Abstract
Reconstructions for paediatric bone tumours of the shoulder girdle and humerus are intended to optimize placement of the hand in space. Given the longevity of paediatric survivors of sarcoma, durability is an important planning consideration. Here, I review a subset of approaches based on anatomical site with an emphasis on function and longevity. Often, biological reconstructions that combine living bone with tendon repairs and transfers best address those goals.
Introduction
Preservation of the upper extremity and bimanual function are nearly always achievable despite tumours that arise from the shoulder and humerus in children and adolescents. Reconstructions of tissue gaps resulting from tumour resections are usually intended to preserve a normal hand and a near normal elbow. At the shoulder itself, the aims are to maximize movement and stability and minimize the need for revisions.
Although four articulations contribute to shoulder movement, most attention is rightly paid to the important glenohumeral and scapulothoracic articulations that often have to be compromised to achieve a sufficient resection margin. Fortunately, preservation or optimization of one of these sites of movement can result in sufficient day-to-day function. It is often challenging to achieve very wide arcs of shoulder rotation and elevation, but not many activities require two hands behind the back, far out to the sides or overhead. A roughly 90° arc of shoulder rotation that allows placement of the hand on the belly (at the extreme of internal rotation)1 to just beyond neutral position (forearm forward at the extreme of external rotation) is arguably the most functionally useful range of movement in that axis. Achieving that range of active rotation allows for midline (dressing, buttoning, perineal care), bimanual and desktop function. Achieving active abduction and flexion of 30° to 40° represents a substantial functional benefit over no elevation. Combined with rotation, that degree of elevation allows placement of the hand in a wider zone of useful space in front of the child and permits reaching the mouth and side of the head. These day-to-day functions are often achievable despite large resections by combining movement at one of the major shoulder articulations with tendon repairs and transfers.
The longevity of skeletal reconstructions is likely related to whether living bone or nonviable materials are employed. Although we have evidence for reasonably good survival of certain allograft and endoprosthetic reconstructions over one to two decades, perhaps we should consider several decades as a relevant time scale for paediatric sarcoma survivors. The selective reviews of site-specific reconstructive options that follow are discussed from that perspective.
Clavicle
Soft-tissue masses arising from tumours of the clavicle commonly displace the subclavian vessels and even the brachial plexus. Fortunately, the most common malignant tumour of the clavicle, Ewing sarcoma, responds fairly reliably to neoadjuvant chemotherapy and recedes from those structures prior to surgery, thereby diminishing the need for vascular or nerve resection. As the only skeletal link between the upper limb and the appendicular skeleton, the clavicle may seem at first glance to be important for limb function. However, as has been suggested by observation of those who congenitally lack the clavicle, the wide arc of movement at the shoulder does not substantially require stability afforded by the clavicle. Reconstructions of the clavicle with allograft or vascularized fibular grafts supported by hardware such as hook plates offer the potential to maintain the breadth of the shoulder.2 However, reconstruction is associated with greater complications and may even delay functional recovery of the shoulder.3-5 For these reasons, most surgeons would probably agree that no reconstruction is a suitable option in most circumstances.
Scapula
The scapula may be resected as an isolated procedure for a primary scapular tumour or as part of a resection for a large tumour arising from the proximal humerus. For primary scapular tumours, the axillary nerve and deltoid can fortunately often be safely preserved, while the rotator cuff and teres major muscle bellies are commonly resected. Therefore, restoration of elevation requires reapproximation of the posterior deltoid origin to the posterior edge of superior trapezius. Active internal rotation is provided by pectoralis major, while restoration of external rotation requires transfer of latissimus dorsi to the posterior aspect of the proximal humerus.
An important distinction for primary scapular tumours is whether a total or glenoid-sparing subtotal resection is required (Figs 1a and 1b). The latter situation offers more options with potentially superior function.6 Simple humeral suspension from the clavicle or ribs is associated with low complications and is functionally somewhat improved by reattachment of the rotator cuff and biceps tendon when that is possible.7,8 Comparatively, scapular replacement using recycled bone9,10 or an endoprosthesis may offer superior elevation (37° to 90°).11,12 A constrained endoprosthesis may lower the complication rate and results in variable function.13,14
Fig. 1.
