Abstract
In compliance with Article 43 of Regulation (EC) No 396/2005, EFSA received from the European Commission a mandate to provide its reasoned opinion on the toxicological properties and maximum residue levels (MRLs) for the benzimidazole substances carbendazim and thiophanate‐methyl. Specifically, EFSA was asked to assess whether thiophanate‐methyl or carbendazim have clastogenic potential and, in case clastogenic potential can be excluded, to derive toxicological reference values necessary for consumer risk assessment and assessment of maximum residue levels (MRLs). Although these active substances are no longer authorised within the European Union, MRLs were established by the Codex Alimentarius Commission (codex maximum residue limits; CXLs), and import tolerances are in place. Based on the assessment of the available data, toxicological reference values and MRL proposals were derived and a consumer risk assessment was carried out. Some information required by the regulatory framework was found to be missing and a possible acute risk to consumers was identified. Hence, the consumer risk assessment is considered indicative only, all MRL proposals derived by EFSA still require further consideration by risk managers and measures for reduction of the consumer exposure should also be considered.
Keywords: benzimidazole substances, carbendazim, thiophanate‐methyl, MRL, Regulation (EC) No 396/2005, consumer risk assessment
Summary
Carbendazim was firstly included in Annex I to Directive 91/414/EEC in 2006 by Commission Directive 2006/135/EC. After the first approval, EFSA published in 2009 a reasoned opinion on the refined risk assessment regarding certain MRLs of concern for the active substance. Carbendazim was then evaluated by EFSA during the peer review for renewal of approval in 2010, in the framework of Commission Regulation (EC) No 1107/2009. On 10 May 2011, the approval of carbendazim was renewed by Commission Directive 2011/58/EU. Following the renewal of the approval, EFSA published two reasoned opinions, including the one on the review of the all existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/2005. On 11 March 2015, carbendazim was included in the list of candidates for substitution by Commission Implementing Regulation (EU) 2015/408, due to its classification as toxic for reproduction category 1B, in accordance with the provisions of Regulation (EC) No 1272/2008. Carbendazim is also classified as mutagenic category 1B. In 2019, the European Chemicals Agency (ECHA) published several opinions from the Biocidal Products Committee (BPC) for carbendazim as product types 7 (P7; film preservatives), 9 (P9; fibre, leather, rubber and polymerised materials preservatives) and 10 (P10; construction material preservatives). Carbendazim is currently not approved in the European Union for uses as pesticide.
Thiophanate‐methyl was firstly included in Annex I to Directive 91/414/EEC in 2005 by Commission Directive 2005/53/EC. After the first approval, EFSA published several reasoned opinions on the assessment and modification of the existing maximum residue levels (MRLs) for thiophanate‐methyl, including the assessment of all the existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/2005. The active substance was then evaluated by EFSA during the peer review for renewal of approval in 2018, in the framework of Commission Regulation (EC) No 1107/2009 and according to Commission Implementing Regulation (EU) No 844/2012. On 15 October 2020, the approval of the active substance thiophanate‐methyl was not renewed by Commission Implementing Regulation (EU) 2020/1498. Thiophanate methyl is classified as mutagenic category 2 in accordance with the provisions of Regulation (EC) No 1272/2008 and proposed for classification as carcinogen category 2, based on the latest evaluation by ECHA Committee for risk assessment (RAC) under the classification and labelling (CLH) process (ECHA, 2019b).
Through the different assessments, the two active substances presented clear aneugenic properties, while their clastogenic potential remained outstanding. It is noted that during the re‐assessment of thiophanate‐methyl under the EFSA pesticide peer review, evidence of clastogenicity was found for thiophanate‐methyl and carbendazim. On the other hand, during the assessment by ECHA RAC in 2019 under classification and labelling scheme, which also included the assessment of further data that were not available at the time of the EFSA pesticide peer review, it was confirmed the aneugenic potential of thiophanate‐methyl but not the clastogenic potential.
Based on the above, on 13 November 2020, EFSA received from the European Commission a mandate to deliver, in accordance with Article 43 of Regulation (EC) No 396/2005, a reasoned opinion on the toxicological properties and maximum residue levels (MRLs) for the benzimidazole substances carbendazim and thiophanate‐methyl. EFSA was asked to first assess whether thiophanate‐methyl or carbendazim have clastogenic potential. In case clastogenic potential can be excluded, EFSA shall derive toxicological reference values necessary to perform consumer risk assessment and assessment of MRLs.
The European Commission also asked EFSA to involve ECHA and the respective Rapporteur Member States (Germany for carbendazim and Sweden for thiophanate‐methyl) in the assessment, and to consult with the EU Reference Laboratories for Residues of Pesticides on the achievable limits of analytical determination for benomyl, carbendazim and thiophanate‐methyl in different matrices.
Subsequent to the request from the European Commission, EFSA compiled a master list on genotoxicity studies available, based on the data submitted to EFSA during the pesticides peer review; to ECHA in the context of the CLH process (for thiophanate‐methyl) and for the application for approval of carbendazim as active substance in biocidal products under Reg. (EU) No 528/2012; also including the pertinent studies suggested in the mandate from European Commission and a screening of the published literature available (PubMed). This master list (Appendix F), was further screened for studies relevant to assess the aneugenic and in particular the clastogenic potential of carbendazim and thiophanate‐methyl. The studies identified as relevant to assess these endpoints (Appendices G for carbendazim and H for thiophanate‐methyl) were discussed at the related EFSA experts meeting which was held on 15 January 2021.
In the meantime, EFSA initiated the collection of data in order to gather the most up to date information to review the MRLs of carbendazim and thiophanate methyl. Considering that the two active substances are no longer approved for use as pesticides in EU, Member States (including the two RMSs) and the UK1 were invited to submit by 25 January 2021 Good Agricultural Practices (GAPs) in non‐EU countries for which GAPs for import tolerance (IT) are authorised.
On the basis of the feedback received by Member States and the information submitted by the EU Reference Laboratories for Pesticides Residues (EURLs) and the conclusions derived by EFSA in the framework of Regulation (EC) No 1107/2009, EFSA completed the Pesticide Residues Overview File (PROFile) and prepared in May 2021 a draft reasoned opinion, which was circulated to Member States, ECHA and EURLs for consultation via a written procedure. Comments received by 14 June 2021 were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
The experts of the peer review experts meeting on mammalian toxicology agreed that by considering the new data available to ECHA RAC, the weight of evidence suggests that there is direct evidence in vitro that thiophanate‐methyl is not clastogenic but aneugenic whereas there is indirect evidence in vivo that thiophanate‐methyl is not clastogenic but aneugenic. The majority of experts agreed that the most suitable basis for setting the acceptable daily intake (ADI) and acute reference dose (ARfD) for thiophanate‐methyl is the no observed adverse effect level (NOAEL) of 2 mg/kg body weight (bw) per day for maternal and developmental toxicity in the rabbit and applying an uncertainty factor of 100. The resulting ADI and ARfD is 0.02 mg/kg bw (per day). Regarding carbendazim, the experts agreed that the weight of evidence suggests that there is direct evidence in vitro and in vivo that carbendazim is not clastogenic but aneugenic and agreed to maintain previous ADI and ARfD of carbendazim of 0.02 mg/kg bw (per day).
The metabolism of thiophanate‐methyl and carbendazim in plants was investigated in primary crops. According to the results of the metabolism studies and the available toxicological studies, the residue definitions for enforcement and risk assessment can be proposed as ‘thiophanate‐methyl’ and ‘carbendazim’, separately. A specific residue definition for rotational crops is not deemed necessary considering that only import tolerances were considered in the present assessment. These residue definitions are also applicable to processed commodities. Fully validated analytical methods are available for the separate enforcement of the proposed residue definitions in the main four matrices at the limit of quantification (LOQ) of 0.01 mg/kg. According to the EURLs this LOQ is achievable by using the QuEChERS method in routine analyses. Nevertheless, the EURLs highlighted that during routine analyses, benomyl degrades rapidly to carbendazim and therefore using routine methods is not possible to analyse separately for benomyl and carbendazim.
Available residue trials data were considered sufficient to derive MRL proposals as well as risk assessment values for all commodities under evaluation. Considering that homogenisation of samples leads to a drastically reduced storage stability, pending additional data to ensure that no degradation of thiophanate‐methyl and carbendazim occurred in samples during storage, all the derived MRLs should be considered tentative only.
Thiophanate‐methyl and carbendazim are authorised for use on citrus fruits that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. Based on the uses reported in the framework of this assessment, significant exposure to thiophanate‐methyl and to carbendazim are expected for cattle and swine only; therefore, the nature and magnitude of residues in animals was investigated only in these groups of livestock.
The metabolism of thiophanate‐methyl and carbendazim residues in livestock was investigated in lactating goats and cow at dose rate covering the maximum dietary burdens calculated in this review. For thiophanate‐methyl, the residue definition for enforcement and risk assessment was proposed as parent ‘thiophanate‐methyl’ only. For carbendazim, the relevant residue definition for enforcement in all animal matrices was set as the ‘sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim’. The same residue definition also applies for risk assessment in muscle, fat, liver and kidney while an additional metabolite (4‐hydroxy‐carbendazim) is also included for risk assessment in milk. Available feeding studies performed with thiophanate‐methyl and carbendazim demonstrated that no residues above the LOQ are expected in cattle milk and in cattle and swine tissues following their exposure to thiophanate‐methyl and carbendazim and MRLs for these commodities can be established at the enforcement LOQ.
Fully validated analytical methods using LC‐MS/MS (QuEChERS) are available for the separate enforcement of thiophanate‐methyl, carbendazim and 5‐hydroxy‐carbendazim at the LOQ of 0.01 mg/kg for each compound in all animal matrices.
According to the EURLs, it is expected that this LOQ would be achievable for the separate enforcement of thiophanate‐methyl and carbendazim during routine analyses. Moreover, the same LOQ is also valid for benomyl (measured as carbendazim). Analytical methods for the enforcement of 5‐hydroxy‐carbendazim are currently not available to the EURLs but according to the information shared during the MSC on the draft reasoned opinion they will perform validation experiments in animal matrices to provide LOQs for routine analysis. According to the EURLs the analytical standards for carbendazim, benomyl, thiophanate‐methyl and 5‐hydroxy‐carbendazim are commercially available.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 3.1 of the EFSA PRIMo.
For thiophanate‐methyl, the highest chronic exposure was calculated for German child, representing 8% of the acceptable daily intake (ADI). With regard to the acute exposure, however, an exceedance of the ARfD was identified for oranges, grapefruits, mandarins and papaya, representing 314%, 186%, 140% and 106% of the ARfD, respectively.
For carbendazim, the highest chronic exposure was calculated for Dutch toddler, representing 7% of the acceptable daily intake (ADI) while the highest acute exposure was calculated for mandarins, representing 84% of the ARfD.
Furthermore, before proposing a refinement of the risk assessment, a combined acute risk assessment was performed summing the results from the acute risk assessment of thiophanate‐methyl and carbendazim. According to this calculation, an exceedance of the ARfD was identified for oranges, grapefruits, mandarins, mangoes, papaya and lemons, representing 342%, 203%, 224%, 143%, 133% and 129% of the ARfD. It is, however, noted by EFSA that the approach followed for the combined exposure assessment leads to an overestimation of the exposure in lemons, mandarins and limes, where residues resulting from the use of carbendazim and thiophanate‐methyl have been combined while co‐occurrence of these residues is not expected to occur in practice for these three crops.
A second (scenario EU2, reflecting option 1 in Table 1) and a third (scenario EU3, reflecting option 2 in Table 1) exposure calculation were therefore performed, considering possible fall‐back GAPs and assuming that residues from the uses of carbendazim and thiophanate‐methyl are not co‐occurring in lemons.
Table 1.
Summary table
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: thiophanate‐methyl | |||||
| 110010 | Grapefruits | 6 | – | – | Further consideration neededa Data gap #1 |
| 110020 | Oranges | 6 | 1 | – | Further consideration neededb Data gap #1 |
| 110030 | Lemons | 6 | – | Option 1c: – | Further consideration neededd Data gap #1 |
| Option 2e: 7 | Further consideration neededf Data gap #1 | ||||
| 110040 | Limes | 6 | – | 7 | Further consideration neededf Data gap #1 |
| 110050 | Mandarins | 6 | – | – | Further consideration neededa Data gap #1 |
| 163030 | Mangoes | 1 | 5 | – | Further consideration neededb Data gap #1 |
| 163040 | Papayas | 1 | – | – | Further consideration neededa Data gap #1 |
| 231040 | Okra/lady's fingers | 1 | – | 0.9 | Further consideration neededd Data gap #1 |
| Enforcement residue definition (existing): thiophanate‐methyl and carbendazim, expressed as carbendazim Enforcement residue definition (proposed): thiophanate‐methyl | |||||
| 1011010 | Swine muscle | 0.05* | – | 0.01* | Further consideration neededd Data gap #1 |
| 1011020 | Swine fat tissue | 0.05* | – | 0.01* | Further consideration neededd Data gap #1 |
| 1011030 | Swine liver | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1011040 | Swine kidney | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1012020 | Bovine fat tissue | 0.05* | – | 0.01* | Further consideration neededd Data gap #1 |
| 1012030 | Bovine liver | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1012040 | Bovine kidney | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1015010 | Equine muscle | 0.05* | – | 0.01* | Further consideration neededd Data gap #1 |
| 1015020 | Equine fat tissue | 0.05* | – | 0.01* | Further consideration neededd Data gap #1 |
| 1015030 | Equine liver | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1015040 | Equine kidney | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1020010 | Cattle milk | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| 1020040 | Horse milk | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gap #1 |
| Enforcement residue definition (existing): sum of benomyl and carbendazim, expressed as carbendazim Enforcement residue definition (proposed): carbendazim | |||||
| 110010 | Grapefruits | 0.2 | – | – | Further consideration neededa Data gap #1 |
| 110020 | Oranges | 0.2 | 1 | – | Further consideration neededb Data gap #1 |
| 110030 | Lemons | 0.7 | – | Option 1c: 0.9 | Further consideration neededd Data gap #1 |
| Option 2e: 0.2 | Further consideration neededd Data gap #1 | ||||
| 110040 | Limes | 0.7 | – | 0.9 | Further consideration neededd Data gap #1 |
| 110050 | Mandarins | 0.7 | – | 0.9 | Further consideration neededd Data gap #1 |
| 163030 | Mangoes | 0.5 | 5 | – | Further consideration neededb Data gap #1 |
| 163040 | Papayas | 0.2 | – | – | Further consideration neededa Data gap #1 |
| 231040 | Okra/lady's fingers | 2 | – | 1.5 | Further consideration neededd Data gap #1 |
| Enforcement residue definition (existing): carbendazim and thiophanate‐methyl, expressed as carbendazim Enforcement residue definition (proposed): sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim | |||||
| 1011010 | Swine muscle | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1011020 | Swine fat tissue | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1011030 | Swine liver | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1011040 | Swine kidney | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1012020 | Bovine fat tissue | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1012030 | Bovine liver | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1012040 | Bovine kidney | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1015010 | Equine muscle | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1015020 | Equine fat tissue | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1015030 | Equine liver | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1015040 | Equine kidney | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1020010 | Cattle milk | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2,3 |
| 1020040 | Horse milk | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2,3 |
| – | Other commodities of plant and/or animal origin | See Reg. 559/2011 | – | – | Further consideration neededg |
| Enforcement residue definition (proposed): benomyl | |||||
| – | Commodities of plant and/or animal origin | – | – | – | Further consideration neededg |
MRL: maximum residue level; CXL: codex maximum residue limit.
Indicates that the MRL is set at the limit of quantification.
The residue definition is fat soluble.
GAP evaluated at EU level is not fully supported by data and a risk to consumers cannot be excluded; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination E‐I in Appendix I).
GAP evaluated at EU level is not fully supported by data and a risk to consumers cannot be excluded; CXL is not compatible with EU residue definitions. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination E‐II in Appendix I).
Option 1: MRL based on the authorised use for carbendazim, assuming that the authorised use of thiophanate‐methyl will be withdrawn.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination F‐I in Appendix I). It is noted that carbendazim is classified as toxic for reproduction category 1B in accordance with Regulation (EC) No 1272/2008.
Option 2: MRL based on the authorised use for thiophanate‐methyl, assuming that the authorised use of carbendazim will be withdrawn.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is not compatible with EU residue definitions (combination F‐II in Appendix I). It is noted that carbendazim is classified as toxic for reproduction category 1B in accordance with Regulation (EC) No 1272/2008.
