Abstract
Background:
Sympathetic nervous system plays a central role in the development and persistence of essential hypertension. In recent years renal sympathetic denervation (RSD) has emerged as a promising option for the treatment of patients with hypertension.
Methods:
We conducted a literature search of PubMed, EMBASE, Cochrane library and Clinicaltrials.gov from inception through April 20, 2020. Outcomes of interest were change in 24-hour ambulatory systolic (ASBP) or diastolic blood pressure (ADBP) and change in office systolic (OSBP) or diastolic blood pressure (ODBP). We pooled data from randomized controlled trials (RCTS) comparing RSD to sham procedures in the management of hypertension using the random effect model.
Results:
A total of 1,363 patients from eight studies were included in the current meta-analysis. The mean age of the included patients was 56 ± 2.6 years, 29% were women and the median duration of maximum follow up was 6-month (range 3–12 month). There was more reduction favoring RSD in ASBP (Weighted mean difference [WMD] -3.55; 95% CI -4.91 – -2.19, p < .001, I2 = 0%), ADBP (WMD -1.87; 95% CI -3.07 – -0.66, p = .002, I2 = 43%), OSBP (WMD -5.5; 95% CI -7.59 – -3.40, p < .001, I2 = 7%) and ODBP (WMD -3.20; 95% CI -4.47 – -1.94, p < .001, I2 = 14%).
Conclusion:
The use of RSD for the management of hypertension resulted in effective reduction in the ambulatory and office blood pressure compared to sham procedure. Adequately powered RCTs of RSD are needed to confirm safety, reproducibility and assess the impact on clinical outcomes.
Keywords: hypertension, renal sympathetic denervation, resistant hypertension
1 |. INTRODUCTION
Hypertension is the leading cardiovascular risk factor for mortality and morbidity worldwide1,2 The benefits of intensive blood pressure reduction were reinforced by the systolic blood pressure intervention trial (SPRINT) trial which showed that lower blood pressure targets were associated with significant cardiovascular risk reduction with concomitant drop in all-cause mortality.3 However, despite the availability of multiple anti-hypertensive medications, data from the national health and nutritional survey show that lifelong polypharmacy is failing as a therapeutic strategy due to lack of awareness and compliance with medications.4 The significant cost in terms of morbidity and mortality associated with uncontrolled hypertension calls for nonpharmacologic approaches for management.
Renal sympathetic nerves play a central role in the development and persistence of essential hypertension.5,6 Renal sympathetic denervation(RSD) is being studied as a non-pharmacological alternative both as a standalone and adjunct therapy. Over the past decade several studies looked at the efficacy of RSD for the management of hypertension with conflicting results.7–9 Most recently, the results of Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED) Pivotal trial demonstrated the superiority of RSD compared with a sham procedure in lowering the blood pressure in the absence of antihypertensive medications.10 Previous meta-analysis lacked pertinent subgroups analysis and was conducted before the publications of the recent trials.11 Hence, in the current analysis we conducted a systematic review and meta-analysis of RCTs which investigated the efficacy of RSD in the management of hypertension.
2 |. METHODS
2.1 |. Search strategy
We conducted a literature search of PubMed, EMBASE, Cochrane library, Clinicaltrials.gov and Conference proceedings, from inception through April 20, 2020. The search strategy combined the terms “Hypertension”, “Blood Pressure”“Renal Denervation”, “Renal Sympathetic Denervation” and “Sympathetic Denervation”. We utilized the “related articles” function in PubMed to find relevant articles which were missed by the initial search. In addition, reference lists of included studies were hand searched to further locate relevant articles that were missed by the primary search. Our search and meta-analysis were conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) Statement 2015.12 The protocol of this study was registered at the Open Science Framework (OSF) and is available online for the public (https://osf.io/sbfvc).
2.2 |. Study selection
Titles and abstracts of studies were screened by two authors. The full texts of relevant articles were reviewed to determine if the study met the inclusion criteria. Any discrepancies were resolved by a third author.
2.3 |. Inclusion criteria
To be included in the current analysis, the study has to fulfill all of the following criteria: (a) randomized controlled trial, (b) includes patients with hypertension treated by RSD, (c) control group treated by sham procedure, (d) reported the outcomes of interest, (e) study is in English language and full text published in a peer-review journal.
