Abstract
Background:
Limited evidence is available regarding low (24/26 mg) and middle (49/51 mg) doses of sacubitril/valsartan.
Objectives:
The purpose of this study was to investigate the effect of sacubitril/valsartan dose on heart failure (HF) hospitalization and mortality in patients with HF with reduced ejection fraction (HFrEF).
Methods:
A retrospective multicenter cohort study compared 3 doses of sacubitril/valsartan in patients with HFrEF. The coprimary outcomes were all-cause mortality and rehospitalization for HF. Propensity matching analysis was performed.
Results:
Of 721 eligible patients, propensity matching created a cohort with an effective sample size of 652 (24/26-mg group [n = 326], 49/51-mg group [n = 147], 97/103-mg group [n = 179]). The HF hospitalization rates were 29.14% in the 24/26-mg group, 19.51% in the 49/51-mg group, and 16.10% in the 97/103-mg group (24/26 vs 49/51 mg: HR = 1.56, 95% CI = 1.04–2.34; 24/26 vs 97/103 mg: HR = 1.79, 95% CI = 1.18–2.73; 49/51 vs 97/103 mg: HR = 1.15, 95% CI = 0.70–1.89). All-cause mortality rates were 29.63% in the 24/26-mg group, 17.58% in the 49/51-mg group, and 9.27% in the 97/103-mg group (24/26 vs 49/51 mg: HR = 1.67, 95% CI = 1.07–2.59; 24/26 vs 97/103 mg: HR = 2.56, 95% CI = 1.54–4.24; 49/51 vs 97/103 mg: HR = 1.54, 95% CI = 0.84–2.82).
Conclusion and Relevance:
Sacubitril/valsartan 97/103- or 49/51-mg dose is associated with a lower mortality or hospitalization rate for HF in patients receiving sacubitril/valsartan compared with the 24/26-mg dose group.
Keywords: sacubitril, valsartan, heart failure, dose dependence, neprilysin inhibitor
Introduction
Currently, the American College of Cardiology/American Heart Association/Heart Failure Society of America guideline update in 2017 recommends replacing angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blocker (ARB) with angiotensin receptor-neprilysin inhibitor to further reduce mortality or the heart failure (HF) rehospitalization rate in patients with symptomatic HF with reduced ejection fraction (HFrEF).1 However, little evidence is available regarding low (24/26 mg) and middle (49/51 mg) doses because the PARADIGM-HF trial required dose titration up to a target dose (97/103 mg) before randomization.2 A post hoc analysis of the PARADIGM-HF trial showed that patients who had dose reduction to the low or middle dose of sacubitril/valsartan or enalapril had a significantly higher risk of cardiovascular death or rehospitalization for HF than those maintained on the target dose of either medication.3 However, the PARADIGM investigators did not evaluate the difference in clinical outcomes between the sacubitril/valsartan low versus middle doses. The PIONEER-HF trial evaluated sacubitril/valsartan among patients hospitalized for acute decompensated HF, and the study included patients on lower or middle doses.4 However, the follow-up period was 8 weeks, and the primary outcome was the time-averaged proportional change in the NT-pro-B-type natriuretic peptide (BNP); hospitalization for HF and mortality were just exploratory outcomes. Also, the dose dependence was not investigated in the trial. Thus, the trial results did not answer sacubitril/valsartan dose dependence based on clinical outcomes. In the real-world setting, a contemporary US outpatient HFrEF registry showed that only 14% of patients with HFrEF are on the target dose of sacubitril/valsartan.5 Significantly different sacubitril/valsartan dose patterns may exist between the contemporary real-world setting and the PARADIGM trial, and the significant gaps may imply that a lot of patients may only maximally tolerate sacubitril/ valsartan lower doses than the target dose in the real-world setting. Thus, our real-world analysis was designed to evaluate the effect of sacubitril/valsartan dose (low vs middle vs target dose) on hospitalization for HF and mortality rates in patients with HFrEF.