There are no import tolerances reported at EU level; no CXL is available or CXL is not compatible with EU residue definitions. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I/II in Appendix I).
According to the results of the second calculation (scenario EU2), the highest acute exposure for thiophanate‐methyl is calculated for limes, representing 48% of the ARfD, the highest acute exposure for carbendazim is calculated for mandarins, representing 84% of the ARfD and the highest combined acute exposure is calculated for mandarins, representing 84% of the ARfD.
According to the results of the third calculation (scenario EU3), the highest acute exposure for thiophanate‐methyl is calculated for lemons, representing 81% of the ARfD, the highest acute exposure for carbendazim is calculated for mandarins, representing 84% of the ARfD and the highest combined acute exposure is calculated for lemons, representing 88% of the ARfD.
These calculations show that no risk for consumers is identified for lemons in case residues from the uses of carbendazim and thiophanate‐methyl are not co‐occurring.
In order to perform a combined chronic risk assessment, results from the chronic risk assessment of thiophanate‐methyl and results from the chronic risk assessment of carbendazim from the refined calculations were summed (scenario EU2 and EU3). This calculation has been done for the Dutch diet (toddler), the British diet (infant) and the French diet (toddler) being the diets with the highest estimated exposure.
The highest chronic exposure for scenario EU2 was calculated for the Dutch diet (toddler), representing 10% of the ADI. The highest chronic exposure for scenario EU3 was calculated for the Dutch diet (toddler), representing 9% of the ADI.
Based on these calculations, an acute risk to consumers was identified for the most critical GAPs for thiophanate‐methyl on oranges, grapefruits, mandarins, mangoes and papaya and for lemons, if the residues from the uses of carbendazim and thiophanate‐methyl are co‐occurring. However, fall‐back GAPs were identified for mandarins and lemons, for which a second (scenario EU2) and a third (scenario EU3) risk assessments did not indicate risk to consumers. For the remaining commodities, although some major uncertainties remain due to the data gaps identified, the indicative exposure calculation did not indicate a risk to consumers.
Background
Carbendazim was firstly included in Annex I to Directive 91/414/EEC2 in 2006 by Commission Directive 2006/135/EC3. After the first approval, EFSA published a reasoned opinion on the refined risk assessment regarding certain MRLs of concern for the active substance (EFSA, 2009). Carbendazim was then evaluated by EFSA during the peer review for renewal of approval in the framework of Commission Regulation (EC) No 1107/20094 in 2010 (EFSA, 2010). On 10 May 2011, the approval of carbendazim was renewed by Commission Directive 2011/58/EU5. Following the renewal of the approval, EFSA published two reasoned opinions, including the one on the review of the all existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/20056 (EFSA, 2012, 2014). On 11 March 2015, carbendazim was included in the list of candidates for substitution Commission Implementing Regulation (EU) 2015/4087, due to its classification as toxic for reproduction category 1B, in accordance with the provisions of Regulation (EC) No 1272/20088. Carbendazim is also classified as mutagenic 1B. In 2019, the European Chemicals Agency (ECHA) published several opinions from the Biocidal Products Committee (BPC) for carbendazim as product types 7 (P7; film preservatives), 9 (P9; fibre, leather, rubber and polymerised materials preservatives) and 10 (P10; construction material preservatives) (ECHA, 2019a,c,d). Carbendazim is currently not approved in the European Union for uses as pesticide.
Thiophanate‐methyl was firstly included in Annex I to Directive 91/414/EEC in 2005 by Commission Directive 2005/53/EC9. After the first approval, EFSA published several reasoned opinions on the assessment and modification of the existing maximum residue levels (MRLs) for thiophanate‐methyl, including the assessment of all the existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/2005 (EFSA, 2009, 2012, 2014). The active substance was then evaluated by EFSA during the peer review for renewal of approval in the framework of Commission Regulation (EC) No 1107/2009 and according to Commission Implementing Regulation (EU) No 844/201210 in 2018 (EFSA, 2018a). On 15 October 2020, the approval of the active substance thiophanate‐methyl was not renewed by Commission Implementing Regulation (EU) 2020/149811.Thiophanate methyl is classified as mutagenic category 2 in accordance with the provisions of Regulation (EC) No 1272/2008, and proposed for classification as carcinogen category 2, based on the latest evaluation by ECHA RAC under the classification and labelling (CLH) process (ECHA, 2019b).
Through the different assessments, the two active substances presented clear aneugenic properties, while their clastogenic potential remained outstanding. It is noted that during the re‐assessment of thiophanate‐methyl under the EFSA pesticide peer review, evidence of clastogenicity was found for thiophanate‐methyl and carbendazim. On the other hand, during the assessment by ECHA RAC in 2019 under classification and labelling scheme, which also included the assessment of further data that were not available at the time of the EFSA pesticide peer review, the aneugenic potential of thiophanate‐methyl was confirmed but not the clastogenic potential.
Based on the above, on 13 November 2020, EFSA received from the European Commission a mandate to deliver, in accordance with Article 43 of Regulation (EC) No 396/2005, a reasoned opinion on the toxicological properties and maximum residue levels for the benzimidazole substances carbendazim and thiophanate‐methyl. EFSA was asked to first assess whether thiophanate‐methyl or carbendazim have clastogenic potential. In case clastogenic potential can be excluded, EFSA shall derive toxicological reference values necessary to perform consumer risk assessment and set MRLs.
European Commission also asked EFSA to involve ECHA and the respective Rapporteur Member States (Germany for carbendazim and Sweden for thiophanate‐methyl) in the assessment, and consult with the EU Reference Laboratories for Residues of Pesticides on the achievable limits of analytical determination for benomyl, carbendazim and thiophanate‐methyl in different matrices.
Subsequent to the request from the European Commission, EFSA compiled a master list on genotoxicity studies available, based on the data submitted to EFSA during the pesticides peer review; to ECHA in the context of the CLH process (for thiophanate‐methyl) and for the application for approval of carbendazim as active substance in biocidal products under Reg. (EU) No 528/201212; including the pertinent studies suggested in the mandate from European Commission and a screening of the published literature available (PubMed). This master list (Appendix F) was screened for studies relevant to assess the aneugenic and in particular the clastogenic potential of carbendazim and thiophanate‐methyl (EFSA, 2021a,b). The studies identified as relevant to assess these endpoints (Appendices G for carbendazim and H for thiophanate‐methyl) were discussed at the related experts meeting which was held on 15 January 2021 (EFSA, 2021c).
In the meantime, EFSA initiated on 10 December 2020 the collection of data in order to gather the most up to date information to review the MRLs thiophanate methyl and carbendazim. Considering that the two active substances are no longer approved for use in EU, Member States (including the RMSs) and the UK were invited to submit by 25 January 2021 Good Agricultural Practices (GAPs) in non‐EU countries for which import tolerances (IT) are authorised. In the framework of this consultation, one Member State, Germany, informed EFSA that no additional import tolerances are currently in place for carbendazim and thiophanate methyl, apart from the ones already assessed in the framework of the review of the MRLs for carbendazim and thiophanate‐methyl.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
On the basis of the feedback received by Member States and the information submitted by the EU Reference Laboratories for Pesticides Residues (EURLs) and the conclusions derived by EFSA in the framework of Regulation (EC) No 1107/2009, EFSA completed the Pesticide Residues Overview File (PROFile) and prepared in May 2021 a draft reasoned opinion, which was circulated to Member States, ECHA and EURLs for consultation via a written procedure. Comments received by 14 June 2021 were considered during the finalisation of this reasoned opinion.
The EURLs report on analytical methods (EURLs, 2021), the Member States consultation report (EFSA, 2021d), the exposure calculations for all crops reported in the framework of this assessment performed using the EFSA Pesticide Residues Intake Model (PRIMo) and the PROFiles are considered supporting documents to this reasoned opinion and, thus, made publicly available.
A screenshot of the report sheet of the PRIMo is presented in Appendix C.
Finally, the report of the pesticide peer review experts meeting on mammalian toxicology (TC 39) (EFSA, 2021c), as well as the screening overview tables prepared for carbendazim and thiophanate‐methyl (EFSA, 2021a,b) presenting all the relevant studies used for the discussion, are also made available. The full list of studies considered for the initial screening assessment is available in Appendix F; the resulting list of studies relevant to assess the clastogenic potential of carbendazim is available in Appendix G and for thiophanate‐methyl in Appendix H.
Terms of Reference
According to the specific mandate received from the European Commission in accordance with Article 43 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the toxicological properties of benzimidazole substances carbendazim and thiophanate‐methyl, specifically, to check whether thiophanate‐methyl or carbendazim have clastogenic potential;
In case clastogenic potential can be excluded, EFSA will derive toxicological reference values (TRVs) necessary for the consumer risk assessment and the setting of maximum residue levels;
EFSA should consider the pertinent studies for carbendazim and thiophanate‐methyl as available thorough previous assessments (EFSA, 2010, 2018a; ECHA, 2019a, 2019b, 2019c, 2019d) and as referred in the background section of the mandate;
EFSA will involve the European Chemicals Agency (ECHA) and the respective Rapporteur Member States and consult with the EU Reference Laboratories for Residues of Pesticides on the achievable limits of analytical determination for benomyl, carbendazim and thiophanate‐methyl in different matrices;
EFSA will provide its Reasoned Opinion by 13 July 2021.
The active substance and its use pattern
Carbendazim is the ISO common name for methyl benzimidazol‐2‐ylcarbamate (IUPAC). Carbendazim is a metabolite of thiophanate‐methyl.
Thiophanate‐methyl is the ISO common name for dimethyl (1,2‐phenylenedicarbamothioyl)dicarbamate (IUPAC).
The chemical structure of the active substances and the main metabolites are reported in Appendix E.
The EU MRLs for both active substances are established in Annexes II and III of Regulation (EC) No 396/2005. Codex maximum residue limits (CXLs) for thiophanate‐methyl and carbendazim were also established by the Codex Alimentarius Commission (CAC).
Assessment
EFSA has based its assessment on the following documents:
the PROFile as prepared by EFSA;
the report of the pesticide peer review experts meeting on mammalian toxicology (TC 39) and related background documents (EFSA, 2021a,b,c)
the renewal assessment report (RAR) and its final addendum on the active substance carbendazim, prepared by the rapporteur Member State, Germany, in accordance with Article 5(5) of Council Directive 91/414/EEC (Germany, 2009, 2010)
the renewal Assessment Report (RAR) on the active substance thiophanate‐methyl prepared by the rapporteur Member State, Sweden, in the framework of Commission Implementing Regulation (EU) No 844/201 (Sweden, 2016, 2017);
the conclusion on the peer review of the pesticide risk assessment of the active substance carbendazim (EFSA, 2010);
the conclusion on the peer review of the pesticide risk assessment of the active substance thiophanate‐methyl (EFSA, 2018a);
the ECHA RAC CLH opinion on thiophanate‐methyl (ECHA, 2019b)
the ECHA BPC opinion for carbendazim as product types PT7, PT9 and PT10 (ECHA, 2019a,c,d) and related Competent Authority assessment Report (CAR) (Germany, 2019).
the previous reasoned opinions on the assessment, modification and review of the existing MRLs for carbendazim and thiophanate methyl (EFSA, 2009, 2012, 2014).
The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/201113 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a, 1997b, 1997c, 1997d, 1997e, 1997f, 1997g, 2000, 2010a, 2010b, 2017; OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Mammalian toxicology
Under the remit of the current mandate only conclusions regarding clastogenicity and aneugenicity have been considered with the aim to consider the setting of reference values for the active substances thiophanate‐methyl and carbendazim. The toxicological profile of both substances was discussed during the Pesticide Peer Review TC 39 (15 January 2021).
Regarding thiophanate‐methyl, the experts agreed that by considering the new data available to ECHA RAC (ECHA, 2019b), the weight of evidence suggests that there is direct evidence in vitro that thiophanate‐methyl is not clastogenic but aneugenic whereas there is indirect evidence in vivo that thiophanate‐methyl is not clastogenic but aneugenic.
The majority of experts agreed that the most suitable basis for setting the Acceptable Daily Intake (ADI) and Acute Reference Dose (ARfD) for thiophanate‐methyl is the NOAEL of 2 mg/kg bw per day for maternal and developmental toxicity in the rabbit and applying an uncertainty factor of 100. The resulting ADI and ARfD is 0.02 mg/kg bw (per day).
Regarding carbendazim, the experts agreed that there is no additional data that challenge previous conclusion on the genotoxicity profile of carbendazim as assessed by EFSA (2010) and ECHA (2019a,c,d). Therefore, the experts agreed that the weight of evidence suggests that there is direct evidence in vitro and in vivo that carbendazim is not clastogenic but aneugenic and agreed to maintain previous ADI and ARfD of carbendazim of 0.02 mg/kg bw (per day).
As thiophanate‐methyl and carbendazim share a similar toxicological effect, i.e. aneugenic potential, these compounds can be considered together in a combined risk assessment. The experts noted that there are differences in potency, where thiophanate‐methyl showed a lower potency for aneugenicity compared to carbendazim and that there are also differences in the toxicological profile regarding other toxicity endpoints (e.g. thyroid, as a critical target organ for thiophanate‐methyl). It is also noted that carbendazim is a metabolite of thiophanate‐methyl. The reference values proposed for carbendazim and thiophanate‐methyl are protective of the aneugenic potential of both substances.
2. Residues in plant: residue definitions, analytical methods for enforcement and MRL proposals
The metabolism of thiophanate‐methyl in primary crops has been assessed in the framework of the MRL review for carbendazim and thiophanate methyl (EFSA, 2014). During the peer review for the renewal of thiophanate‐methyl these studies were reassessed versus the current data requirements and additional metabolism studies were considered (EFSA, 2018a).
The metabolism of carbendazim in primary crops has been assessed in the framework of the peer review for the renewal of carbendazim (EFSA, 2010) and in the MRL review for carbendazim and thiophanate methyl (EFSA, 2014).
Primary crop metabolism of thiophanate‐methyl and carbendazim was investigated separately for foliar application in four different crop groups (fruit crops, root crops, pulses and oilseeds and cereals). Additional studies where carbendazim was applied to strawberry plants via hydroponic solution (considered informative only; EFSA, 2010) or thiophanate‐methyl was applied to tomato plants by drip irrigation are also available (EFSA, 2018a). Studies investigating the metabolism of benomyl, another active substance of the group of benzimidazoles, in rice, soyabeans and sugar beets were also taken into account during the MRL review, as this compound shares a similar metabolism with thiophanate‐methyl and it is degraded mainly into carbendazim (EFSA, 2014).
For each active substance, metabolic patterns in the different studies were shown to be similar. After foliar treatments, carbendazim was shown to be a main metabolite of thiophanate‐methyl. The following additional metabolites were also observed: 2‐AB (both in metabolism studies with thiophanate‐methyl and carbendazim) and metabolites FH‐432 and DX‐105 (identified in the metabolism studies with thiophanate‐methyl as intermediate compounds before the cyclisation to form carbendazim).
In particular, following foliar applications of thiophanate‐methyl on apples (relevant for the uses under assessment), parent and carbendazim were the main compounds identified (accounting for up to 64% and for up to 29% total radioactive residue (TRR), respectively). At preharvest intervals (PHIs) of 1 and 7 days, most of the TRR was found in the rinsate (97–93% TRR), with limited translocation into the pulp (3–7% TRR). Metabolites 2‐AB, FH‐432 and DX‐105 were identified in the rinsate but were present at low proportions (accounting for 0.6–1.2% TRR, for 3–5% TRR and for 1–2% TRR, respectively). Following foliar application of thiophanate‐methyl on grapes, at harvest (35 DAT) carbendazim was the main compound identified in berries accounting for 53% TRR. Thiophanate‐methyl, metabolites FH‐432 and DX‐105 were identified at low proportions (accounting for 4% TRR, for 4% and for 0.5% TRR, respectively) while metabolite 2‐AB was only found in the leaves (1.2% TRR) (Sweden, 2016, 2017).
Following foliar application of carbendazim on peaches, TRR was 1 mg eq/kg and 1.27 mg eq/kg immediately after the first and the second treatment, respectively. The extraction procedure removed over 97% of the TRR in the peaches. The only detectable residue in these extracts was carbendazim but its levels were not reported. After treatment with NaOH to release unextractable radioactivity the only residue found was 2‐AB, which is converted from carbendazim. No further metabolites were detected in any sample (Germany, 2009, 2010).
Therefore, the main compounds identified in the available metabolism studies with foliar applications on fruit crops were thiophanate‐methyl and carbendazim while metabolites 2‐AB, FH‐432 and DX‐105 were only present at low proportions.
