 2015 and December 31, 2017 were identified in the NRD using the International Classification of Disease 10th Procedure codes (PCS) 5A0221D and 5A0211D which are used to code for the use of impeller heart pumps. For the in-hospital outcomes, we excluded the following patients: (a) patients who are younger than 18 years, (b) patients who were transferred to a different hospital on the same day of admission, (b) patients with missing mortality or gender data, (c) patients who were treated concomitantly with intra-aortic balloon pump or extra-corporal membrane oxygenation. Furthermore, to calculate the 30-day readmission rates we excluded: (a) patients who died during the index admission, (b) patients who were discharged in the month of December, this was done because the NRD provides data on an annual basis and consequently, patients who are discharged in December will not have 30-day follow-up.
2.3 |. Study endpoints
The primary endpoint of this study was in-hospital major adverse events (MAEs) (a composite of in-hospital mortality, vascular complications, and major bleeding). The secondary endpoints were, in-hospital mortality, vascular complications, major bleeding (defined as post-procedural bleeding requiring blood transfusion), severe disability surrogates (nonhome discharge and need for mechanical ventilation), resources utilization surrogates (length of stay and cost of hospitalization) and 30-day readmission rate.
2.4 |. Statistical analysis
All variables are expressed as weighted national estimates. This was done following the survey analysis method by incorporating the (DISWT) variable as a weight variable, (HOSP_NRD) as a clustering variable and accounting for the different strata in the design of the NRD using the (NRD_STRATUM) as recommended in the AHRQ methods series.11 Categorical variables were expressed as count (percentage) and compared using Scott-Rao Chi-square test. Continuous variables were expressed as median (interquartile range) and compared using the Wilcoxon rank-sum test.
Moreover, a propensity score-matching (PSM) model was calculated using a multivariate logistic regression to derive two matched groups for comparative outcomes analysis (Females vs. Males). We included the discharge weight provided by the HCUP in the propensity score analysis to minimize the differences between the matched cohorts. A nearest neighbor 1:1 variable ratio, parallel, balanced propensity-matching model was applied using a caliper width of 0.01. In addition, we performed several sensitivity analyses as follow: (1) sensitivity analysis by reporting the outcomes among patients in whom the indication for IPVADs was CS. (2) sensitivity analysis by reporting the outcomes in patients who received IPVADs for HRPCI. The HRPCI cohort was isolated by identifying patients who received PCI during the index admission and excluding any patients who had the diagnosis of CS or AMI.
For cost calculation, we used the cost-to-charge ratio files provided by the HCUP to convert the hospital charges to more accurate hospital costs. A p-value of <.05 was considered statistically significant. For calculation of the readmission rates, we followed the methods recommended by the HCUP.10 All statistical analyses were performed using the statistical package for social science (SPSS) version 26 (IBM Corp) and R, version 3.5 for propensity matching.
3 |. RESULTS
A total of 19,278 (Female = 5,456; Male = 13,822) patients qualified to be included in the current analysis (Figure 1). Comparing females to males, female patients were older (70 years [IQR62–79] vs. 68 years [IQR 59–76], p < .01), had higher prevalence of hypertension (79.5 vs. 75.7%, p < .01), obesity (19.2 vs. 15%, p < .01), chronic lung disease (24.2 vs. 21.9; p < .01), coagulopathy (20 vs. 17.3%, p < .01) and chronic anemia (27.7 vs. 20.4%, p < .01). On the other hand, female patients had lower prevalence of chronic kidney disease (28.9 vs. 31.5%, p = .01), atrial fibrillation (24.4 vs. 28.5%, p < .01) and smoking (12.9 vs. 17.7%, p < .01). Detailed baseline characteristics of the two groups are shown in (Table 1).
FIGURE 1.
