Abstract
The recognition, diagnosis and management of mild traumatic brain injuries are difficult and confusing. It is unclear how the severity and number of injuries sustained relate to brain injuries, such as diffuse axonal injury, diffuse vascular injury and progressive neurodegeneration. Advances in neuroimaging techniques enable the investigation of neuropathologies associated with acute and long-term effects of injury. Head injuries are the most commonly reported injury seen during professional rugby. There is increased vigilance for the immediate effects of these injuries in matches, but there has been surprisingly little research investigating the longer-term effects of rugby participation. Here, we present a longitudinal observational study investigating the relationship of exposure to rugby participation and sub-acute head injuries in professional adult male and female rugby union and league players using advanced MRI. Diffusion tensor imaging and susceptibility weighted imaging was used to assess white matter structure and evidence of axonal and diffuse vascular injury. We also studied changes in brain structure over time using Jacobian Determinant statistics extracted from serial volumetric imaging. We tested 41 male and 3 female adult elite rugby players, of whom 21 attended study visits after a head injury, alongside 32 non-sporting controls, 15 non-collision-sport athletic controls and 16 longitudinally assessed controls. Eighteen rugby players participated in the longitudinal arm of the study, with a second visit at least 6 months after their first scan. Neuroimaging evidence of either axonal injury or diffuse vascular injury was present in 23% (10/44) of players. In the non-acutely injured group of rugby players, abnormalities of fractional anisotropy and other diffusion measures were seen. In contrast, non-collision-sport athletic controls were not classified as showing abnormalities. A group level contrast also showed evidence of sub-acute injury using diffusion tensor imaging in rugby players. Examination of longitudinal imaging revealed unexpected reductions in white matter volume in the elite rugby players studied. These changes were not related to self-reported head injury history or neuropsychological test scores and might indicate excess neurodegeneration in white matter tracts affected by injury. Taken together, our findings suggest an association of participation in elite adult rugby with changes in brain structure. Further well-designed large-scale studies are needed to understand the impact of both repeated sports-related head impacts and head injuries on brain structure, and to clarify whether the abnormalities we have observed are related to an increased risk of neurodegenerative disease and impaired neurocognitive function following elite rugby participation.
Keywords: concussion, TBI, mTBI, imaging, DTI
Using advanced neuroimaging techniques to study a group of professional rugby players, Zimmerman et al. report evidence of both cross-sectional and longitudinal imaging abnormalities in the white matter. Their findings suggest an association of participation in elite adult rugby with changes in brain structure.
Graphical Abstract
Graphical Abstract.
Introduction
Mild traumatic brain injuries (mTBIs) are common, with a global incidence of 45–54 million per year.1 Sport-related mTBIs account for 15–20% of these injuries requiring treatment in the USA.2,3 They are often considered relatively harmless. However, symptoms can persist after injuries that appear relatively trivial4–6 and animal models show persistent brain injury after mTBI.7 Some types of neuropathology, such as diffuse axonal injury (DAI), are under-recognised by conventional diagnostic imaging and repeated mTBI has been associated with neurodegenerative diseases, such as chronic traumatic encephalopathy (CTE).8–11 Hence, detailed investigation of the long-term effects of repeated sports mTBI on brain health is important.
Rugby is a high-intensity collision team sport played in over 120 countries by over 9 million people12 and is split into Rugby Union and Rugby League. In professional rugby union in the UK, mTBI is the most common reported match injury (∼20% of injuries) with an incidence of 20.4 per 1000 h.13 In Australia, the rate of mTBI in the National Rugby League (NRL) was estimated to be 14.8 per 1000 player match hours.14 Despite relatively high rates of head injury and an increasing focus on prevention in the form of law variations15 and player preparation,16 there has been relatively little research investigating the long-term effects of rugby participation. Previous work in retired rugby players has found evidence of a limited effect of higher self-reported rates of depression and mild cognitive impairment compared to controls.17–19 However, rates of dementia in the retired rugby player population are not necessarily different from control levels.20 CTE has recently been reported in a small number of ex-players: two in rugby league21 and four in rugby union.22,23 Therefore, further research is needed to understand whether exposure to rugby participation and head injuries experienced during a rugby career are sufficient to lead to lasting brain injury.
Neuroimaging is key to the investigation of traumatic brain injury (TBI). Standard CT and MRI often appear normal after mTBI.5 However, white matter injuries can be more sensitively identified using advanced magnetic resonance imaging (MRI) sequences, such as diffusion MRI and susceptibility weighted imaging (SWI).24–26 DAI causes many of the long-term effects of TBI. It can be sensitively identified by diffusion MRI. Abnormalities in the directionality of water diffusion, most commonly quantified using changes in fractional anisotropy (FA), provide information about the location and severity of DAI. White matter FA increases acutely and subsequently reduces in the presence of longer-term damage.27–29 FA and other diffusion metrics provide: (i) a validated measure of white matter integrity after TBI in animals models of TBI e.g. Mac Donald et al.30; (ii) increased sensitivity to DAI compared to conventional MRI24; and (iii) information that helps predict long-term clinical outcome after TBI,31 including the type of post-traumatic problems that develop.26,32–34
Diffusion abnormalities have been demonstrated in a range of sporting mTBI types, however, the direction of change is inconsistent. In mixed sport cohorts, ice hockey players and American Footballers, FA has been shown to increase acutely after a sports mTBI.35–38 This elevation has been attributed to increased anisotropy caused by cerebral oedema.27,39 In several studies, these changes persist in the chronic stage,37,40,41 particularly in cohorts of younger athletes. In others, including studies of boxers, FA is observed to be decreased relative to controls in the chronic stages after a sports mTBI,42–45 similar to changes observed in non-sport mTBI and moderate-severe TBI. Several studies do not find abnormalities in FA as a result of sports mTBI.46–49 Few studies investigate solely a population of rugby players with diffusion tensor imaging (DTI). In 73 non-injured female varsity50 and 11 retired rugby league players,51 evidence of reduced FA as well as elevated diffusivity was observed in both studies.
SWI provides additional information about the presence of diffuse vascular injury (DVI).52 Gross intracerebral haemorrhage identifiable on CT is relatively uncommon following sports mTBI. However, more subtle DVI can occur and may be missed on conventional imaging. DVI produces small peri-venular haemorrhages that are seen as petechial microhaemorrhages on SWI. Post-traumatic microhaemorrhages are associated with more cognitive impairment and worse clinical outcomes.53,54 However, studies utilizing SWI in a sporting context are very limited. The majority of studies in boxing, ice hockey and American Football have found little to no evidence of abnormal microhaemorrhage prevalence in athletes.55–58 Currently, there exist no studies of rugby players using SWI.
In addition to the acute effects of sport mTBI, repeated head impacts can lead to progressive neurodegeneration.7,10,59 Therefore, an important question is to what extent different sports accelerate neurodegeneration and increase the risk of dementia. Most recent research has focussed on sports, such as American Football and boxing, where the long-term risks of CTE and other types of dementia have been clearly demonstrated.10,11,22,60 The neurodegenerative risks of other sports are less clear and the prevalence of sports related dementia is generally uncertain as is the dose of head injury exposure needed to accelerate neurodegeneration. Recent work has demonstrated a surprisingly large increase in the risk of dementia, in particular Alzheimer’s disease, in deceased association football players.61 In rugby, CTE pathology has been reported in small case studies of ex-rugby players,22,23 but the prevalence and consequences of neurodegenerative pathology in rugby players are unclear.
The presence of neurodegeneration can be studied using serial MRI. Brain atrophy is a common end-point of various types of neurodegeneration. This is routinely used in the assessment of dementia and can be sensitively measured using longitudinal brain scanning. Serial structural imaging can be used to generate metrics of volume change over time, such as the Jacobian Determinant (JD).62 White matter volume changes are highly dependent on age. Volumes increase in children and young adults, before declining in older age.63,64 The normal development and ageing of the white matter can be affected by TBI. Atrophy is increased after single moderate-severe TBI,65 is more prominent in the depths of sulci where CTE pathology occurs66 and is associated with poorer clinical outcomes.67–70 In other neurodegenerative diseases, atrophy measured by MRI has been histologically validated at post-mortem.71 Hence, serial MRI provides a method of sensitively assessing whether neurodegeneration is taking place and mapping its spatial pattern, which may give an indication of the underlying pathological process.
Here, we report a longitudinal observational study of 41 male and 3 female elite rugby union and league players using repeated diffusion, susceptibility weighted and volumetric MRI to measure DAI, DVI and brain atrophy, respectively. We test the hypotheses that participation in elite rugby will be associated with: (i) evidence of DAI measured by diffusion MRI; (ii) evidence of DVI measured by SWI; and (iii) evidence of abnormal white matter tract development and/or increased brain atrophy, measured by longitudinal changes in brain volume. To investigate the acute and long-term impact of rugby participation, players were recruited with or without a recent history of mTBI. Players were compared against sporting and non-sporting controls to investigate whether imaging abnormalities were associated with participation in high intensity sporting activity.
