Abstract
Purpose
Although malignant bone tumours in children are infrequent, it is important to know how to properly diagnose and stage them, in order to establish an adequate treatment.
Methods
We present a review of the diagnostic workflow of malignant bone tumours in children, including history and clinical examination, imaging, laboratory tests and biopsy techniques. Moreover, the two most commonly used staging systems are reviewed.
Results
History, clinical examination and laboratory tests are nonspecific for diagnosing malignant bone tumours in children. Radiographs remain the mainstay for initial diagnosis, with MRI the modality of choice for local assessment and staging. Fluorine-18 labelled fluoro-deoxy-glucose-positron emission tomography scans provide a noninvasive method to assess the aggressiveness of the tumour and to rule out metastasis and is replacing the use of the bone scintigraphy. Biopsy must be always performed under the direction of the surgeon who is to perform the surgical treatment and after all diagnostic evaluation has been done. Staging systems are useful to study the extent of the tumour and its prognosis. They are expected to evolve as we better understand new molecular and genetic findings.
Conclusion
When a malignant bone tumour is suspected in a child, it is essential to make a correct diagnosis and referral to an experienced centre. Following an appropriate workflow for diagnosis and staging facilitates, prompt access to treatment improves outcomes.
Level of Evidence
Level V Expert opinion
Keywords: malignant bone tumours, bone tumours diagnosis, bone tumours imaging techniques, bone tumours biopsy, bone tumours staging
Introduction
What is common? What do I have to think about?
Malignant bone tumours in children are very rare. In fact, most physicians will see few patients with symptoms of an undiagnosed primary bone tumour throughout their careers. Nevertheless, the paediatric orthopaedic surgeon should be able to guide the clinical workup of a paediatric bone tumour and know the relevant differential diagnoses. This review will outline the most common malignant bone lesions in children and provide a basic guide to diagnosis and staging.
The two leading primary malignant bone tumours in children are the osteosarcoma and lesions from the Ewing sarcoma family.1
Osteosarcoma is the most common primary bone sarcoma in children. The incidence is one to three per million with a slight male predominance.2 Histologically it is defined as a spindle cell neoplasm that produces osteoid. The characteristic localizations are the metaphyses of the long bones in the region of the knee, the proximal humerus and the proximal femur.3 Osteosarcoma rarely occurs in children less than five years of age. However, when this is the case, it is most commonly associated with Li-Fraumeni syndrome. Other syndromes with an associated risk of osteosarcoma include retinoblastoma 1 gene, Rothmund-Thomson syndrome or RAPADILINO syndrome. Osteosarcoma metastasizes mainly to the lung and bone. About 10% to 20% of patients already have pulmonary metastases at initial presentation. Long-term survival following multimodal chemotherapy and wide resection lies between 60% and 70%.2,3
The Ewing sarcoma family of tumours includes Ewing sarcoma of bone, extraskeletal Ewing sarcoma, peripheral primitive neuroectodermal tumours of bone and soft tissue and Askin tumour. It is the second most common primary bone and soft-tissue tumour in children and adolescents with an incidence of 2.5 to three per million. Histology typically presents a distinctive small, blue round-cell sarcoma. The most frequent sites are the pelvis and the diaphyseal or metadiaphyseal regions of long bones. Unlike other primary bone tumours, approximately 20% arise in the soft tissue. The Ewing sarcoma is characterized by a chromosomal translocation of the EWSR1 gene on chromosome 22 t(11;22)(q24;q12). Treatment includes chemotherapy, radiation therapy and wide surgical resection. Five-year overall survival is about 85% for patients with localized Ewing sarcoma and 27% for patients with metastatic disease, respectively.2
The major differential diagnoses for paediatric bone lesions include infection (i.e. osteomyelitis, septic arthritis or brodie abscess), lymphoma, Langerhans cell histiocytosis (LCH or histiocytosis X) and benign lesions like aneurysmal bone cysts, osteoid osteoma, osteoblastoma or osteochondroma. In the spine, location may be helpful to distinguish between entities. Malignancies as well as infection or LCH more often occur in the anterior elements (vertebral body), whereas benign tumours are more often located in the posterior elements.4
A meticulous diagnostic workup is important for implementation of the correct diagnosis and successful treatment. This is an interdisciplinary process that includes clinical examination, adequate imaging, correct biopsy and histopathological analysis.