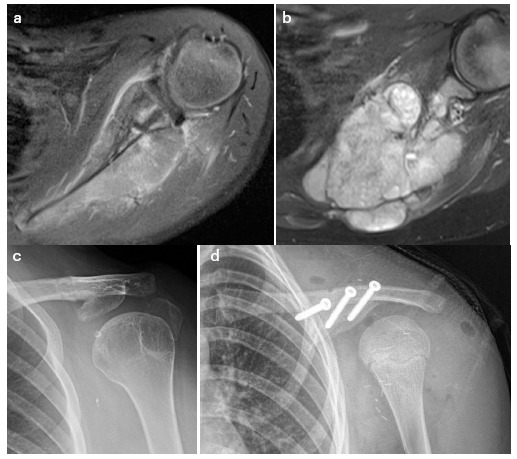
Ewing sarcoma requiring total (a) and potentially subtotal glenoid surface preserving (b) resection. Glenoid fragment fixed to the clavicle (c). An inner table iliac autograft fixed to the undersurface of the clavicle (d).
The glenoid fragment can be secured to the clavicle to offer a stable platform for elevation (Fig. 1c). In the absence of a glenoid fragment, a superomedial buttress using, for example, the inner table of the iliac crest (Fig. 1d) may offer a similar restraint against medial displacement of the humeral head during deltoid contraction, thereby levering the humerus to a greater extent. Anecdotally, these approaches have a low complication profile and similar range of elevation as reported for endoprosthetic reconstruction, but have not been examined relative to other suspensions.
Proximal humerus
The proximal humerus is the most common site of primary malignant tumours around the shoulder. Most paediatric cases involve intraarticular or extraarticular resection of the proximal humerus, but often do not require en bloc resection of the scapula and lateral clavicle or other variants of the wider Tikhoff-Linberg approach.15 Although numerous reconstructive variations have been described to deal with the proximal humeral defect, most of them fall into one of two categories: glenohumeral fusion or mobile glenohumeral reconstruction. Perhaps the most important consideration is whether a functioning deltoid can be preserved, including the axillary nerve and a majority of the muscle belly. Although active shoulder rotation can often be restored, elevation by superior trapezius transfer has been less reliable.16 Other considerations include whether the patient is of juvenile or adolescent age, whether the resection will preserve the glenoid (intraarticular) or not (extraarticular), and whether the rotator cuff can be repaired (Fig. 2a).
Fig. 2.
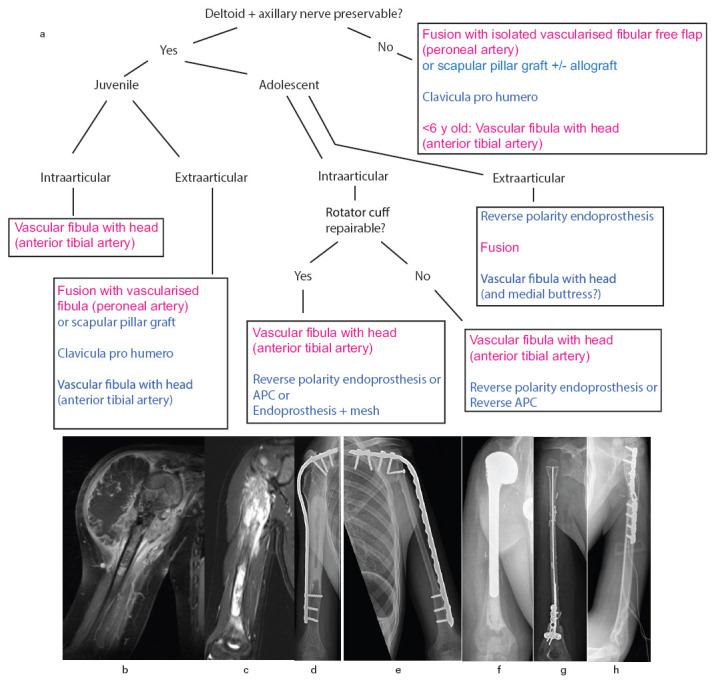
a) A proposed algorithm for proximal humeral reconstruction based on key parameters; b) a tumour for which the deltoid and axillary nerve require resection. Author’s preferences are in magenta; c) a tumour for which the axillary nerve might be resectable (after review of other images), but the deep aspect of deltoid (together with the subepimysial course of the motor branches) would best be resected; d) scapulohumeral fusion with allograft (and intramedullary cement); e) scapulohumeral fusion with vascular fibular autograft; f) a proximal humeral endoprosthesis; g) vascular fibular epiphyseal-diaphyseal autograft articulating with the glenoid; h) clavicula pro-humero reconstruction.
An insufficient deltoid margin can increase the likelihood of local recurrence.17 Factors on preoperative MRI that help to determine whether the deltoid can safely be preserved include whether a continuous fat plane can be visualized deep to the muscle, and whether the axillary nerve is visible throughout its course.18 We should note that the nerve supply courses close to the deep deltoid epimysium as it emerges posteriorly from the humeral neck. Therefore, resecting the deep aspect of the muscle en bloc with the tumour may denervate the superficial portion that is preserved. Anecdotally, functional deltoid preservation is a relatively uncommon circumstance (Figs 2b and 2c).