Methods
Study Design and Population
A retrospective, multicenter cohort study was conducted to compare 3 doses of sacubitril/valsartan (24/26, 49/51, and 97/103 mg) in patients with HFrEF. Patients >18 years old who were diagnosed with HFrEF (ejection fraction [EF] ≤ 40%) and received sacubitril/valsartan from July 2015 to December 2019 were included in this retrospective cohort study. The follow-up period was at least 1 year. Patients who died within 1 year while on sacubitril/valsartan were included if they received sacubitril/valsartan for at least 1 month. Both inpatients and outpatients were included. Patients receiving sacubitril/valsartan for less than 1 year or undergoing dialysis were excluded. All sacubitril/valsartan pharmacy claims during the follow-up period were reviewed to assign the patients to each dose group. The most frequent dose during the follow-up period was used to assign the patients to each dose group. The study was approved by the West Virginia University (WVU) institutional review board.
Data Collection
Data were obtained from West Virginia Clinical and Translational Science Institute database developed at WVU Medicine that includes patients’ demographics and clinical data sets to conduct a clinical research. Investigators also did a retrospective chart review to collect supplemental patient information. Patient demographics and comorbidities, baseline medication history, laboratory data, and EF data were collected from the closest date prior to the index date. EF was measured by transthoracic or transesophageal echocardiogram.
Outcomes
The coprimary outcomes were all-cause mortality and hospitalization for HF. Outcome events were counted if events occurred at the WVU Medicine affiliated institutions or events were documented in electronic records. Events over 3.5 years following the index date were recorded. If the death record was not found in the medical records, the observational medical outcomes partnership database was also searched for survival status verification. Hospitalization for HF was defined as at least 24-hour inpatient stay with the primary diagnosis of worsening HF treated with an additional diuretic, intravenous inotrope, or intravenous vasodilator.
Statistical Analysis
ANOVA for continuous variables and a χ2 test for categorical variables were used to analyze the baseline characteristics. To examine the association between the sacubitril/ valsartan doses and hospitalization for HF or all-cause mortality, propensity score matching was conducted first, and then a weighted multivariable Cox proportional-hazard model was run where age, body mass index (BMI), BNP, creatinine, EF, sex, systolic blood pressure, prior ACE inhibitor or ARB use, β-blocker, mineralocorticoid receptor antagonist, and loop diuretics were accounted for. The selection of these confounders was based on both the corresponding P values from the baseline characteristics between the groups and the investigators’ clinical judgment. The significance level was set at 0.05. Statistical analyses were performed using R 4.0.3 (R Core Team, Vienna, Austria, 2020) and the twang (v1.6; Ridgeway et al, 2020; https://cran.r-project.org/web/packages/twang/index.html) package.
Results
A total of 351 patients were included in the 24/26-mg group, 165 patients in the 49/51-mg group, and 205 patients in the 97/103-mg group. The propensity score matching generated a cohort of 652 patients (24/26-mg group [n = 326], 49/51-mg group [n = 147], and 97/103-mg group [n = 179]; Figure 1). The baseline characteristics are shown in Table 1. In the unmatched cohorts, age, systolic blood pressure, BMI, serum creatinine, BNP, and loop diuretic use were significantly different between the 3 groups (Table 1). After propensity matching, all baseline characteristics were well balanced between the 3 groups (Table 1). The mean age (±SD) was 65.23 ± 12.70 years; 69.2% were male, and 95.7% were Caucasian. The mean systolic blood pressure was 121.06 ± 19.23 mm Hg, and the mean EF was 26.25 ± 8.59%. The median BNP was 631.5 pg/mL (interquartile range, 227.5–1465.0). The mean follow-up period was 1.82 ± 0.77 years.
Figure 1.