Diagnosis
History and clinical examination
Early diagnosis of a malignant bone tumour may not only increase the chance of survival, but also the possibility of performing a limb-sparing resection. Unfortunately, the initial symptoms are unspecific but pain is usually a constant finding. Initially, the pain can be intermittent and related to activity and loading.5-7 Pain that worsens at night has been described as characteristic of malignancy but this is not a constant finding. With time the pain becomes constant without relief and gets worse with activity.5-8 Sometimes a lump is present, but usually, when it is evident, the disease is already advanced.6-8 Bone tumours can weaken the bone and can present as a pathological fracture.9 Pelvic and spine tumours can present with neurological deficits.5,6 Past history of malignancy and history of some specific predisposed genetic conditions must be investigated. In children, it is mandatory to make a differential diagnosis with an infection, as this is far more common than a sarcoma.10
The clinical examination must include the inspection and palpation of the area of tenderness, swelling or pain. If a mass is present, its size, consistency, position and mobility must be assessed. Local lymph nodes should be palpated.7,8
Imaging
Different imaging techniques are useful to evaluate a bone lesion. Radiography remains the mainstay for initial diagnosis and other advanced imaging modalities, such as MRI, CT scans, bone scintigraphy and positron emission tomography (PET) are useful in evaluating the extent of the lesion and its local and distant staging.
Radiographs
Conventional radiographs are still the first and the most valuable imaging technique to study a bone tumour.11-16 It is available in every hospital and it is a relatively inexpensive technique. Orthogonal radiographs of the area should be completed for all lesions. Although a radiologist’s report may be helpful, all orthopaedic surgeons should have the basic knowledge to be able to discern a concerning lesion from a benign one. Benign lesions have well delineated borders, sclerotic edges, do not break the cortical and do not usually have periosteal reaction or a soft-tissue mass (Fig. 1a). On the contrary, malignant lesions have undefined borders with a speckled pattern, break the cortical, usually have a soft-tissue mass and periosteal reaction can be observed (Fig. 1b).
Fig. 1.
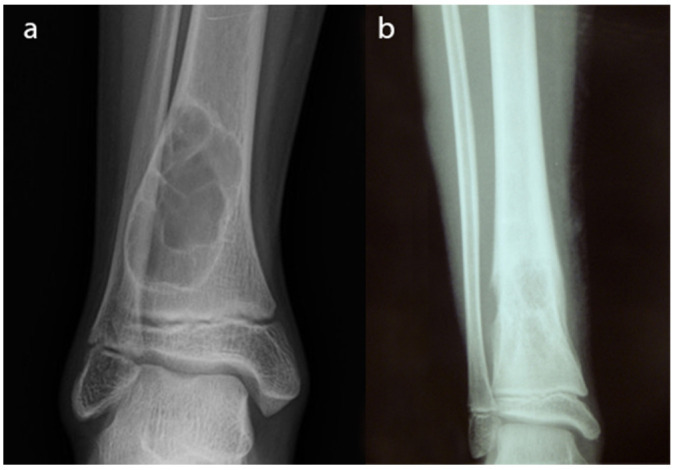
Radiographs of a non-ossifying fibroma in the distal tibia. This is a benign lesion. Well delineated borders and sclerotic edges can be appreciated. There is no cortical breakage and no soft-tissue mass; b) radiographs of an Ewing sarcoma of the distal tibia. This is a malignant bone lesion with undefined borders and a speckled pattern. Breakage of the cortical is well observed.
Enneking suggested questions to be asked when a bone tumour is suspected in a plain radiograph:17
Where is the lesion? It is important where the lesion is located, but also which part of the bone. For example, osteosarcoma is usually located in the metaphysis of the long bones.
What is the lesion doing to the bone? Three patterns of bone destruction help us to identify the aggressiveness of the bone lesion.11,18 Type 1, ‘geographic bone destruction’, when a lesion has borders well defined with sclerotic margins (1A), without sclerotic margins but with a narrow zone of transition (1B) and lesions with ill-defined and indistinct margins (1C). This pattern implies benign or less aggressive lesions. Type II, ‘moth-eaten appearance’, when a lesion has areas of bone destruction with ragged edges, indicative of a malignant process rapidly expanding into the bone. Type III, ‘permeative appearance’, when the tumour moves through the bone without destroying all the trabeculae with a zone of transition between pathological and normal bone.
What is the bone doing to the lesion? The response of the bone depends on the tumour histology and grade and the rapidity of its growth. ‘Sunbursts’ or ‘hair-on-end’ periosteal reactions and the ‘Codman triangle’ are typical in malignant lesions.13,19
What is in the lesion? The content of the lesion can give us a clue to the histological diagnosis.
The information provided by the radiograph is limited when the lesion is located in complex anatomical locations such as in the iliac bones and spine. In posterior elements of vertebrae, the overlapping of structures on 2D planes limits the radiographic interpretation11,14 (Fig. 2a). Also, the radiograph has limitations concerning the evaluation of soft tissue and the precise extent of medullary involvement.11,14
Fig. 2.