Materials and methods
Subjects
Thirty-seven elite male and three female rugby union players, and four male rugby league players were recruited from professional rugby clubs in the UK [mean age 25.2, standard deviation (SD) = 3.5, 41 males, 3 females]. Twenty-eight players had baseline study visits without an acute injury, while 21 players attended study visits shortly after a head injury as determined by medical professionals in line with Rugby Union and Rugby League guidelines. All head injuries met the Mayo Clinic classification of mild—probable TBI 72. Post-injury study visits for injured players were performed a maximum of 7 days after injury [mean days 4.7, SD = 1.2]. All study visits included an MRI assessment and battery of neuropsychological tests. Five players had both a baseline non-injury study visit and a study visit following a head injury (Fig. 1). Eighteen rugby players attended a second assessment a mean of 12.1 months later. Inclusion criteria were age 16–45, active rugby players with exclusion criteria comprising other major neurological or psychiatric issues (e.g. substance abuse, previous moderate-severe TBI) or contraindication to MRI.
Figure 1.
Overview of study cohorts. Thirty-seven elite male and three female rugby union players, and 4 male rugby league players were recruited from professional rugby clubs in England. Twenty-eight players had baseline study visits without an acute injury, while 21 players attended study visits shortly after a head. Post-injury study visits for acutely injured players were performed a maximum of 7 days after injury. Five players had both a baseline non-injury study visit and a study visit following an acute head injury, and 18 rugby players attended a second assessment. Three control groups were recruited to the study, including a Non-Sport and Sport Control group for cross-sectional comparison, and a separate group of Longitudinal Controls for longitudinal comparison. Longitudinal Controls only completed the structural imaging components of the study.
Injury characteristics, including the total numbers of symptoms after injury and symptomatic days as well as the duration the player remained in the return to play protocol were recorded. Any head injuries in rugby union that met criteria for permanent immediate removal from play (e.g. loss of consciousness or ataxia as previously described73) were documented. Acutely injured players completed the SCAT-5 which includes a cognitive and neurological screen, and a symptom evaluation (symptom severity score out of 132). For all players, head injury history and length of professional career were collected from the professional sporting body, and where gaps existed, from players and the club. Self-reported alcohol intake was also recorded (Supplementary Table 1).
Healthy controls were screened according to the same exclusion criteria and were required to additionally have no self-reported history of mTBI. Three control groups were recruited to the study, including a Non-Sport and Sport Control group for cross-sectional comparison, and a separate group of Longitudinal Controls for longitudinal comparison. The Non-Sport Control group were a cohort of controls not playing sports at university or professional competition level. The Sport Controls group were a cohort of non-collision sport athletes recruited from university sport clubs. Inclusion criteria for the Sport Controls were age 16–45, active athletes at least 6 h of exercise per week with additional exclusion criteria of history of playing collision sports competitively. This included representing their high school competitively in rugby or soccer. Sport Controls comprised of 3 swimmers, 1 water polo player, 9 rowers, 1 cyclist and 1 weight lifter, with a median hours of exercise per week of 12 (IQR = 6.9). The Longitudinal Control group were the third cohort of controls and comprised of individuals who had participated in a longitudinal imaging study of outcomes after TBI which used aligned imaging and exclusion protocols.65 Longitudinal Controls only completed the structural imaging components of the study.
Recruitment was coordinated by local research facilities at the Hammersmith Hospital. All study participants provided written informed consent in accordance with the Declaration of Helsinki. Ethical approval was approved by the University College London research ethics committee (7385/001).
Neuropsychological testing
Established paper neuropsychological tests sensitive to impairments after TBI74 were administered during study visits, including the Test of Premorbid Functioning (TOPF), Trail Making Test (TMT), Delis-Kaplan Executive Function System (D-KEFS Stroop), Hopkins Verbal Learning-Test (HVLT) and the Brief Visuospatial Memory Test-Revised (BVMT). Participants also completed a battery of cognitive tasks on an IPad as part of a subset of previously studied and described tasks.75,76 These included Visuospatial Working Memory, Paired Associates and Self-Ordered Search, Feature Match, Odd One Out and Spatial Planning. A visuospatial memory Fractals task on a touchscreen computer was also completed following a previously described protocol.77
MRI acquisition
Participants were scanned with the following sequences: resolution structural T1-weighted MPRAGE with voxel dimensions of 1 mm3 (160 slices, matrix = 208 × 208, repetition time = 2300 ms, echo time = 2.98 ms, flip angle = 9°, FOV = 256 × 256 mm, GRAPPA = 2), T2 FLAIR (Fluid attenuated inversion recovery), T2 SWI (120 1.2-mm-thick transverse slices, TR = 28 ms, TE = 20 ms, FA = 15°, in-plane resolution = 0.8 × 0.6 mm, field of view = 225 × 225 mm) and DTI using a multishell 99 direction protocol (66 slices, isotropic 2 mm3 voxels, field of view = 25.6 cm × 25.6 cm, TR = 5000 ms, TE = 85 ms, b-value 1 = 700 s/mm2 with 30 directions, b-value 2 = 2000 s/mm2 with 60 directions, 6 direction b-value = 0 s/mm2 and 6 reverse phase encoding direction b-value = 0 s/mm2 volumes).
Longitudinal controls were scanned with the same structural sequences. Participants were all scanned on a Siemens 3T Verio (Siemens Healthcare) scanner using a 32-channel head coil. All scans were examined by a consultant neuroradiologist for the presence of focal lesions or microbleeds.
Neuroimaging analysis
Diffusion data processing
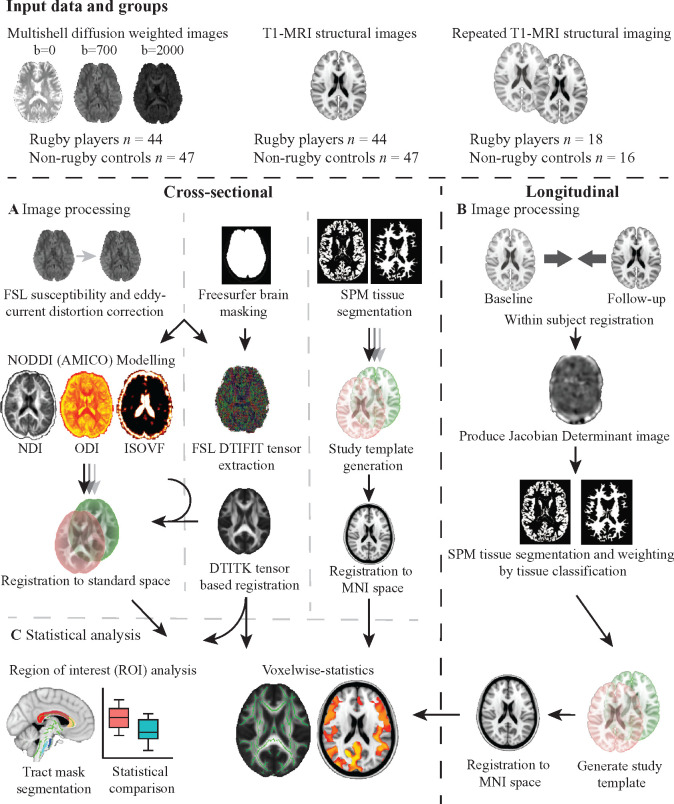
Diffusion weighted images were processed following the standard TBSS pipeline in the FMRIB Software Library (version 5.0.8),78 including correcting susceptibility and eddy current induced distortions79 and diffusion tensor fitting (Fig. 2). Freesurfer80 was used to skull strip brains and remove non-brain voxels from the field map. Tensor-based registration was performed using DTI-TK81 involving the creation of a group template using affine and non-linear diffeomorphic registrations followed by registration of participant diffusion imaging to the template. Images were warped to 1 mm isotropic space and the mean FA map produced was thresholded at 0.2 to produce a white matter skeleton. Subject FA and mean diffusivity (MD) data were projected onto the mean FA skeleton and tract level data generated using the Johns Hopkins University (JHU) white matter atlas. Neurite density index (NDI), orientation dispersion index (ODI) and isotropic volume fraction (ISOVF) maps were generated using NODDI modelling using the Accelerated Microstructure Imaging via Convex Optimization (AMICO) framework.82 NDI, ODI and ISOVF maps were registered to DTI-TK-space using the transformation matrix generated during the DTI-TK pipeline. Images were visually inspected at the brain extraction, eddy current correction and tensor registration stage. Subjects with poor quality data or un-correctable artefacts were removed.
Figure 2.