In cases where a functioning deltoid cannot be preserved, scapulo-humeral fusion offers superior function compared with suspension of some type of humeral replacement from the glenoid, clavicle or ribs.19-21 With a fusion, thoraco-humeral elevation of at least 30° is generated by periscapular muscles including the upper trapezius. Some children can achieve apparent flexion and abduction approaching 90° by adaptive maneuvers such as laterally arching their spine. Allograft is a traditional material to achieve fusion (Fig. 2d) and complications such as resorption, fracture and infection can be mitigated by a composite graft that includes a free vascular fibular diaphysis (on the peroneal vessels),19 or potentially avoided altogether by using an isolated vascularized fibula22 (Fig. 2e). An alternative option for short-segment fusion is a pedicled lateral scapular pillar graft supplemented by allograft bone.23 The position of a spanning plate has traditionally been over-top of the acromion but a sub-acromial position23 places the hardware deeper (Fig. 2e), making it less likely to compromise the flap and to be exposed in case of a wound gap. Living-bone fusions are likely to provide the greatest longevity with the fewest late complications.
For cases in which a functioning deltoid can be preserved, a mobile glenohumeral reconstruction can be useful. Traditional options include an osteoarticular allograft19,20,24,25 and recycled autogenous bone (extracorporeally irradiated or pasteurized),26,27 both of which result in relatively high revision rates due to resorption, fracture and infection.
Endoprosthetic replacement (Fig. 2f) has been reported with variable and sometimes unsatisfactory function secondary to problems such as subluxation.19,26 Allograft (or recycled bone)28-prosthetic composites (APC), endoprostheses and osteoarticular allografts result in similar function. However, APCs exhibit lower fracture and revision risks compared with osteoarticular allografts and lower subluxation than endoprostheses.29,30 Subluxation can be minimized by applying synthetic mesh to restore a restraining joint capsule31 or by employing a reverse-polarity type of endoprosthesis, especially when the rotator cuff cannot be preserved.32-37 Extendable proximal humeral endoprostheses result in over 50% subluxation, especially in children under nine, with implant survival about 75% at ten years.38 A substantial portion of those implants are not actually lengthened as planned,38 and it is unclear whether a modest humeral lengthening is worth the downsides of extendable implants.
A free vascular fibular epiphyseal-diaphyseal autograft has a small amount of cartilage and can articulate loosely with the glenoid39-42 (Fig. 2g). This free flap survives best when it is raised on the anterior tibial vessels which serve both the head and shaft of the fibula, whereas the peroneal vessels only reliably serve the shaft. Potential problems include fracture, which generally heals well and may be mitigated by placing an intramedullary Kirschner-wire, and resorption of the fibular head. An additional advantage of this graft is the proximal fibular physis can grow up to a little over 1 cm per year. It should be noted that dissection of the anterior tibial vessels for this purpose causes at least a transient foot drop. Also, the long-term outcome of the fibular-glenoid articulation has not been well documented. Nonetheless, this is the only mobile gleno-humeral reconstruction that provides living bone and, therefore, has the potential for a minimal long-term revision rate. For juvenile aged children, it may be tempting to consider this free flap to restore growth. However, if an intact deltoid cannot be preserved, the shoulder will function as a suspension and fusion will offer a superior functional outcome despite the anticipated undergrowth of the arm.
An alternate type of reconstruction is clavicula pro-humero suspension.43-46 The clavicle is divided medially and turned inferiorly to join the humerus, thereby suspending the upper limb on the acromioclavicular joint (Fig. 2h). The length of the gap one can restore is limited to about 12 cm in skeletally mature individuals and nonunion can be a problem, but good function with 30° to 90° of elevation is possible with an intact deltoid. This option could be especially useful for the unusual circumstance in which the deltoid, but not the glenoid and scapular neck, can be spared.
Although the choice of mobile or fixed glenohumeral joint hinges substantially on the deltoid, the function of both types of reconstruction is improved by restoration of active rotation (through the glenohumeral or scapulothoracic articulations, respectively). A useful goal is to achieve an approximately 90° arc of rotation from hand-to-belly to neutral (straight ahead) position which allows for most daily activities, especially in combination with at least 30° of elevation. Pectoralis major reattachment provides competent internal rotation, and transfer of teres major and/or latissimus dorsi to the postero-superior aspect of the humeral head or plate provides sufficient external rotation.