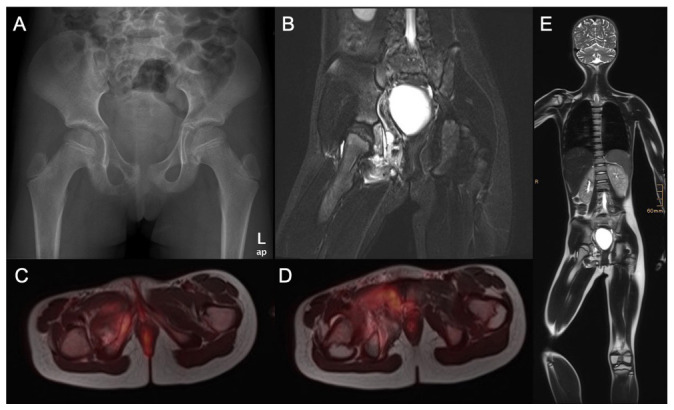
A nine-year-old girl presented with pain in the right hip and thigh for two months: a) the anteroposterior pelvis radiograph shows an osteolytic lesion of the right pubic bone (ramus inferior ossis pubis). Iliac crest biopsy was consecutively performed and Ewing sarcoma was diagnosed; b) coronal MRI scans of the pelvis show a tumour mass extending from the os pubis and infiltrating the obturator externus muscle; c) and d) full body positron emission tomography MRI scans were performed to complete staging; e) full body MRI scan.
CT scan
CT scans allow precise anatomical delineation and evaluation of the lesions in complex anatomical location.12-15 Also, CT allows better visualization of bony changes.12,13,15,16 It can be very useful, especially 3D evaluation, when it comes to planning reconstructive surgery and is commonly used to guide biopsy.12,14-16 Moreover, CT can also be very useful in osteolytic lesions when the fracture risk must be evaluated (Fig. 3).
Fig. 3.
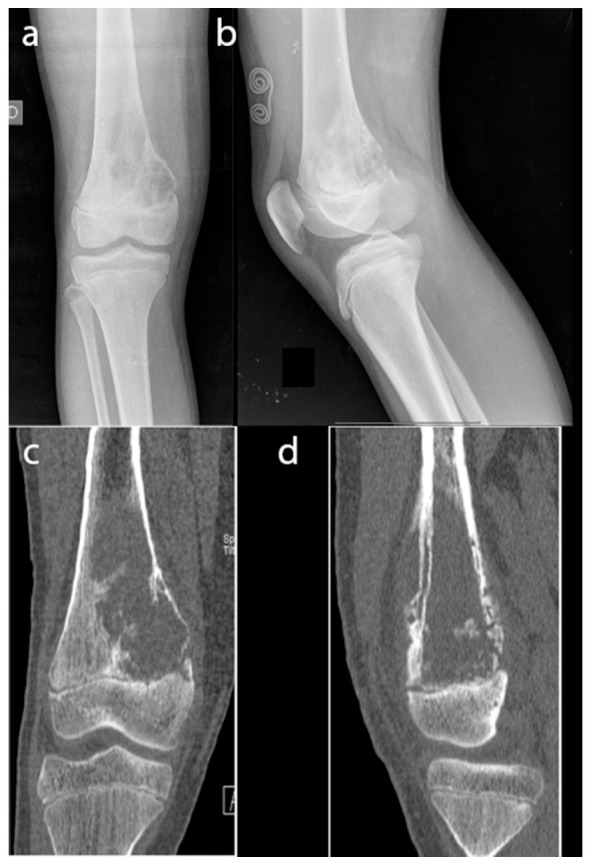
Right femur osteosarcoma of a 12-year-old boy: a) and b) on the radiographs an osteolytic lesion in the distal metaphysis can be appreciated; c) and d) the CT study shows a large osteolytic lesion with a high risk of pathological fracture.
When malignancy is suspected, a chest CT scan is mandatory to rule out lung metastasis.13,20
MRI
MRI is the modality of choice for local assessment and staging of a malignant bone tumour.11-16,20 It is recommended to have this study performed by a radiologist with experience in malignant bone tumours and in the institution where the patient will have treatment.
MRI is much more sensitive than conventional radiographs. Lytic bone lesions can be seen on plain radiographs only when there is > 30% to 50% loss of mineralization12,16 (Figs 2b and 4). If a patient continues to have symptoms and radiographs do not show any abnormality, MRI is the preferred modality to assess bone marrow, soft-tissue mass and the involvement of neurovascular structures and adjacent joints.12-15 But MRI should be interpreted only with concurrent radiographs. The MRI study must include the two joints on either side of the tumour; careful attention must be paid to any epiphyseal or articular invasion, and it should include the entire bone where the lesion is settled in to detect skip lesions13,16,21 (Fig. 4). The short tau inversion recovery sequence is the most sensitive, but these sequences should not be used to measure tumour extension, since they lead to overestimation in both intramedullary and soft tissue. T1 sequences with intravenous contrast and especially when combined with a fat-suppressed sequence are more appropriate14,16 (Fig. 5). Moreover, MRI must always be performed before the biopsy and it is essential to plan it. Any follow-up with MRI should use the same protocol every time, as it will enable better comparison in different occasions.15
Fig. 4.