Overview of study methods. (A) Image processing of diffusion data followed the standard TBSS pipeline, including correcting for susceptibility and eddy current induced distortions and diffusion tensor fitting. Freesurfer was used to skull strip brains and remove non-brain voxels from the field map. Tensor-based registration was performed using DTI-TK involving the creation of a group template using affine and non-linear diffeomorphic registrations followed by registration of participant diffusion imaging to the template. Images were all warped to 1 mm isotropic space and the mean fractional anisotropy map produced was thresholded at 0.2 to produce a white matter skeleton. Subject fractional anisotropy and mean diffusivity data were projected onto the skeleton and tract level data generated using the Johns Hopkins University white matter atlas. Neurite density index (NDI), orientation dispersion index (ODI) and isotropic volume fraction (ISOVF) maps were generated using NODDI modelling using the Accelerated Microstructure Imaging via Convex Optimization (AMICO) framework. NDI, ODI and ISOVF maps were registered to DTI-TK-space using the transformation matrix generated during the DTI-TK pipeline. Images were visually inspected at the brain extraction, eddy current correction and tensor registration stage for quality control checks. Image processing of cross-sectional structural data used SPM to segment T1 images into tissue probability maps. A study-specific template was generated using DARTEL registration for non-linear spatial normalization and then affine registered to MNI152 space. All images were then normalized to MNI152 space via the study template. (B) Processing of longitudinal structural images involved within-subject registration of baseline and follow-up images and generation of a within-subject temporal average image and Jacobian determinant image. The Jacobian determinant image represented a voxelwise statistic of spatial expansion and contraction required to match baseline and follow-up images. Average images were then segmented into grey and white matter and Jacobian determinant images normalized by tissue probability. A study-specific longitudinal template was generated with DARTEL and affine registered to MNI152 space. Individual average images were then normalized to MNI152 space via the longitudinal study template. (C) Statistical analysis was carried out in both a region of interest and voxelwise manner. Region of interest analysis involved sampling of mean diffusion and structural metrics of interest within pre-defined regions of interest in spatially standardized images. Voxelwise analysis was carried out using Randomise (FSL) with 5000 permutations using the threshold-free cluster enhancement and adjusting for age, gender and intracranial volume (cross-sectional structural only).
Subject-level diffusion analysis
Mean FA was calculated for selected large white matter tracts based on tract size, reproducibility and sensitivity.83 The mean and standard deviation of FA for each tract region of interest (ROI) across the Non-Sport Control cohort was then used to calculate subject Z-scores for each ROI. Z-scores were converted to P-values (two-tailed, with 95% confidence interval) and corrected for multiple comparisons across all tract ROIs using FDR. Abnormalities in subjects’ FA were determined by tracts that have significantly lower or higher FA in the subject compared to the control group, P < 0.05, FDR corrected. Subject-level and ROI analysis was only conducted for FA, and not other diffusion metrics.
Voxelwise diffusion analysis
Voxelwise analysis of skeletonised diffusion metrics was conducted using the general linear model with non-parametric permutation testing (5000) in FSL Randomise.84 Diffusion weighted voxelwise results reported are corrected for multiple comparisons at a threshold of P < 0.05 with age and gender included as a nuisance covariate.
Cross-sectional structural data processing and analysis
Volumetric data were analysed using standard approaches65 (Fig. 2). SPM 12 (UCL) was used to segment T1 images into grey matter, white matter and CSF, and to calculate volumes of these tissue classes at baseline and follow up. Cross-sectional analysis involved the generation of a study template using SPM DARTEL non-linear registration85 before affine registration of segmented images to MNI152 space, with normalization of volume and smoothing using an 8 mm kernel. Tract level data were generated using eroded JHU white matter atlas’ masks to sample tissue volumes. Cross-sectional voxelwise analyses were conducted using non-parametric permutation testing (5000) with age, gender and intracranial volume included as nuisance covariates.
Longitudinal structural data processing and analysis
Longitudinal volumetric analysis was performed using the SPM12 longitudinal registration tool. Baseline and follow-up T1 images were iteratively co-registered to produce a temporal average image, with the transformation required to move from the baseline to follow-up image encoded in the JD of the expansion/contraction at a voxel-wise level.62 JD was then weighted by the interval between baseline and follow-up to give an annualized rate of change and further weighted by tissue class. A study-specific template was generated using SPM DARTEL’s non-linear registration and tissue-specific JD images normalized to group space before affine registration to MNI152 space with normalization of volume and smoothing using an 8 mm kernel. Tract level data were generated using skeletonised JHU white matter atlas’ masks in MNI152 space. Voxelwise analysis was conducted using non-parametric permutation testing (5000) with age included as a nuisance covariate and results reported are corrected for multiple comparisons at a threshold of P < 0.1. Voxelwise correlation between JD and DTI metrics were conducted after registration of DTI images to MNI152 space, using non-parametric permutation testing (5000).
Statistical analysis
Whole brain summary and voxelwise measures were calculated by adding grey and white matter tissue data. Summary measures of brain volume were normalized for participant head-size by dividing them into total intracranial volume (grey matter + white matter + CSF). Statistical tests were completed in the open-source software package R 86. Planned analyses included the comparison of demographics, neuropsychological performance and diffusion metrics in rugby players and all controls as a group, and sub-analysis between sub-acutely injured players and non-acutely injured players (Fig. 1). An individualized assessment of FA was planned to identify abnormalities in DTI using methods previously described 83. An analysis of brain atrophy comparing rugby players and a sub-group of controls with longitudinal data were also planned using previously described methods.65
Non-sport and sport control groups were combined as an ‘All controls’ group for comparisons with the largest N. This was done for a comparison of demographics, neuropsychological test performance and voxelwise diffusion and cross-sectional volumetric imaging analyses. Further sub-analyses were performed using the Sport and Non-Sport Control groups individually to investigate the effect of sports participation and test if the group-wise differences in diffusion measures remained. Rugby players were also split into sub-acutely and non-acutely injured players to investigate the effects of a sub-acute injury on neuropsychological performance and imaging metrics. Longitudinal controls, who were not screened for sports participation, were used for longitudinal volumetric imaging analyses comparisons against a group of both sub-acutely and non-acutely injury players, combined to maintain large enough group sizes for powering. A subgroup analysis was performed using only male Rugby Union players and utilised the same methodology as the main analyses but included only male non-sport and sport controls for cross-sectional comparisons.
Group differences between all rugby players and all controls in demographics and neuropsychological performances were investigated using t-tests, with effect sizes reported using Cohen’s d. The effect of sub-acute injury and sports participation on neuropsychological test performance was assessed using an analysis of variance with Tukey multiple post hoc comparisons of means. FDR correction for multiple comparisons was used. Details on neuroimaging statistical analysis are detailed in the neuroimaging section. Pearson’s correlation was used to test the relationship between JD and FA, and between the neuropsychological test score performances and neuroimaging metrics. Categorical variables were compared using the Chi-squared test and effect sizes reported using Cramer’s V. In cases where the normality assumption was violated, the Wilcoxon test was used. The data were not manipulated to account for missing data and design and analysis not prespecified publically.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Subjects
From 26 July 2017 until 1 March 2019, 44 elite level rugby players were recruited into the study. Twenty-one players were assessed shortly after a mild TBI that was sustained whilst playing professional rugby. On average these players were 4.7 days post-injury. Sub-acutely injured players presented with a mean symptom severity score of 10.3 on the SCAT-5 during their pitch side assessment at the time of injury and had an average return to play duration of 7.2 days (Supplementary Table 1). Demographic and physical characteristics were well matched between sub-acutely injured and non-acutely injured rugby players. Eighteen rugby players attended a second assessment a mean of 12.1 months later and players who returned for a follow-up were not significantly different in demographics, baseline neuropsychological test performance or any imaging measures compared to players who did not return for follow-up.
Rugby players were compared to three control groups. A non-collision sport cross-sectional (Sport Controls), a non-sport cross-sectional (Non-Sport Controls) and non-sport longitudinal control group (Longitudinal Controls). Sport Controls were younger than rugby players (t = −3.44, P = 0.0022, d = 1.05) with no difference in proportion of males to females. Non-Sport Controls were well matched for age, but had a lower proportion of males to female (X2= 10.7, P = 0.001, V = 0.42) than the rugby players. Longitudinal Controls were older than the rugby players who returned for their longitudinal assessment (t = 6.28, P < 0.001, d = 2.16) but were matched for gender. Alcohol intake was not significantly different between rugby players and controls.
Rugby players showed lower neuropsychological test performance at baseline
Rugby players as a group showed lower visual memory immediate recall (t = 3.64, P = 0.0083, d = 0.99) and lower premorbid intelligence scores (FSIQ; t = 3.2, P = 0.030, d = 0.99) compared to Non-Sport and Sport controls as a single group (Non-Rugby controls) (Fig. 3). Rugby players also showed trends towards slower performance on two tests of processing speed (Trails B, Trails B minus A) and poorer performance on verbal memory immediate recall, however, these did not survive correction for multiple comparisons. There were no differences between players and controls in computerized testing performance or on measures of executive function.