2.4 |. Outcomes and definitions
The outcomes of interest included: (a) change in 24-hour ambulatory systolic blood pressure (ASBP), (b) change in 24-hour ambulatory diastolic blood pressure (ADBP), (c) change in office systolic blood pressure (OSBP), (d) change in office diastolic blood pressure(ODBP). For the primary analysis all the outcomes were analyzed at the maximum follow up provided by the included RCTs.
2.5 |. Quality assessment
The methodological quality and risk of bias of included studies was evaluated using the Cochrane Collaboration's tool for risk of bias.
2.6 |. Statistical analysis
The meta-analysis outcomes were pooled using Mantel-Hansel random effects model. DerSimonian and Laird method was used for estimation of heterogeneity. We preferred random effects model to account for potential statistical heterogeneity. Summary estimates were calculated as weighted mean difference (WMD) with 95% confidence intervals (CI). Sensitivity analysis was performed on primary outcomes by exclusion of one trial at a time. Heterogeneity between studies was explored by Cochran Q statistic (p < .05) and I-squared (I2) statistic. All statistical tests were two-sided and P values <.05 were considered significant.
We also performed the following subgroup analyses (a) based on medication status by dividing the studies into those which allowed the patients to be on antihypertensive medications (ON Medication) versus studies which mandated all patients to be off antihypertensive medications (OFF Medication) and (b) based on the whether a first generation monopolar catheter versus second generation multipolar catheter was used (First versus Second generation trials). Furthermore, to study the effect of difference in duration of follow up between the trials, we performed an additional analysis looking at the heterogeneity of the treatment effect by dividing the studies into two groups, studies which reported outcomes at >3 month of follow-up versus studies reporting the outcomes at ≤3 month of follow