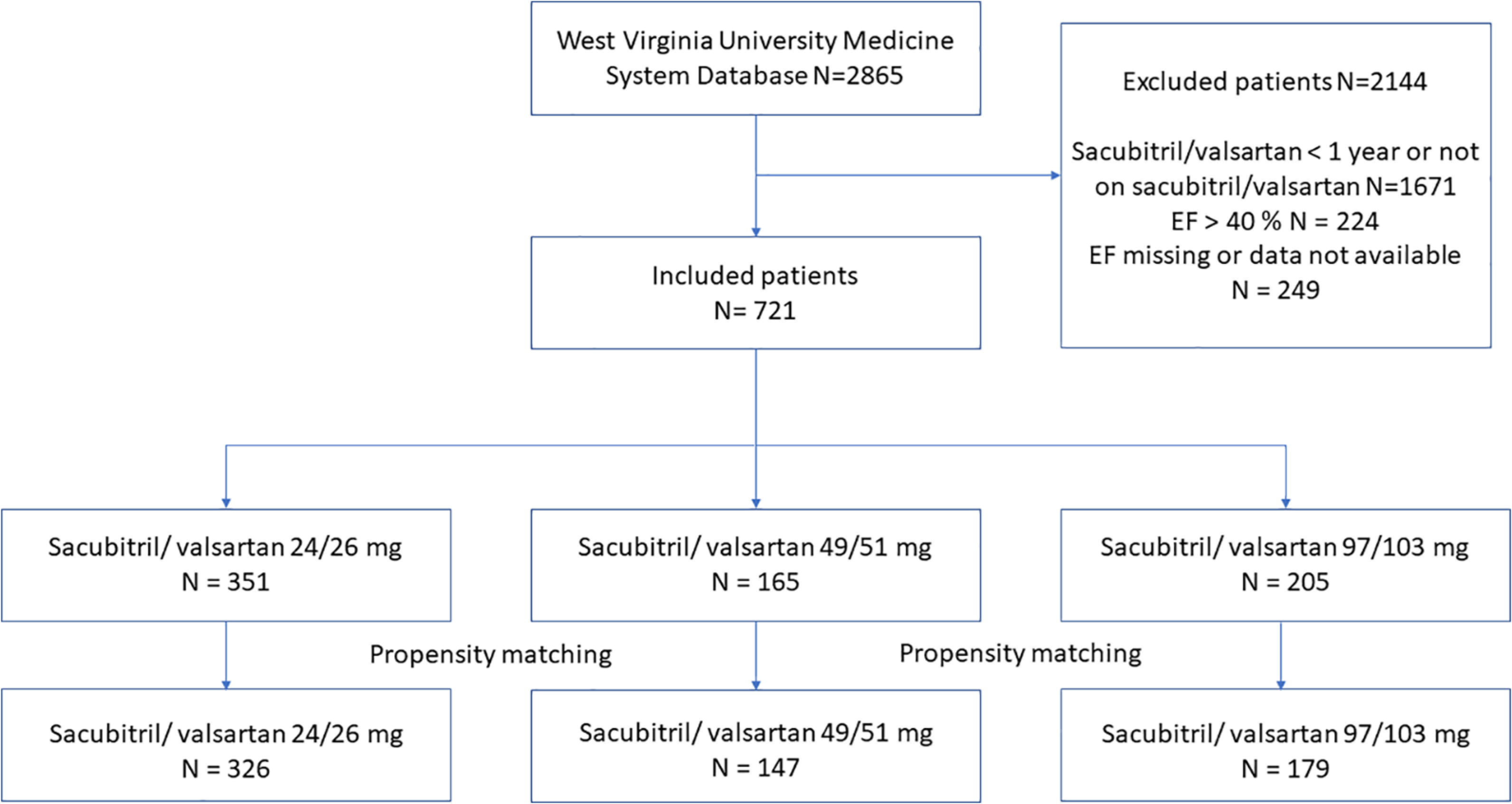
Patient selection flow diagram.
Abbreviation: EF, ejection fraction.
Table 1.
Baseline Characteristics in Unmatched and Propensity Score-Matched Groups.