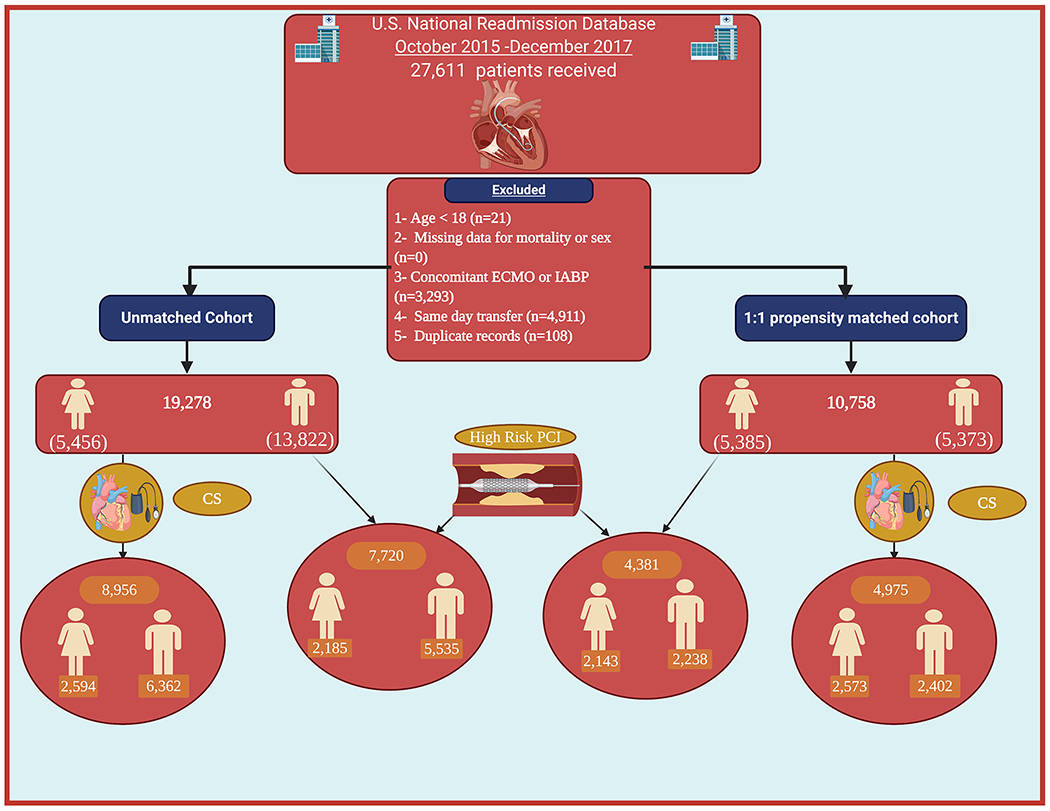
Flow chart of the study. CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; PCI, percutaneous coronary interventio006E
TABLE 1.
Baseline characteristics of the patients included in the analysis before and after propensity score matching
| Unmatched cohorts |
Matched cohorts |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables (%) | Female (n = 5,456) | Male (n = 13,822) | Total (n = 19,278) | p-value | Female (n = 5,385) | Male (n = 5,373) | Total (n = 10,758) | p-value |
| Age. median (25th–75th IQR) | 70 (62–79) | 68 (59–76) | 69 (60–77) | <.01 | 70 (62–79) | 70 (61–78) | 70 (62–78) | .12 |
|
| ||||||||
| Diabetes mellitus | 44.8 | 43.2 | 43.6 | .15 | 44.8 | 45.1 | 44.9 | .80 |
|
| ||||||||
| Hypertension | 79.5 | 75.7 | 76.7 | <.01 | 79.3 | 78.8 | 79 | .59 |
|
| ||||||||
| Peripheral vascular disease | 201 | 18.8 | 19.2 | .17 | 19.9 | 20.2 | 20.1 | .77 |
|
| ||||||||
| Chronic anemia | 27.7 | 20.4 | 22.4 | <.01 | 27.1 | 26.7 | 26.9 | .78 |
|
| ||||||||
| Chronic heart failure | 68 | 70.1 | 69.5 | .057 | 68.2 | 67.8 | 68 | .77 |
|
| ||||||||
| Chronic kidney disease | 28.9 | 31.5 | 30.7 | .01 | 28.9 | 30.9 | 29.9 | .09 |
|
| ||||||||
| Chronic liver disease | 3.1 | 3.2 | 3.1 | .81 | 3.1 | 2.7 | 2.9 | .36 |
|
| ||||||||
| Chronic lung disease | 24.2 | 21.9 | 22.5 | .03 | 24 | 23.9 | 23.9 | .96 |
|
| ||||||||
| Atrial fibrillation | 24.4 | 28.5 | 27.3 | <.01 | 24.6 | 24.2 | 24.4 | .73 |
|
| ||||||||
| Smoking | 12.9 | 17.7 | 16.3 | <.01 | 12.9 | 13.7 | 13.3 | .42 |
|
| ||||||||
| Coagulopathy | 20 | 17.3 | 18 | <.01 | 19.8 | 18.7 | 19.2 | .29 |
|