It is noted that thiophanate‐methyl is also authorised for post‐harvest dip treatment on citrus fruits, for which no representative metabolism study is available. Nevertheless, considering that in the available studies on fruit crops thiophanate‐methyl was applied close to the harvest, a different metabolism is not expected following post‐harvest treatment according to the authorised use and no additional studies are required.
Since thiophanate‐methyl and carbendazim are no longer authorised for uses as plant protection products in EU and only import tolerances were considered in the present assessment, there is no need to investigate the nature and magnitude of residues in rotational crops.
The nature of residues in processed commodities was investigated and evaluated in previous EFSA assessments (EFSA, 2010, 2014, 2018a). Thiophanate‐methyl was shown to be stable to pasteurisation. Substantial breakdown was observed following conditions simulating boiling/brewing/baking and sterilisation. Carbendazim was the major degradation product in both cases, accounting for maximum amounts of 14.2% (boiling/brewing/baking) and 92.0% (sterilisation) of the applied radioactivity. 2‐AB was formed under sterilisation conditions only, accounting for 10.3% of the radioactivity (EFSA, 2014, 2018a). Carbendazim was shown to be stable during pasteurisation, cooking, brewing and sterilisation (EFSA, 2010, 2014). Based on the above data, it is concluded that thiophanate‐methyl and carbendazim are the relevant compounds to be included in the residue definition for processed commodities.
In the framework of the peer review for the renewal of thiophanate‐methyl, storage stability of thiophanate‐methyl and carbendazim was demonstrated for a period of 12 months at −18 °C in high acid content (grapes), high oil content (rapeseeds), high protein content (dry peas) and high starch content commodities (wheat grain). Nevertheless, this stability was only observed when samples were not homogenised before storage, whereas homogenisation of samples leads to a drastically reduced storage stability (EFSA, 2018a). In the framework of a previous MRL application, storage stability of thiophanate‐methyl was demonstrated for a period of 36 months at –18°C in commodities with high water content (apples) (EFSA, 2012). In this study, the apples were cut in half before storage and further homogenised before analysis. In the framework of the peer review for carbendazim, storage stability of carbendazim was demonstrated for a period of 30 months at –18°C in high water content commodities (tomatoes) and for a period of 18 months at –18°C in high oil content commodities (soyabean oil) (EFSA, 2010).
Based on the results from the studies on the nature of residues of thiophanate‐methyl in primary, rotational crops and processed commodities, during the peer review for the renewal of this active substance, metabolites 2‐AB, FH‐432, DX‐105 were tentatively proposed for inclusion in the residue definition for risk assessment, pending confirmation on their toxicological profiles (EFSA, 2018a). Nevertheless, considering the uses under assessment are for fruit crops only, where the main components of the TRR were identified as thiophanate‐methyl and its metabolite carbendazim and considering that the crops under assessment are expected to be consumed as peeled and/or are minor crops, the residue definition for both enforcement and risk assessment can be limited to parent ‘thiophanate‐methyl’ and its metabolite ‘carbendazim’. It is underlined that this conclusion is limited to the present assessment and might need to be reconsidered for different uses and crops. Considering the different toxicological properties of carbendazim and thiophanate‐methyl (i.e. differences in potency regarding aneugenic potential and differences in the toxicological profile regarding other toxicity endpoints, see Section 1), separate residue definitions are recommended. It is noted that the residue definition for carbendazim currently set in the Regulation also includes the active substance benomyl. Nevertheless, considering that the toxicological assessment of benomyl was never carried out at EU level, it is not considered any longer appropriate to include benomyl in the residue definition.
Analytical methods for the enforcement of thiophanate‐methyl and carbendazim were submitted and evaluated in previous EFSA assessments (EFSA, 2014, 2018a). Fully validated analytical methods using LC‐MS/MS (QuEChERS) are available for the separate enforcement of thiophanate‐methyl and carbendazim at the LOQ of 0.01 mg/kg in high water content, high acid content, high oil content and dry matrices (EFSA, 2018a).
According to the information submitted by the EURLs, this LOQ is achievable for the separate enforcement of thiophanate‐methyl and carbendazim during routine analyses. Moreover, the same LOQ is also valid for benomyl (measured as carbendazim) (EURLs, 2021). Furthermore, the EURLs highlighted that during routine analyses, benomyl degrades rapidly to carbendazim and therefore using routine methods is not possible to analyse separately for benomyl and carbendazim.
Based on the feedback received by Germany during the data call, no additional import tolerances are currently in place for carbendazim and thiophanate methyl, apart from the ones already assessed in the framework of the review of the MRLs for carbendazim and thiophanate‐methyl.
Therefore, to assess the magnitude of residues in primary crops, EFSA considered all the residue trials relevant for the crops under assessment reported in the framework of the review of the existing MRLs for carbendazim and thiophanate‐methyl (EFSA, 2014).
The number of residue trials and extrapolations were evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2017).
The available data were sufficient to derive MRLs and risk assessment values for all crops under assessment, taking note of the following considerations:
Mangoes and okra (lady fingers): results from the available trials supporting the authorised use of thiophanate‐methyl on these crops are reported as sum of thiophanate‐methyl and carbendazim, expressed as carbendazim or as thiophanate‐methyl. Although the derived MRLs and risk assessment values are expected to be overestimated, EFSA deemed it acceptable considering that mangoes and okra are only very minor crops. Nevertheless, 4 residue trials on mangoes and 4 residue trials on okra (lady fingers) compliant with the import tolerance GAPs for thiophanate‐methyl, are still desirable (minor deficiency).
Citrus fruits (post‐harvest use for thiophanate‐methyl): as the MRL derived by the OECD calculator can be overestimated for these types of treatments, the proposed MRL was based on the mean plus 4 times the standard deviation in line with the EFSA guidelines on residues trials and MRL calculations (EFSA, 2015).
During the MRL review, no information was given on whether samples were homogenised prior storage or after and this information is still required (data gap). Considering that homogenisation of samples leads to a drastically reduced storage stability, pending additional data to ensure that no degradation of thiophanate‐methyl and carbendazim occurred in samples during storage, all the derived MRLs should be considered tentative only.
The magnitude of residues of thiophanate‐methyl and carbendazim in processed commodities was also investigated. Robust processing factors for enforcement and risk assessment were derived for several processed commodities in the framework of a previous MRL assessment (EFSA, 2009), during the review of the existing MRLs for thiophanate‐methyl and carbendazim (EFSA, 2014) and in the framework of the peer review for the renewal for the approval for thiophanate‐methyl (EFSA, 2018a). The processing factors relevant for the present assessment are reported in Appendix B.2.2.4.
Considering the outcome of the risk assessment (see Section 4), additional processing studies may be useful to refine the risk assessment, especially for papayas for which no peeling factor could be derived. In addition, if further robust processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed for the other processed commodities where a tentative processing factor is derived.
3. Residues in livestock: residue definitions, analytical methods for enforcement and MRL proposals
Thiophanate‐methyl and carbendazim are authorised for use on citrus fruits that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance (OECD, 2013), which has now also been agreed upon at European level. The input values for all relevant commodities are summarised in Appendix D.1.
The dietary burden calculations were performed for thiophanate‐methyl and for carbendazim, separately, in line with the proposed residue definitions for risk assessment (RD‐RA 1 and RD‐RA 2). For carbendazim, residues arising from the use of thiophanate‐methyl and residues arising from the use of carbendazim were compared and the highest values were used for the calculation of the dietary burden. This approach is valid only assuming that crops are not treated with both thiophanate‐methyl and carbendazim during the same crop cycle. For lemons, lime and mandarin (dry pulp), the residues arising from treatment with carbendazim were higher than the residues arising from treatment with thiophanate‐methyl (see footnote (a) in Appendix D.1).
Based on the uses reported in the framework of this assessment, significant exposures to thiophanate‐methyl and to carbendazim are expected for cattle and swine only; therefore, the nature and magnitude of residues in animals was investigated only in these groups of livestock.
The metabolism of thiophanate‐methyl in lactating ruminants (goat) was assessed in the MRL review and during the peer review for the renewal (EFSA, 2018a). According to the available study, the metabolism of thiophanate‐methyl is extensive and releases several compounds. During the peer review it was proposed to include in the residue definition for risk assessment for ruminants thiophanate‐methyl, 4‐hydroxy‐carbendazim (4‐OH‐MBC), 5‐hydroxy‐carbendazim (5‐OH‐MBC) and 5‐hydroxy‐carbendazim sulfate (5‐OH‐MBC‐S). Furthermore, it was flagged that plant metabolites FH‐432 and DX‐105 were not recovered in the animal metabolic pathways and thus, their fate in the animals was considered not addressed by the available studies. Consequently, during the peer review it was not possible to conclude on the relevant compounds to be monitored in animal matrices (EFSA, 2018a).
In the framework of the present assessment, however, none of the compounds identified in the metabolism study is likely to be present at significant levels considering the calculated exposure of ruminants to thiophanate‐methyl. This conclusion is confirmed by the results of the available feeding studies performed with thiophanate‐methyl (see Section B.3.2.1) which were considered in the MRL review and re‐assessed during the peer review for the renewal of thiophanate‐methyl (EFSA, 2014, 2018a). Therefore, under the framework of this assessment, parent compound only is considered a sufficient marker for enforcement and risk assessment of thiophanate‐methyl residues and MRLs for cattle and swine tissues and for cattle milk can be established at the LOQ of 0.01 mg/kg. It is underlined that this conclusion is limited to the present assessment and might need to be reconsidered for different uses and crops. As poultry and sheep are not expected to be exposed to significant levels of thiophanate‐methyl residues, residue definition and MRLs for poultry and sheep commodities are not needed.
The storage stability of thiophanate‐methyl covering the conditions of the samples from the feeding study was investigated during the peer‐review for the renewal of thiophanate‐methyl where storage stability data for 4‐hydroxy‐carbendazim residues in animal matrices was identified as a data gap (EFSA, 2018a). Considering that at the calculated dietary burden thiophanate‐methyl is expected to be a sufficient marker for enforcement and risk assessment and that livestock feeding studies were only considered to confirm the results of the metabolism study, no additional storage stability study is required in the present assessment. Nevertheless, pending confirmation that samples from trials on plants were not homogenised, the derived MRLs should be considered tentative only.
The metabolism of carbendazim in lactating ruminants (cow and goat) was assessed in the framework of the peer review for the renewal of carbendazim and during the MRL review. Based on these studies, EFSA concluded that the residue definition for enforcement in ruminants should be set as the ‘sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim’. The same residue definition was proposed for risk assessment in muscle, fat, liver and kidney. For risk assessment in milk however, the residue definition should also include the metabolite 4‐hydroxy‐carbendazim (EFSA, 2010, 2014). These residue definitions are still considered valid in the present assessment, noting that residue definitions and MRLs for poultry and sheep commodities are not needed since these livestock are not expected to be exposed to significant levels of carbendazim residues.
Analytical methods for the enforcement of thiophanate‐methyl, carbendazim and 5‐hydroxy‐carbendazim were submitted and evaluated during the peer review for the renewal of thiophanate‐methyl (EFSA, 2018a). According to the information available, fully validated analytical methods using LC‐MS/MS (QuEChERS) are available for the separate enforcement of these compounds at the LOQ of 0.01 mg/kg in all animal matrices (EFSA, 2018a).
According to the EURLs, screening methods are available for livestock commodities suggesting that this LOQ would be achievable for the separate enforcement of thiophanate‐methyl and carbendazim during routine analyses. Moreover, the same LOQ is also valid for benomyl (measured as carbendazim). Analytical methods for the enforcement of 5‐hydroxy‐carbendazim are currently not available to the EURLs (EURLs, 2021) but, according to the information shared during the MSC on the draft reasoned opinion, validation experiments in animal matrices to provide LOQs for routine analysis (EFSA, 2021d) will be performed.
According to the results of the livestock feeding studies performed with carbendazim and assessed in the framework of the peer review for the renewal of carbendazim and during the MRL review (EFSA, 2010, 2014), no residues above the combined LOQ of 0.02 mg/kg are expected in cattle tissues and milk and in swine tissues following their exposure to carbendazim. Therefore, MRLs for these commodities can be established at the combined enforcement LOQ of 0.02 and the conversion factor from enforcement to risk assessment in milk can be proposed as 1.
Since the storage conditions of the samples from the livestock feeding studies were not reported and storage stability data for metabolite 4‐hydroxy‐carbendazim (metabolite relevant for the risk assessment of milk) are not available, and pending confirmation that samples from trials on plants were not homogenised, the derived MRLs should be considered tentative only.
4. Consumer risk assessment
In the framework of this assessment, only the uses of thiophanate‐methyl reported in Appendix A were considered, however these uses of thiophanate‐methyl and carbendazim were previously also assessed by the JMPR (FAO, 1994, 1998, 2003). The CXLs, resulting from these assessments by JMPR and adopted by the CAC, are now international recommendations that need to be considered by European risk managers when establishing MRLs. It is however noted that a different residue definition for enforcement and risk assessment has been derived by the JMPR (FAO, 1998) as the ‘sum of thiophanate‐methyl, carbendazim and benomyl, expressed as carbendazim’. Based on the incompatibility of the residue definitions and considering as well that benomyl has never been evaluated at EU level, is not approved for use in Europe and there are no import tolerances currently in place for this active substance, the existing CXLs were not considered further in this assessment and should not be recommended.
Since carbendazim and thiophanate methyl share a similar toxicological effect (see Section 1), EFSA proposes to perform the risk assessment of carbendazim and thiophanate methyl separately and then to sum the results from the two single assessments to obtain their combined exposures. This approach allows to evaluate the effect of a combined exposure still considering the respective toxicological reference values.
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 3.1 of the EFSA PRIMo (EFSA, 2018b, 2019). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where a tentative MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009).
For carbendazim, residues arising from the use of thiophanate‐methyl and residues arising from the use of carbendazim were compared and the highest values were used for the calculation of the exposure. This approach is valid only assuming that crops are not treated with both thiophanate‐methyl and carbendazim during the same crop cycle. Furthermore, considering the effect of processing on the nature of the residue observed in the hydrolysis study on thiophanate‐methyl (see Section 2), values from residue trials have been adjusted assuming that, following boiling/brewing/baking, thiophanate‐methyl levels would be reduced by 15% and converted to carbendazim. According to the OECD guidelines on the magnitude of pesticide residues in processed commodities (OECD, 2008) and in line with approach followed during the MRL review (EFSA, 2014), the effect of boiling/brewing/baking has been considered relevant for mangoes and papaya (that can be consumed as jam and marmalades) and for okra that is usually consumed cooked. Additionally, thiophanate‐methyl residues were expressed as carbendazim considering that the ratio between the two molecular weights is 0.56. It is acknowledged by EFSA that this approach may overestimate the exposure calculations for carbendazim in raw agricultural commodities. However, in the absence of more adequate data for refinement of the exposure calculations, the most conservative approach was applied. For citrus fruit and mangoes, the peeling factors derived in Section 2 have also been considered. For the commodities of animal origin, considering that no residues of carbendazim and thiophanate‐methyl are expected in the raw commodities, the effect of processing was not deemed relevant.
All input values included in the exposure calculations are summarised in Appendix D.2.
The calculated exposure values were compared with the toxicological reference values for thiophanate‐methyl and for carbendazim (ADI of 0.02 mg/kg bw per day and ARfD of 0.02 mg/kg bw), derived or confirmed in this assessment.
For thiophanate‐methyl, the highest chronic exposure was calculated for German child, representing 8% of the acceptable daily intake (ADI). With regard to the acute exposure, however, an exceedance of the ARfD was identified for oranges, grapefruits, mandarins and papaya, representing 314%, 186%, 140% and 106% of the ARfD, respectively.
For carbendazim, the highest chronic exposure was calculated for Dutch toddler, representing 7% of the acceptable daily intake (ADI) while the highest acute exposure was calculated for mandarins, representing 84% of the ARfD.
Furthermore, before proposing a refinement of the risk assessment, a combined acute risk assessment was performed summing the results from the acute risk assessment of thiophanate‐methyl and carbendazim. This approach is considered valid provided that carbendazim and thiophanate methyl are not used together on the same crop in the same season. According to this calculation, an exceedance of the ARfD was identified for oranges, grapefruits, mandarins, mangoes, papaya and lemons, representing 342%, 203%, 224%, 143%, 133% and 129% of the ARfD. It is however noted by EFSA that the approach followed for the combined exposure assessment leads to an overestimation of the exposure in lemons, mandarins and limes, where residues resulting from the use of carbendazim and thiophanate‐methyl have been combined while co‐occurrence of these residues is not expected to occur in practice for these three crops.