Humeral diaphysis
A humeral shaft defect can be bridged by a number of materials. The use of intercalary allograft is a traditional approach (Fig. 3a) that may require further surgery due to problems such as an approximately 50% nonunion rate at diaphyseal junctions, fractures, resorption, and infection.47 Filling the allograft medulla with antibiotic-loaded cement can potentially decrease the fracture and infection risk.48,49 Endoprosthetic diaphyseal implants may require short-segment compressive fixation methods and have an approximately 50% revision rate at ten years owing to mechanical failure, loosening and infection.50-52 If the resected diaphysis has sufficient structural integrity, it can be reimplanted after treatment by liquid nitrogen or radiation, for example. This approach suffers from problems in common with allograft including fracture, nonunion and infection.53,54 Although there is less experience published for Masquelet-induced membrane restoration of intercalary defects for tumours, the method can be successful for defects over 20 cm.55 That approach simplifies the primary resection with provisional stabilization but mandates a second substantial procedure.
Fig. 3.
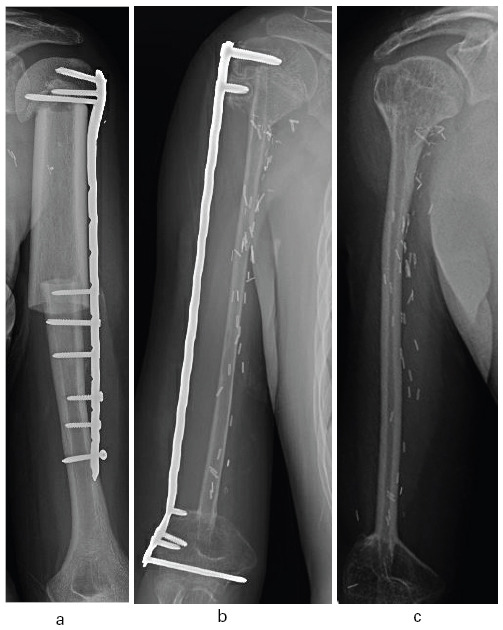
a) Proximal humeral metadiaphyseal intercalary allograft; b) pan-humeral diaphyseal intercalary reconstruction with vascular fibular autograft; c) early hypertrophy following plate removal.
Vascularized fibular diaphyseal free flaps can be used in an isolated manner or as part of a Capanna-style allograft-fibula composite reconstruction.56-59 The isolated fibula is at risk of early, usually minimally displaced, fracture but the junctions tend to heal relatively quickly in about three months, whereas the allograft portion is susceptible to delayed union (13 months average), resorption, fracture and infection. A healed, isolated fibular graft likely has a low long-term complication rate (Figs 3b and 3c).
Distal humerus
The distal humerus is an uncommon site for primary bone tumours. Resection of the distal humeral articular surface becomes necessary in the unusual circumstance in which there is tumour or unresolving edema in the trochlear or capitellar epiphysis (Fig. 4a).
Fig. 4.
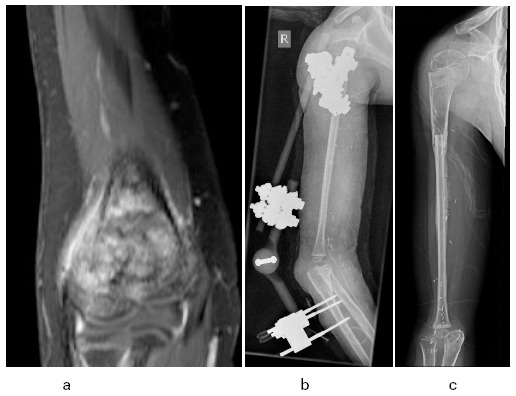
a) Distal humeral osteosarcoma with epiphyseal signal changes that did not resolve following neoadjuvant chemotherapy; b) vascular fibular epiphyseal-diaphyseal autograft articulating with the proximal forearm bones. Initial stabilization was with a hinged external fixator; c) following removal of the fixator and early hypertrophy of the graft.
In adolescents approaching skeletal maturity, a reasonably straight-forward option is to reconstruct the elbow with an endoprosthesis. Unfortunately, for distal or total humeral implants, whether they are traditional, compressive or extendable, there is a high (~50%) complication and revision rate.52,60,61 A rarely employed, but potentially durable, alternative, especially for younger children, is proximal fibular epiphyseal-diaphyseal free flap such that the fibular head articulates with the proximal ulna and radius. This approach has been described for an adult patient62 and we applied it for a seven-year-old by transferring the proximal fibular head and shaft (on the anterior tibial vessels) to the distal humerus. We stabilized the bony junction and the elbow with a hinged external fixator for 2.5 months in order to promote sagittal hinge movement while repressing coronal instability (Figs 4b and 4c). That patient has maintained an active range of 20° to 110° 15 months since surgery and has moderate coronal instability that does not interfere markedly with daily function. We will observe the long-term outcome of elbow movement, distal humeral (fibular) growth (which may be excessive) and articular/pseudarthrosis health with interest. Vascular diaphyseal fibular or allograft pseudarthrosis or fusion of the elbow are other potential approaches for skeletal reconstruction.