up, differences between groups were assessed by formal p- for interaction testing, a p for interaction value of ≤.05 was deemed statistically significant. We did not examine publication bias due to small number of studies (<10). All outcomes were analyzed according to the intention to treat method. All analyses were conducted using “Admetan” commands from Stata, 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
3 |. RESULTS
The initial database search retrieved 5,929 articles. After excluding duplicates, a total of 4,485 articles were screened for eligibility by reading the title and abstract of the study. A total of 18 articles were then screened using the predetermined inclusions criteria to assess eligibility. Details of the study selection process are reported following the PRISMA-P guidelines (Figure 1). The PRISMA checklist of the meta-analysis is shown in (eTable 1). The risk of bias table and summary graph for the included trials are shown in the online supplement (eTable 2 and eFigure 1).
FIGURE 1.
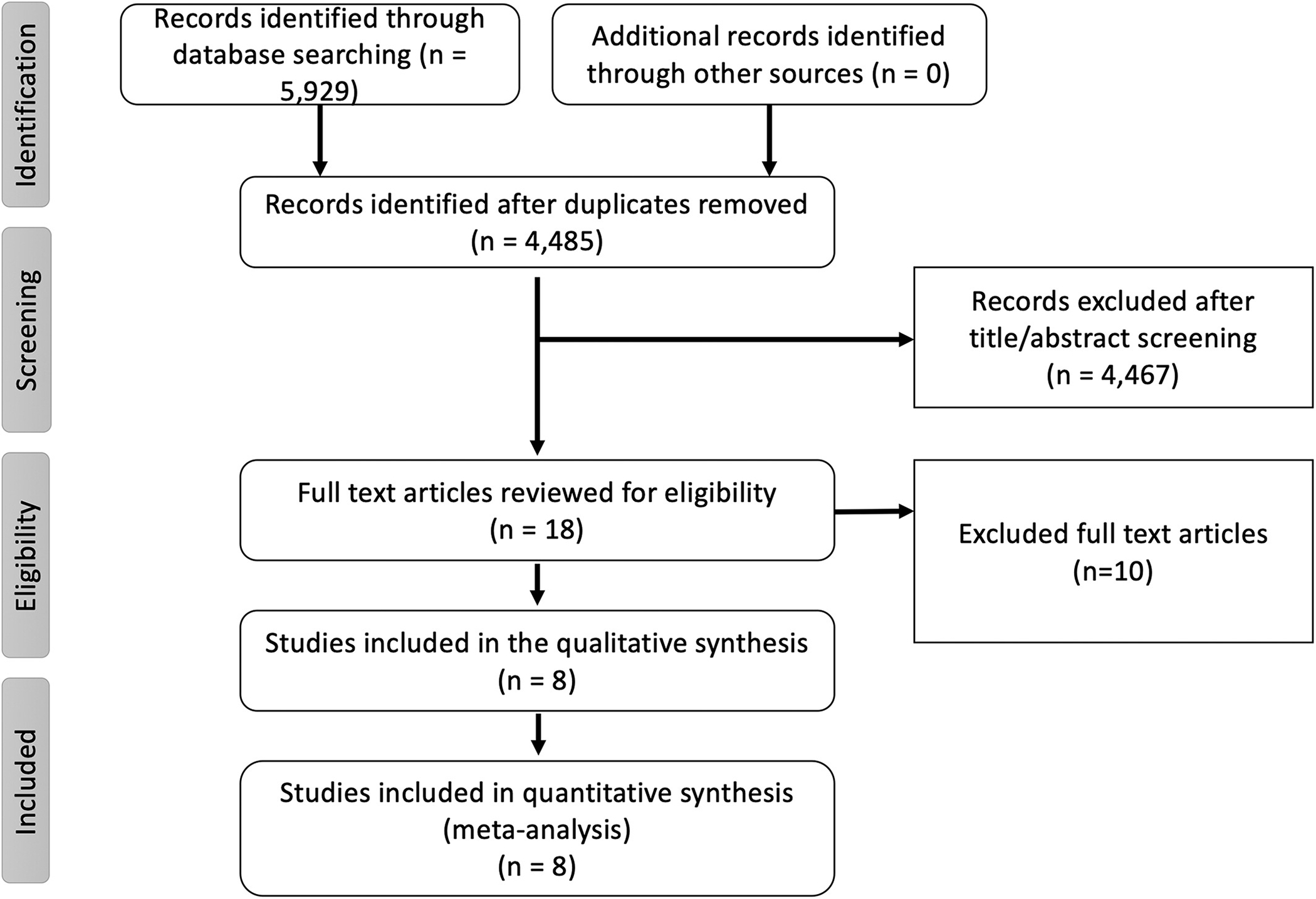
Flow chart of the meta-analysis as per the preferred reporting system of systematic review and meta-analysis (PRISMA)
A total of 1,363 patients from 8 studies were included in the current meta-analysis.7–9,13–17 The mean age of the included patients was 56 ± 2.6 years, 29% were women and the median duration of maximum follow up was 6 month (range 3–12 month). The details of the baseline characteristics of the included studies are shown in eTable 3.
For the ASBP, data was available from all the included studies compromising 1,363 patients (RSD = 785, Sham = 578). There was more reduction in the systolic blood pressure favoring RSD versus sham (WMD-3.55; 95% CI -4.91 – -2.19, p < .001, I2 = 0%) (Figure 2). This persisted when the studies were divided into groups based on ON medication versus OFF medication status (p for interaction = .79) and first-generation studies versus second generation studies (p for interaction = .20) (Figure 3).
FIGURE 2.