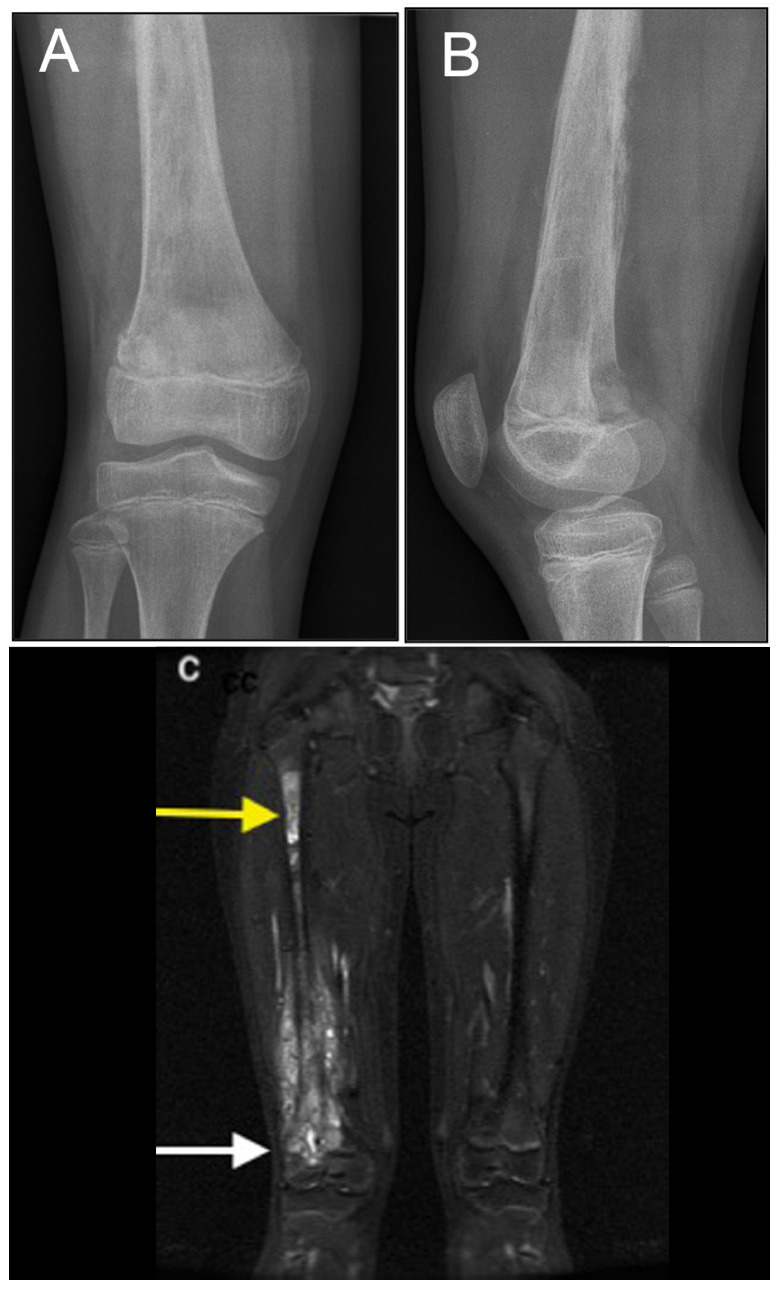
Right femur osteosarcoma in a 11-year-old girl: a) and b) on the radiographs the lesion cannot be well delimited; c) the MRI study reveals a large lesion with epiphyseal extension (white arrow) and a skip lesion in the proximal diaphysis (yellow arrow).
Fig. 5.