| ||||||||
| Obesity | 19.2 | 15 | 16.2 | <.01 | 18.7 | 20.4 | 19.6 | .14 |
|
| ||||||||
| STEMI | 26.8 | 27.9 | 27.6 | .33 | 27 | 26.4 | 26.7 | .64 |
|
| ||||||||
| Percutaneous coronary intervention (PCI) | 75.4 | 74.5 | 74.7 | .38 | 75.3 | 74.9 | 75.1 | .73 |
|
| ||||||||
| Multivessel PCI | 38.5 | 38.8 | 38.7 | .76 | 38.5 | 40.2 | 39.4 | .18 |
|
| ||||||||
| Drug eluting stent | 66.5 | 65.2 | 65.6 | .24 | 66.6 | 65.7 | 66.1 | .49 |
|
| ||||||||
| Bare metal stent | 6.3 | 6.7 | 6.6 | .46 | 6.1 | 5.9 | 6 | .79 |
|
| ||||||||
| Coronary artery bypass grafting | 5.4 | 6.4 | 6.1 | .07 | 5.4 | 5.9 | 5.7 | .44 |
|
| ||||||||
| Hospital bed size | ||||||||
|
| ||||||||
| Small | 8.7 | 8.8 | 8.8 | .11 | 8.7 | 10.2 | 9.4 | .09 |
|
| ||||||||
| Medium | 26.6 | 24.5 | 25.1 | 26.6 | 24.9 | 25.7 | ||
|
| ||||||||
| Large | 64.7 | 66.7 | 66.1 | 64.7 | 64.9 | 64.8 | ||
Abbreviations: IQR, interquartile range: STEMI; ST-segment elevation myocardial infarction.
Before PSM, among all-comers, comparing females to males, females had higher rates of in-hospital MAEs (38 vs. 31.9%, p < .01), in-hospital mortality (31 vs. 27.6%, p < .01), vascular complications (3.3 vs. 2.2%; p < .01), and major bleeding (7.8 vs. 4.3%, p < .01). Moreover, females had higher nonhome discharges (21.7 vs. 16%; p < .01)] compared to males (Table 2). Length of stay was longer in the female group compared to the male group (7 days [IQR 2–12] vs. 6 days [IQR 2–12], p < .01). After discharge from the hospital and among survival of index hospitalization, females had higher 30-day readmission rate compared to males (20.5 vs. 16.6%, p < .01).
TABLE 2.