In planning distal humeral resection, one commonly considers whether the ulnar nerve, among other neurovascular structures, needs to be resected en bloc with the tumour. Nerve grafts to bridge the defect or nerve transfers to bypass it succeed relatively reliably in children and are very worthwhile. Radial and median nerve deficits can also be managed in this way, although extrinsic muscle functions can be partially restored by tendon transfers. However, practically the only way to restore intrinsic muscles and sensation in the hand is by nerve grafts and/or transfers. For example, intrinsic motor function can be restored by transfer of a median nerve branch to the ulnar nerve at the elbow,63 and sensation can be restored by lateral antebrachial cutaneous to ulnar nerve transfer64 with recoveries taking from six months to well over one year. Ultimately, the elbow is a vehicle for placing the hand in space, so preserving or restoring hand function is more important than achieving a particular quality of elbow movement.
Closing perspective
A structural or functional deficit of one upper extremity can greatly affect quality of life. Bone tumours may grow at a similar rate regardless of age, but the relatively small volume of host tissue makes resection and reconstruction arguably more challenging in children. For sarcomas of the upper limb, the surgical team is challenged to balance sufficient resection margins while providing reliable and durable reconstruction. Given the spatial constraints of childrens’ anatomy, multi-centimetre margins are not possible circumferentially around a tumour. It is, therefore, most important to understand which tissue planes are reliable tumour barriers and how individual muscles are innervated and supplied by vessels to plan a rationale and safe resection. The next most important aspect of sarcoma surgery around the shoulder and humerus is preservation or restoration of hand function. Finally, optimizing shoulder and elbow function is the third broad goal.
Adult-style approaches to reconstruction may succeed in adolescents but they are generally not reliable for younger children. For all young patients, avoidance of inevitable revisions and longevity on the order of several decades is probably best achieved by not depending too heavily on endoprostheses. Many surgeons have pioneered biological approaches that are promising alternatives. Combining biological skeletal reconstruction with tendon transfers and nerve grafting or transfers in collaboration with plastic surgeons requires a tremendous investment of time and effort but may provide profound long-term benefits.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Compliance with ethical standards
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical statement
Ethical approval: This topical review and opinion manuscript does not contain any studies with human participants or animals performed by the author and does not require formal review by the Research Ethics Board of the author’s institution.
Informed consent: Informed consent was not required for this work.
ICMJE Conflict of interest statement
The author has no conflict of interest to declare
Author Contributions
SH wrote the manuscript.
References
- 1.Russo SA, Kozin SH, Zlotolow DA, Nicholson KF, Richards JG. Motion necessary to achieve mallet internal rotation positions in children with brachial plexus birth palsy. J Pediatr Orthop 2019;39:14-21. [DOI] [PubMed] [Google Scholar]
- 2.Guo Z, Wang Z, Wang Z, Zhang Y. Allograft of clavicle for reconstruction of bone defect after tumor resection. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2008;22:102-105. [PubMed] [Google Scholar]
- 3.Li J, Wang Z, Fu J,. et al. Surgical treatment of clavicular malignancies. J Shoulder Elbow Surg 2011;20:295-300. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor S, Tiwari A, Kapoor S. Primary tumours and tumorous lesions of clavicle. Int Orthop 2008;32:829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez Martin J, Pretell Mazzini J, Viña Fernandez R, Marti Ciruelos R, Curto de la Mano A. Ewing sarcoma of clavicle in children: report of 5 cases. J Pediatr Hematol Oncol 2009;31:820-824. [DOI] [PubMed] [Google Scholar]