A second (scenario EU2) and a third (scenario EU3) exposure calculation were therefore performed, as described below and assuming that residues from the uses of carbendazim and thiophanate‐methyl are not co‐occurring in lemons.
Scenario EU2 (reflecting option 1 in Table 1): excluding the uses of thiophanate‐methyl on oranges, grapefruits, mandarins, lemons, mangoes and papaya and considering as a fall‐back GAPs for mandarins and lemons the uses of carbendazim. No fall‐back GAPs could be identified for oranges, grapefruits, papaya and mangoes. According to the results of this second calculation, the highest acute exposure for thiophanate‐methyl is calculated for limes, representing 48% of the ARfD, the highest acute exposure for carbendazim is calculated for mandarins, representing 84% of the ARfD and the highest combined acute exposure is calculated for mandarins, representing 84% of the ARfD.
Scenario EU3 (reflecting option 2 in Table 1): excluding the uses of thiophanate methyl on oranges, grapefruits, mandarins, mangoes and papaya and the use of carbendazim on lemons and considering as a fall‐back GAP for mandarins the use of carbendazim and as fall‐back GAP for lemons the use of thiophanate‐methyl. As in scenario EU2, no fall‐back GAPs could be identified for oranges, grapefruits, papaya and mangoes. According to the results of this third calculation, the highest acute exposure for thiophanate‐methyl is calculated for lemons, representing 81% of the ARfD, the highest acute exposure for carbendazim is calculated for mandarins, representing 84% of the ARfD and the highest combined acute exposure is calculated for lemons, representing 88% of the ARfD.
These calculations show that no risk for consumers is identified for lemons in case residues from the uses of carbendazim and thiophanate‐methyl are not co‐occurring.
In order to perform a combined chronic risk assessment, results from the chronic risk assessment of thiophanate‐methyl and results from the chronic risk assessment of carbendazim from the refined calculations were summed (for scenario EU2 and EU3, respectively). This calculation has been done for the Dutch diet (toddler), the British diet (infant) and the French diet (toddler) being the diets with the highest estimated exposure.
The highest chronic exposure for scenario EU2 was calculated for the Dutch diet (toddler), representing 10% of the ADI. The highest chronic exposure for scenario EU3 was calculated for the Dutch diet (toddler), representing 9% of the ADI.
Based on these calculations, an acute risk to consumers was identified for the most critical GAPs for thiophanate‐methyl on oranges, grapefruits, mandarins, mangoes and papaya and for lemons, if the residues from the uses of carbendazim and thiophanate‐methyl are co‐occurring. However, fall‐back GAPs were identified for mandarins and lemons, for which a second (scenario EU2) and a third risk (scenario EU3) assessment did not indicate risk to consumers. For the remaining commodities, although some major uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculation did not indicate a risk to consumers.
Conclusions
The experts of the peer review experts meeting on mammalian toxicology agreed that by considering the new data available to ECHA RAC, the weight of evidence suggests that there is direct evidence in vitro that thiophanate‐methyl is not clastogenic but aneugenic whereas there is indirect evidence in vivo that thiophanate‐methyl is not clastogenic but aneugenic. The majority of experts agreed that the most suitable basis for setting the Acceptable Daily Intake (ADI) and Acute Reference Dose (ARfD) for thiophanate‐methyl is the NOAEL of 2 mg/kg bw per day for maternal and developmental toxicity in the rabbit and applying an uncertainty factor of 100. The resulting ADI and ARfD is 0.02 mg/kg bw (per day). Regarding carbendazim, the experts agreed that the weight of evidence suggests that there is direct evidence in vitro and in vivo that carbendazim is not clastogenic but aneugenic and agreed to maintain previous ADI and ARfD of carbendazim of 0.02 mg/kg bw (per day).
The metabolism of thiophanate‐methyl and carbendazim in plants was investigated in primary crops. According to the results of the metabolism studies and the available toxicological studies, the residue definitions for enforcement and risk assessment can be proposed as ‘thiophanate‐methyl’ and ‘carbendazim’, separately. A specific residue definition for rotational crops is not deemed necessary considering that only import tolerances were considered in the present assessment. These residue definitions are also applicable to processed commodities. Fully validated analytical methods are available for the separate enforcement of the proposed residue definitions in the main four matrices at the LOQ of 0.01 mg/kg. According to the EURLs this LOQ is achievable by using the QuEChERS method in routine analyses. Nevertheless, the EURLs highlighted that during routine analyses, benomyl degrades rapidly to carbendazim and therefore using routine methods is not possible to analyse separately for benomyl and carbendazim.
Available residue trials data were considered sufficient to derive MRL proposals as well as risk assessment values for all commodities under evaluation. Considering that homogenisation of samples leads to a drastically reduced storage stability, pending additional data to ensure that no degradation of thiophanate‐methyl and carbendazim occurred in samples during storage, all the derived MRLs should be considered tentative only.
Thiophanate‐methyl and carbendazim are authorised for use on citrus fruits that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. Based on the uses reported in the framework of this assessment, significant exposure to thiophanate‐methyl and to carbendazim are expected for cattle and swine only; therefore, the nature and magnitude of residues in animals was investigated only in these groups of livestock.
The metabolism of thiophanate‐methyl and carbendazim residues in livestock was investigated in lactating goats and cow at dose rate covering the maximum dietary burdens calculated in this review. For thiophanate‐methyl, the residue definition for enforcement and risk assessment was proposed as parent ‘thiophanate‐methyl’ only. For carbendazim, the relevant residue definition for enforcement was set as the ‘sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim’. The same residue definition also applies for risk assessment in muscle, fat, liver and kidney while an additional metabolite (4‐hydroxy‐carbendazim) is also included for risk assessment in milk. Available feeding studies performed with thiophanate‐methyl and carbendazim demonstrated that no residues above the LOQ are expected in cattle milk and in cattle and swine tissues following their exposure to thiophanate‐methyl and carbendazim and MRLs for these commodities can be established at the enforcement LOQ.
Fully validated analytical methods using LC‐MS/MS (QuEChERS) are available for the separate enforcement of thiophanate‐methyl, carbendazim and 5‐hydroxy‐carbendazim at the LOQ of 0.01 mg/kg for each compound in all animal matrices.
According to the EURLs, it is expected that this LOQ would be achievable for the separate enforcement of thiophanate‐methyl and carbendazim during routine analyses. Moreover, the same LOQ is also valid for benomyl (measured as carbendazim). Analytical methods for the enforcement of 5‐hydroxy‐carbendazim are currently not available to the EURLs but according to the information shared during the MSC on the draft reasoned opinion they will perform validation experiments in animal matrices to provide LOQs for routine analysis. According to the EURLs the analytical standards for carbendazim, benomyl, thiophanate‐methyl and 5‐hydroxy‐carbendazim are commercially available.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 3.1 of the EFSA PRIMo.
For thiophanate‐methyl, the highest chronic exposure was calculated for German child, representing 8% of the acceptable daily intake (ADI). With regard to the acute exposure, however, an exceedance of the ARfD was identified for oranges, grapefruits, mandarins and papaya, representing 314%, 186%, 140% and 106% of the ARfD, respectively.
For carbendazim, the highest chronic exposure was calculated for Dutch toddler, representing 7% of the acceptable daily intake (ADI) while the highest acute exposure was calculated for mandarins, representing 84% of the ARfD.
Furthermore, before proposing a refinement of the risk assessment, a combined acute risk assessment was performed summing the results from the acute risk assessment of thiophanate‐methyl and carbendazim. According to this calculation, an exceedance of the ARfD was identified for oranges, grapefruits, mandarins, mangoes, papaya and lemons, representing 342%, 203%, 224%, 143%, 133% and 129% of the ARfD. It is however noted by EFSA that the approach followed for the combined exposure assessment leads to an overestimation of the exposure in lemons, mandarins and limes, where residues resulting from the use of carbendazim and thiophanate‐methyl have been combined while co‐occurrence of these residues is not expected to occur in practice for these three crops.
A second (scenario EU2, reflecting option 1 in Table 1) and a third (scenario EU3, reflecting option 2 in Table 1) exposure calculations were therefore performed, considering possible fall‐back GAPs and assuming that residues from the uses of carbendazim and thiophanate‐methyl are not co‐occurring in lemons.
According to the results of the second calculation (scenario EU2), the highest acute exposure for thiophanate‐methyl is calculated for limes, representing 48% of the ARfD, the highest acute exposure for carbendazim is calculated for mandarins, representing 84% of the ARfD and the highest combined acute exposure is calculated for mandarins, representing 84% of the ARfD.
According to the results of the third calculation (scenario EU3), the highest acute exposure for thiophanate‐methyl is calculated for lemons, representing 81% of the ARfD, the highest acute exposure for carbendazim is calculated for mandarins, representing 84% of the ARfD and the highest combined acute exposure is calculated for lemons, representing 88% of the ARfD.
These calculations show that no risk for consumers is identified for lemons in case residues from the uses of carbendazim and thiophanate‐methyl are not co‐occurring.
In order to perform a combined chronic risk assessment, results from the chronic risk assessment of thiophanate‐methyl and results from the chronic risk assessment of carbendazim from the refined calculations were summed (scenario EU2 and EU3). This calculation has been done for the Dutch diet (toddler), the British diet (infant) and the French diet (toddler) being the diets with the highest estimated exposure.
The highest chronic exposure for scenario EU2 was calculated for the Dutch diet (toddler), representing 10% of the ADI. The highest chronic exposure for scenario EU3 was calculated for the Dutch diet (toddler), representing 9% of the ADI.
Based on these calculations, an acute risk to consumers was identified for the most critical GAPs for thiophanate‐methyl on oranges, grapefruits, mandarins, mangoes and papaya and for lemons, if the residues from the uses of carbendazim and thiophanate‐methyl are co‐occurring. However, fall‐back GAPs were identified for mandarins and lemons, for which a second (scenario EU2) and a third (scenario EU3) risk assessments did not indicate risk to consumers. For the remaining commodities, although some major uncertainties remain due to the data gaps identified, the indicative exposure calculation did not indicate a risk to consumers.
Recommendations
MRL recommendations were derived in compliance with the decision tree reported in Appendix I of the reasoned opinion (see Table 1). None of the MRL values listed in the table are recommended for inclusion in Annex II to the Regulation as they are not sufficiently supported by data. In particular, all tentative MRLs need to be confirmed by the following data:
information on whether samples from residue trials were homogenised prior or after storage;
information on the storage condition of the samples from the livestock feeding studies performed with carbendazim;
storage stability study for metabolite 4‐hydroxy‐carbendazim in milk.
Moreover, it is highlighted that an exceedance of the ARfD was observed for oranges, grapefruits, mandarins, lemons, mangoes and papaya. Consequently, risk managers should consider measures for reduction of the consumer exposure. Furthermore, in order to avoid decline of residues during storage of food samples, enforcement laboratories are recommended not to homogenise samples prior to storage.
To inform further risk management discussions, it is noted that carbendazim is classified as toxic for reproduction category 1B in accordance with Regulation (EC) No 1272/2008.
It is noted that the residue definition for carbendazim currently in the Regulation also includes the active substance benomyl. Nevertheless, considering that a toxicological assessment of benomyl was never carried out at EU level, it is not considered any longer appropriate to include benomyl in the residue definition. As the use of benomyl is no longer authorised within the EU, this change of residue definition will only have consequences for food products treated with benomyl that may be imported from third countries. Hence, if no need to establish import tolerances for benomyl is identified by risk managers, MRLs for benomyl may be established at a specific LOQ or at the default MRL of 0.01 mg/kg. It is also underlined for further considerations by risk managers that, according to the EURLs, it is not possible to analyse separately for benomyl and carbendazim using routine methods.