| Unmatched groups |
Propensity score-matched groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| 24/26 mg (n = 351) | 49/51 mg (n = 165) | 97/103 mg (n = 205) | P value | 24/26 mg (n = 326) | 49/51 mg (n = 147) | 97/103 mg (n = 182) | P value | |
|
| ||||||||
| Age (years) | 68.02 ±12.16 | 64.40 ± 12.54 | 61.1 1 ± 12.57 | <0.001 | 66.16 ± 12.34 | 64.83 ± 12.32 | 63.60 ± 12.29 | 0.229 |
| Male sex (%) | 66.7 | 72.7 | 70.7 | 0.325 | 68.1 | 72.8 | 72.9 | 0.258 |
| Ethnicity (%) | 0.859 | |||||||
| White | 96.6 | 95.2 | 95.1 | |||||
| Black | 2.0 | 3.0 | 2.9 | |||||
| Hispanic | 0.6 | 0.0 | 0.5 | |||||
| Unknown | 0.9 | 1.8 | 1.5 | |||||
| HTN (%) | 84.0 | 81.8 | 77.6 | 0.162 | 83.4 | 79.8 | 79.4 | 0.261 |
| DM (%) | 53.6 | 50.9 | 47.3 | 0.363 | 52.1 | 49.9 | 48.5 | 0.438 |
| SBP, mm Hg | 117.63 ± 18.56 | 125.28 ± 18.83 | 123.55 ± 19.74 | <0.001 | 120.06 ± 18.91 | 122.01 ± 18.13 | 122.16 ± 18.80 | 0.496 |
| BMI (kg/m2) | <0.001 | 0.189 | ||||||
| ≤24.9 | 21.4% | 17.0% | 13.7% | 19.8% | 20.0% | 15.2% | ||
| 25–29.9 | 28.8% | 34.5% | 21.0% | 27.9% | 31.5% | 26.0% | ||
| ≥30.0 | 49.9% | 48.5% | 65.4% | 52.6% | 48.5% | 58.8% | ||
| Creatinine, mg/dL | 1.22 ± 0.44 | 1.24 ± 0.52 | 1.12 ± 0.36 | 0.014 | 1.20 ± 0.42 | 1.20 ± 0.47 | 1.17 ± 0.39 | 0.728 |
| Potassium (mEq/L) | 4.38 ± 0.73 | 4.34 ± 0.49 | 4.24 ± 0.53 | 0.457 | ||||
| BNP, pg/mL | 2144.70 ± 5208.46 | 1463.98 ± 2323.71 | 1072.34 ± 1781.50 | 0.012 | 1793.82 ± 4402.81 | 1523.73 ± 2449.53 | 1 197.34 ± 1792.54 | 0.262 |
| Ejection fraction (%) | 26.42 ± 8.89 | 26.80 ± 8.29 | 25.53 ± 8.29 | 0.326 | 26.21 ± 8.65 | 26.51 ± 8.54 | 25.68 ± 8.45 | 0.700 |
| BB (%) | 91.1 | 88.2 | 86.1 | 0.326 | 93.9 | 94.2 | 92.7 | 0.481 |
| Prior ACE inhibitor/ARB (%) | 65.1 | 76.5 | 73.0 | 0.072 | 79.2 | 85.6 | 83.9 | 0.210 |
| MRA (%) | 66.0 | 65.0 | 68.0 | 0.632 | 66.8 | 59.9 | 68.6 | 0.438 |
| Loop use (%) | 79.1 | 70.6 | 63.5 | 0.004 | 84.4 | 85.3 | 80.8 | 0.418 |
| Digoxin (%) | 15.3 | 15.7 | 14.6 | 0.971 | ||||
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, [3-blocker; BMI, body mass index; BNP, B-type natriuretic peptide; DM, diabetes mellitus; HTN, hypertension; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure.
Univariate analysis in unmatched groups showed that the HF hospitalization rates were 29.14% in the 24/26-mg group, 19.51% in the 49/51-mg group, and 16.10% in the 97/103-mg group (24/26 vs 97/103 mg: HR = 2.17, 95% CI = 1.46–3.21, P < 0.001; 49/51 vs 97/103 mg: HR = 1.23, 95% CI = 0.75–2.00, P = 0.41; 24/26 vs 49/51 mg: HR = 1.77, 95% CI = 1.19–2.63, P = 0.005; Table 2; Figure 2). The all-cause mortality rates were 29.63% in the 24/26-mg group, 17.58% in the 49/51-mg group, and 9.27% in the 97/103-mg group (24/26 vs 97/103 mg: HR = 3.67, 95% CI = 2.25–5.99, P < 0.001; 49/51 vs 97/103 mg: HR = 1.89, 95% CI = 1.06–3.37, P = 0.032; 24/26 vs 49/51 mg: HR = 1.95, 95% CI = 1.29–2.94, P = 0.002; Table 2; Figure 2).
Table 2.
Hospitalization and Mortality Before and After Propensity Matching by Sacubitril/Valsartan Dose Status.