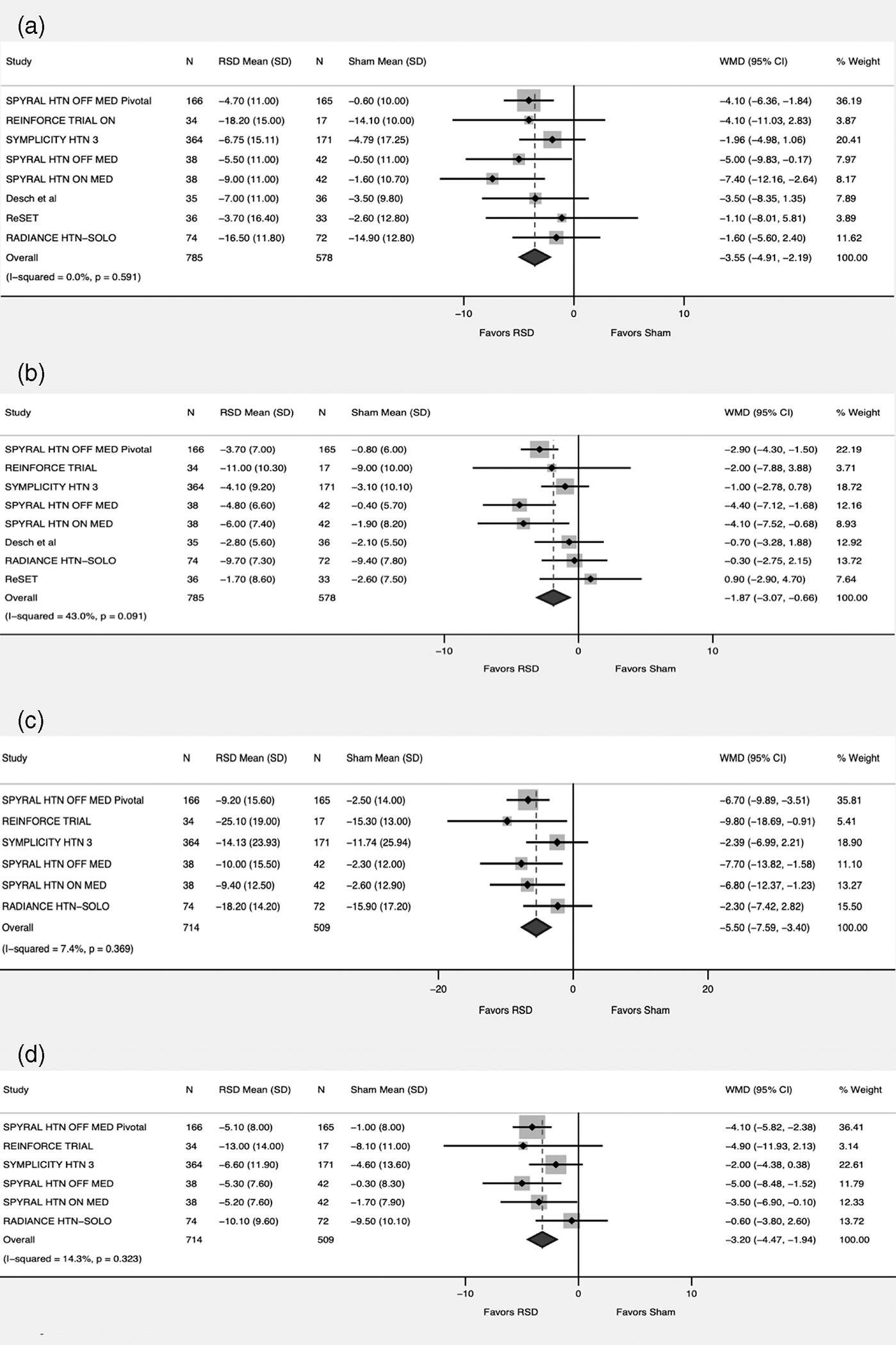
Forest plot comparing the outcomes between renal sympathetic denervation (RSD) and sham procedure. (a) Ambulatory systolic blood pressure. (b) Ambulatory diastolic blood pressure. (c) Office systolic blood pressure. (d) Office diastolic blood pressure
FIGURE 3.