Minor deficiencies were also identified in the assessment, but these deficiencies are not expected to impact either on the validity of the MRLs derived. The following data are therefore considered desirable but not essential:
Additional residue trials on mangoes and on okra (lady fingers) compliant with the import tolerance GAPs for thiophanate‐methyl with samples analysed separately for thiophanate‐methyl and carbendazim.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CIRCA
(EU) Communication & Information Resource Centre Administrator
- CS
capsule suspension
- CV
coefficient of variation (relative standard deviation)
- CXL
codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DM
dry matter
- DP
dustable powder
- DS
powder for dry seed treatment
- DT90
period required for 90% dissipation (define method of estimation)
- EDI
estimated daily intake
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐FID
gas chromatography with flame ionisation detector
- GC‐MS
gas chromatography with mass spectrometry
- GC‐MS/MS
gas chromatography with tandem mass spectrometry
- GS
growth stage
- HPLC
high‐performance liquid chromatography
- HPLC‐MS
high‐performance liquid chromatography with mass spectrometry
- HPLC‐MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LC
liquid chromatography
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- MW
molecular weight
- NEDI
national estimated daily intake
- NESTI
national estimated short‐term intake
- NOAEL
no observed adverse effect level
- NTMDI
national theoretical maximum daily intake
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged and Safe (analytical method)
- RA
risk assessment
- RD
residue definition
- RAC
raw agricultural commodity
- RD
residue definition
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- SL
soluble concentrate
- SP
water soluble powder
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- WHO
World Health Organization
Appendix A – Summary of authorised uses considered for the review of MRLs
A.1. Import tolerances – thiophanate methyl
| Crop and/or situation | MS or country | F G or I(a) | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)(d) | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages and seasonc | Number min–max | Interval between application (min) | a.s./hL min–max | Water L/ha min–max | Rate and unit | ||||||
| Grapefruits | Non‐EU | I | Penicillium | SC | 500 g/L | Post‐harvest treatment – dipping | N.a. | 1–1 | – | – | 0.18 kg a.i./hL | 3 | ||
| Oranges | Non‐EU | I | Penicillium | SC | 500 g/L | Post‐harvest treatment – dipping | n.a. | 1–1 | – | – | 0.18 kg a.i./hL | 3 | ||
| Lemons | Non‐EU | I | Penicillium | SC | 500 g/L | Post‐harvest treatment – dipping | n.a. | 1–1 | – | – | 0.18 kg a.i./hL | 3 | ||
| Limes | Non‐EU | I | Penicillium | SC | 500 g/L | Post‐harvest treatment – dipping | n.a. | 1–1 | – | – | 0.18 Kg a.i./hL | 3 | ||
| Mandarins | Non‐EU | I | Penicillium | SC | 500 g/L | Post‐harvest treatment – dipping | n.a. | 1–1 | – | – | 0.18 Kg a.i./hL | 3 | ||
| Mangoes | Non‐EU | F | Alternaria, Cercospora Dothiorella Collelotrichum gloeosporioies Botryodiplodia theobromae | SC | 500 g/L | Foliar treatment – spraying | 81–86 | 1–2 | 10 | – | – | 0.075 kg a.i./hL | 14 | |
| Papayas | Non‐EU | F | Anthrachnosis | WP | 700 g/kg | Foliar treatment – spraying | N.a. | 5–5 | 14 | – | – | 0.7 kg a.i./ha | 3 | |
| Okra | Non‐EU | F | Leaf spot | EC | 500 g/L | Foliar treatment – spraying | n.a. | 1–2 | 14 | – | – | 0.49 kg a.i./ha | 2 | |
MS: Member State.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system. Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
A.2. Import tolerances – carbendazim
| Crop and/or situation | MS or country | F G or I(a) | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)(d) | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type(b) | Conc. a.s. | Method kind | Range of growth stages and season(c) | Number min–max | Interval between application (min) | a.s./hL min–max | Water L/ha min–max | Rate and unit | ||||||
| Lemons | South Africa | I | Penicillium | SC | 500 g/L | Foliar treatment – spraying | n.a. | 2 | – | – | 0.013 kg a.i./hL | 60 | ||
| Limes | South Africa | I | Penicillium | SC | 500 g/L | Foliar treatment – spraying | n.a. | 2 | – | – | 0.013 kg a.i./hL | 60 | ||
| Mandarins | South Africa | I | Penicillium | SC | 500 g/L | Foliar treatment – spraying | n.a. | 2 | – | – | 0.013 kg a.i./hL | 60 | ||
MS: Member State.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system. Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
Appendix B – List of end points
B.1. Mammalian Toxicology
B.1.1. Thiophanate‐methyl
Genotoxicity

Summary
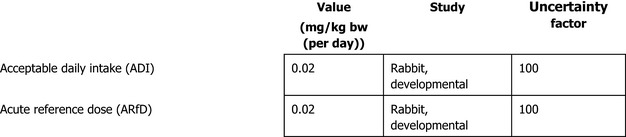
B.1.2. Carbendazim
Genotoxicity
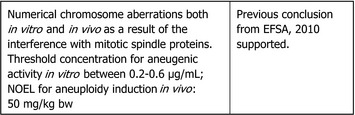
Summary
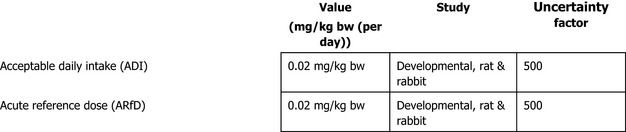
B.2. Residues in plants
B.2.1. Nature of residues and methods of analysis in plants
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Thiophanate methyl | |||||
|---|---|---|---|---|---|
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
| Fruit crops | Apples | Foliar, 3 × 3.9 kg/ha | 1, 7 | Radiolabelled active substance: 14C‐phenyl ‐a.s. (EFSA, 2014, 2018a) | |
| Grapes | Foliar, 1 × 1.042 kg a.s./ha | 0, 14, 35 | 14C‐phenyl (EFSA, 2018a) | ||
| Tomatoes | Drip irrigation, 1 × 0.702; 1 × 1.386; 1 × 2.314 kg/ha | 7 | 14C‐phenyl (Sweden 2016, 2017) | ||
| Root crops | Sugar beets | Foliar, 3 × 0.39 kg/ha | 0, 21 | 14C‐thiocarbonyl (EFSA, 2014, 2018a) | |
| Cereals/grass | Wheat | Foliar, 1 × 0.75 kg/ha | 0, 28, 69 | 14C‐thiocarbonyl (EFSA, 2014; Sweden 2016, 2017) | |
| Pulses/oilseeds | Lima beans | Foliar, 2 × 1.18 kg/ha | 28, 35 | 14C‐thiocarbonyl (EFSA, 2014, 2018a) Supportive, not acceptable as a standalone study | |
| Soyabeans | Run‐off, 1 × 700 mg/l | 0, 7, 14 | 14C‐thiophanate‐methyl (label position not given) mixed with non labelled thiophanate‐ methyl (EFSA, 2018a) | ||
| Green beans | Run‐off (assumed), 1 × 50 mg/L | 14 | 14C‐thiophanate‐methyl (label position not given) (EFSA, 2018a) | ||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | Carrots | Bare soil, 1 × 1.6 kg/ha | 30, 120, 365 | EFSA (2014, 2018a) Studies available but not relevant since only import tolerances under assessment. | |
| Leafy crops | Lettuce | Bare soil, 1 × 1.6 kg/ha | 30, 120, 365 | EFSA (2014, 2018a) Same comment as above. | |
| Cereal (small grain) | Wheat | Bare soil, 1 × 1.6 kg/ha | 30, 120, 365 | EFSA (2014, 2018a) Same comment as above. |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | EFSA (2014, 2018a) | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | No | Thiophanate‐methyl degraded to carbendazim that accounted 14.2% (EFSA 2014, 2018a) | |
| Sterilisation (20 min, 120°C, pH 6) | No | Thiophanate‐methyl degraded to carbendazim (92%) and to metabolite 2‐AB (10.3%) (EFSA 2014, 2018a) |
| Carbendazim | |||||
|---|---|---|---|---|---|
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
| Fruit crops | Peaches | Foliar, 2 × 1.12 kg/ha, interval of 14 days between applications | –14, 0 | Sampling after each treatment. Study performed with 14C‐phenyl carbendazim (EFSA, 2010, 2014) | |
| Strawberries | Hydroponic, 1 × 0.182 kg as/L | 36, 88 | Study performed with 14C‐imidazole carbendazim Informative only (EFSA, 2010, 2014) | ||
| Root crops | Sugar beet | Foliar, 3 × 0.55 kg/ha Foliar, 5 × 0.55 kg/ha | 21 133 | Study performed with 14C‐phenyl benomyl (FAO, 1998) | |
| Cereals/grass | Rice | Foliar, 2 × 2.25 kg/ha | –14, 0, 35 | Study performed with 14C‐phenyl benomyl (EFSA, 2010, 2014) | |
| Pulses/oilseeds | Beans | Foliar, 2 × 1.12 kg/ha | 0, 7, 14, 21, 28 | Study performed with 14C‐imidazole carbendazim Residues analysed in plants and beans (EFSA, 2010, 2014) | |
| Soyabeans | Foliar, 2 × 1.1 kg/ha | –14, 0, 35 | Study performed with 14C‐phenyl benomyl (FAO, 1998; EFSA, 2014) | ||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
| Root/tuber crops | Beet | Bare soil, 1.12 kg/ha | 30 | [2‐14C]‐carbendazim (EFSA, 2010, 2014) Studies available but not relevant since only import tolerances under assessment. | |
| Bare soil, 3.36 kg/ha | 120 | ||||
| Radish | 3 mg carbendazim/kg soil | 224 | 14C‐carbendazim (EFSA, 2010, 2014) | ||
| Leafy crops | Cabbages | Bare soil, 1.12 kg/ha | 30 | [2‐14C]‐carbendazim (EFSA, 2010, 2014) Same comment as above. | |
| Bare soil, 3.36 kg/ha | 120 | ||||
| Lettuce | 3 mg carbendazim/kg soil | 224 | 14C‐carbendazim (EFSA, 2010, 2014) Same comment as above. | ||
| Pulses and oilseeds | Soybean | Bare soil, 2.24 kg/ha | 60 | 80:20 mix of 14C‐labelled carbendazim and 2‐AB (EFSA, 2010, 2014) Same comment as above. | |
| Alfalfa | Bare soil, 2.24 kg/ha | 60 | |||
| Cereal (small grain) | Barley | Bare soil, 1.12 kg/ha | 30 | [2‐14C]‐carbendazim (EFSA, 2010, 2014) Same comment as above. | |
| Bare soil, 3.36 kg/ha | 145 | ||||
| Rye grass | Bare soil, 2.24 kg/ha | 60 | 80:20 mix of 14C‐labelled carbendazim and 2‐AB (EFSA, 2010, 2014) Same comment as above. | ||
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | EFSA (2010, 2014) | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | EFSA (2010, 2014) | |||
| Sterilisation (20 min, 120°C, pH 6) | Yes | EFSA (2010, 2014) | |||
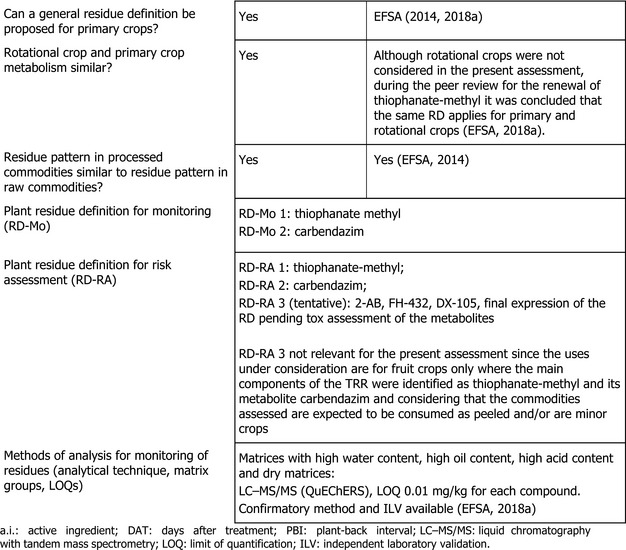
B.2.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Apples, cut in half | –18 | 36 | Months | Thiophanate‐methyl | EFSA (2012) | |
| Tomatoes | –18 | 30 | Months | Carbendazim | EFSA (2010) | ||
| High oil content | Rapeseeds, intact | –18 | 12 | Months | Thiophanate‐methyl Carbendazim | EFSA (2018a) | |
| Rapeseeds, homogenised | –18 | 1 | Month | Thiophanate‐methyl | EFSA (2018a) | ||
| Rapeseeds, homogenised | –18 | 3 | Months | Carbendazim | EFSA (2018a) | ||
| High protein content | Dry peas, intact | –18 | 12 | Months | Thiophanate‐methyl Carbendazim | EFSA (2018a) | |
| Dry peas, homogenised | –18 | 3 | Months | Thiophanate‐methyl Carbendazim | EFSA (2018a) | ||
| High starch content | Wheat, intact | –18 | 12 | Months | Thiophanate‐methyl Carbendazim | EFSA (2018a) | |
| Wheat, homogenised | –18 | 2 | Weeks | Thiophanate‐methyl | EFSA (2018a) | ||
| Wheat, homogenised | –18 | 3 | Months | Carbendazim | EFSA (2018a) | ||
| High acid content | Grapes, intact | –18 | 12 | Months | Thiophanate‐methyl Carbendazim | EFSA (2018a) | |
| Grapes, homogenised | –18 | < 10 | Days | Thiophanate‐methyl | EFSA (2018a) | ||
| Grapes, homogenised | –18 | 1 | Month | Carbendazim | EFSA (2018a) | ||
| Strawberries, intact | –18 | 9 | Months | Thiophanate‐methyl | EFSA (2018a) | ||
| Strawberries, intact | –18 | 12 | Months | Carbendazim | EFSA (2018a) | ||
| Processed commodities | Soyabeans, oil | –18 | 18 | Months | Carbendazim | EFSA (2010) | |
B.2.2. Magnitude of residues in plants
B.2.2.1. Summary of residues data from the supervised residue trials performed with thiophanate methyl – Primary crops
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| RD‐Mo 1: thiophanate methyl RD‐RA 1: thiophanate methyl | ||||||
| Citrus fruits | Import | Oranges: 1.4; 1.7; 2.6; 2.9 Mandarins: 2.0; 2.4; 3.1; 4.3 | Combined dataset on oranges and mandarins compliant with GAP for post‐harvest treatment of citrus fruits (EFSA, 2014). MRL based on mean + 4 SD (6.21) | 7 (tentative)d | 4.3 | 2.5 |
| Mangoes | Import | < 0.1; 0.2; 0.2; 0.6 | Trials on mangoes compliant with GAP. Residues determined as sum of thiophanate‐methyl and carbendazim, expressed as thiophanate‐methyl, deemed acceptable for a minor crop (EFSA, 2014). MRLOECD = 1.16 | 1.5 (tentative)d | 0.6 | 0.2 |
| Papaya | Import | 0.3; 0.39; 0.42; 0.59 | Trials on papaya compliant with GAP (EFSA, 2014). MRLOECD = 1.28 | 1.5 (tentative)d | 0.59 | 0.41 |
| Okra, lady's fingers | Import | 0.03; 0.07; 0.15; 0.23; 0.26; 0.48 | Trials on okra compliant with GAP. Residues determined as sum of thiophanate‐methyl and carbendazim, expressed as thiophanate‐methyl, deemed acceptable for a minor crop (EFSA, 2014). MRLOECD = 0.85 | 0.9 (tentative)d | 0.48 | 0.19 |
| RD‐Mo 2: carbendazim RD‐RA 2: carbendazim | ||||||
| Citrus fruits | Import | Oranges: 0.06; 0.06; 0.08; 0.09 Mandarins: 0.08; 0.08; 0.08; 0.09 | Combined dataset on oranges and mandarins compliant with GAP for post‐harvest treatment of citrus fruits (EFSA, 2014). MRL based on mean + 4 SD (0.124) | 0.2 (tentative)d | 0.09 | 0.08 |
| Mangoes | Import | < 0.05; 0.12; 0.12; 0.35 | Trials on mangoes compliant with GAP. Residues determined as sum of thiophanate‐methyl and carbendazim, expressed as carbendazim deemed acceptable for a minor crop (EFSA, 2014). MRLOECD = 0.68 | 0.7 (tentative)d | 0.35 | 0.12 |
| Papaya | Import | 0.03; 0.07; 0.08; 0.08 | Trials on papaya compliant with GAP (EFSA, 2014). MRLOECD = 0.20 | 0.2 (tentative)d | 0.08 | 0.08 |
| Okra, lady's fingers | Import | 0.05; 0.13; 0.27; 0.42; 0.46; 0.87 | Trials on okra compliant with GAP. Residues determined as sum of thiophanate‐methyl and carbendazim, expressed as carbendazim deemed acceptable for a minor crop (EFSA, 2014) MRLOECD = 1.54 | 1.5 (tentative)d | 0.87 | 0.35 |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
Indicates that the MRL is proposed at the limit of quantification.
Mo: residue levels expressed according to the monitoring residue definition; RA: residue levels expressed according to risk assessment residue definition.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Although a sufficient number of data is available, MRL proposal is tentative because it was not reported whether or not the analysed samples used to derive MRL and risk assessment values were homogenised prior storage (see also body text).
B.2.2.2. Summary of residues data from the supervised residue trials performed with carbendazim – Primary crops
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| RD‐Mo 2: carbendazim RD‐RA 2: carbendazim | ||||||
| Lemons Lime Mandarins | Import (SA) | 0.05; 0.15; 0.15; 0.20; 0.22; 0.24; 0.24; 0.24; 0.27; 0.27; 0.30; 0.31; 0.34; 0.35; 0.44; 0.60 | Combined dataset on oranges (8) and lemons (8). Extrapolation to mandarins and limes possible. MRLOECD = 0.82 | 0.9 (tentative)d | 0.60 | 0.26 |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
Indicates that the MRL is proposed at the limit of quantification.
Mo: residue levels expressed according to the monitoring residue definition; RA: residue levels expressed according to risk assessment residue definition.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Although a sufficient number of data is available, MRL proposal is tentative because it was not reported whether or not the analysed samples used to derive MRL and risk assessment values were homogenised prior storage (see also body text).
B.2.2.3. Residues in rotational crops

B.2.2.4. Processing factors
| Processed commodity | Number of valid studiesa | Processing Factor (PF) | Comment/Source | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| RD Mo 1: thiophanate‐methyl RD RA 1: thiophanate‐methyl | ||||
| Mangoes, peeled | 4 | 0.17; 0.29; 0.5; 0.6 | 0.40 | Processing factors based on the sum of thiophanate‐methyl and carbendazim (EFSA, 2009, 2014). |
| Citrus fruits, peeled | 10 | Not available | 0.11 | EFSA (2009, 2014) |
| Orange, juice | 4 | 4 × 0.03 | 0.03 | 1 balance study and 3 follow up studies (EFSA, 2014) |
| Citrus fruits, dry pomace | 4 | 0.75; 1.49; 1.53; 1.66 | 1.51 | 1 balance study and 3 follow up studies (EFSA, 2014) |
| Orange, marmalade | 4 | 0.23; 0.26; 0.74; 0.93 | 0.50 | 1 balance study and 3 follow up studies (EFSA, 2014) |
| Citrus fruits, wet pomace | 1 | 1.23 | 1.23 | Tentativeb 1 balance study (EFSA, 2014) |
| RD Mo 2: carbendazim RD RA 2: carbendazim | ||||
| Mangoes, peeled | 4 | 0.17; 0.29; 0.5; 0.6 | 0.40 | Processing factors based on the sum of thiophanate‐methyl and carbendazim, expressed as thiophanate‐methyl (EFSA, 2009, 2014) |
| Citrus fruits, peeled | 16 | 0.53; 0.50; 0.35; 0.43; 0.50; 0.30; 2 × 0.40; 0.80; 0.33; 0.63; 0.60; 0.45; 0.46; 0.47; 0.63 | 0.47 | EFSA (2009, 2014) |
| Orange, juice | 4 | 3 × < 0.04; < 0.05 | < 0.04 | 1 balance study and 3 follow up studies (EFSA, 2014) |
| Citrus fruits, dry pomace | 4 | 24.6; 25.2; 26.3; 43.3 | 25.7 | 1 balance study and 3 follow up studies (EFSA, 2014) |
| Orange, marmalade | 4 | 0.4; 0.41; 0.62; 0.63 | 0.51 | 1 balance study and 3 follow up studies (EFSA, 2014) |
| Citrus fruits, wet pomace | 1 | 1.28 | 1.28 | Tentativeb 1 balance study (EFSA, 2014) |
PF: Processing factor (=Residue level in processed commodity expressed according to RD‐Mo/Residue level in raw commodity expressed according to RD‐Mo);
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
A tentative PF is derived based on a limited dataset.