Figure 3.
Neuropsychological performance in all controls and rugby players. Grouped violin plots of rugby player and non-rugby control performance in select neuropsychological tests. The width of the coloured area represents the distribution of scores, with the mean defined by the black horizontal lines. Independent samples t-tests were conducted, with * = uncorrected P < 0.05, ** = uncorrected P < 0.01, *** = uncorrected P < 0.001. Premorbid FSIQ is a composite score of intelligence, Trails B and Trails B minus A are tests of speed of processing, Stroop InhSwi versus CombiBase is a composite score indicative of executive function, BVMT Total Recall and HVLT Total Recall are scores of immediate memory. BVMT, Brief Visuospatial Memory Test; CombiBase, Combined Baseline; FSIQ, Full Scale Intelligence Quotient; HVLT, Hopkins Verbal Learning Test; InhSwi, Inhibition Switch.
Neuropsychological test performance in rugby players was not influenced by sub-acute injury
To assess if these differences were present in both sub-acutely injured players and non-acutely injured players, and if there was an effect of sport participation in controls, we compared baseline performance between the 4 groups. A significant effect of group was found for premorbid intelligence scores (FSIQ; F(3,37) = 5.74, P = 0.03). Post hoc tests showed that these effects were driven by lower FSIQ scores in both sub-acutely injured rugby players (P = 0.0035) and non-acutely injured players (P = 0.0095) when compared to Non-Sport Controls. There were trends towards effects of group on visual memory immediate recall and verbal memory immediate recall, however, these did not survive correction for multiple comparisons. There were no differences between neuropsychological test scores of non-acutely and sub-acutely injured rugby players at baseline, nor any differences between baseline and follow-up scores within players who returned for a second visit.
Abnormal white matter integrity in rugby players
White matter structure was assessed using diffusion, volumetric and susceptibility weighted MRI scans. Three baseline DTI scans of rugby players and 2 Sport Control DTI scans were discarded after quality control checks. We investigated the white matter structure at the individual level by calculating the mean FA from a number of large white matter tract and the whole white matter skeleton and comparing results to control ranges. This approach is illustrated in the case study presented in Fig. 4. Conventional MRI of this rugby player with a sub-acute injury showed no evidence of abnormalities. In contrast, FA averaged across specific white matter tracts showed abnormalities in the genu of the corpus callosum, and left and right corticospinal tract, with low FA characteristic of white matter abnormality.
Figure 4.

Subject level analysis of fractional anisotropy: Case study 1. (A) Routine MRI scans including T1-MPRAGE (magnetization-prepared rapid acquisition with gradient echo), SWI (susceptibility weighted imaging) and FLAIR (fluid attenuated inversion recovery) of the player showed no abnormalities. (B) An individualized approach to DTI analysis. Mean FAs from specific white matter tracts are plotted; highlighted in red and inset above are the areas sampled corresponding to the left corticospinal tract. The boxplots represent the values found using the Non-Sport Control cohort (n = 32). Single points on the plot represent the individuals mean FA value within the specific tract. Black dots represent where a value is not abnormal, while red points indicate where an individual has a significantly abnormal FA compared to the control cohort. Abnormal FA is defined by using a z-scoring approach, comparing the individual to the Non-Sport Control cohort, P < 0.05, 95% confidence interval, FDR corrected. CC, corpus callosum; CR, corona radiata; CST, corticospinal tract; ILF, inferior longitudinal fasiculus; MCP, middle cerebellar peduncle; WM Skel, whole white matter skeleton.
Across the whole cohort, there was a higher proportion of rugby players with DTI abnormalities defined in this way than controls (X2 = 3.97, P = 0.046, V = 0.21). Seventeen per cent of rugby players (7/42) had abnormalities in mean FA in at least one of the sampled tracts, compared to 3% of Non-Sport Controls (1/32). Sport Controls showed no abnormality in FA (Fig. 5). The prevalence of abnormalities was highest in the corpus callosum (3/7, 43%) and the corticospinal tracts (5/7, 71%). There were no differences in alcohol intake between players with diffusion abnormalities and those without.
Figure 5.
Results of individualized analysis of fractional anisotropy across the groups. Mean FAs from select white matter tracts where abnormalities were observed are plotted. Single points on the plot represent an individuals’ mean FA value within the specific tract. Black dots represent where a value is not abnormal, while red points indicate where an individual has a significantly abnormal FA compared to the Non-Sport Control cohort. Individuals with at least one abnormal value across the tracts are determined to be DTI abnormal. Across the groups, a higher proportion of rugby players with abnormalities compared to controls can be observed.
Voxelwise group comparisons of diffusion MRI also provided evidence for altered white matter integrity in rugby players. Voxelwise cross-sectional comparison of all rugby players to all controls (Non-Sport and Sport Controls combined) showed reductions in FA in the posterior thalamic radiation, left posterior corona radiata, left posterior limb of internal capsule and the superior cerebellar peduncle bilaterally (Fig. 6A). No significant differences were observed for the other DTI metrics investigated (MD, NDI, ODI and ISOVF).
Figure 6.

Voxelwise comparison of diffusion metrics between groups. Voxelwise comparisons were conducted using FSL Randomise with age and gender added as covariates. Green denotes the white matter skeleton and areas in red indicate a result where corrected P < 0.05. (A) Comparisons of fractional anisotropy (FA) between all rugby players (n = 42) and all controls (n = 45) showed regions of lower FA in rugby players compared to controls. No differences were seen in other diffusion metrics. (B) Regions of lower FA, higher mean diffusivity (MD), and lower neurite density index (NDI) were observed in non-acutely injured rugby players (n = 27) when compared to all controls. (C) Elevated FA was observed in sub-acutely injured players (n = 19) when compared to non-acutely injured players (n = 24) excluding multiple observations of the same player. No differences were seen in other DTI metrics. (D) When splitting the control group, non-acutely injured rugby players still showed areas of reduced FA relative to Non-Sport Controls (n = 32). No differences were observed in other DTI metrics, nor between Non-Sport Controls and sub-acutely injured players or between Sport Controls (n = 13) and either rugby group.
Differences in white matter integrity between non-acutely and sub-acutely injured players
To disentangle the effects of a sub-acute injury, we investigated diffusion changes in sub-acute and non-acutely injured players separately. Out of the 7 players with DTI abnormalities, 5 were non-acutely injured and 2 were scanned sub-acutely after injury. Voxelwise analysis showed that imaging abnormalities were most prominent in non-acutely injured players. Compared to all controls, reductions in FA in the non-acutely injured group were seen in the corona radiata and sagittal stratum, as well as elevations of MD in the genu of the corpus callosum, corona radiata, posterior thalamic radiation, retrolenticular part of internal capsule and sagittal stratum. Furthermore, lower NDI was seen in widespread white matter regions in the non-acutely injured players (Fig. 6B). In contrast, sub-acutely-injured rugby players showed no difference in diffusion metrics when compared to all controls.
We then compared the sub-acutely injured players with non-acutely injured rugby players. MD was reduced in sub-acutely-injured players compared to non-acutely injured players within the genu of the corpus callosum, retrolenticular part of the internal capsule, corona radiata and external capsule (Fig. 6C). There were no differences in the other DTI metrics.
We split the control groups to investigate the effect of sporting activity on diffusion measures. There were no differences between observed in any of the DTI metrics between the Non-Sport Controls and Sport Controls. We investigated if the diffusion differences were still present using only Sport or Non-Sport Controls. Reductions in FA were still observed in non-acutely injured rugby players when compared to only Non-Sport Controls in the corona radiata, posterior thalamic radiation, sagittal stratum and corticospinal tract (Fig. 6D). There were no reductions in other DTI metrics, differences between sub-acutely injured players and Non-Sport Controls nor differences when comparing the Sports Control group with either rugby group.
Microbleed evidence for DVI
Three rugby players had microbleeds, 2 of whom were sub-acutely injured, while none were present in Non-Sport Controls (Fig. 7). One Sport Control showed microbleeds. FA abnormalities were not observed in the players with microbleeds. Hence, 23% of players (10/44) had evidence of diffuse axonal or DVI. Longitudinal SWI was stable in individuals with follow-up scans, with no change in microbleed incidence or evolution of microbleeds on SWI scans.
Figure 7.

Presence of microbleeds on susceptibility weighted imaging: Case study 2. (A) A player with a sub-acute injury presented with no abnormalities on routine structural MRI scans, but had evidence of microbleeds (highlighted in the magnified imaged) on susceptibility weighted imaging (SWI). (B) Utilising the individualized approach to DTI analysis, no abnormalities were found in FA for this player. CC, corpus callosum; CR, corona radiata; CST, corticospinal tract; ILF, inferior longitudinal fasiculus; MCP, middle cerebellar peduncle; WM Skel, whole white matter skeleton.