In-hospital outcomes of the patients included in the analysis before and after the propensity score matching
| Unmatched cohorts |
Matched cohorts |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables no. (%) | Female (n = 5,456) | Male (n = 13,822) | Total (n = 19,278) | p-value | Female (n = 5,385) | Male (n = 5,373) | Total (n = 10,758) | p-value |
| In-hospital outcomes | ||||||||
| Major adverse events | 38 | 31.9 | 33.6 | <.01 | 38 | 32.6 | 35.3 | <.01 |
| Death | 31 | 27.6 | 28.6 | <.01 | 31 | 28 | 29.5 | .02 |
| Vascular compilations | 3.3 | 2.2 | 2.5 | <.01 | 3.3 | 2.1 | 2.7 | <.01 |
| Major bleeding | 7.8 | 4.3 | 5.3 | <.01 | 7.8 | 4.8 | 6.3 | <.01 |
|
| ||||||||
| Severe disability surrogates | ||||||||
| Nonhome discharge | 21.7 | 16 | 17.6 | <.01 | 21.6 | 16.3 | 18.9 | <.01 |
| Mecharccal ventilation | 35.3 | 33.3 | 33.8 | .06 | 35.4 | 32.4 | 33.9 | .02 |
|
| ||||||||
| Resources utilization | ||||||||
| Length of hospitalization | 7 | 6 | 6 | <.01 | 7 | 6 | 6 | .02 |
| Median days (25th–75th IQR) | 2-12 | 2-12 | 2-12 | – | 2-12 | 2-12 | 2-12 | – |
| Cost of hospitalization | 59,090 | 58,997 | 59,024 | .91 | 59,071 | 58,789 | 58,963 | .63 |
| Median $ (25th–75th IQR) | (42,898–84,043) | (42,462–84,932) | (42,574–84,533) | – | (42,908–84,075) | (41,990–84.354) | (42,492–84,224) | – |
Abbreviation: IQR, interquartile range.
After PSM, two pairs of well-matched female and male groups were compared (Female = 5,385, Male = 5,373) (Table 2). After PSM, standardized mean differences were reduced to <10% for all variables indicating an adequate balanced population (Figure 2). Females continued to show higher rates of MAEs (38 vs. 32.6%, p < .01), in-hospital mortality (31 vs. 28%, p < .01), vascular complications (3.3 vs. 2.1%, p < .01) and major bleeding (7.8 vs. 4.8%, p < .01). Moreover, females continued to have higher rate of nonhome discharges (21.6 vs. 16.3%; p < .01) (Table 2). The length of stay continued to be longer in the female group (7 days [IQR 2–12] vs. 6 days [IQR 2–12], p = .02). There was no difference in term of hospitalization costs for females versus males (59,071$ [IQR 42,908–84,075] vs. 58,789$ [IQR41,990–84,354]; p = .63). Females continued to show higher 30-day readmission rate compared to males (20.5 vs. 16.4%, p < .01).
FIGURE 2.
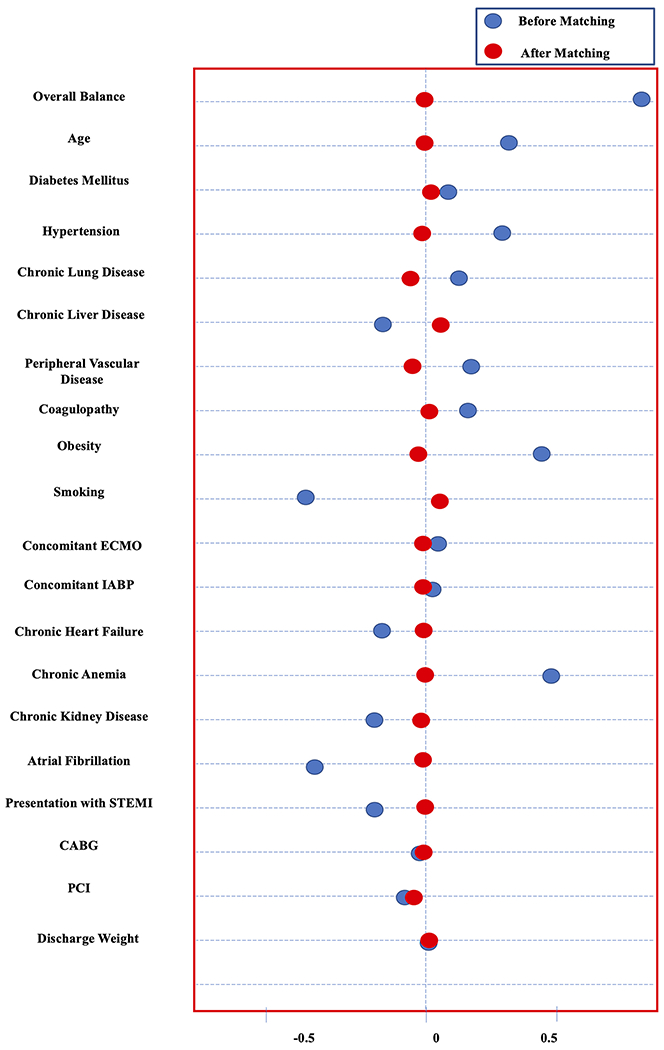
Dot plot showing the balance before and after propensity score matching. CABG, coronary artery bypass grafting; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction
In the sensitivity analysis, by only including patients who received impella for HRPCI, females continued to show higher rates of MAEs (13.2 vs. 8.7%, p < .01), major bleeding (5.5 vs. 2.1%, p < .01), nonhome discharge (19.9 vs. 12.6%, p < .01), longer length of stay (6 days [IQR 3–11] vs. 5 days [IQR 2–9], p < .01), and higher cost of hospitalization (55,010$ [IQR 41,604–73,011] vs. 52,653$ [IQR 39,327–69,854]; p < .01). Similar in-hospital mortality (6.7 vs. 5.5% p = .11) was observed (Table 3).