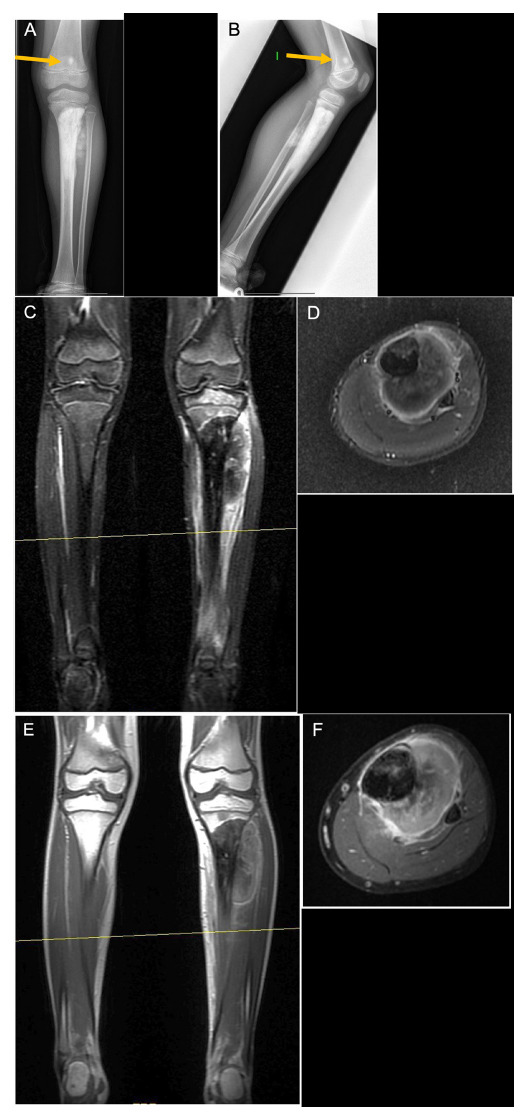
Left tibia osteosarcoma in a seven-year-old boy: a) and b) radiographs show an osteoblastic lesion in the proximal tibia, with a skip lesion in the distal femur (yellow arrow). Biopsy showed a high-grade osteosarcoma with osteoblastic predominance; c) and d) in short tau inversion recovery images of the lesion, it is difficult to distinguish between tumour and oedema; a) and f) T1 fat saturation images with contrast, where the tumour can be better delineated.
More recently, new MRI modalities, like dynamic contrast-enhanced MRI scanning (perfusion MRI), diffusion-weighted imaging and MR spectroscopy, have been developed, adding advantages in tissue characterization and in staging of bone tumours.12,14,15
Bone scintigraphy
The technetium-99m bone scan is highly sensitive but with a low specificity as it only measures osteoblast activity. It can help us to detect active lesions and distant bone metastasis.12-14,16,20 Nowadays, the use of PET is preferred when it is available.
PET
PET scans provide a non-invasive method to assess the aggressiveness of tumours. It is useful for staging the tumour, to rule out distant bony and soft-tissue (lung or lymph nodal) metastasis and to assess skip lesions in equivocal conditions.13,19,20,22-24 Benign diseases are glucose avid but in less quantity than a malignant process. PET scans have a 97% specificity to rule out malignant disease.23,25 Nevertheless, numerous exceptions exist.19 Therefore, interpreting the morphological characteristics of the tumours is important and PET findings should be always interpreted in conjunction with other imaging studies.
PET/CT is an advanced examination method that provides functional and metabolic information (PET) and detailed anatomical and pathological information (CT).22-24 The images can be evaluated separately or fused together. This technique can locate the lesion more accurately, detect the smaller lesion and distinguish the benign, malignant and different stages of bone tumours. Particularly, fluoro-deoxy-glucose (FDG) PET/TC is a whole-body imaging modality that has been found to be successful in the staging of malignancies.19,20,23,24
New modalities of PET are emerging like FDG PET/CT dual-time-point imaging which consists of registering images in two different moments. It has been used to differentiate malignant lesions with a prolonged uptake from benign lesions with a shorter uptake.19,22 However, the use of dual-time-point imaging is still not a routine practice because of prolonged scheduling time requirements.19 FDG PET/MRI is another emerging imaging modality, which results in reduced radiation with increased anatomical resolution (Figs 2c and 2d). PET/MRI has been proven superior to PET/CT in the evaluation of the brain, the soft-tissue component of the lesions and also in bone marrow evaluation.19
Laboratory tests
If malignancy is suspected, laboratory tests should be performed: full blood count, inflammatory markers and a bone profile. An association between an elevated C-reactive protein and erythrocyte sedimentation rate and worse overall survival has been described by some authors.26 A meta-analysis by Ren et al27 concluded that a high-serum alkaline phosphatase level is associated with poor overall survival and presence of metastasis at diagnosis in osteosarcoma patients.
Nevertheless, these findings are non-specific, and their value limited.