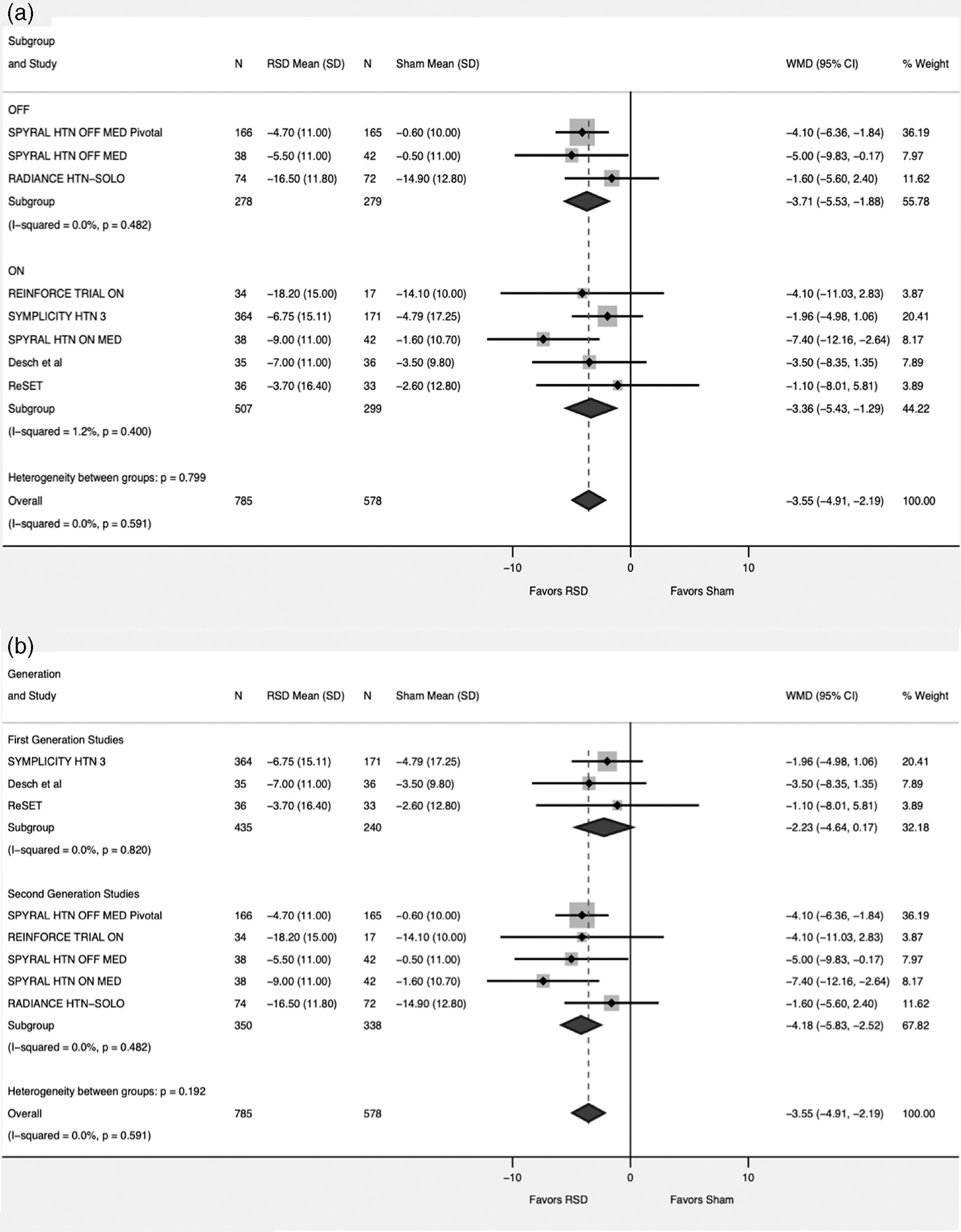
Forest plot comparing the ambulatory systolic blood pressure between renal sympathetic denervation (RSD) and sham procedure. (a) subgroup analysis based on medications status (ON = studies allowing patients to continue on antihypertensive medications, OFF = Studies discontinuing antihypertensive medications). (b) subgroup analysis based on First versus Second generation studies
For the ADBP, data was available from all the included studies compromising 1,363 patients (RSD = 785, Sham = 578). There was more reduction favoring RSD versus sham (WMD -1.87; 95% CI -3.07 – -0.66, p = .002, I2 = 43%) (Figure 2).In the subgroup analysis we observed heterogeneity of the treatment effect when studies were divided into two groups based on the antihypertensive medications status (p for interaction .009). While RSD continued to have favorable effect on ADBP in the OFF-Medication group (WMD -3.22; 95% CI -4.46 – -1.97, p < .001, I2 = 0%), this was lost in the ON group (WMD -1; 95% CI -2.11–-0.11, p = .07, I2 = 0%). Moreover, the analysis based on first versus second generation studies there was significant heterogeneity between the two groups (p for interaction =0.02). Second generation studies showed favorable effect of RSD versus sham (WMD -2.73; 95% CI -4.17 – -1.29, p < .001, I2 = 43%). This did not hold true in the subgroup including first generation studies (WMD -0.67; 95% CI -2.04 – 0.7, p = .33, I2 = 0%). (Figure 4).