TABLE 3.
Sensitivity analysis including patients who received Impella for high-risk percutaneous coronary intervention
| Unmatched cohorts |
Matched cohorts |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables no. (%) | Female (n = 2,185) | Male (n = 5,535) | Total (n = 7,720) | p-value | Female (n = 2,143) | Male (n = 2,238) | Total (n = 4,381) | p-value |
| In-hospital outcomes | ||||||||
| Major adverse events | 13.2 | 7.9 | 9.4 | <.01 | 13.2 | 8.7 | 10.9 | <.01 |
| Death | 6.7 | 4.6 | 5.2 | .01 | 6.7 | 5.5 | 6.1 | .19 |
| Vascular complications | 2.7 | 2.3 | 2.4 | .50 | 2.7 | 2 | 2.3 | .34 |
| Major bleeding | 5.6 | 2.1 | 3.1 | <.01 | 5.5 | 2.1 | 3.8 | <.01 |
|
| ||||||||
| Severe disability surrogates | ||||||||
| Nonhome discharge | 20.1 | 11.5 | 13.9 | <.01 | 19.9 | 12.6 | 16.1 | <.01 |
| Mechanical ventilation | 7.6 | 6.9 | 7.1 | .50 | 7.6 | 7 | 7.3 | .62 |
|
| ||||||||
| Resources utilization | ||||||||
| Length of hospitalization | 6 | 5 | 5 | <.01 | 6 | 5 | 5 | <.01 |
| Median days (25th–75th IQR) | 3-11 | 2-9 | 2-9 | – | 3-11 | 2-9 | 2-10 | – |
| Cost of hospitalization | 55,036 | 52,000 | 52,692 | <.01 | 55,010 | 52,653 | 53,743 | .01 |
| Median $ (25th–75th IQR) | (41,571–73,092) | (39,209–68,369) | (39,726–69,801) | – | (41,604–73,011) | (39,327–69,854) | (40,427–71,540) | – |
Abbreviation: IQR, interquartile range.
In the sensitivity analysis, by including only patients with the diagnosis of CS, comparing females to males, females had similar MAEs (56.7 vs. 53.7%, p = .12), major bleeding (9.3 vs. 7.3%, p = .09) and in-hospital mortality (50.1 vs. 48%, p = .29). Female patients with CS had lower cost of hospitalization compared to males (64,274$ [IQR 44,553–93,886] vs. 67,994$ [IQR 46,956–101,288]; p = .02) (Table 4) and (Figure 3).
TABLE 4.