Biopsy
Histopathological evaluation is the final step in the diagnostic path and is part of the staging process. A biopsy must be performed after all diagnostic imaging evaluation has been done, as the post-biopsy changes can alter the radiological appearance of the lesion.12,13,15,16,20,24,28 The biopsy must be always performed by or under the guidance of the surgeon who is to perform the surgical treatment.12-16,29 It is a known fact that biopsies performed in a non-specialist centre can lead to diagnostic errors, can cause a change in the treatment plan, increased local recurrence and may also result in unnecessary amputation.13-15,29
Biopsy techniques may be percutaneous, incisional or excisional. Excisional biopsy is not recommended for malignant bone tumours. Percutaneous biopsy is the usual technique employed when a malignant bone lesion is suspected. It can be performed with a true-cut or a needle. The biopsy must be discussed by the interventional radiologist, the tumour surgeon and the histopathologist, in order to plan the site, the entry point, direction and the target of the needle biopsy, in order to obtain a representative tissue to allow the diagnosis. If malignant disease is confirmed the biopsy tract requires excision at the time of surgery.12,14-16,28,29 A percutaneous biopsy can be carried out accurately using ultrasound, CT or MRI guidance. In a specialist centre, 98% diagnostic accuracy has been described.14,16,28 When more pathological tissue is needed, or the lesion cannot be safely accessed percutaneously, an incisional biopsy can be performed. Open biopsy increases the risk of local contamination and clinical morbidity, with a worse prognosis in 8% of the cases.28 There are strict oncological principles regarding biopsy surgery, of which the surgeon must be cognizant.14,15,28,29 Proper location and orientation of the biopsy incision as well as meticulous hemostasis are required to avoid a haematoma. No compartmental barrier, anatomical plane, joint space or tissue area around a neurovascular bundle should be open.28,29
Microscopic examination of the haematoxylin-eosin-stained tissue sections, together with the integration of the clinical and radiological data, is still the basis for establishing a correct diagnosis when dealing with bone tumours.20,28 The use of the World Health Organization classification system is recommended, which incorporates morphological and genetic data and provides a uniform classification and nomenclature for the diagnosis of bone tumours.30 In certain cases, the use of additional techniques such as immunohistochemistry and/or molecular pathology is required for a definitive diagnosis.15,28 The pathology report of a biopsy should include histological type and grade, as well as the results of ancillary studies.15
Staging
Staging a malignant bone tumour is a standardized way of assessing a patient’s prognosis at the time of initial diagnosis and helps to guide treatment. Furthermore, staging allows meaningful comparisons to be done among groups of patients.13,20 Two main staging systems are currently used: the one proposed by Enneking and adapted by the Musculoskeletal Tumor Society (MSTS)31 and the one proposed by The American Joint Committee on Cancer (AJCC), based on TNM (primary tumour site and size, lymph node involvement, metastastatic spread).32,33
The Enneking system,31 described in the 1980s, is more surgically oriented and is the most widely used among orthopaedic surgeons. According to this system, malignant bone tumours are staged based on histological grade, the intraosseous or extraosseous extent of the tumour and the presence of distant metastases. The system was evaluated and endorsed by the MSTS. According to this system, low-grade tumours were classified as stage I, high-grade tumours were classified as stage II, and metastatic tumours were classified as stage III. These stages were subclassified based on the local extent of growth of the primary tumour, A intracompartmental and B extracompartmental31 (Table 1).
Table 1.
Musculoskeletal Tumour Society staging system
| Stage | Grade | Local extent | Metastases |
|---|---|---|---|
| IA | G1 | T1 | M0 |
| IB | G1 | T2 | M0 |
| IIA | G2 | T1 | M0 |
| IIB | G2 | T2 | M0 |
| III | G1 or G2 | T1 or T2 | M1 |
G1, low grade; G2, high grade; T1, tumour is intracompartmental; T2, extracompartmental; M0, no regional or distant metastases; M1, metastases
The previous AJCC staging system for primary malignancies of bone was almost identical to the MSTS system, except for metastatic disease being classified as stage IV (to remain consistent with the AJCC staging systems for other cancers), while stage III was left undefined. Also, stage IV was subdivided based on the presence of lymph node metastasis only (stage IV-A) or the presence of distant metastases (stage IV-B).13 Since its description, some revisions to the system have been carried out. Rather than using the intraosseous or extraosseous extent of the tumour, its size was suggested as a better prognostic indicator. Tumours associated with skip metastases were classified separately as stage III and later on, this stage III was reserved for grade 3 (poorly differentiated) and grade 4 (undifferentiated tumours). Stage IV tumours were subdivided based on the presence of pulmonary metastases only (stage IV-A) or metastases to other locations including bone (stage IV-B). In the last edition33 spine/pelvis segments were added as part of the ‘T’ classification of primary bone tumours, as these locations are associated with worse prognosis.34-36 Another notable change in the eighth edition is the migration from a four-tier to three-tier grading system to maintain uniformity in the way the bone and soft-tissue sarcomas are staged (Table 2).
Table 2.