Rugby players show abnormal white matter volume change over time
We assessed volumetric differences between the rugby players and controls. Cross-sectional comparisons were made between the groups at baseline. A voxelwise analysis of grey and white matter volumes showed no significant differences at baseline between players and all controls. There were no differences between the control groups, or between non-acutely injured and sub-acutely injured rugby players.
We then investigated whether there was any evidence for abnormal brain volume changes in rugby players using a sensitive measure of brain volume change over time (Jacobian Determinant, ‘JD’). Voxelwise comparison of JD maps between rugby players who attended 2 visits and the Longitudinal Control group showed lower white matter JD in rugby players, specifically within the lateral occipital cortex and post central gyri (Fig. 8A). A lower JD suggests that either the rate of volume expansion was lower in rugby players than controls, or players were losing volume at a higher rate than controls. Longitudinal Controls were slightly older than the rugby players (mean age in years 33.3 versus 24.8, t = 6.28, P < 0.001), and so would be expected to have lower JD values than the rugby players. However, the reverse was true. Sampling the mean JD values from these regions revealed that 50% of players (9/18) had mean JD values below zero (indicating brain volume loss) compared to 25% of controls (4/16) (Fig. 8B). These results suggest that the rugby players had an abnormal trajectory of white matter volume change for individuals of that age (Fig. 8C). Mean JD within the grey, white matter or whole brain did not correlate significantly with head injury history.
Figure 8.

Longitudinal changes in volume in rugby players. Voxelwise comparisons were conducted using FSL Randomise with age as a covariate and results shown for areas where corrected P < 0.1 (one tailed t-test). (A) Lower annualized Jacobian Determinant (JD) in rugby players (n = 18) compared to Longitudinal Controls (n = 16) was observed in the white matter. (B) When plotting the JD values within the significant areas, values between rugby players (blue) and Longitudinal Controls (red) look similar. (C) However, when plotting against age, a proportion of rugby players have JD values that appear lower than what would be expected for individuals of that age, suggesting there are altered trajectories for white matter volume change in rugby players.
White matter integrity and longitudinal volume change in rugby players
We tested whether areas of the white matter showing evidence of abnormal volume changes over time (low JD) in rugby players were associated with abnormalities in white matter integrity (low FA). We sampled the diffusion metrics and JD values within the white matter tracts in areas where there was a significant difference in JD between players and controls and found no correlation between baseline FA, MD or NDI with JD in these areas. Additionally, there were no significant correlations when restricting analysis only to non-acutely injured rugby players.
White matter volume changes, white matter integrity and neuropsychological performance
We also explored the relationship between atrophy rates, white matter integrity and cognitive function. Investigating tasks which showed differences at baseline, no correlations were found between the mean FA, MD or NDI in white matter tract ROI’s and neuropsychological test performance. Rugby players who showed abnormalities in at least one white matter tract ROIs (n = 7) did not perform significantly differently in neuropsychological tests, including premorbid intelligence scores, compared to players not classified as abnormal on their individualized DTI. No correlations were found either with mean JD in grey, white matter or whole brain and neuropsychological test scores. We investigated if premorbid intelligence influenced neuroimaging parameters. We removed 3 non-sport controls at the higher range of FSIQ scores so that the non-sport controls (n = 29) and rugby players (n = 42) were matched for premorbid intelligence. Running the individualized DTI assessment with FSIQ matched controls, players previously identified as abnormal on DTI remained abnormal. Furthermore, FSIQ scores were not correlated with mean FA within rugby players or controls.
Subgroup imaging analysis in male Rugby Union players only
We investigated if there were any differences in the results when looking at scans from male rugby union players only (n = 37) and included only male controls for the cross-sectional comparison. A similar pattern of abnormalities was observed, with 11% of rugby players (4/35) with DTI abnormalities and 8% with microbleeds (3/37). In total, 19% of players (7/37) had evidence of diffuse axonal or DVI. Voxelwise comparisons of diffusion metrics showed similar results, with reductions in FA and NDI in non-acutely injured rugby players persisting. With 3 players excluded from the longitudinal volumetric analysis (n = 15), the area of abnormality in JD within the white matter remained but was slightly reduced in size.
Discussion
Our longitudinal observational study shows abnormalities of brain structure associated with elite rugby participation. DTI and SWI were used to assess white matter structure and evidence of axonal injury was seen in several players studied. Across the group of rugby players studied abnormalities of FA and other diffusion measures were seen. In contrast, sport playing controls were not classified as showing abnormalities of FA. We also studied changes in brain structure over time. Abnormal trajectories of white matter volume were seen in elite rugby players, suggesting an impact of elite rugby participation on brain development. To our knowledge, this is the first study to investigate MR imaging changes in active elite rugby players.
Diffusion imaging abnormalities of FA, neurite density and MD were seen in the rugby group. Similar directions of change are seen in other mild TBI patients scanned in the chronic stage40,45,87 and longitudinally88 after an injury. We used an individualized approach to DTI analysis to identify players with neuroimaging abnormalities.83 This allowed us to investigate the structure of a number of large white matter tracts in a focussed way. Twenty-three per cent of the rugby players showed signs of either diffuse axonal or DVI. The majority of the players with evidence of DAI showed abnormalities within the corticospinal tract. Reductions in FA are associated with abnormalities of motor function in other contexts.89,90 Although this was not explicitly assessed, the players studied did not show any evident abnormalities of motor function and all players were successfully competing at an elite level. However, retired rugby players have been shown to display reduced fine motor control ability.18,91 Therefore, it is possible that reductions of FA within the corticospinal tract might be significant in terms of future motor function.
Abnormalities of white matter structure are correlated with clinical features of injury severity and neuropsychological test performance following moderate-severe TBI, with evidence of DAI (low FA) usually associated with cognitive impairment.24,34,65 Although the rugby players we studied showed did show poorer performance on a number of cognitive tests, cognitive performance did not correlate with white matter abnormalities. Players with white matter abnormalities did not perform significantly worse than those classified as normal. Rather than effect of a rugby participation or sub-acute injury, the difference in performance observed across a select number of neuropsychological tests may have been related to group differences in IQ and appears unrelated to injury or imaging abnormalities within this study.
Multi-shell diffusion-MRI was acquired. This allowed Neurite Orientation Dispersion and Density Imaging (NODDI) to be performed. NODDI is an advanced diffusion MRI technique was used to calculate a range of diffusion metrics that potentially provide more sensitive and specific diffusion metrics of white matter pathology. NODDI separates the diffusion signal from three different tissue compartments: intra-neurite (restricted diffusion), extra-neurite (hindered diffusion) and cerebrospinal fluid (free diffusion) to extract a NDI, ODI and ISOVF.92 Sub-acutely after a mild TBI, studies have observed reduced NDI,93 which has correlated with reductions in FA and increased symptom load.42 In athletes not acutely injured, but with a history of injury, studies have found abnormalities in FA which were negatively correlated to ODI.40,94 We extend these findings by showing widespread reductions in NDI in our non-acutely injured rugby players, but not in the sub-acutely injured players, with no differences in ODI or ISOVF. These results are suggestive of lower white matter tissue density and that the FA and MD findings are not primarily driven by variations in free water volume.
Moderate-severe TBI produces a progressive loss of brain volume, particularly affecting the white matter.65,95 This is a marker of the progressive neurodegenerative process that can be triggered by TBI.96 Abnormal white matter volume changes were observed in the rugby group. Reductions in a region of white matter volume, as defined by a negative JD, were seen in around 50% of the rugby players studied, and a higher rate of white matter volume loss was seen across the group when compared to a control group who were slightly older. White matter volume is expected to increase until the age of around 40,64,97 and this pattern was seen in our control group who had longitudinal imaging. The rugby players studied had an average age of 25 years old and the oldest was 31 years old. In this age range reductions of white matter volume are abnormal and may be an early sign of an active neurodegenerative process that might increase the risk of neurodegenerative disease in later life. The pathological correlates of abnormal white matter volume we detected with advanced neuroimaging in rugby players is unknown and requires more investigation. However, evidence of CTE in retired professional rugby players has been previously observed,21–23 and in deceased professional Scottish football players where head injury risk is lower but exposure to heading the football is seen as a unique sport-specific risk factor, a five-fold increased risk of developing Alzheimer’s disease was identified.61
The current focus of head injury management programmes in sport is the identification of defined observable signs, such as loss of consciousness and ataxia,98 and assessment for the presence of characteristic symptoms and alterations from baseline in cognitive function and balance. Effective surveillance at the elite and community levels of Rugby Union is overseen in the UK by the Rugby Football Union and any signs or symptoms of mTBI routinely lead to a player’s removal from play and a graduated return to play once these have resolved. Animal studies show that early markers of neurodegeneration such as tau pathology, vascular damage and neuroinflammation can be produced by mild injuries of the type frequently experienced by professional rugby players.7,99–102 However, an important observation is that this pathology can be seen in the absence of concussive symptoms. Players in a range of sports are repeatedly exposed to head impacts in matches and training that may in themselves be sufficient to result in the subsequent development of neurodegenerative pathology. However, these impacts would not trigger removal from play and are not routinely measured over a player’s career. Further studies of both active and retired rugby players are needed to investigate whether these subthreshold impacts as well as reported mTBIs are associated with evidence of brain injury and to ascertain how cumulative injury burden relates to neurodegeneration and ongoing brain health.