Sensitivity analysis including only patients with diagnoses of cardiogenic shock
| Unmatched cohorts |
Matched cohorts |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables no. (%) | Female (n = 2,594) | Male (n = 6,362) | Total (n = 8,956) | p-value | Female (n = 2,573) | Male (n = 2,402) | Total (n = 4,975) | p-value |
| In-hospital outcomes | ||||||||
| Major adverse events | 56.8 | 52 | 53.4 | <.01 | 56.7 | 53.7 | 55.2 | .12 |
| Death | 50.2 | 47.1 | 48 | .07 | 50.1 | 48 | 49.1 | .29 |
| Vascular complications | 3.1 | 1.7 | 2.1 | .02 | 3.1 | 1.9 | 2.5 | .06 |
| Major bleeding | 9.2 | 6.2 | 7.1 | <.01 | 9.3 | 7.3 | 8.3 | .09 |
|
| ||||||||
| Severe disability surrogates | ||||||||
| Nonhome discharge | 22.2 | 19.7 | 20.4 | .20 | 22.4 | 19.7 | 21.1 | .08 |
| Mechanical ventilation | 58.1 | 57.3 | 57.5 | .95 | 58.1 | 55.8 | 57 | .26 |
|
| ||||||||
| Resources utilization | ||||||||
| Length of hospitalization | 7 | 7 | 7 | <.01 | 8 | 8 | 7 | .047 |
| Median days (25th-75th IQR) | 2-14 | 3-14 | 2-14 | – | 2-15 | 3-16 | 2-14 | – |
| Cost of hospitalization | 64,208 | 67,960 | 66,743 | <.01 | 64,274 | 67,994 | 65,722 | .02 |
| Median $ (25th–75th IQR) | (44,521–93,928) | (47,484–101,747) | (46,696–99,538) | – | (44,553–93,886) | (46,956–101,288) | (45,769–97,147) | – |
Abbreviation: IQR, interquartile range.
FIGURE 3.
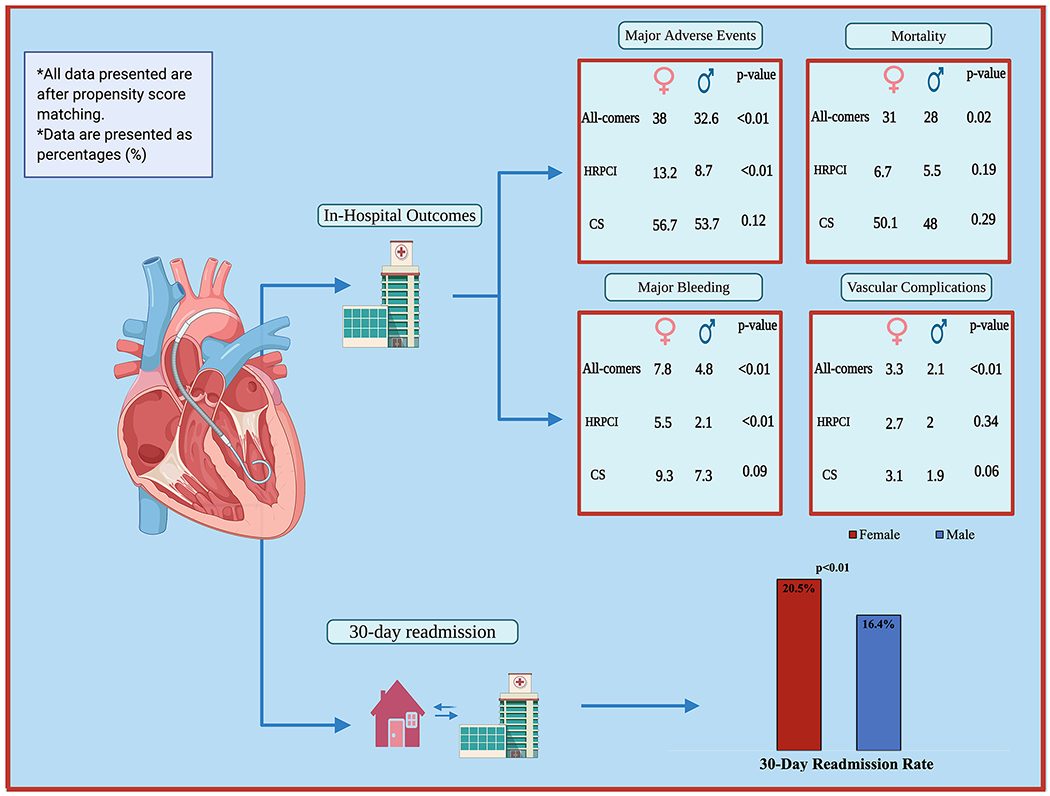
Summary of the main findings from the study. CS, cardiogenic shock; HRPCI, high-risk percutaneous coronary intervention
4 |. DISCUSSION
In the current study, evaluating the outcomes of patients who were treated with IPVADs from a nationally representative database, significant gender-based differences were observed. First, among all patients who received IPVADs irrespective of the indication (HRPCI or CS), females had higher rates of in-hospital MAEs, including mortality, vascular complications, and major bleeding, these differences persisted after PSM. Second, female patients also had a higher 30-day all-cause readmission rate compared to male patients. Third, among patients who received IPVADs for HRPCI, females continued to have higher risk of MAEs, which was driven mainly by higher rates of major bleeding. Fourth, among patients who received IPVADs for CS, females and males had comparable outcomes.