B.3. Residues in livestock
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroupa | Most critical commodityb | Trigger exceeded (Y/N) | Comments | |||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | |||||||
| Median | Maximum | Median | Maximum | |||||
| Thiophanate‐methyl | ||||||||
| Cattle (all) | 0.032 | 0.032 | 0.83 | 0.83 | Dairy cattle | Grapefruits, dried pulp | Y | – |
| Cattle (dairy only) | 0.032 | 0.032 | 0.83 | 0.83 | Dairy cattle | Grapefruits, dried pulp | Y | – |
| Sheep (all & ewe only) | – | – | – | – | – | – | N | – |
| Swine (all) | 0.014 | 0.014 | 0.62 | 0.62 | Swine (breeding) | Grapefruits, dried pulp | Y | – |
| Poultry (all & layer only) | – | – | – | – | – | – | N | – |
| Carbendazim | ||||||||
| Cattle (all) | 0.055 | 0.055 | 1.44 | 1.44 | Dairy cattle | Lemons, dried pulp | Y | Based on the uses of both thiophanate‐methyl and carbendazim |
| Cattle (dairy only) | 0.055 | 0.055 | 1.44 | 1.44 | Dairy cattle | Lemons, dried pulp | Y | Based on the uses of both thiophanate‐methyl and carbendazim |
| Sheep (all & ewe only) | – | – | – | – | – | – | N | – |
| Swine (all) | 0.025 | 0.025 | 1.08 | 1.08 | Swine (breeding) | Lemons, dried pulp | Y | Based on the uses of both thiophanate‐methyl and carbendazim |
| Poultry (all & layer only) | – | – | – | – | – | – | N | – |
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.3.1. Nature of residues and methods of analysis in livestock
B.3.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw/d) | Duration (days) | Comment/Source |
|---|---|---|---|---|
| Thiophanate‐methyl | ||||
| Laying hen | 2.9–3.5 | 10 | 14C‐phenyl ring label, Hens (EFSA, 2014, 2018a) | |
| Lactating ruminants | 1.15–1.19 | 5 | 14C‐phenyl ring, Goat (EFSA, 2014, 2018a) | |
| Pig | – | – | Not available and not required since the metabolism in ruminants and rat is similar (EFSA, 2014) | |
| Carbendazim | ||||
| Laying hen | 0.37 8.8a | 6 | [2‐14C]‐carbendazim, Hens (EFSA, 2010, 2014) | |
| Lactating ruminants | 2.1(b) 1.8c | 5 30 | [2‐14C]‐carbendazim, Cow (EFSA 2010, 2014) 14C‐phenyl, Goat (EFSA 2010, 2014) | |
| Pig | – | – | Not available and not required since the metabolism in ruminants and rat is similar (EFSA, 2014) | |
In the study summary, the administrated dose was only expressed in mg/kg feed as received (5 and 120 mg/kg feed as received). Based on this information, EFSA derived theoretical administrated doses, assuming a body weight of 1.9 kg, a daily intake of 0.12 kg of feed (dry matter basis) and feed composed of maize grain and pulses.
In the study summary, the administrated dose was only expressed in mg/kg feed as received (50 mg/kg feed as received). Based on this information, EFSA derived a theoretical administrated dose, assuming a body weight of 550 kg, a daily intake of 20 kg of feed (dry matter basis) and feed only composed of hay.
In the study summary, the administrated dose was only expressed in mg/animal per day (73 mg/animal per day). Based on this information, EFSA derived a theoretical administrated dose, assuming a body weight of 40 kg.
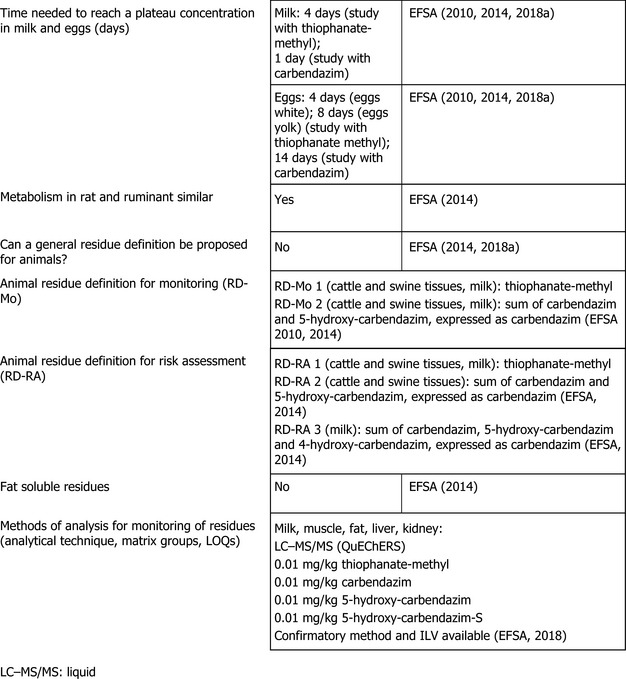
B.3.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| Bovine | Muscle | –20 ± 10 | 8 | Months | Thiophanate‐methyl Carbendazim | No info on the storage stability of metabolites 5‐OH‐MBC and 5‐OH‐MBC‐S (EFSA, 2018a) | |
| Bovine | Liver | –20 ± 10 | 7 | Months | Carbendazim 5‐OH‐MBC | No info on the storage stability of thiophanate‐methyl and 5‐OH‐MBC‐S (EFSA, 2018a) | |
| Bovine | Milk | –20 ± 10 | 8 | Months | Carbendazim 5‐OH‐MBC‐S | No info on the storage stability of thiophanate‐methyl, 4‐OH‐MBC and 5‐OH‐MBC (EFSA, 2018a) | |
| Poultry | Muscle | –25 ca. | 8 | Months | Carbendazim 5‐OH‐MBC | No info on the storage stability of thiophanate‐methyl and 5‐OH‐MBC‐S (EFSA, 2018a) | |
| Poultry | Liver | –25 ca. | 8 | Months | Thiophanate‐methyl 5‐OH‐MBC | No information on the storage stability of carbendazim and 5‐OH‐MBC‐S (EFSA, 2018a) | |
| Poultry | Eggs | –25 ca. | 10 | Months | Carbendazim 5‐OH‐MBC | No info on the storage stability of 5‐OH‐MBC‐S (EFSA, 2018a) | |
| Poultry | Eggs | –25 ca. | 9 | Months | Thiophanate‐methyl | ||
B.3.2. Magnitude of residues in livestock
B.3.2.1. Summary of the residue data from livestock feeding studies
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | CFc | ||
|---|---|---|---|---|---|---|
| Mean | Highest | STMRMo a (mg/kg) | HRMo b (mg/kg) | |||
| Thiophanate‐methyl | ||||||
| Cattle (all) – Closest feeding level (2.6 mg/kg bw; 81.25N rate)d | ||||||
| Muscle | n.r. | < 0.05 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Fat | n.r. | < 0.05 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Liver | n.r. | 0.20 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Kidney | n.r. | 0.38 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Cattle (dairy only) – Closest feeding level (2.6 mg/kg bw; 81.25N rate)d | ||||||
| Milkf | n.r. | 0.23 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Sheep (all)/Sheep (ewe only) – No need to set MRLs since sheep are not expected to be exposed to significant levels of thiophanate residues | ||||||
| Swine (all)g – Closest feeding level (2.6 mg/kg bw; 186N rate)(d) | ||||||
| Muscle | n.r. | < 0.05 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Fat | n.r. | < 0.05 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Liver | n.r. | 0.20 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| kidney | n.r. | 0.38 | < 0.01 | < 0.01 | 0.01* (tentative)e | 1 |
| Poultry (all)/Poultry (layer only) – No need to set MRLs since poultry are not expected to be exposed to significant levels of thiophanate residues | ||||||
| Carbendazim | ||||||
| Cattle (all) – Closest feeding level (0.09 mg/kg bw; 1.64N rate)d | ||||||
| Muscle | < 0.02 | < 0.02 | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Fat | 0.03h | 0.03h | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Liver | < 0.02 | < 0.02 | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Kidney | < 0.02 | < 0.02 | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Cattle (dairy only) – Closest feeding level (0.09 mg/kg bw; 1.64N rate)d | ||||||
| Milkf | < 0.02 | n.a. | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Sheep (all)/Sheep (ewe only) – No need to set MRLs since sheep are not expected to be exposed to significant levels of carbendazim residues | ||||||
| Swine (all)g – Closest feeding level (0.09 mg/kg bw; 3.6N rate)(d) | ||||||
| Muscle | < 0.02 | < 0.02 | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Fat | 0.03 | 0.03 | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Liver | < 0.02 | < 0.02 | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Kidney | < 0.02 | < 0.02 | < 0.02 | < 0.02 | 0.02* (tentative)i | 1 |
| Poultry (all)/Poultry (layer only) – No need to set MRLs since poultry are not expected to be exposed to significant levels of carbendazim residues | ||||||
Indicates that the MRL is proposed at the limit of quantification.
n.a.: not applicable; n.r. : not reported.
Median residues expressed according to the residue definition for monitoring (sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim), recalculated at the 1N rate for the median dietary burden.
Highest residues covering the sum of all relevant compounds and expressed as parent (thiophanate‐methyl) or highest residues expressed according to the residue definition for monitoring (sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim) re‐calculated at the 1N rate for the maximum dietary burden.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
Closest feeding level and N dose rate related to the maximum dietary burden.
Pending confirmation that samples from trials on plants were not homogenised, the derived MRLs should be considered tentative only.
For milk, mean was derived from samplings performed from day 1 to day 28 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in swine.
5‐hydroxy‐carbendazim was quantified in the renal fat of one animal at 0.02 mg/kg. Nevertheless, considering that no residues of this compound were detected in renal fat from the two higher dose groups, this value is considered to be an outlier and is reported only for completeness.
Pending confirmation that samples from trials on plants were not homogenised, information on the storage conditions of the samples from the livestock feeding studies and storage stability data for 4‐hydroxy‐carbendazim (metabolite relevant for the risk assessment of carbendazim in milk), the derived MRLs should be considered tentative only.
B.4. Consumer risk assessment


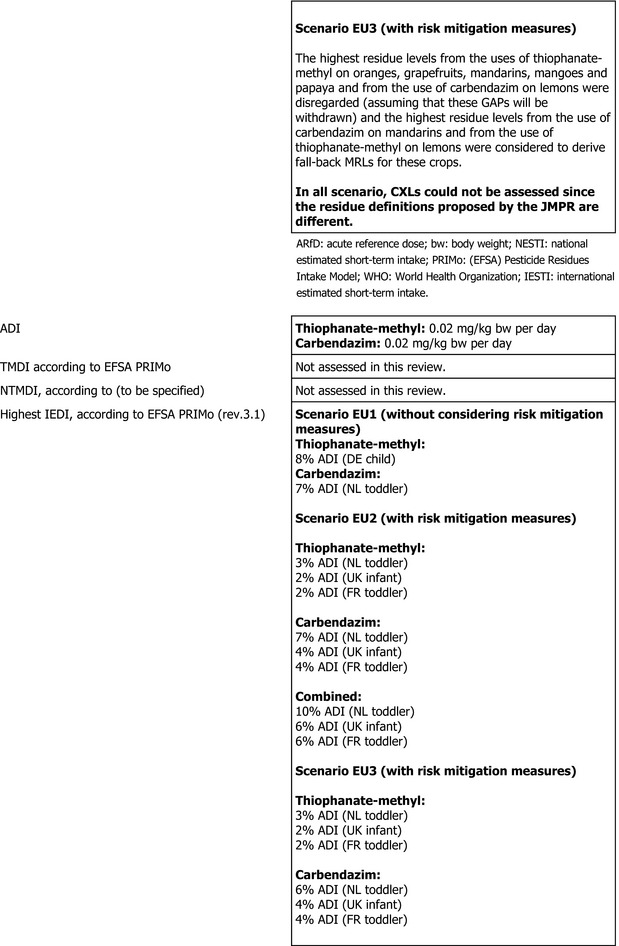

B.5. Proposed MRLs
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: thiophanate‐methyl | |||||
| 110010 | Grapefruits | 6 | – | – | Further consideration neededa Data gap #1 |
| 110020 | Oranges | 6 | 1 | – | Further consideration neededb Data gap #1 |
| 110030 | Lemons | 6 | – | Option 1c: – | Further consideration neededd Data gap #1 |
| Option 2e: 7 | Further consideration neededd Data gap #1 | ||||
| 110040 | Limes | 6 | – | 7 | Further consideration neededd Data gap #1 |
| 110050 | Mandarins | 6 | – | – | Further consideration neededa Data gap #1 |
| 163030 | Mangoes | 1 | 5 | – | Further consideration neededb Data gap #1 |
| 163040 | Papayas | 1 | – | – | Further consideration neededa Data gap #1 |
| 231040 | Okra/lady's fingers | 1 | – | 0.9 | Further consideration neededd Data gap #1 |
| Enforcement residue definition (existing): thiophanate‐methyl and carbendazim, expressed as carbendazim Enforcement residue definition (proposed): thiophanate‐methyl | |||||
| 1011010 | Swine muscle | 0.05* | – | 0.01* | Further consideration neededd Data gaps #1 |
| 1011020 | Swine fat tissue | 0.05* | – | 0.01* | Further consideration neededd Data gaps #1 |
| 1011030 | Swine liver | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1011040 | Swine kidney | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1012020 | Bovine fat tissue | 0.05* | – | 0.01* | Further consideration neededd Data gaps #1 |
| 1012030 | Bovine liver | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1012040 | Bovine kidney | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1015010 | Equine muscle | 0.05* | – | 0.01* | Further consideration neededd Data gaps #1 |
| 1015020 | Equine fat tissue | 0.05* | – | 0.01* | Further consideration neededd Data gaps #1 |
| 1015030 | Equine liver | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1015040 | Equine kidney | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1020010 | Cattle milk | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| 1020040 | Horse milk | 0.05* | 0.05* | 0.01* | Further consideration neededf Data gaps #1 |
| Enforcement residue definition (existing): sum of benomyl and carbendazim, expressed as carbendazim Enforcement residue definition (proposed): carbendazim | |||||
| 110010 | Grapefruits | 0.2 | – | – | Further consideration neededa Data gap #1 |
| 110020 | Oranges | 0.2 | 1 | – | Further consideration neededb Data gap #1 |
| 110030 | Lemons | 0.7 | – | Option 1(c): 0.9 | Further consideration neededd Data gap #1 |
| Option 2(e): 0.2 | Further consideration neededd Data gap #1 | ||||
| 110040 | Limes | 0.7 | – | 0.9 | Further consideration neededd Data gap #1 |
| 110050 | Mandarins | 0.7 | – | 0.9 | Further consideration neededd Data gap #1 |
| 163030 | Mangoes | 0.5 | 5 | – | Further consideration neededb Data gap #1 |
| 163040 | Papayas | 0.2 | – | – | Further consideration neededa Data gap #1 |
| 231040 | Okra/lady's fingers | 2 | – | 1.5 | Further consideration neededd Data gap #1 |
| Enforcement residue definition (existing): carbendazim and thiophanate‐methyl, expressed as carbendazim Enforcement residue definition (proposed): sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim | |||||
| 1011010 | Swine muscle | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1011020 | Swine fat tissue | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1011030 | Swine liver | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1011040 | Swine kidney | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1012020 | Bovine fat tissue | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1012030 | Bovine liver | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1012040 | Bovine kidney | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1015010 | Equine muscle | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1015020 | Equine fat tissue | 0.05* | – | 0.02* | Further consideration neededd Data gaps #1,2 |
| 1015030 | Equine liver | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1015040 | Equine kidney | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2 |
| 1020010 | Cattle milk | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2,3 |
| 1020040 | Horse milk | 0.05* | 0.05* | 0.02* | Further consideration neededf Data gaps #1,2,3 |
| – | Other commodities of plant and/or animal origin | See Reg. 559/2011 | – | – | Further consideration needed(g) |
| Enforcement residue definition (proposed): benomyl | |||||
| – | Commodities of plant and/or animal origin | – | – | – | Further consideration needed(g) |
MRL: maximum residue level; CXL: codex maximum residue limit.
Indicates that the MRL is set at the limit of quantification.
The residue definition is fat soluble.
GAP evaluated at EU level is not fully supported by data and a risk to consumers cannot be excluded; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination E‐I in Appendix I).
GAP evaluated at EU level is not fully supported by data and a risk to consumers cannot be excluded; CXL is not compatible with EU residue definitions. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination E‐II in Appendix I).
Option 1: MRL based on the authorised use for carbendazim, assuming that the authorised use of thiophanate‐methyl will be withdrawn.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination F‐I in Appendix I). It is noted that carbendazim is classified as toxic for reproduction category 1B in accordance with Regulation (EC) No 1272/2008.