| Prematch | Sacubitri l/valsartan | Hazard ratio | 95% Cl | P value |
|
| ||||
| Heart failure | 24/26 vs 97/103 | 2.167 | 1.46–3.21 | <0.001 |
| hospitalization | 49/51 vs 97/103 | 1.227 | 0.75–2.00 | 0.410 |
| 24/26 vs 49/51 | 1.766 | 1.19–2.63 | 0.005 | |
| All-cause | 24/26 vs 97/103 | 3.671 | 2.25–5.99 | <0.001 |
| mortality | 49/51 vs 97/103 | 1.887 | 1.06–3.37 | 0.032 |
| 24/26 vs 49/51 | 1.945 | 1.29–2.94 | 0.002 | |
|
| ||||
| Postmatch | Sacubitri l/valsartan | Hazard ratio | 95% Cl | P value |
|
| ||||
| Heart failure | 24/26 vs 97/103 | 1.79 | 1.18–2.73 | 0.006 |
| hospitalization | 49/51 vs 97/103 | 1.15 | 0.70–1.89 | 0.588 |
| 24/26 vs 49/51 | 1.56 | 1.04–2.34 | 0.031 | |
| All-cause | 24/26 vs 97/103 | 2.56 | 1.54–4.24 | <0.001 |
| mortality | 49/51 vs 97/103 | 1.54 | 0.84–2.82 | 0.166 |
| 24/26 vs 49/51 | 1.67 | 1.07–2.59 | 0.024 | |
Figure 2.
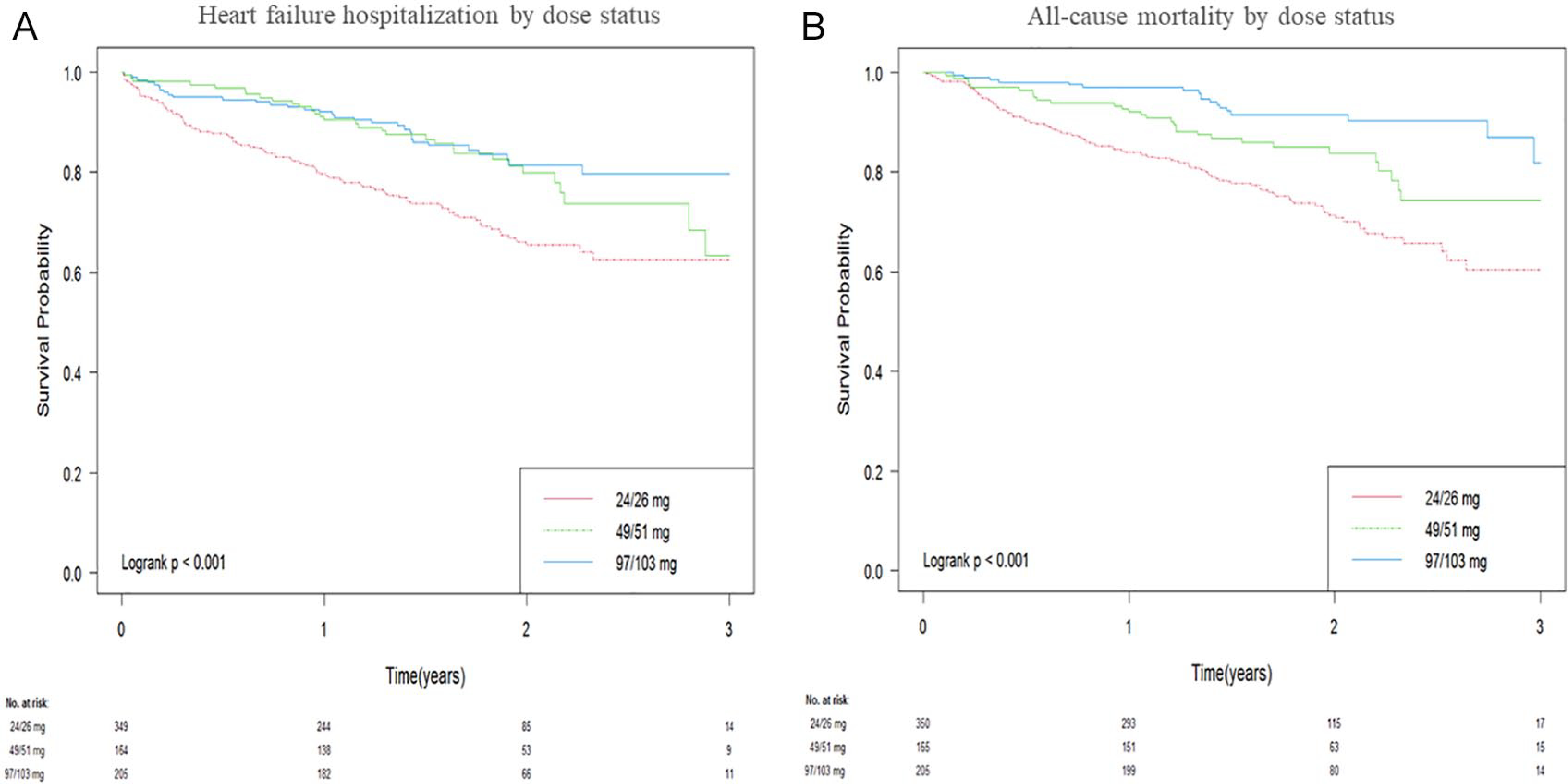
Kaplan-Meier curve for time-to-first (A) hospitalization for heart failure and (B) all-cause mortality, by sacubitril/valsartan dose status.