The adoption of shock protocols relying on hemodynamic guided early IPVADs placement in patients with CS has shown promising results in the early reports from National Cardiogenic Shock Initiative.4 The early use of IPVADs is a central part of the current shock protocols; this has led to a significant uptrend in the use of IPVADs in recent years.12,13 The current analysis is the largest to date to shed some light on the outcomes of IPVADs among females. Our analysis showing higher hospital mortality, vascular complications, and bleeding complications among all-comers females receiving IPVADs is consistent with previous literature showing that females undergoing invasive procedures have a significantly higher risk of vascular complications and bleeding than males.14,15 Even the use of intra-aortic balloon pump (IABP) which typically uses a smaller sheath compared to IPVADs has been associated with a higher risk of serious vascular complications among females.16 Females often have smaller iliofemoral vessels compared to males, this difference persists even after adjusting for height, weight, and other comorbidities known to affect vascular anatomy, this could potentially account for the higher risk of complications among females.17 As the innovation in device technology continues, a newer version of IPVADs is currently under investigation under the name Impella-ECP (Expandable Cardiac Power). The new device will be able to be introduced with a small nine French sheath, which expands to 15 French in the descending aorta eliminating the need for a larger diameter for vascular access. Though yet to be studied, this can lead to a decrease in vascular and bleeding complications especially among females, which are often access site related. A downtrend in access site complications has been noted with other large-bore sheath access procedures like transcatheter aortic valve replacement, in part due to the decrease in sheath size.18
Interestingly, while females’ outcomes were comparable to the matched male cohort when IPVADs were used in CS, we observed worse outcomes among females with the use of IPVADs as an adjunct for HRPCI. The worse MAEs in females were mainly driven by higher rates of major bleeding compared to males. Bleeding has been the Achilles heel of IPVADs use. Bleeding arises as a complication not only due to the procedure and due to the use of large sheath size but also secondary to post-procedure bleeding risk due to the need for anticoagulation. This reiterates the value of risk stratification to guide optimum patient selection and proper planning for the procedure. A recent report from the PROTECT III trial on HRPCI, showed that when IPVADs were used as bailout strategy without prior planning, in-hospital mortality was significantly higher compared to a planned prophylactic approach (41.9 vs. 4.3%; p < .01). Moreover, more females were treated with the bailout strategy, which may explain the worse outcomes among females compared to males in the HRPCI cohort.19 Further studies should focus on the underlying mechanisms behind the discrepancies in the outcomes between males and females.
5 |. STUDY LIMITATIONS
The current study has several limitations that need to be acknowledged. First, the NRD is an administrative data, which comes with uncertainty pertaining to some diagnoses as we rely on the appropriate capture of ICD 10 codes. Second, the NRD does not provide data on medications such as antiplatelets and anticoagulants and hence we could not adjust for these in the current analysis. Third, the NRD only identify readmissions to the same original state and hence we could not identify patients who were readmitted to out of state hospitals. Fourth, any management that could have been done in the outpatient settings would not be captured by the NRD since the database only captures in-patient admissions.