- 6.Mayil Vahanan N, Mohanlal P, Bose JC, Gangadharan R, Karthisundar V. The functional and oncological results after scapulectomy for scapular tumours: 2-16-year results. Int Orthop 2007;31:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi K, Iwata S, Ogose A,. et al. Factors that influence functional outcome after total or subtotal scapulectomy: Japanese Musculoskeletal Oncology Group (JMOG) study. PLoS One 2014;9:e100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi K, Karita M, Yamamoto N,. et al. Functional outcomes after total scapulectomy for malignant bone or soft tissue tumors in the shoulder girdle. Int J Clin Oncol 2011;16:568-573. [DOI] [PubMed] [Google Scholar]
- 9.El Ghoneimy AM, Zaghloul MS, Zaky I,. et al. Reconstruction of the scapula in pediatric and adolescent patients after total scapulectomy. A report of 10 patients treated by extracorporeal irradiation and reimplantation of the scapula. J Pediatr Orthop 2018;38:e91-e96. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar CR, Mohammed R, Grimer RJ. Extracorporeally irradiated scapula as autograft in tumor surgery. J Shoulder Elbow Surg 2009;18:e28-e32. [DOI] [PubMed] [Google Scholar]
- 11.Wittig JC, Bickels J, Wodajo F, Kellar-Graney KL, Malawer MM. Constrained total scapula reconstruction after resection of a high-grade sarcoma. Clin Orthop Relat Res 2002;397:143-155. [DOI] [PubMed] [Google Scholar]
- 12.Puchner SE, Panotopoulos J, Puchner R,. et al. Primary malignant tumours of the scapula—a review of 29 cases. Int Orthop 2014;38:2155-2162. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Wu Q, Zhang Z, Liu J, Shao Z. Reconstruction with constrained scapular prosthesis after total scapulectomy for scapular malignant tumor. J Surg Oncol 2018;118:177-183. [DOI] [PubMed] [Google Scholar]
- 14.Savvidou OD, Zampeli F, Georgopoulos G,. et al. Total scapulectomy and shoulder reconstruction using a scapular prosthesis and constrained reverse shoulder arthroplasty. Orthopedics 2018;41:e888-e893. [DOI] [PubMed] [Google Scholar]
- 15.Linberg BE. Interscapulo-thoracic resection for malignant tumors of the shoulder joint region. 1928. Clin Orthop Relat Res 1999;358:3-7. [PubMed] [Google Scholar]
- 16.Gosheger G, Hardes J, Ahrens H, Gebert C, Winkelmann W. Endoprosthetic replacement of the humerus combined with trapezius and latissimus dorsi transfer: a report of three patients. Arch Orthop Trauma Surg 2005;125:62-65. [DOI] [PubMed] [Google Scholar]
- 17.Gupta GR, Yasko AW, Lewis VO,. et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009;115:3767-3773. [DOI] [PubMed] [Google Scholar]
- 18.Cladière-Nassif V, Bourdet C, Audard V, Babinet A, Anract P, Biau D. Is it safe to preserve the deltoid when resecting the proximal humerus for a primary malignant bone tumour? A comparative study. J Bone Joint Surg [Br] 2017;99-B:1244-1249. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor MI, Sim FH, Chao EY. Limb salvage for neoplasms of the shoulder girdle. Intermediate reconstructive and functional results. J Bone Joint Surg [Am] 1996;78-A:1872-1888. [DOI] [PubMed] [Google Scholar]
- 20.Probyn LJ, Wunder JS, Bell RS, Griffin AM, Davis AM. A comparison of outcome of osteoarticular allograft reconstruction and shoulder arthrodesis following resection of primary tumours of the proximal humerus. Sarcoma 1998;2:163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Shen J, Dickinson IC. Functional outcome of arthrodesis with a vascularized fibular graft and a rotational latissimus dorsi flap after proximal humerus sarcoma resection. Ann Surg Oncol 2011;18:1852-1859. [DOI] [PubMed] [Google Scholar]
- 22.Mimata Y, Nishida J, Sato K, Suzuki Y, Doita M. Glenohumeral arthrodesis for malignant tumor of the shoulder girdle. J Shoulder Elbow Surg 2015;24:174-178. [DOI] [PubMed] [Google Scholar]
- 23.Padiolleau G, Marchand JB, Odri GA, Hamel A, Gouin F. Scapulo-humeral arthrodesis using a pedicled scapular pillar graft following resection of the proximal humerus. Orthop Traumatol Surg Res 2014;100:177-181. [DOI] [PubMed] [Google Scholar]
- 24.Ogink PT, Teunissen FR, Massier JR,. et al. Allograft reconstruction of the humerus: complications and revision surgery. J Surg Oncol 2019;119:329-335. [DOI] [PubMed] [Google Scholar]
- 25.Squire G, Grundy TJ, Ferran NA, Harper WM, Ashford RU. Long-term survival of proximal humerus allografts for reconstruction following resection of malignant bone tumours. Acta Orthop Belg 2013;79:260-265. [PubMed] [Google Scholar]