After propensity matching, the significant difference in the HF hospitalization between the 24/26- and 49/51- or 97/103-mg groups persisted (24/29 vs 97/103 mg: HR = 1.79, 95% CI = 1.18–2.73, P = 0.006; 24/26 vs 49/51 mg: HR = 1.56, 95% CI = 1.04–2.34, P = 0.031; Table 2), but there were still no significant differences in the hospitalization for HF between the 49/51- and 97/103-mg groups (49/51 vs 97/103 mg: HR = 1.15, 95% CI = 0.70–1.89, P = 0.588; Table 2). The significant differences in the all-cause mortality rates between the 24/26- and 49/51- or 97/103-mg groups were also retained (24/26 vs 97/103 mg: HR = 2.56, 95% CI = 1.54–4.24, P < 0.001; 24/26 vs 49/51 mg: HR = 1.66, 95% CI = 1.07–2.59, P = 0.024), whereas there was no significant difference in the all-cause mortality rate between the 49/51- and 97/103-mg groups (HR = 1.54, 95% CI = 0.84–2.82, P = 0.166; Table 2).
Discussion
This is the first large-scale real-world study showing sacubitril/valsartan dose dependence on clinical outcomes in patients with HFrEF. The 24/26-mg dose was associated with significantly higher event rates of all-cause mortality and rehospitalization for HF compared with the 49/51- or 97/103-mg dose. However, there were no significant differences in either all-cause mortality or HF rehospitalization rates between the 49/51- and 97/103-mg groups.
In practice, sacubitril/valsartan doses are uptitrated up to 97/103 mg twice daily, unless there are adverse effects such as hypotension, hyperkalemia, or angioedema, on the basis of the results of a pivotal clinical trial, which support the use of 97/103 mg twice daily in patients with HFrEF.2 However, the external validity of the PARADIGM trial needs to be interpreted cautiously because it included only randomized patients who were able to tolerate 97/103 mg twice daily during a run-in period before randomization. By contrast, the present study included a real-world patient population who received 3 different doses of sacubitril/valsartan. The inability for many patients to tolerate the maximal dose of sacubitril/valsartan and subsequent need for lower maintenance dosing are more realistic and representative of the real-world setting.5 Therefore, our current analysis may be more applicable to the patients with HFrEF in the real-world setting.
Contemporary data on lower doses (24/26 and 49/51 mg) are very sparse. The only large-scale clinical data are the post hoc study of the PARADIGM trial, which showed that any dose reduction group receiving either enalapril or sacubitril/valsartan had a significantly higher event rate of cardiovascular death or hospitalization for HF compared with the target dose group; however, in that study, the 24/26- and 49/51-mg doses were combined into 1 dose reduction group.3 Additionally, this dose reduction group included patients who temporarily or permanently stopped sacubitril/ valsartan. Thus, the study did not provide an answer regarding whether there was a sacubitril/valsartan dose dependence between the 3 dose groups. In contrast, our present study showed that the 24/26-mg dose was associated with a significantly higher event rate of all-cause mortality or hospitalization for HF compared with the 49/51- or 97/103-mg dose, but there was no significant difference between the 49/51- and 97/103-mg groups. Although further investigation with higher level of evidence is needed, our study results showed sacubitril/valsartan dose dependence on clinical outcomes in patients with HFrEF.
Several small-scale real-world studies evaluated the effect of sacubitril/valsartan doses on clinical outcomes in patients with HFrEF.6,7 The study by Chang et al6 found that sacubitril/valsartan dose escalation was associated with lower cardiovascular mortality and hospitalization for HF compared with the subtarget stable dose or dose de-escalation group. This study investigated the effect of dose change but did not specifically evaluate the effect of the sacubitril/ valsartan dose. Another study compared the sacubitril/valsartan target dose group with a suboptimal dose group. However, only 68 patients were included in the study; moreover, the 24/26- and 49/51-mg groups were combined into 1 group.7 Although the study concludes that there was no significant difference with regard to all-cause mortality or HF hospitalization between the 2 groups, type 2 error is highly probable.