We compared rugby players with a recent history of mTBI to those without. Reduced MD was seen in sub-acutely injured rugby players compared to non-acutely injured players, although there were no differences when compared to the control group. This is in keeping with several previous studies of sport mTBI, which have shown reduced diffusivity alongside elevated FA in recently injured players.42,103,104 Other diffusion metrics did not show any abnormalities sub-acutely after injury. Previous studies have described the dynamic nature of diffusion changes seen in the early stages of TBI where FA increases initially in the acute to sub-acute period.27 However, sub-acutely injured players did not show abnormalities of FA compared to controls. Similar findings have been described in other studies of non-acutely injured contact sport athletes.105–107 In the context of reduced FA observed in our non-acutely injured players, the absence of evidence for diffusion changes in sub-acutely injured players may be due to the acute effects being occluded by longer term reductions in FA.
Three players also had microbleeds visible on their SWI, an abnormality not seen in any of our non-sporting controls, but observed in 1 sporting control. Microbleeds are a marker of DVI and are produced by small perivascular haemorrhage that can be missed by conventional imaging and that remains visible on SWI because of the persistence of haemosiderin laden macrophages in the perivsascular region.52,108 Previous studies of have reported the presence of microbleeds after mild TBI, including in contact sport athletes.56,109 We extend these findings by showing that microbleeds can occur in the absence of diffusion abnormalities and vice versa. The distinct information identified by these two imaging methods has recently been shown in moderate-severe TBI.52 This illustrates that DVI and DAI can occur independently after TBI and that SWI and diffusion MRI provide complementary information about the presence of subtle brain injury.
There are some limitations to our study. Our longitudinal control group were non-sport controls, meaning that the effect of exercise was not controlled for in our longitudinal analyses. However, we do not believe this likely to have a large effect on the comparison as evidence suggests exercise should lead to higher grey and white matter volume.110–112 A further limitation to our study was that we had gender and age differences in our control groups when compared to the rugby players. We minimised this effect by modelling age in all groupwise analyses, and gender where appropriate. One potential limitation is that diffusion metrics such as FA have been shown to be related to premorbid intelligence scores,113,114 which were higher in our control group. This did not appear to be a major confound for our results, as a sensitivity analysis showed no difference in classification of DTI abnormalities in rugby players when the groups were matched for FSIQ, and intelligence scores were not correlated to FA. Our non-collision sport control group were also not professional athletes and not matched for BMI which limits our ability to exclude the effect of high-level sporting participation on our results. We only had a small sample of players who had a followed-up visit, meaning we were not well powered to investigate the effect of a sub-acute injury on longitudinal volume change, which may be influenced by the resolution of oedema or inflammation, and lacked longitudinal DTI to help interpret the volumetric changes. Our sports control group was small and may have been underpowered to detect differences in a group wise analysis. We therefore utilised an individualized analysis to detect abnormalities in FA, and did not observe any abnormalities in our sports control group compared to 17% of rugby players.
It is important to note that our results in adult professional rugby union and league players are not directly comparable to play at the community or youth levels. The overall health benefit of participating in sports and physical exercise have been well established including the reduction in mortality and chronic diseases such as dementia,115,116 and have been explored in detail within rugby.117 In the general professional sport setting, evidence of long-term health benefits includes lower all-cause mortality.61,118 The clinical implications on an individual level of the imaging changes associated with elite rugby participation are unclear. In retired rugby players, a lower risk of cardiovascular disease, with no evidence of exposure to repetitive head injuries nor participation in elite rugby affecting later life mental health, social or work functioning was found.18
In conclusion, our study shows diffusion and brain volume abnormalities in adult male and female rugby union and league players competing at an elite level. The results are in keeping with the presence of axonal injury, potentially related to repeated head impacts. Unexpected reductions in brain volume were seen in around 50% of the rugby players studied, which might indicate excess neurodegeneration in white matter tracts affected by injury. Rugby governing bodies, including the Rugby Football Union, are increasingly active collaborators in a range of head injury prevention and management projects as well as cross-sectional studies looking at brain health in retired players.119,120 To date these have rarely had a major focus on reporting neuroimaging changes. Further longitudinal imaging research in active and retired rugby players is needed to understand the impact of both repeated sports-related head impacts and head injuries on brain structure, and to clarify whether the abnormalities we have observed are related to an increased risk of neurodegenerative disease and impaired neurocognitive function following elite rugby participation.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The Drake Rugby Biomarker Study was supported by the Rugby Football Union (RFU) and the NIHR Imperial College Biomedical Research Centre. The authors thank all players, teams and study participants who took part in the study. The authors thank Keith Stokes for his review and edits of the manuscript when in preparation.
Funding
The Drake Rugby Biomarker Study was supported by the James Drake and The Drake Foundation. The research was also supported by a European Research Area Network (ERA-NET) grant (MR/R004528/1), National Institute of Health Research Professorship (NIHR-RP-011-048) and Medical Research Council through a Clinician Scientist Fellowship awarded to D.J.S., and by the National Institute of Health Research Clinical Research Facility and Biomedical Research Centre at Imperial College Healthcare NHS Trust. D.J.S. is also funded by the Care Research and Technology Centre, UK Dementia Research Institute and the Royal British Legion Centre for Blast Injury Studies. J.H. is supported by the UK Dementia Research Institute which receives its funding from Dementia Research Institute Ltd, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. N.G. was supported by an Alzheimer’s Research UK Clinical Research Fellowship (ARUK-CRF2017A-1).
Competing interests
D.J.S. has received an honorarium from the Rugby Football Union for participation in an expert concussion panel, which was used to support his research. He has also received an honorarium from the Wellcome Trust. H.R.M. is employed by UCL. In the last 24 months, he reports paid consultancy from Biogen, UCB, Abbvie, Denali, Biohaven and Lundbeck; lecture fees/honoraria from Biogen, UCB, C4X Discovery, GE-Healthcare, Wellcome Trust and Movement Disorders Society; Research Grants from Parkinson’s UK, Cure Parkinson’s Trust, PSP Association, CBD Solutions, Drake Foundation and Medical Research Council. H.R.M. is a co-applicant on a patent application related to C9ORF72—Method for diagnosing a neurodegenerative disease (PCT/GB2012/052140). S.K. is employed as the Medical Services Director for the Rugby Football Union. All other authors have no competing interests.
Glossary
- CTE =
chronic traumatic encephalopathy
- DAI =
diffuse axonal injury
- DTI =
diffusion tensor imaging
- DVI =
diffuse vascular injury
- FA =
anisotropy
- ISOVF =
isotropic volume fraction
- JD =
Jacobian Determinant
- MD =
mean diffusivity
- mTBI =
mild traumatic brain injury
- NDI =
neurite density index
- NODDI =
neurite orientation dispersion and density imaging
- ODI =
orientation dispersion index
- ROI =
region of interest
- SWI =
susceptibility weighted imaging
- TBI =
traumatic brain injury
References
- 1.Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease C. Prevention. Nonfatal traumatic brain injuries from sports and recreation activities–United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733–737. [PubMed] [Google Scholar]
- 3.Faul M, Wald MM, Xu L, Coronado VG. Traumatic brain injury in the United States; emergency department visits, hospitalizations, and deaths, 2002-2006; Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 4.Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A.. When a minor head injury results in enduring symptoms: A prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(2):217–223. [DOI] [PubMed] [Google Scholar]
- 5.Sharp DJ, Jenkins PO.. Concussion is confusing us all. Pract Neurol. 2015;15(3):172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903–909. [DOI] [PubMed] [Google Scholar]
- 7.Tagge CA, Fisher AM, Minaeva OV, et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141(2):422–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavett BE, Stern RA, Cantu RC, Nowinski CJ, McKee AC.. Mild traumatic brain injury: A risk factor for neurodegeneration. Alzheimers Res Ther. 2010;2(3):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. discussion 719–26. [DOI] [PubMed] [Google Scholar]
- 10.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318(4):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Rugby. Global Participation Map. 2018. https://resources.world.rugby/worldrugby/document/2020/07/28/212ed9cf-cd61-4fa3-b9d4-9f0d5fb61116/P56-57-Participation-Map_v3.pdf. Accessed 18 June 2021.