- 26.Liu T, Zhang Q, Guo X,. et al. Treatment and outcome of malignant bone tumors of the proximal humerus: biological versus endoprosthetic reconstruction. BMC Musculoskelet Disord 2014;15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takenaka S, Araki N, Ueda T,. et al. Clinical outcomes of osteoarticular extracorporeal irradiated autograft for malignant bone tumor. Sarcoma 2020;2020:9672093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran M, Stalley PD. Reconstruction of the proximal humerus with a composite of extracorporeally irradiated bone and endoprosthesis following excision of high grade primary bone sarcomas. Arch Orthop Trauma Surg 2009;129:1339-1345. [DOI] [PubMed] [Google Scholar]
- 29.Lozano-Calderón SA, Chen N. Proximal humerus allograft prosthetic composites: technique, outcomes, and pearls and pitfalls. Curr Rev Musculoskelet Med 2015;8:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nota S, Teunis T, Kortlever J,. et al. Functional outcomes and complications after oncologic reconstruction of the proximal humerus. J Am Acad Orthop Surg 2018;26:403-409. [DOI] [PubMed] [Google Scholar]
- 31.Tang X, Guo W, Yang R, Tang S, Ji T. Synthetic mesh improves shoulder function after intraarticular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res 2015;473:1464-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassab M, Dumaine V, Babinet A,. et al. Les reconstructions après résection tumorale de l’extrémité supérieure de l’humérus. Rev Chir Orthop Repar Appar Mot 2005;91:15-23. [DOI] [PubMed] [Google Scholar]
- 33.De Wilde LF, Plasschaert FS, Audenaert EA, Verdonk RC. Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orthop Relat Res 2005;430:156-162. [DOI] [PubMed] [Google Scholar]
- 34.Houdek MT, Bukowski BR, Athey AG,. et al. Comparison of reconstructive techniques following oncologic intraarticular resection of proximal humerus. J Surg Oncol 2021;123:133-140. [DOI] [PubMed] [Google Scholar]
- 35.King JJ, Nystrom LM, Reimer NB,. et al. Allograft-prosthetic composite reverse total shoulder arthroplasty for reconstruction of proximal humerus tumor resections. J Shoulder Elbow Surg 2016;25:45-54. [DOI] [PubMed] [Google Scholar]
- 36.Maclean S, Malik SS, Evans S, Gregory J, Jeys L. Reverse shoulder endoprosthesis for pathologic lesions of the proximal humerus: a minimum 3-year follow-up. J Shoulder Elbow Surg 2017;26:1990-1994. [DOI] [PubMed] [Google Scholar]
- 37.Streitbuerger A, Henrichs M, Gosheger G,. et al. Improvement of the shoulder function after large segment resection of the proximal humerus with the use of an inverse tumour prosthesis. Int Orthop 2015;39:355-361. [DOI] [PubMed] [Google Scholar]
- 38.Tsuda Y, Fujiwara T, Stevenson JD, Parry MC, Tillman R, Abudu A. The long-term results of extendable endoprostheses of the humerus in children after the resection of a bone sarcoma. Bone Joint J 2020;102-B:64-71. [DOI] [PubMed] [Google Scholar]
- 39.Ejiri S, Tajino T, Kawakami R, Hakozaki M, Konno S. Long-term follow-up of free vascularized fibular head graft for reconstruction of the proximal humerus after wide resection for bone sarcoma. Fukushima J Med Sci 2015;61:58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erdmann D, Garcia RM, Blueschke G, Brigman BE, Levin LS. Vascularized fibula-based physis transfer for pediatric proximal humerus reconstruction. Plast Reconstr Surg 2013;132:281e-287e. [DOI] [PubMed] [Google Scholar]
- 41.Innocenti M, Ceruso M, Manfrini M, et al. Free vascularized growth-plate transfer after bone tumor resection in children. J Reconstr Microsurg 1998;14:137-143. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson JD, Doxey R, Abudu A, Parry M, Evans S, Peart F, Jeys L. Vascularized fibular epiphyseal transfer for proximal humeral reconstruction in children with a primary sarcoma of bone. J Bone Joint Surg [Br] 2018;100-B:535-541. [DOI] [PubMed] [Google Scholar]
- 43.Barbier D, De Billy B, Gicquel P, Bourelle S, Journeau P. Is the clavicula pro humero technique of value for reconstruction after resection of the proximal humerus in children? Clin Orthop Relat Res 2017;475:2550-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvert GT, Wright J, Agarwal J, Jones KB, Randall RL. Is claviculo pro humeri of value for limb salvage of pediatric proximal humerus sarcomas? Clin Orthop Relat Res 2015;473:877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkelmann WW. Clavicula pro humero—a new surgical method for malignant tumors of the proximal humerus. Z Orthop Ihre Grenzgeb 1992;130:197-201. [DOI] [PubMed] [Google Scholar]