Option 2: MRL based on the authorised use for thiophanate‐methyl, assuming that the authorised use of carbendazim will be withdrawn.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is not compatible with EU residue definitions (combination F‐II in Appendix I). It is noted that carbendazim is classified as toxic for reproduction category 1B in accordance with Regulation (EC) No 1272/2008.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available or CXL is not compatible with EU residue definitions. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I/II in Appendix I).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
• PRIMo (Scenario EU1) thiophanate‐methyl – without risk mitigation measures
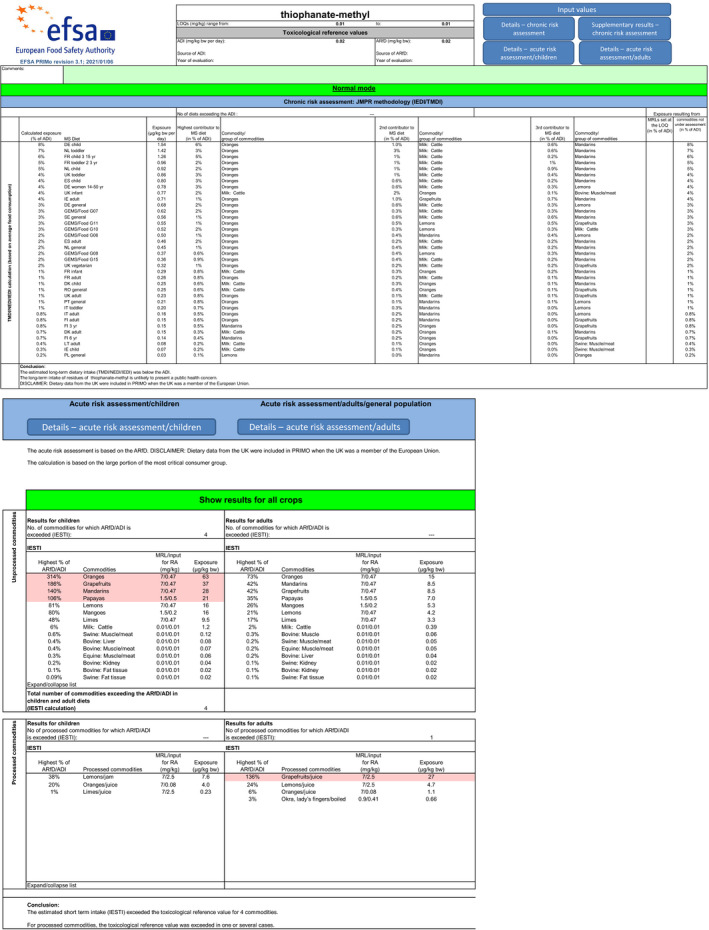
• PRIMo (Scenario EU1) carbendazim – without risk mitigation measures
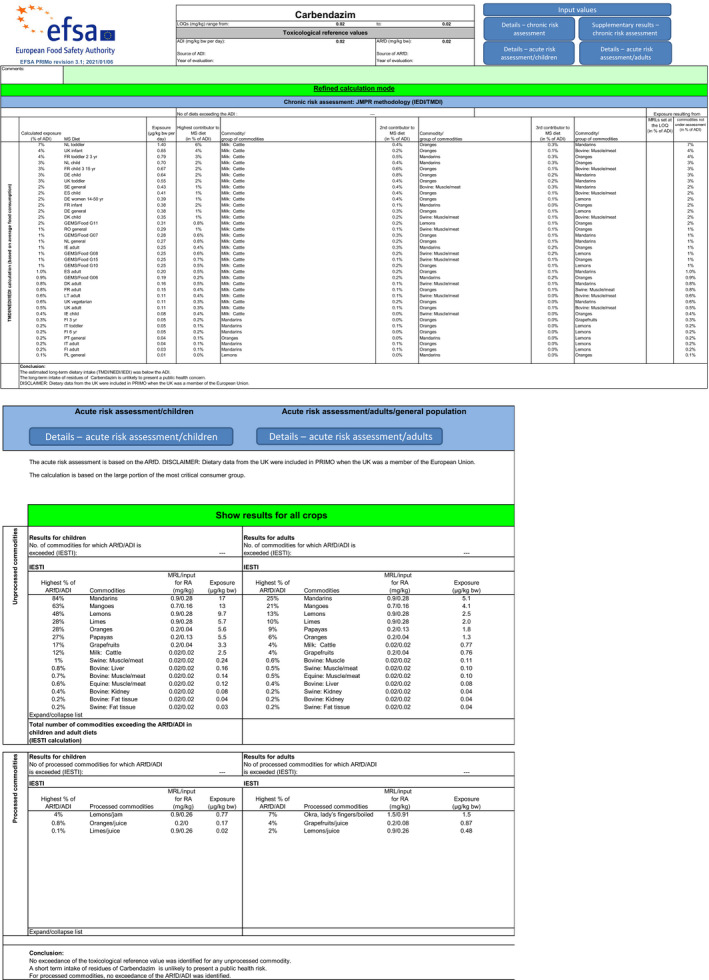
• PRIMo (Scenario EU2) thiophanate‐methyl – with risk mitigation measures
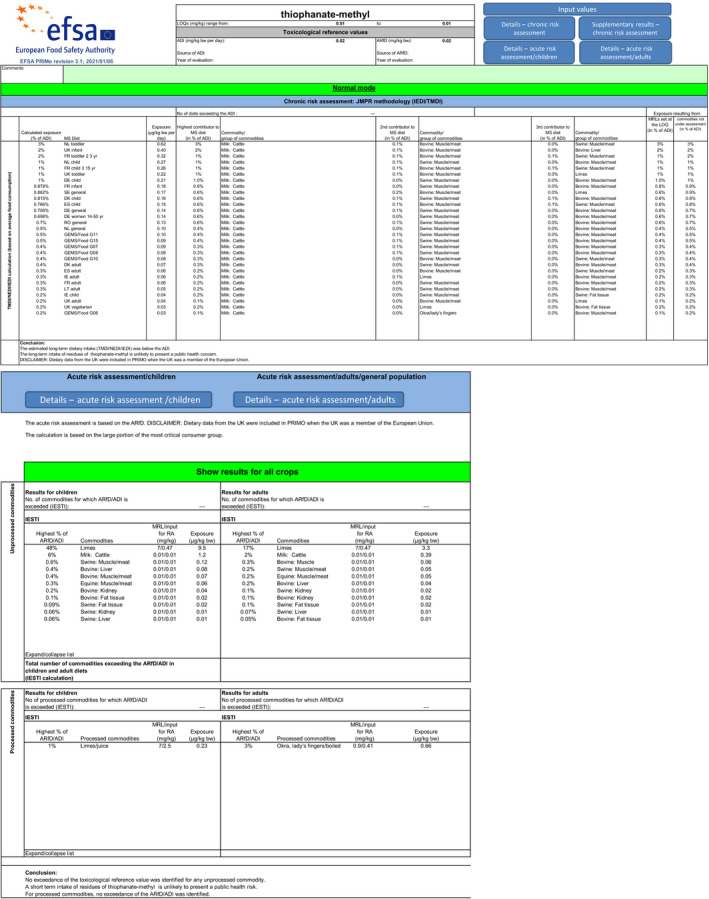
• PRIMo (Scenario EU2) carbendazim – with risk mitigation measures
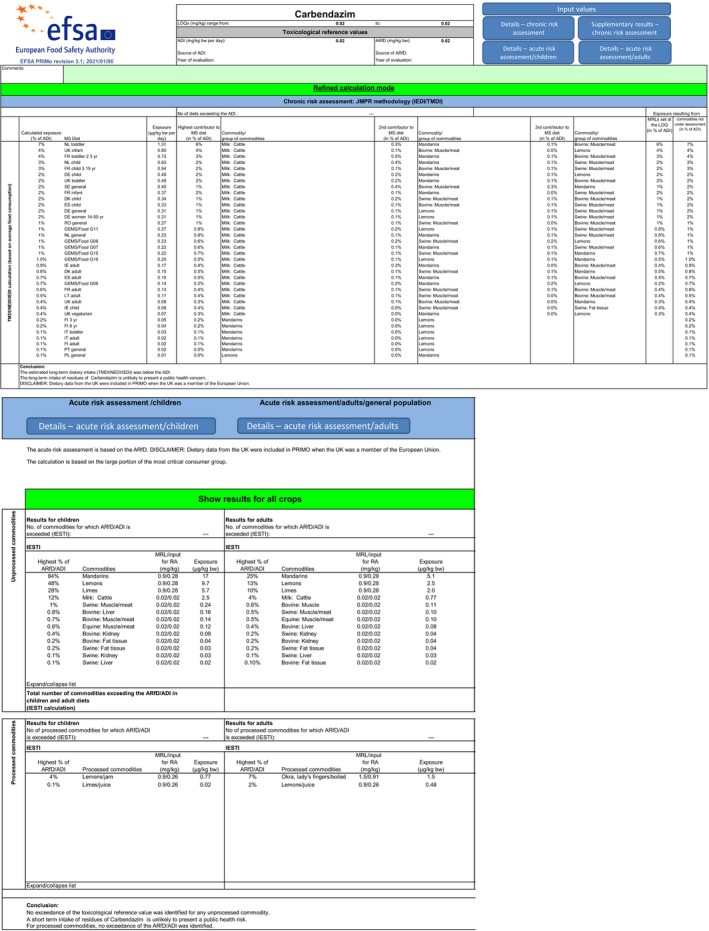
• PRIMo (Scenario EU3) thiophanate‐methyl – with risk mitigation measures
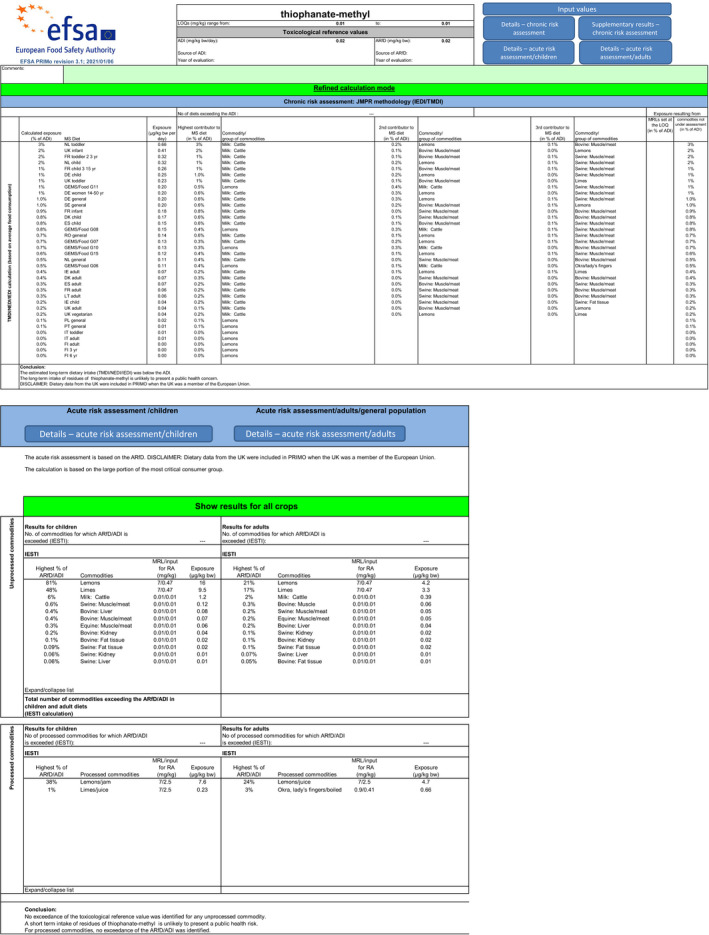
• PRIMo (Scenario EU3) carbendazim – with risk mitigation measures
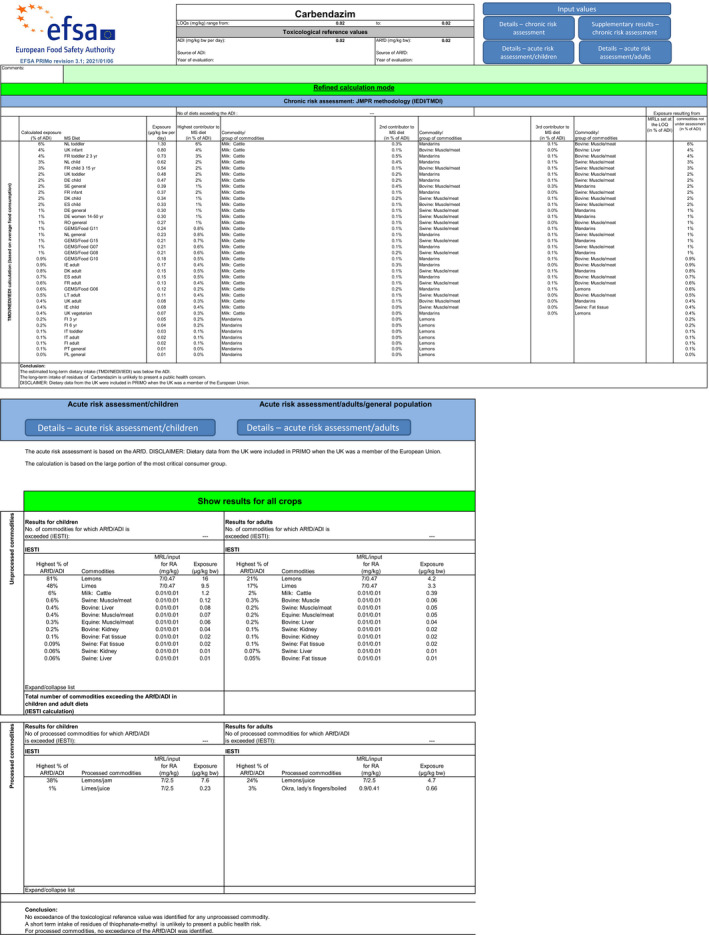
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| RD‐RA 1: thiophanate‐methyl | ||||
| Citrus, dry pulp | 3.8 | STMR × PF (1.51) | 3.8 | STMR × PF (1.51) |
| RD‐RA 2: carbendazim | ||||
| Grapefruit, orange, dry pulp | 2.06 | STMR × PF (25.7) | 2.06 | STMR × PF (25.7) |
| Lemon, lime and mandarin, dry pulp | 6.55a | STMR × PF (25.7) | 6.55a | STMR × PF (25.7) |
STMR: supervised trials median residue; PF: processing factor.