ACE inhibitor and ARB dose dependences were also evaluated in multiple clinical trials.8,9 The ATLAS trial compared the low-dose lisinopril group with the high-dose lisinopril group in patients with HFrEF.8 It found that the higher dose of lisinopril was more effective than the lower dose to reduce hospitalization for HF, but there was no significant difference in all-cause mortality between the 2 groups. Losartan dose dependence was also investigated in patients with HFrEF.9 The primary outcome was the composite of all-cause mortality and hospitalization for HF. The high-dose losartan group had a significantly lower event rate of the primary outcome compared with the lower-dose losartan group, but there was no significant difference in all-cause mortality. The higher-dose group had significantly lower rate of hospitalization for HF than the lower-dose group. Taken together, ACE inhibitor/ARB higher dose could lead to further reducing hospitalization rate for HF but no further mortality benefits. Thus, the sacubitril component could have produced conflicting results between our present study (significantly lower all-cause mortality rate in the higher-dose group of sacubitril/valsartan) and previous studies for ACE inhibitor/ARB.
This study has multiple limitations. First, in this study, we divided the whole cohort into 3 dose groups based on the most frequent dose during the follow-up period. When guideline-directed dose titration is attempted in clinical practice, patients rarely stay on only 1 dose throughout the follow-up. Therefore, these investigators felt that this assignment of dose groups was most appropriate, but a dose group assignment based on stable maintenance dose would be ideal. Second, some clinical data were limited by availability of our EMR: for example, device therapies such as implantable cardioverter device or cardiac resynchronization therapy and New York Heart Association functional classification were not included in the current analysis. Third, authors could not access cause of death, and cardiovascular mortality rate was not reported. Fourth, sacubitril/ valsartan dosing instructions were speculated based on pharmacy claims, which include the tablet strength and dispensed quantities. Thus, actual dosing instructions could not be confirmed with patients because of the retrospective study design. Fifth, authors did not calculate a sample size between each dose group because authors decided to collect all eligible patients on sacubitril/valsartan in the WVU system. However, there is a risk for type 2 error between the middle and high doses. Sixth, although our study conducted propensity score matching to adjust variables and the baseline characteristics in the postmatched cohort were statistically well balanced between groups, other unrecognized variables may have influenced the results. Also, patients in the lower-dose group were numerically older, and BNP was numerically higher in the lower-dose group. Although the baseline characteristics were statistically adjusted well, there is a possibility that sicker patients could have been included in the lower-dose group. Randomization is the only way to overcome this possibility completely. Finally, this is a retrospective study and can only show association. These real-world data certainly deserve further investigation with future prospective randomized trials.
Conclusion and Relevance
The results indicate that the sacubitril/valsartan 49/51- or 97/103-mg dose is associated with a lower HF hospitalization or mortality rate in patients receiving sacubitril/valsartan compared with the 24/26-mg dose group. There was no significant difference in the HF hospitalization or mortality rates between the 49/51- and 97/103-mg groups. This analysis suggests that persistent attempts at uptitration of the sacubitril/valsartan dose should be undertaken to improve HF outcomes.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: George Sokos was a speaker for a Novartis Pharmaceutical sponsored presentation. Other authors declare no conflicts of interest.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/ HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 3.Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/ valsartan vs enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Morrow DA, DeVore AD, et al. ; PIONEER-HF Investigators. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 5.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 6.Chang HY, Feng AN, Fong MC, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74:372–380. doi: 10.1016/j.jjcc.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 7.De Vecchis R, Ariano C, Di Biase G, Noutsias M. In HFREF patients, sacubitril/valsartan, given at relatively low doses, does not lead to increased mortality or hospitalization: a retrospective cohort study. Herz. 2019;44:651–658. doi: 10.1007/s00059-018-4690-6 [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999;100:2312–2318. doi: 10.1161/01.cir.100.23.2312 [DOI] [PubMed] [Google Scholar]
- 9.Konstam MA, Neaton JD, Dickstein K, et al. ; HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9 [DOI] [PubMed] [Google Scholar]