FIGURE 4.
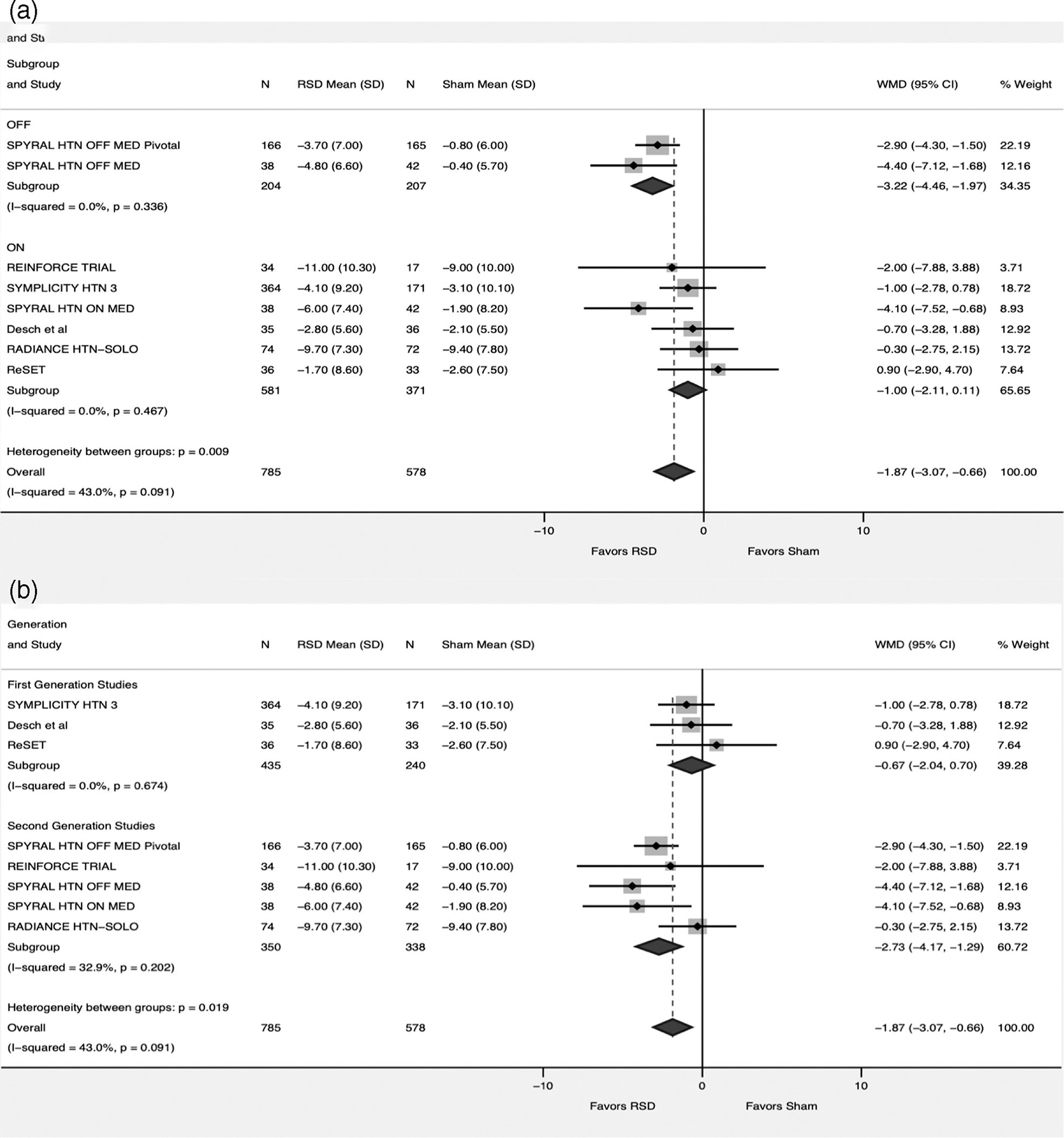
Forest plot comparing the ambulatory diastolic blood pressure between renal sympathetic denervation (RSD) and sham procedure. (a) subgroup analysis based on medications status (ON = studies allowing patients to continue on antihypertensive medications, OFF = Studies discontinuing antihypertensive medications). (b) subgroup analysis based on First versus Second generation studies
For the OSBP, data was available from six studies compromising 1,223 patients (RSD = 714, Sham = 509). There was more reduction favoring RSD versus sham (WMD -5.5; 95% CI -7.59 – -3.40, p < .001, I2 = 7%)(Figure 2). This persisted when the studies were divided into groups based on ON-medication versus OFF-medication status (p for interaction = 0.17) (eFigure 2). We were not able to conduct an analysis for this outcome based on first generation trials versus second generation trials due to low number of first-generation trials reporting the outcome.