Residues arising from the use of carbendazim.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| RD‐RA 1: thiophanate‐methyl | ||||
| Grapefruits Oranges Mandarins | 0.28 | STMR × PF (0.11) (tentative) (scenario EU1) | 0.47 | HR × PF (0.11) (tentative) (scenario EU1) |
| – | No fall‐back GAP available scenario EU2/EU3) | – | No fall‐back GAP available (scenario EU2/EU3) | |
| Lemons | 0.28 | STMR × PF (0.11) (tentative) (scenario EU1) | 0.47 | HR × PF (0.11) (tentative) (scenario EU1) |
| – | No fall‐back GAP available (Scenario EU2) | – | No fall‐back GAP available (Scenario EU2) | |
| 0.28 | STMR × PF (0.11) (tentative) (Scenario EU3) | 0.47 | HR × PF (0.11) (tentative) (Scenario EU3) | |
| Limes | 0.28 | STMR × PF (0.11) (tentative) | 0.47 | HR × PF (0.11) (tentative) |
| Mangoes | 0.07 | 0.85a × STMR × PF (0.4) (tentative) (scenario EU1) | 0.20 | 0.85a × HR × PF (0.4) (tentative) (scenario EU1) |
| – | No fall‐back GAP available (scenario EU2/EU3) | – | No fall‐back GAP available (scenario EU2/EU3) | |
| Papaya | 0.34 | 0.85a × STMR (tentative) (scenario EU1) | 0.50 | 0.85a × HR (tentative) (scenario EU1) |
| – | No fall‐back GAP available (scenario EU2/EU3) | – | No fall‐back GAP available (scenario EU2/EU3) | |
| Okra, lady's fingers | 0.16 | 0.85a × STMR (tentative) | 0.41 | 0.85a × HR (tentative) |
| Swine, bovine and equine meat | 0.01* | STMR muscle (tentative) | 0.01* | HR muscle (tentative) |
| Swine, bovine and equine fat | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| Swine, bovine and equine liver | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| Swine, bovine and equine kidney | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| Cattle and horse milk | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| RD‐RA 2: carbendazim | ||||
| Grapefruit, Oranges | 0.04 | STMR CBZ × PF (0.47) (tentative) (scenario EU1) | 0.04 | HR CBZ × PF (0.47) (tentative) (scenario EU1) |
| – | No fall‐back GAP available (scenario EU2/EU3) | – | No fall‐back GAP Available (scenario EU2/EU3) | |
| Lemons | 0.12b | STMR CBZ × PF (0.47) (tentative) (scenario EU1/EU2) | 0.28b | HR CBZ × PF (0.47) (tentative) (scenario EU1/EU2) |
| 0.04 | STMR CBZ × PF (0.47) (tentative) (scenario EU3) | 0.04 | HR CBZ × PF (0.47) (tentative) (scenario EU3) | |
| Limes, Mandarins | 0.12b | STMR CBZ × PF (0.47) (tentative) | 0.28b | HR CBZ × PF (0.47) (tentative) |
| Mangoes | 0.06 | STMR TM × PF (0.4) × 0.15 × 0.56a + STMR CBZ × PF (0.4) (tentative) (scenario EU1) | 0.16 | HR TM × PF (0.4) × 0.15 × 0.56a + HR CBZ × PF (0.4) (tentative) (scenario EU1) |
| – | No fall‐back GAP available (scenario EU2/EU3) | – | No fall‐back GAP available (scenario EU2/EU3) | |
| Papaya | 0.11 | STMR TM × 0.15 × 0.56a + STMR CBZ (tentative) (scenario EU1) | 0.13 | HR TM × PF × 0.15 × 0.56a + HR CBZ (tentative) (scenario EU1) |
| – | No fall‐back GAP available (scenario EU2/EU3) | – | No fall‐back GAP available (scenario EU2/EU3) | |
| Okra, lady's fingers | 0.36 | STMR TM × 0.15 × 0.56a + STMR CBZ (tentative) | 0.91 | HR TM × 0.15 × 0.56a + HR CBZ (tentative) |
| RD‐RA 3: sum of carbendazim and 5‐hydroxy‐carbendazim, expressed as carbendazim | ||||
| Swine, bovine and equine meat | 0.02* | STMR muscle (tentative) | 0.02* | HR muscle (tentative) |
| Swine, bovine and equine fat | 0.02* | STMR (tentative) | 0.02* | STMR (tentative) |
| Swine, bovine and equine liver | 0.02* | STMR (tentative) | 0.02* | STMR (tentative) |
| Swine, bovine and equine kidney | 0.02* | STMR (tentative) | 0.02* | STMR (tentative) |
| RD‐RA 4: sum of carbendazim, 5‐hydroxy‐carbendazim and 4‐hydroxy‐carbendazim, expressed as carbendazim | ||||
| Cattle and horse milk | 0.02* | STMR (tentative) | 0.02* | STMR (tentative) |
Indicates that the input value is proposed at the limit of quantification.
TM: thiophanate‐methyl; CBZ: carbendazim.
Values derived from residue trials have been adjusted assuming that, following boiling/brewing/baking, thiophanate‐methyl would be reduced by 15% and converted into carbendazim. Additionally, thiophanate‐methyl residues were expressed as carbendazim considering that the ratio between the two molecular weights is 0.56.
Residues arising from the use of carbendazim.
Appendix E – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| Thiophanate‐methyl | dimethyl (1,2‐phenylenedicarbamothioyl)dicarbamate S=C(Nc1ccccc1NC(=S)NC(=O)OC)NC(=O)OC QGHREAKMXXNCOA‐UHFFFAOYSA‐N |
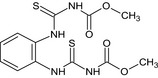
|
| Carbendazim MBC, CF‐27 | methyl 1H‐benzimidazol‐2‐ylcarbamate O=C(OC)Nc1nc2ccccc2[NH]1 TWFZGCMQGLPBSX‐UHFFFAOYSA‐N |
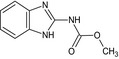
|
| 2‐AB | 1H‐benzimidazol‐2‐amine Nc1nc2ccccc2[NH]1 JWYUFVNJZUSCSM‐UHFFFAOYSA‐N |
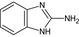
|
| FH‐432 | dimethyl (1,2‐phenylenedicarbamoyl)biscarbamate O=C(Nc1ccccc1NC(=O)NC(=O)OC)NC(=O)OC ASZYYQWGTGVAMG‐UHFFFAOYSA‐N |
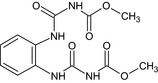
|
| DX‐105 | methyl [(2‐{[(methoxycarbonyl)carbamothioyl]amino}phenyl)carbamoyl]carbamate S=C(Nc1ccccc1NC(=O)NC(=O)OC)NC(=O)OC NPQZXKVOYZCOW‐UHFFFAOYSA‐N |
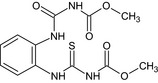
|
| 4‐hydroxy‐carbendazim 4‐OH‐MBC | methyl (4‐hydroxy‐1H‐benzimidazol‐2‐yl)carbamate O=C(OC)Nc1nc2c(cccc2O)[NH]1 GQINHLNACVSEKE‐UHFFFAOYSA‐N |
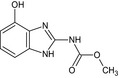
|
| 5‐hydroxy‐carbendazim 5‐OH‐MBC FH 622 | methyl (5‐hydroxy‐1H‐benzimidazol‐2‐yl)carbamate O=C(OC)Nc1nc2cc(O)ccc2[NH]1 UINGPWWYGSJYAY‐UHFFFAOYSA‐N |
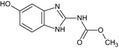
|
| 5‐hydroxy‐carbendazim sulfate 5‐hydroxy‐carbendazim‐S 5‐OH-MBC‐S | methyl [5‐(sulfooxy)‐1H‐benzimidazol‐2‐yl]carbamate O=S(=O)(O)Oc1cc2nc(NC(=O)OC)[NH]c2cc1 ZRHUZHWZOGOGOT‐UHFFFAOYSA‐N |
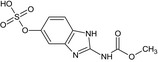
|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.3 ACD/Labs 2019 Release (File version N05E41, Build 111418, 3 September 2019).
ACD/ChemSketch 2019.1.3 ACD/Labs 2019 Release (File version C05H41, Build 111302, 27 August 2019).
Appendix F – Reference list of genotoxicity studies for Thiophanate‐methyl and Carbendazim
1.
The reference list in full is provided as background document to the output.
Appendix G – Carbendazim (MBC) reference list of studies relevant to assess clastogenicity
1.
The reference list in full is provided as background document to the output.
Appendix H – Thiophanate‐methyl reference list of studies relevant to assess clastogenicity
1.
The reference list in full is provided as background document to the output.
Appendix I – Decision tree for deriving MRL recommendations
1.
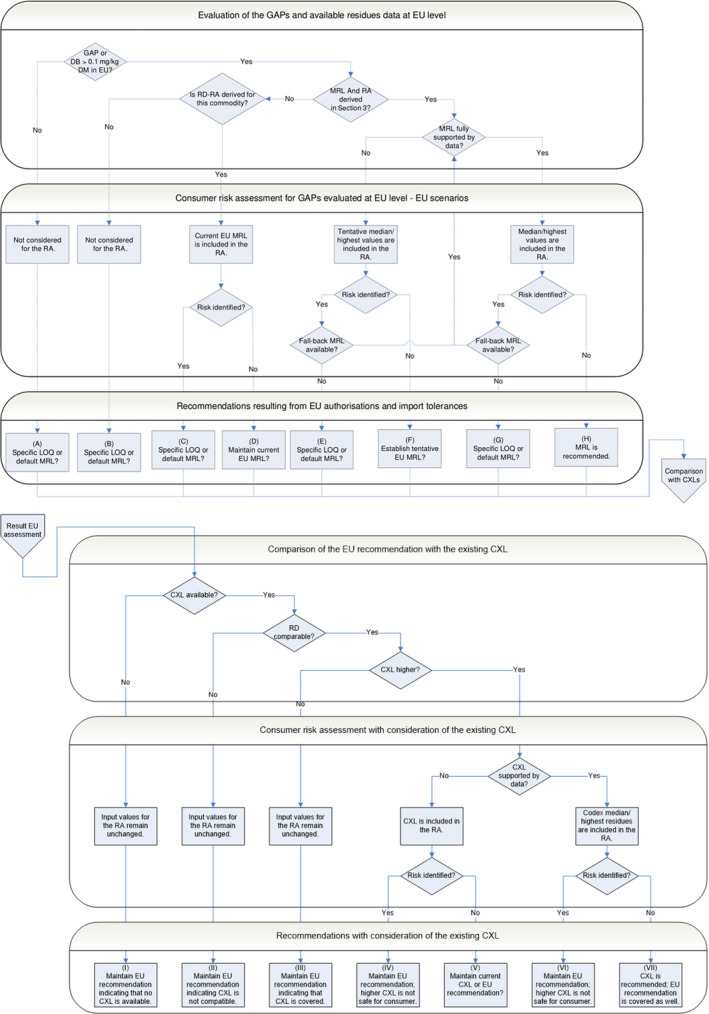
Suggested citation:EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Carrasco Cabrera L, Ferreira L, Giner G, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Ruocco S, Santos M, Scarlato AP, Theobald A, Vagenende B and Verani A, 2021. Reasoned opinion on the toxicological properties and maximum residue levels (MRLs) for the benzimidazole substances carbendazim and thiophanate‐methyl. EFSA Journal 2021;19(7):6773, 66 pp. 10.2903/j.efsa.2021.6773
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00751
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgement: EFSA wishes to thank Stathis Anagnos, Laszlo Bura, Andrea Mioč, Marta Szot, Aikaterini Vlachou for the support provided to this scientific output.
Approved: 5 July 2021
Notes
The United Kingdom withdrew from EU on 1 February 2020. In accordance with the Agreement on the Withdrawal of the United Kingdom from the EU, and with the established transition period, the EU requirements on data reporting also apply to the United Kingdom data collected until 31 December 2020’.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Commission Directive 2006/135/EC of 11 December 2006 amending Council Directive 91/414/EEC to include carbendazim as active substance.OJ L 349, 12.12.2006, p. 37–41.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Directive 2011/58/EU of 10 May 2011 amending Council Directive 91/414/EEC to renew the inclusion of carbendazim as active substance.OJ L 122, 11.5.2011, p. 71–75.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Implementing Regulation (EU) 2015/408 of 11 March 2015 on implementing Article 80(7) of Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and establishing a list of candidates for substitution.OJ L 67, 12.3.2015, p. 18–22.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006.OJ L 353, 31.12.2008, p. 1–1355.
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.OJ L 252, 19.9.2012, p. 26–32.
Commission Directive 2005/53/EC of 16 September 2005 amending Council Directive 91/414/EEC to include chlorothalonil, chlorotoluron, cypermethrin, daminozide and thiophanate‐methyl as active substances. OJ L 241, 17.9.2005, p. 51–56.
Commission Implementing Regulation (EU) 2020/1498 of 15 October 2020 concerning the non‐renewal of approval of the active substance thiophanate‐methyl, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. C/2020/7017. OJ L 342, 16.10.2020, p. 5–7.
Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. OJ L 167, 27.6.2012, p. 1–123.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- ECHA (European Chemicals Agency), 2019a. Biocidal Products Committee (BPC) Opinion on the application for approval of the active substance: Carbendazim, Product type: 9 ECHA/BPC/218/2019. Available online: www.echa.europa.eu [Accessed: 27 February 2019].
- ECHA (European Chemicals Agency), 2019b. Committee for Risk Assessment (RAC) opinion proposing harmonised classification and labelling at EU level of thiophanate‐methyl (ISO); dimethyl (1,2‐ phenylenedicarbamothioyl)biscarbamate; dimethyl 4,4′‐(o-phenylene)bis(3‐ thioallophanate). CLH‐O-0000001412‐86-281/F. Available online: www.echa.europa.eu [Accessed: 15 March 2019].
- ECHA (European Chemicals Agency), 2019c. Biocidal Products Committee (BPC) Opinion on the application for approval of the active substance: Carbendazim, Product type: 7. ECHA/BPC/234/2019. Available online: www.echa.europa.eu [Accessed: 10 December 2019].
- ECHA (European Chemicals Agency), 2019d. Biocidal Products Committee (BPC) Opinion on the application for approval of the active substance: Carbendazim, Product type: 10. ECHA/BPC/235/2019. Available online: www.echa.europa.eu [Accessed: 10 December 2019].
- EFSA (European Food Safety Authority), 2009. Reasoned opinion of EFSA on the refined risk assessment regarding certain MRLs of concern for the active substances carbendazim and thiophanate‐methyl. Prepared by EFSA Pesticide Risk Assessment Peer Review (PRAPeR) Unit. EFSA Journal 2009;7(6):289r, 29 pp. 10.2903/j.efsa.2008.289r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance carbendazim. EFSA Journal 2010;8(5):1598, 76 pp. 10.2903/zj.efsa.2010.1598 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Reasoned opinion on the modification of the existing MRLs for thiophanate‐methyl and carbendazim in apples and pears. EFSA Journal 2012; 10(4):2685, 36 pp. 10.2903/j.efsa.2012.2685 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for thiophanate‐methyl and carbendazim according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(12):3919, 118 pp. 10.2903/j.efsa.2014.3919 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. EFSA guidelines on residues trials and MRL calculations. Proposals for a harmonised approach for the selection of the trials and data used for the estimation of MRL, STMR and HR. 10 pp. September 2015. Available online: www.ec.europa.eu
- EFSA (European Food Safety Authority), Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bu‐ra L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2018a. Conclusion on the peer re‐view of the pesticide risk assessment of the active substance thiophanate‐methyl. EFSA Journal 2018;16(1):5133, 31 pp. 10.2903/j.efsa.2018.5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018b. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Leuschner R, Kazocina A, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1. EFSA supporting publication 2019;16(3):EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI]
- EFSA (European Food Safety Authority), 2021a. Overview of the genotoxicity studies selected for the assessment of carbendazim, 15 January 2021. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2021b. Overview of the genotoxicity studies selected for the assessment of thiophanate‐methyl, 15 January 2021. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2021c. Report of the pesticide peer review experts meeting on mammalian toxicology (TC 39), held on 15 January 2021. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2021d. Member States consultation report on the opinion on the toxicological properties and maximum residue levels (MRLs) for the benzimidazole substances carbendazim and thiophanate‐methyl prepared by EFSA in the framework of Article 43 of Regulation (EC) No 396/2005, 28 June 2021. Available online: www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010-rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.3, June 2017.
- EURLs (European Union Reference Laboratories for Pesticide Residues), 2021. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for carbendazim and thiophanate‐methyl. January 2021. Available online: www.efsa.europa.eu
- FAO (Food and Agriculture Organization of the United Nations), 1994. Thiophanate‐methyl, carbendazim and benomyl. In: Pesticide residues in food – 1994. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues. FAO Plant Production and Protection. September 1994, 270 pp.
- FAO (Food and Agriculture Organization of the United Nations), 1998. Thiophanate‐methyl, carbendazim and benomyl. In: Pesticide residues in food – 1998. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues. FAO Plant Production and Protection Paper 148, 276 pp.
- FAO (Food and Agriculture Organization of the United Nations), 2003. Thiophanate‐methyl and carbendazim. In: Pesticide residues in food – 2003. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues. FAO Plant Production and Protection Paper 176, 317 pp.
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 264 pp.
- Germany , 2009. Renewal Reassessment Report on the active substance carbendazim, prepared by the rapporteur Member State Germany in accordance with Article 5(5) of Council Directive 91/414/EEC, July 2009. Available online: www.efsa.europa.eu
- Germany , 2010. Final Addendum to Assessment Report on carbendazim, compiled by EFSA, March 2010. Available online: www.efsa.europa.eu
- Germany , 2019. Competent Authority assessment Report on carbendazim, Product‐type 7 (Film Preservative) and 10 (Construction Material Preservative), November 2019. Available online: www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2008. Guidance document on the magnitude of pesticide residues in processed commodities. In: Series of Testing and Assessment No 96. ENV/JM/MONO(2008)23, 29 July 2008.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 4 September 2013.
- Sweden , 2016. Renewal Assessment Report (RAR) on the active substance thiophanate‐methyl prepared by the rapporteur Member State Sweden, in the framework of Commission Implementing Regulation (EU) No 844/2012, October 2016. Available online: www.efsa.europa.eu
- Sweden , 2017. Revised Renewal Assessment Report (RAR) on thiophanate‐methyl prepared by the rapporteur Member State Sweden in the framework of Regulation (EU) No 844/2012, October 2017. Available online: www.efsa.europa.eu