American Joint Committee on Cancer staging system
| Stage | Tumour | Lymph node | Metastases | Grade |
|---|---|---|---|---|
| IA | T1 | N0 | M0 | G1 or GX |
| IB | T2 or T3 | N0 | M0 | G1 or GX |
| IIA | T1 | N0 | M0 | G2 or G3 |
| IIB | T2 | N0 | M0 | G2 or G3 |
| III | T3 | N0 | M0 | G2 or G3 |
| IVA | Any T | N0 | M1a | Any G |
| IVB | Any T | N1 | Any M | Any G |
| Any T | Any N | M1b | Any G |
T1, tumour 8 cm or less in greatest dimension; T2, tumour > 8 cm in greatest dimension; T3, discontinuous tumours in the primary bone site; N0, no regional lymph node metastasis; N1, regional lymph node metastasis; M0, no distant metastasis; M1, distant metastasis; M1a, lung; M1b, other distant sites; GX, grade cannot be assessed; G1, well differentiated (low grade); G2, moderately differentiated (high grade); G3, poorly differentiated (high grade)
There are other prognostic factors not included in the staging systems. Histological response to chemotherapy is a well-demonstrated prognostic variable, especially in osteosarcoma34,36 and Ewing sarcoma37 but it cannot be evaluated at the time of diagnosis and it is still unclear how this fact should impact the treatment of individual patients.13 Finally, multiple studies have shown that molecular abnormalities can be correlated with outcome in patients with osteosarcoma and Ewing sarcoma.13,38,39
In the near future, emerging PET modalities, age and molecular and genetic findings will play a role in the next big leap in malignant tumour staging.13,16,20,39
Conclusion
Although malignant bone tumours are not common in children, every orthopaedic surgeon should be able to recognize a malignant lesion in a radiograph and if malignancy is suspected the patient should be referred to a specialized centre. A complete and systematic diagnosis study should be performed before deciding the best treatment. As new imaging techniques and molecular and genetic tests are arising, they will be become part of our diagnostic protocol.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Compliance with ethical standards
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical statement
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent: Informed consent was not required.
ICMJE Conflict of interest statement
All authors declare that they have no conflicts of interest related to this work.
Author Contributions
MS: writing of manuscript
MW: writing of manuscript
CC: writing of manuscript
JMGA: review of manuscript
RW: review of manuscript
IS: review of manuscript
References
- 1.Larsson SE, Lorentzon R. The incidence of malignant primary bone tumours in relation to age, sex and site. A study of osteogenic sarcoma, chondrosarcoma and Ewing’s sarcoma diagnosed in Sweden from 1958 to 1968. J Bone Joint Surg [Br] 1974;56-B:534-540. [PubMed] [Google Scholar]
- 2.Jackson TM, Bittman M, Granowetter L. Pediatric malignant bone tumors: a review and update on current challenges, and emerging drug targets. Curr Probl Pediatr Adolesc Health Care 2016;46:213-228. [DOI] [PubMed] [Google Scholar]
- 3.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol 2010;21(suppl 7):vii320-vii325. [DOI] [PubMed] [Google Scholar]
- 4.Damron TA. Orthopaedic surgery essentials. 7th ed. Oncology and Basic Science, 2008. [Google Scholar]
- 5.Garg S, Dormans JP. Tumors and tumor-like conditions of the spine in children. J Am Acad Orthop Surg 2005;13:372-381. [DOI] [PubMed] [Google Scholar]
- 6.Kadhim M, Oyoun NA, Womer RB, Dormans JP. Clinical and radiographic presentation of pelvic sarcoma in children. SICOT J 2018;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg [Am] 2000;82-A:667-674. [DOI] [PubMed] [Google Scholar]
- 8.Messerschmitt PJ, Garcia RM, Abdul-Karim FW, Greenfield EM, Getty PJ. Osteosarcoma. J Am Acad Orthop Surg 2009;17:515-527. [DOI] [PubMed] [Google Scholar]
- 9.Scully SP, Ghert MA, Zurakowski D, Thompson RC Jr, Gebhardt MC. Pathologic fracture in osteosarcoma : prognostic importance and treatment implications. J Bone Joint Surg [Am] 2002;84:49-57. [PubMed] [Google Scholar]
- 10.McCarville MB. The child with bone pain: malignancies and mimickers. Cancer Imaging 2009;9:S115-S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costelloe CM, Madewell JE. Radiography in the initial diagnosis of primary bone tumors. AJR Am J Roentgenol 2013;200:3-7. [DOI] [PubMed] [Google Scholar]
- 12.Goyal N, Kalra M, Soni A, Baweja P, Ghonghe NP. Multi-modality imaging approach to bone tumors - State-of-the art. J Clin Orthop Trauma 2019;10:687-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heck RK Jr, Peabody TD, Simon MA. Staging of primary malignancies of bone. CA Cancer J Clin 2006;56:366-375. [DOI] [PubMed] [Google Scholar]