- 46.Tsukushi S, Nishida Y, Takahashi M, Ishiguro N. Clavicula pro humero reconstruction after wide resection of the proximal humerus. Clin Orthop Relat Res 2006;447:132-137. [DOI] [PubMed] [Google Scholar]
- 47.Pazourek L, Tomáš T, Mahdal M,. et al. Use of solid intercalary allografts for reconstruction following the resection of primary bone tumors. Acta Chir Orthop Traumatol Cech 2018;85:171-178. [PubMed] [Google Scholar]
- 48.Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. Intramedullary, antibiotic-loaded cemented, massive allografts for skeletal reconstruction. 26 cases compared with 19 uncemented allografts. Acta Orthop Scand 1997;68:387-391. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S, Kafchinski LA, Gundle KR,. et al. Intercalary allograft augmented with intramedullary cement and plate fixation is a reliable solution after resection of a diaphyseal tumour. J Bone Joint Surg [Br] 2017;99-B:973-978. [DOI] [PubMed] [Google Scholar]
- 50.McGrath A, Sewell MD, Hanna SA,. et al. Custom endoprosthetic reconstruction for malignant bone disease in the humeral diaphysis. Acta Orthop Belg 2011;77:171-179. [PubMed] [Google Scholar]
- 51.Aldlyami E, Abudu A, Grimer RJ, Carter SR, Tillman RM. Endoprosthetic replacement of diaphyseal bone defects. Long-term results. Int Orthop 2005;29:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goulding KA, Schwartz A, Hattrup SJ,. et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clin Orthop Relat Res 2017;475:1702-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Yang Y, Huang Z,. et al. Bone defect reconstruction with autologous bone inactivated with liquid nitrogen after resection of primary limb malignant tumors: an observational study. Medicine (Baltimore) 2020;99:e20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puri A, Gulia A, Agarwal M, Jambhekar N, Laskar S. Extracorporeal irradiated tumor bone: A reconstruction option in diaphyseal Ewing’s sarcomas. Indian J Orthop 2010;44:390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chotel F, Nguiabanda L, Braillon P,. et al. Induced membrane technique for reconstruction after bone tumor resection in children: a preliminary study. Orthop Traumatol Surg Res 2012;98:301-308. [DOI] [PubMed] [Google Scholar]
- 56.Petersen MM, Hovgaard D, Elberg JJ,. et al. Vascularized fibula grafts for reconstruction of bone defects after resection of bone sarcomas. Sarcoma 2010;2010:524721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Germain MA, Mascard E, Dubousset J, Nguefack M. Free vascularized fibula and reconstruction of long bones in the child—our evolution. Microsurgery 2007;27:415-419. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Chen G, Lu Y,. et al. Factors influencing osseous union following surgical treatment of bone tumors with use of the Capanna technique. J Bone Joint Surg [Am] 2019;101-A:2036-2043. [DOI] [PubMed] [Google Scholar]
- 59.Sainsbury DCG, Liu EH, Alvarez-Veronesi MC,. et al. Long-term outcomes following lower extremity sarcoma resection and reconstruction with vascularized fibula flaps in children. Plast Reconstr Surg 2014;134:808-820. [DOI] [PubMed] [Google Scholar]
- 60.Benevenia J, Patterson F, Beebe K,. et al. Results of 20 consecutive patients treated with the Repiphysis expandable prosthesis for primary malignant bone. Springerplus 2015;4:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wafa H, Reddy K, Grimer R,. et al. Does total humeral endoprosthetic replacement provide reliable reconstruction with preservation of a useful extremity? Clin Orthop Relat Res 2015;473:917-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamal AF, Putra A, Widodo W. Vascularized fibular graft as a surgical option for osteosarcoma of distal humerus: a case report. Int J Surg Case Rep 2017;39:280-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bertelli JA, Soldado F, Rodrígues-Baeza A, Ghizoni MF. Transferring the motor branch of the opponens pollicis to the terminal division of the deep branch of the ulnar nerve for pinch reconstruction. J Hand Surg Am 2019;44:9-17. [DOI] [PubMed] [Google Scholar]
- 64.Ruchelsman DE, Price AE, Valencia H, Ramos LE, Grossman JA. Sensory restoration by lateral antebrachial cutaneous to ulnar nerve transfer in children with global brachial plexus injuries. Hand (N Y) 2010;5:370-373. [DOI] [PMC free article] [PubMed] [Google Scholar]