For the ODBP, data was available from six studies compromising 1,223 patients (RSD = 714, Sham = 509). There was more reduction favoring RSD versus sham (WMD -3.20; 95% CI -4.47 – -1.94, p < .001, I2 = 14%) (Figure 2). This persisted when the studies were divided into groups based on ON-medication versus OFF-medication status (p for interaction = .06) (eFigure 2). We were not able to conduct an analysis for this outcome based on first generation trials versus second generation trials as only one study from the first-generation trial that reported this outcome.
In the analysis based on different durations of follow-up studies were divided into two group, studies reporting outcomes at >3 month of follow-up and studies reporting outcomes at ≤3 month of follow up. The effect of RSD on all the outcomes persisted regardless of the duration of follow-up (eFigure 3, 4).
4 |. DISCUSSION
In the current meta-analysis of eight studies comparing RSD to sham procedure in more than 1,000 patients, we observed several findings. First, RSD resulted in significant reduction in systolic blood pressure (both in ambulatory and office setting) compared to sham procedure. Second, RSD resulted in albeit smaller but statistically significant reduction in diastolic blood pressure (both ambulatory and office setting) compared to sham procedure. Third, the favorable effect of RSD on systolic blood pressure persisted regardless of the medication's status or duration of follow-up.
The interest in the field of catheter based sympathetic renal denervation was generated by early open label, nonblinded and nonrandomized trials using monopolar radiofrequency ablation catheter showing a rather large magnitude of reduction in primary end point of office systolic and diastolic blood pressure with RSD.13,14 Despite promising results from small studies, catheter based RSD suffered a major setback with results of the first-generation trials. The largest first-generation pivotal trial was SYMPLICITY HTN 3 trial which was the first major prospective randomized blinded sham-controlled trial.8 The trial failed to meet its primary and secondary efficacy end points of reduction in office systolic blood pressure and 24 hour ambulatory blood pressure with RSD.8 However, SYMPLICITY HTN 3 trial had several shortcomings including ineffective and incomplete renal denervation, use of first generation unipolar radiofrequency ablation catheter that failed to achieve circumferential ablation, ablation of only the main renal arteries without ablation of branch vessels, and lack of consistency between operators and confounding introduced by variable drug adherence and changes in the antihypertensive regimen during the course of the trial.8,15,16 Furthermore, the trial included patients with multiple comorbidities, long standing resistant hypertension and isolated systolic hypertension.8,16 Vascular stiffness may play the predominant role in this subset and sympathetic drive is less likely to play an important role in driving the elevation in blood pressure.15
Insights from the SIMPLICITY HTN 3, understanding of renal nerve anatomy and histology from cadaveric studies, along with changes in trial design, patient selection and ablation techniques led to several second-generation trials.7,17–21 The second-generation renal denervation trials used a multi-electrode flexible catheter which was able to achieve circumferential ablation and ablation extending into the branches of the renal arteries. The SPYRAL HTN OFF med proof of concept trial, was the first among the second-generation trials to use the multielectrode catheter and showed benefit of renal denervation compared to sham control, followed by SPYRAL HTN ON med and RADIANCE-HTN SOLO proof of concept trials.7,9,17
Patient selection in the second-generation trials differed compared to first-generation trials with RSD used an alternative to medical therapy or adjunct therapy. In the alternative therapy strategy, patients were completely taken off medications to help isolate the effect of renal denervation and to remove the confounding introduced by variable drugs adherence and changes in medications during the trial. In the adjunct strategy, standardized protocol for medication adherence testing and titration was performed during the study period. Moreover, the second generation trials used the change in 24 hour ambulatory blood pressure as primary outcome of interest which is a more reliable marker of efficacy of treatment, and has been shown to be a better predictor of cardiovascular events compared to office blood pressure measurements.22