6 |. CONCLUSIONS
Among all-comers who received IPVADs, females had higher rates of in-hospital MAEs, including mortality, vascular complications and bleeding complications with a higher 30-day readmission rate. In addition, higher morbidity derived mainly by higher rates of major bleeding was seen among females who received IPVADs for the hemodynamic support during HRPCI and comparable outcomes were observed when the IPVADs was used for cardiogenic shock. Further studies targeting gender discrepancies in the outcomes are warranted.
ACKNOWLEDGEMENTS
All figures were created at https://biorender.com/.
Abbreviations:
- AHRQ
agency for healthcare research and quality
- CS
cardiogenic shock
- HRPCI
high-risk percutaneous coronary intervention
- IPVADs
impellor pumps percutaneous ventricular assist devices
- NRD
national readmission database
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015;65:e7–e26. [DOI] [PubMed] [Google Scholar]
- 2.Members WC, Levine GN, Bates ER, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33–38. [DOI] [PubMed] [Google Scholar]
- 4.Basir MB, Kapur NK, Patel K, et al. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93:1173–1183. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126:1717–1727. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty MP, Moses JW, Westenfeld R, et al. Impella support and acute kidney injury during high-risk percutaneous coronary intervention: the global cVAD renal protection study. Catheter Cardiovasc Interv. 2020;95:1111–1121. [DOI] [PubMed] [Google Scholar]
- 7.Alasnag M, Truesdell AG, Williams H, et al. Mechanical circulatory support: a comprehensive review with a focus on women. Curr Atheroscler Rep. 2020;22:11. [DOI] [PubMed] [Google Scholar]
- 8.Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38:3523–3531. [DOI] [PubMed] [Google Scholar]
- 9.Basir MB, Schreiber T, Dixon S, et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the D etroit cardiogenic shock initiative. Catheter Cardiovasc Interv. 2018;91:454–461. [DOI] [PubMed] [Google Scholar]
- 10.HCUP National Readmission Database (NRD). Healthcare Cost and Utilization Project (HCUP). 2015-2017. Agency for Healthcare Research and Quality R, MD. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. [PubMed] [Google Scholar]
- 11.Yoon FSM, Jiang HJ, Steiner CA, Barrett ML. Calculating Nationwide readmissions database (NRD) variances. HCUP methods. Series Report # 2017–01 ONLINE. U.S. Agency for Healthcare Research & Quality. Published January24, 2017. [Google Scholar]
- 12.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141:273–284. [DOI] [PubMed] [Google Scholar]
- 13.Shah M, Patnaik S, Patel B, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107:287–303. [DOI] [PubMed] [Google Scholar]
- 14.Mwipatayi BP, Picardo A, Masilonyane-Jones TV, et al. Incidence and prognosis of vascular complications after transcatheter aortic valve implantation. J Vasc Surg. 2013;58:1028–1036.e1. [DOI] [PubMed] [Google Scholar]
- 15.Blomkalns AL, Chen AY, Hochman JS, et al. Gender disparities in the diagnosis and treatment of non–ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the American College of Cardiology/American Heart Association guidelines) national quality improvement initiative. J Am Coll Cardiol. 2005;45:832–837. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson JJ, Cohen M, Freedman RJ, et al. The current practice of intra-aortic balloon counterpulsation: results from the benchmark registry. J Am Coll Cardiol. 2001;38:1456–1462. [DOI] [PubMed] [Google Scholar]
- 17.Tran K, Dorsey C, Lee JT, Chandra V. Gender-related differences in iliofemoral arterial anatomy among abdominal aortic aneurysm patients. Ann Vasc Surg. 2017;44:171–178. [DOI] [PubMed] [Google Scholar]
- 18.Scarsini R, De Maria GL, Joseph J, et al. Impact of complications during transfemoral transcatheter aortic valve replacement: how can they be avoided and managed? J Am Heart Assoc. 2019;8:e013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.William W, O’Neill HFH, Moses JW, Popma JJ. United States; Columbia University medical center, United StatesOutcomes of impella use as prophylacticversus bailout strategy in patients undergoing non-emergent percutaneous coronary intervention. Catheter Cardiovasc Interv. 2020;95(suppl 2):S54–S55. [Google Scholar]