- 13.West S, Starling L, Kemp SPT, et al. Trends in match injury risk in professional male rugby union—A 16-season review of 10 851 match injuries in the English Premiership (2002-2019). The Professional Rugby Injury Surveillance Project. Br J Sports Med. 2021;55(12):676–682. [DOI] [PubMed] [Google Scholar]
- 14.Gardner AJ, Iverson GL, Quinn TN, et al. A preliminary video analysis of concussion in the National Rugby League. Brain Inj. 2015;29(10):1182–1185. [DOI] [PubMed] [Google Scholar]
- 15.Stokes KA, Locke D, Roberts S, et al. Does reducing the height of the tackle through law change in elite men's rugby union (The Championship, England) reduce the incidence of concussion? A controlled study in 126 games. Br J Sports Med. 2019;55(4):220–225. [DOI] [PubMed] [Google Scholar]
- 16.Attwood MJ, Roberts SP, Trewartha G, England ME, Stokes KA.. Efficacy of a movement control injury prevention programme in adult men's community rugby union: A cluster randomised controlled trial. Br J Sports Med. 2017;52(6):368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decq P, Gault N, Blandeau M, et al. Long-term consequences of recurrent sports concussion. Acta Neurochir (Wien). 2016;158(2):289–300. [DOI] [PubMed] [Google Scholar]
- 18.McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: Rugby union players. J Neurol Neurosurg Psychiatry. 2017;88(6):505–511. [DOI] [PubMed] [Google Scholar]
- 19.Gallo V, Motley K, Kemp SPT, et al. Concussion and long-term cognitive impairment among professional or elite sport-persons: A systematic review. J Neurol Neurosurg Psychiatry. 2020;91(5):455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies MAM, A DJ, Delmestri A, et al. Health amongst former rugby union players: A cross-sectional study of morbidity and health-related quality of life. Sci Rep. 2017;7(1):11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckland ME, Sy J, Szentmariay I, et al. Chronic traumatic encephalopathy in two former Australian National Rugby League players. Acta Neuropathol Commun. 2019;7(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart W, McNamara PH, Lawlor B, Hutchinson S, Farrell M.. Chronic traumatic encephalopathy: A potential late and under recognized consequence of rugby union? QJM. 2016;109(1):11–15. [DOI] [PubMed] [Google Scholar]
- 23.Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W.. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol. 2019;138(3):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt 2):449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D.. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27(44):11869–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnelle V, Ham TE, Leech R, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Research Support, Non-U.S. Gov't. Proc Natl Acad Sci U S A. 2012;109(12):4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eierud C, Craddock RC, Fletcher S, et al. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin. 2014;4:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–955. [DOI] [PubMed] [Google Scholar]
- 29.Croall ID, Cowie CJ, He J, et al. White matter correlates of cognitive dysfunction after mild traumatic brain injury. Neurology. 2014;83(6):494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL.. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205(1):116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain. 2008;131(2):559–572. [DOI] [PubMed] [Google Scholar]
- 32.Bonnelle V, Leech R, Kinnunen KM, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31(38):13442–13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellyer PJ, Leech R, Ham TE, Bonnelle V, Sharp DJ.. Individual prediction of white matter injury following traumatic brain injury. Ann Neurol. 2012;73(4):489–499. [DOI] [PubMed] [Google Scholar]
- 34.Sharp DJ, Scott G, Leech R.. Network dysfunction after traumatic brain injury. Research Support, Non-U.S. Gov't Review. Nat Rev Neurol. 2014;10(3):156–166. [DOI] [PubMed] [Google Scholar]
- 35.Henry LC, Tremblay J, Tremblay S, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28(10):2049–2059. [DOI] [PubMed] [Google Scholar]
- 36.Jing M, McGinnity TM, Coleman S, Fuchs A, Kelso JA.. Temporal changes of diffusion patterns in mild traumatic brain injury via group-based semi-blind source separation. IEEE J Biomed Health Inform. 2015;19(4):1459–1471. [DOI] [PubMed] [Google Scholar]
- 37.Meier TB, Bergamino M, Bellgowan PS, et al. Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum Brain Mapp. 2016;37(2):833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manning KY, Schranz A, Bartha R, et al. Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology. 2017;89(21):2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asken BM, DeKosky ST, Clugston JR, Jaffee MS, Bauer RM.. Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): A systematic critical review. Brain Imaging Behav. 2018;12(2):585–612. [DOI] [PubMed] [Google Scholar]
- 40.Churchill NW, Caverzasi E, Graham SJ, Hutchison MG, Schweizer TA.. White matter microstructure in athletes with a history of concussion: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum Brain Mapp. 2017;38(8):4201–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki T, Pasternak O, Mayinger M, et al. Hockey Concussion Education Project, Part 3. White matter microstructure in ice hockey players with a history of concussion: A diffusion tensor imaging study. J Neurosurg. 2014;120(4):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churchill NW, Caverzasi E, Graham SJ, Hutchison MG, Schweizer TA.. White matter during concussion recovery: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum Brain Mapp. 2019;40(6):1908–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremblay S, Henry LC, Bedetti C, et al. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain. 2014;137(Pt 11):2997–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chappell MH, Uluğ AM, Zhang L, et al. Distribution of microstructural damage in the brains of professional boxers: A diffusion MRI study. J Magn Reson Imaging. 2006;24(3):537–542. [DOI] [PubMed] [Google Scholar]
- 45.Fakhran S, Yaeger K, Alhilali L.. Symptomatic white matter changes in mild traumatic brain injury resemble pathologic features of early Alzheimer dementia. Radiology. 2013;269(1):249–257. [DOI] [PubMed] [Google Scholar]
- 46.Meier TB, Bellgowan PS, Bergamino M, Ling JM, Mayer AR.. Thinner cortex in collegiate football players with, but not without, a self-reported history of concussion. J Neurotrauma. 2016;33(4):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S.. Are functional deficits in concussed individuals consistent with white matter structural alterations: Combined FMRI & DTI study. Exp Brain Res. 2010;204(1):57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strain J, Didehbani N, Cullum CM, et al. Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology. 2013;81(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Multani N, Goswami R, Khodadadi M, et al. The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J Neurol. 2016;263(7):1332–1341. [DOI] [PubMed] [Google Scholar]
- 50.Manning KY, Brooks JS, Dickey JP, et al. Longitudinal changes of brain microstructure and function in nonconcussed female rugby players. Neurology. 2020;95(4):e402–e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright DK, Gardner AJ, Wojtowicz M, et al. White matter abnormalities in retired professional rugby league players. J Neurotrauma. 2020;38(8):983–988. [DOI] [PubMed] [Google Scholar]
- 52.Griffin AD, Turtzo LC, Parikh GY, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain. 2019;142(11):3550–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheid R, Walther K, Guthke T, Preul C, von Cramon DY.. Cognitive sequelae of diffuse axonal injury. Arch Neurol. 2006;63(3):418–424. [DOI] [PubMed] [Google Scholar]
- 54.Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: Clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56(1):36–50. [DOI] [PubMed] [Google Scholar]
- 55.Hahnel S, Stippich C, Weber I, et al. Prevalence of cerebral microhemorrhages in amateur boxers as detected by 3T MR imaging. AJNR Am J Neuroradiol. 2008;29(2):388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasiloglu ZI, Albayram S, Selcuk H, et al. Cerebral microhemorrhages detected by susceptibility-weighted imaging in amateur boxers. AJNR Am J Neuroradiol. 2011;32(1):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jarrett M, Tam R, Hernandez-Torres E, et al. A prospective pilot investigation of brain volume, white matter hyperintensities, and hemorrhagic lesions after mild traumatic brain injury. Front Neurol. 2016;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luther N, Niogi S, Kutner K, et al. Diffusion tensor and susceptibility-weighted imaging in concussion assessment of national football league players. Br J Sports Med. 2013;47(5):e1. [Google Scholar]
- 59.Smith DH, Johnson VE, Stewart W.. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat Rev Neurol. 2013;9(4):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH.. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. discussion 128–34. [DOI] [PubMed] [Google Scholar]
- 61.Mackay DF, Russell ER, Stewart K, MacLean JA, Pell JP, Stewart W.. Neurodegenerative disease mortality among former professional soccer players. N Engl J Med. 2019;381(19):1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashburner J, Ridgway GR.. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2012;6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guttmann CR, Jolesz FA, Kikinis R, et al. White matter changes with normal aging. Neurology. 1998;50(4):972–978. [DOI] [PubMed] [Google Scholar]
- 64.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW.. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. [DOI] [PubMed] [Google Scholar]