- 14.Plant J, Cannon S. Diagnostic work up and recognition of primary bone tumours: a review. EFORT Open Rev 2017;1:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redondo A, Bagué S, Bernabeu D,. et al. Malignant bone tumors (other than Ewing’s): clinical practice guidelines for diagnosis, treatment and follow-up by Spanish Group for Research on Sarcomas (GEIS). Cancer Chemother Pharmacol 2017;80:1113-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacy GS, Mahal RS, Peabody TD. Staging of bone tumors: a review with illustrative examples. AJR Am J Roentgenol 2006;186:967-976. [DOI] [PubMed] [Google Scholar]
- 17.Enneking WF. Clinical musculoskeletal pathology. Gainesville, Florida: Storer Printing Co, 1970. [Google Scholar]
- 18.Lodwick GS, Wilson AJ, Farrell C, Virtama P, Dittrich F. Determining growth rates of focal lesions of bone from radiographs. Radiology 1980;134:577-583. [DOI] [PubMed] [Google Scholar]
- 19.Costelloe CM, Chuang HH, Madewell JE. FDG PET/CT of primary bone tumors. AJR Am J Roentgenol 2014;202:W521-31 [DOI] [PubMed] [Google Scholar]
- 20.Steffner RJ, Jang ES. Staging of bone and soft-tissue sarcomas. J Am Acad Orthop Surg 2018;0:1-10. [DOI] [PubMed] [Google Scholar]
- 21.Sajadi KR, Heck RK, Neel MD,. et al. The incidence and prognosis of osteosarcoma skip metastases. Clin Orthop Relat Res 2004;426:92-96. [DOI] [PubMed] [Google Scholar]
- 22.Behzadi AH, Raza SI, Carrino JA,. et al. Applications of PET/TC and PET/MRI imaging in primary bone malignancies. PET Clin 2018;13:623-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggiero A, Lanni V, Librizzi A,. et al. Diagnostic accuracy of 18F-FDG PET/TC in the staging and assessment of response to chemotherapy in children with Ewing sarcoma. J Pediatr Hematol Oncol 2018;40:277-284. [DOI] [PubMed] [Google Scholar]
- 24.Huang T, Li F, Yan Z,. et al. Effectiveness of 18F-FDG PET/CT in the diagnosis, staging and recurrence monitoring of Ewing sarcoma family of tumors: a meta-analysis of 23 studies. Medicine (Baltimore) 2018;97:e13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin DS, Shon OJ, Han DS,. et al. The clinical efficacy of (18)F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008;22:603-609. [DOI] [PubMed] [Google Scholar]
- 26.Jettoo P, Tan G, Gerrand CH, Rankin KS.. Role of routine blood tests for predicting clinical outcomes in osteosarcoma patients. J Orthop Surg (Hong Kong) 2019;27:2309499019838293. [DOI] [PubMed] [Google Scholar]
- 27.Ren HY, Sun LL, Li HY, Ye ZM. Prognostic significance of serum alkaline phosphatase level in osteosarcoma: a meta-analysis of published data. Biomed Res Int 2015;ID160835:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traina F, Errani C, Toscano A,. et al. Current concepts in the biopsy of musculoskeletal tumors. J Bone Joint Surg [Am] 2015; 97-A: e7(1-6). [PubMed] [Google Scholar]
- 29.Interiano RB, Malkan AD, Loh AH,. et al. Initial diagnostic management of pediatric bone tumors. J Pediatr Surg 2016;51:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (). WHO classification of tumors of soft tissue and bone. IARC. Lyon, 2013:244-365. [Google Scholar]
- 31.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980;106-120. [PubMed] [Google Scholar]
- 32.Sobbin LH, Gospodarowicz MK, Wittekind CH (). TNM classification of malignant tumors. 7th ed. Oxford: Wiley, 2009:153-156. [Google Scholar]
- 33.Amin MB, Edge S, Greene F,. et al. AJCC cancer staging manual. 8th ed. Switzerland: Springer International Publishing, 2017. [Google Scholar]
- 34.Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res 2004;429:286-291. [DOI] [PubMed] [Google Scholar]
- 35.Argon A, Basaran M, Yaman F,. et al. Ewing’s sarcoma of the axial system in patients older than 15 years: dismal prognosis despite intensive multiagent chemotherapy and aggressive local treatment. Jpn J Clin Oncol 2004;34:667-672. [DOI] [PubMed] [Google Scholar]
- 36.Bielack SS, Kempf-Bielack B, Delling G,. et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20: 776-790. [DOI] [PubMed] [Google Scholar]
- 37.Marcus RB Jr, Berrey BH, Graham-Pole J, Mendenhall NP, Scarborough MT. The treatment of Ewing’s sarcoma of bone at the University of Florida: 1969 to 1998. Clin Orthop Relat Res 2002;397:290-297. [DOI] [PubMed] [Google Scholar]
- 38.Chibon F, Lagarde P, Salas S,. et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med 2010;16:781-787. [DOI] [PubMed] [Google Scholar]
- 39.Smrke A, Anderson PM, Gulia A,. et al. Future directions in the treatment of osteosarcoma. Cells. 2021;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]