Another concept that has emerged from these trials is that the benefit of renal denervation accrues over a longer period of follow up as shown by the six month results of RADIANCE HTN SOLO trial and REINFORCE trial which showed benefit of RSD at 12 month but not at 8 weeks of follow-up.17,21
The results of our meta-analysis confirm the results of second-generation RSD trials. Although the observed magnitude of blood pressure reduction achieved by RSD may appear small, studies have shown that even a 2-mmHg reduction in mean systolic blood pressure is associated with 10% reduction in stroke mortality and 7% reduction in cardiovascular mortality.23
4.1 |. Limitations
There are several limitations of this analysis that need to be acknowledged. First, there was significant heterogeneity in patient population enrolled in these trials and difference in the technologies used. Second, among the included studies, five were relatively small with less than 100 patients included in each study which may underestimate the true effect of RSD. Third, this was a study level meta-analysis and hence we did not have access to patienťs level data to adjust for comorbidities. Despite these limitations this updated meta-analysis confirms proof of principle that renal denervation produces sustained, statistical and clinically significant reduction in blood pressure.
5 |. CONCLUSION
In the current meta-analysis of RCTs, RSD had favorable reduction in both ambulatory and office systolic and diastolic blood pressure compared to sham procedure. The favorable effect of RSD on blood pressure persisted regardless of medication use and duration of follow-up. Adequately powered RCTs of RSD are needed to confirm safety, reproducibility and assess the impact on clinical outcomes.
Supplementary Material
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable requests.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2.Lemesle G, Bauters A, Bauters C. Antithrombotic therapy in diabetic patients with coronary artery disease. Panminerva Med. 2015;57:87–99. [PubMed] [Google Scholar]
- 3.Wright JT Jr, Williamson JD, Whelton PK, et al. Randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. [DOI] [PubMed] [Google Scholar]
- 5.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. [DOI] [PubMed] [Google Scholar]
- 6.Schlaich MP, Lambert E, Kaye DM, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. [DOI] [PubMed] [Google Scholar]
- 7.Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017; 390:2160–2170. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370: 1393–1401. [DOI] [PubMed] [Google Scholar]
- 9.Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 10.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015; 373:929–938. [DOI] [PubMed] [Google Scholar]
- 11.Sardar P, Bhatt DL, Kirtane AJ, et al. Sham-controlled randomized trials of catheter-based renal denervation in patients with hypertension. J Am Coll Cardiol. 2019;73:1633–1642. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 14.Investigators SH. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. The Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 15.Esler M. Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8:593–598. [DOI] [PubMed] [Google Scholar]
- 16.Kandzari DE, Bhatt DL, Brar S, et al. Predictors of bl response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 18.Bohm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. [DOI] [PubMed] [Google Scholar]
- 19.Desch S, Okon T, Heinemann D, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202–1208. [DOI] [PubMed] [Google Scholar]
- 20.Peters CD, Mathiassen ON, Vase H, et al. The effect of renal denervation on arterial stiffness, central blood pressure and heart rate variability in treatment resistant essential hypertension: a substudy of a randomized sham-controlled double-blinded trial (the ReSET trial). Blood Press. 2017;26:366–380. [DOI] [PubMed] [Google Scholar]
- 21.Weber MA, Kirtane AJ, Weir MR, et al. The REDUCE HTN: REINFORCE: randomized, sham-controlled trial of bipolar radiofrequency renal denervation for the treatment of hypertension. JACC Cardiovasc Interv 2020;13:461–470. [DOI] [PubMed] [Google Scholar]
- 22.Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. [DOI] [PubMed] [Google Scholar]
- 23.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable requests.