- 65.Cole JH, Jolly A, de Simoni S, et al. Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain. 2018;141(3):822–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghajari M, Hellyer PJ, Sharp DJ.. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain. 2017;140(2):333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brezova V, Moen KG, Skandsen T, et al. Prospective longitudinal MRI study of brain volumes and diffusion changes during the first year after moderate to severe traumatic brain injury. Neuroimage Clin. 2014;5:128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bendlin BB, Ries ML, Lazar M, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42(2):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacKenzie JD, Siddiqi F, Babb JS, et al. Brain atrophy in mild or moderate traumatic brain injury: A longitudinal quantitative analysis. AJNR Am J Neuroradiol. 2002;23(9):1509–1515. [PMC free article] [PubMed] [Google Scholar]
- 70.Trivedi MA, Ward MA, Hess TM, et al. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: Relationship with duration of coma. J Neurotrauma. 2007;24(5):766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 1999;95(3):721–725. [DOI] [PubMed] [Google Scholar]
- 72.Malec JF, Brown AW, Leibson CL, et al. The Mayo Classification System for Traumatic Brain Injury Severity. Journal of Neurotrauma. 2007;24(9):1417–1424. 10.1089/neu.2006.0245 [DOI] [PubMed] [Google Scholar]
- 73.Fuller GW, Kemp SP, Raftery M.. The accuracy and reproducibility of video assessment in the pitch-side management of concussion in elite rugby. J Sci Med Sport. 2017;20(3):246–249. [DOI] [PubMed] [Google Scholar]
- 74.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70(3):374–383. [DOI] [PubMed] [Google Scholar]
- 75.Hampshire A, Highfield RR, Parkin BL, Owen AM.. Fractionating human intelligence. Neuron. 2012;76(6):1225–1237. [DOI] [PubMed] [Google Scholar]
- 76.Daws RE, Hampshire A.. The negative relationship between reasoning and religiosity is underpinned by a bias for intuitive responses specifically when intuition and logic are in conflict. Front Psychol. 2017;8:2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pertzov Y, Miller TD, Gorgoraptis N, et al. Binding deficits in memory following medial temporal lobe damage in patients with voltage-gated potassium channel complex antibody-associated limbic encephalitis. Brain. 2013;136(Pt 8):2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 79.Andersson JLR, Sotiropoulos SN.. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dale AM, Fischl B, Sereno MI.. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Avants BB, Yushkevich PA, et al. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Trans Med Imaging. 2007;26(11):1585–1597. [DOI] [PubMed] [Google Scholar]
- 82.Daducci A, Canales-Rodríguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran J-P.. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage. 2015;105:32–44. [DOI] [PubMed] [Google Scholar]
- 83.Jolly AB, Azor M, Zimmerman A. et al. Detecting axonal injury in individual patients after traumatic brain injury. Brain. 2020;144(1):92–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE.. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ashburner J.A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 86.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. Available at: https://www.R-project.org. Accessed 18 June 2021. [Google Scholar]
- 87.Veeramuthu V, Narayanan V, Kuo TL, et al. Diffusion tensor imaging parameters in mild traumatic brain injury and its correlation with early neuropsychological impairment: A longitudinal study. J Neurotrauma. 2015;32(19):1497–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu YC, Harezlak J, Elsaid NMH, et al. Longitudinal white-matter abnormalities in sports-related concussion: A diffusion MRI study. Neurology. 2020;95(7):e781–e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caeyenberghs K, Leemans A, Geurts M, et al. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil Neural Repair. 2011;25(6):492–502. [DOI] [PubMed] [Google Scholar]
- 90.Ressel V, O'Gorman Tuura R, Scheer I, van Hedel HJA.. Diffusion tensor imaging predicts motor outcome in children with acquired brain injury. Brain Imaging Behav. 2017;11(5):1373–1384. [DOI] [PubMed] [Google Scholar]
- 91.Pearce AJ, Rist B, Fraser CL, Cohen A, Maller JJ.. Neurophysiological and cognitive impairment following repeated sports concussion injuries in retired professional rugby league players. Brain Inj. 2018;32(4):498–505. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC.. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Research Support, Non-U.S. Gov't. Neuroimage. 2012;61(4):1000–1016. [DOI] [PubMed] [Google Scholar]
- 93.Wu YC, Mustafi SM, Harezlak J, Kodiweera C, Flashman LA, McAllister TW.. Hybrid diffusion imaging in mild traumatic brain injury. J Neurotrauma. 2018;35(20):2377–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mayer AR, Ling JM, Dodd AB, Meier TB, Hanlon FM, Klimaj SD.. A prospective microstructure imaging study in mixed-martial artists using geometric measures and diffusion tensor imaging: Methods and findings. Brain Imaging Behav. 2017;11(3):698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cole JH, Leech R, Sharp DJ, Alzheimer's Disease Neuroimaging Initiative. Alzheimer's Disease Neuroimaging I. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015;77(4):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Graham NS, Sharp DJ.. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry. 2019;90(11):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J.. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(5):461–465. [DOI] [PubMed] [Google Scholar]
- 98.McCrory P, Meeuwisse W, Dvorak J. et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. [DOI] [PubMed] [Google Scholar]
- 99.Laitinen T, Sierra A, Bolkvadze T, Pitkanen A, Grohn O.. Diffusion tensor imaging detects chronic microstructural changes in white and gray matter after traumatic brain injury in rat. Front Neurosci. 2015;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Glushakova OY, Johnson D, Hayes RL.. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J Neurotrauma. 2014;31(13):1180–1193. [DOI] [PubMed] [Google Scholar]
- 101.Kane MJ, Angoa-Pérez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM.. A mouse model of human repetitive mild traumatic brain injury. J Neurosci Methods. 2012;203(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamberger A, Viano DC, Saljo A, Bolouri H.. Concussion in professional football: Morphology of brain injuries in the NFL concussion model–part 16. Neurosurgery. 2009;64(6):1174–1182; discussion 1182. [DOI] [PubMed] [Google Scholar]
- 103.Virji-Babul N, Borich MR, Makan N, et al. Diffusion tensor imaging of sports-related concussion in adolescents. Pediatr Neurol. 2013;48(1):24–29. [DOI] [PubMed] [Google Scholar]
- 104.Churchill NW, Hutchison MG, Di Battista AP, Graham SJ, Schweizer TA.. Structural, functional, and metabolic brain markers differentiate collision versus contact and non-contact athletes. Front Neurol. 2017;8:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin W, Mahmoud SY, Sakaie K, et al. Diffusion measures indicate fight exposure-related damage to cerebral white matter in boxers and mixed martial arts fighters. AJNR Am J Neuroradiol. 2014;35(2):285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268(3):850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schneider DK, Galloway R, Bazarian JJ, et al. Diffusion tensor imaging in athletes sustaining repetitive head impacts: A systematic review of prospective studies. J Neurotrauma. 2019;36(20):2831–2849. [DOI] [PubMed] [Google Scholar]
- 108.Scheid R, Preul C, Gruber O, Wiggins C, von Cramon DY.. Diffuse axonal injury associated with chronic traumatic brain injury: Evidence from T2-weighted gradient-echo imaging at 3 T. AJNR Am J Neuroradiol. 2003;24(6):1049–1056. [PMC free article] [PubMed] [Google Scholar]
- 109.Huang YL, Kuo YS, Tseng YC, Chen DY, Chiu WT, Chen CJ.. Susceptibility-weighted MRI in mild traumatic brain injury. Neurology. 2015;84(6):580–585. [DOI] [PubMed] [Google Scholar]
- 110.Erickson KI, Leckie RL, Weinstein AM.. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35(Suppl 2):S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wittfeld K, Jochem C, Dorr M, et al. Cardiorespiratory fitness and gray matter volume in the temporal, frontal, and cerebellar regions in the general population. Mayo Clin Proc. 2020;95(1):44–56. [DOI] [PubMed] [Google Scholar]
- 112.Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H.. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. 2016;131:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK.. Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Hum Brain Mapp. 2005;26(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu C, Li J, Liu Y, et al. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. Neuroimage. 2008;40(4):1533–1541. [DOI] [PubMed] [Google Scholar]
- 115.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4(11):705–711. [DOI] [PubMed] [Google Scholar]
- 116.Warburton DE, Nicol CW, Bredin SS.. Health benefits of physical activity: The evidence. CMAJ. 2006;174(6):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Griffin SA, Perera NKP, Murray A, et al. The relationships between rugby union, and health and wellbeing: a scoping review. Br J Sports Med. 2021;55(6):319–326. [DOI] [PubMed] [Google Scholar]
- 118.Lemez S, Baker J.. Do elite athletes live longer? A systematic review of mortality and longevity in elite athletes. Sports Med Open. 2015;1(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gallo V, McElvenny D, Hobbs C, et al. BRain health and healthy AgeINg in retired rugby union players, the BRAIN Study: Study protocol for an observational study in the UK. BMJ Open. 2017;7(12):e017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yakoub KM, O'Halloran P, Davies DJ, et al. Study of Concussion in Rugby Union through MicroRNAs (SCRUM): a study protocol of a prospective, observational cohort study. BMJ Open. 2018;8(11):e024245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.