Abstract
Background:
Progenitor cells serve as a promising source of regenerative potential in a variety of tissue types yet remain underutilized in tendinopathy. Tendon-derived progenitor cells (TDPCs) have previously been isolated from hamstring tendon but only as part of a concomitant medical procedure. Determining the presence of TDPCs in patellar tendon may facilitate clinical utilization of these cells because of the relative accessibility of this location for tissue harvest.
Purpose:
To characterize TDPCs in human patellar tendon samples.
Study Design:
Descriptive laboratory study.
Methods:
Human patellar tendon samples were obtained during elective knee surgery. TDPCs were isolated and seeded at an optimal low cell density and subcultured to confluence for up to 2 passages. Flow cytometry was used to analyze for the expression of CD90+, CD105+, CD44+, and CD31–, CD34–, and CD45– markers. The multilineage differentiation potential of TDPCs was tested in vitro via adipogenic, osteogenic, and chondrogenic culture with subsequent cytochemical staining for Oil Red O, Alizarin Red, and Alcian Blue, respectively. Enzyme-linked immunosorbent assay was used to quantify the amount of adiponectin, alkaline phosphatase, and SRY-box transcription factor 9 secreted into cell culture supernatant for further confirmation of lineage differentiation. Results were analyzed statistically using the 2-tailed Student t test.
Results:
TDPCs demonstrated near-uniform expression of CD90, CD105, and CD44 with minimal expression of CD34, CD31, and CD45. Adipogenic, osteogenic, and chondrogenic differentiation of TDPCs was confirmed using qualitative analysis. The expression of adiponectin, alkaline phosphatase, and SRY-box transcription factor 9 were significantly increased in differentiated cells versus undifferentiated TDPCs (P < .05).
Conclusion:
TDPCs can be successfully isolated from human patellar tendon samples, and they exhibit characteristics of multipotent progenitor cells.
Clinical Relevance:
These data demonstrate the promise of patellar tendon tissue as a source of progenitor cells for use in biologic therapies for the treatment of tendinopathy.
Keywords: stem cell, progenitor cell, tendinopathy, tendon, patellar tendon, regeneration
Introduction
Tendinopathy is a degenerative tendon disorder characterized by chronic pain, decreased strength, localized tenderness, and reduced function.56 Approximately 30% of all general practitioner musculoskeletal visits are attributed to symptoms of tendinopathy, costing an estimated US$30 billion in annual health care utilization in the United States alone.2,37 Traditional treatments for tendinopathy include activity modification, physical therapy consisting mostly of eccentric exercises, injections, and extracorporeal shockwave therapy.8,21,26,54,61 However, these modalities often do not resolve symptoms, as many patients are unable to return to their prior levels of physical activity and work.39
Although biologic treatments have shown promise in other musculoskeletal conditions, biologic treatments for tendinopathy have not advanced to the same degree.20 Human progenitor cells hold promise for their ability to regenerate many types of tissue and ameliorate disease burden. Bone marrow–derived and adipose-derived mesenchymal progenitor/stromal cells (BMSCs and ADSCs) have been the most studied in musculoskeletal regenerative medicine because of their relative ease of isolation and multilineage potential.3,4 Cells derived from these sources have been utilized to treat chondral defects, fractures, and osteonecrosis, among others.14,15,18,19,24,27,50
In order to regenerate tendon, however, it would seem most advantageous to harvest progenitor cells from a tendon source. This would take advantage of the fact that progenitor cells from tendons are more phenotypically committed or “primed” for tenogenesis than are cells from other tissues.13 Bi et al1 demonstrated this when they first isolated tendon-derived progenitor cells (TDPCs) and reported that progenitor cells derived from tendon were phenotypically distinct from bone marrow–derived progenitor cells. Additional investigations have shown TDPCs to have the ability to regenerate tendon after in vitro expansion and subsequent implantation.1,25,36,42,45
The majority of literature to date describing the isolation of TDPCs from human samples has utilized hamstring tendon as the source tissue.1,43,46 Hamstring tendon samples, however, are typically only available as part of a concomitant medical procedure, such as anterior cruciate ligament (ACL) reconstruction. The patellar tendon, in contrast, is nearly subcutaneous, could be easily accessed in both the operative and nonoperative settings, and has been described as part of autologous tenocyte injection treatment for lateral epicondylitis.51,52
There are few reports of isolation of TDPC from human patellar tendon.22,38 If sufficient TDPCs exist within patellar tendon, these cells could be easily harvested and provide a biological regenerative advantage in the treatment of tendinopathy. The purpose of this investigation was to determine the presence of TDPCs in human patellar tendon samples. The authors hypothesized that TDPCs could be consistently isolated from human patellar tendon samples.
Methods
Institutional review board approval was obtained before study initiation. Samples of patellar tendons were obtained during elective knee surgery from 2 healthy human donors undergoing ACL reconstruction (1 man: age, 32 years; 1 woman: age, 26 years). To assess for the presence of TDPCs, we utilized the definition of multipotent mesenchymal stromal cells as put forth by the International Society of Cellular Therapy (ISCT): adherent to plastic when maintained in standard culture conditions, trilineage (osteoblasts, adipocytes, and chondroblasts) differentiation in vitro, and having the specified cell surface marker expression profiles.12
Cell Isolation and Expansion
Tissue was minced and washed twice in 50 mL of phosphate-buffered saline (PBS; Gibco) and 1% penicillin-streptomycin (Gibco) under sterile conditions and centrifuged at 400g at 22°C for 5 minutes. The supernatant was collected, and the tissue fragments were digested in 3 mg/mL of collagenase type I from Clostridium histolyticum and 4 mg/mL of dispase II from Bacillus polymyxa (Sigma) in Dulbecco’s Modified Eagle Medium (DMEM; Sigma-Aldrich) for 2 hours at 37°C, with gentle shaking every 30 minutes. The resulting cell suspension was diluted in PBS, filtered via a 75-µM strainer to remove debris, and centrifuged at 400g at 22°C for 10 minutes to obtain a cell pellet. The supernatant was aspirated without disturbing the pellet. The cell count and viability were performed using a hemocytometer and trypan blue. The isolated viable nucleated cells were seeded at an optimal low cell density (500 cells/cm2) and resuspended in DMEM/Nutrient Mixture F-12 (DMEM/F-12; Gibco) supplemented with 20% fetal bovine serum (Gibco) and 1% penicillin-streptomycin. They were then cultured at 37°C and 5% CO2 to form colonies for the isolation of TDPCs. Subcultured cells were expanded at 500 cells/cm2 when they reached 90% confluence up to 2 passages and then subjected to the in vitro characterization following the previously described guidelines for the definition of mesenchymal stromal cells.12
Expression of Progenitor Cell Surface Markers
Flow cytometry was performed on cells obtained from the digestion after the second passage. An amount of 1 × 106 of cells were harvested using trypsin-EDTA (Gibco), filtered via a 70-µm strainer, and washed in PBS. The cells were suspended in 100 µL of PBS, stained with Zombie Aqua Fixable Viability (BioLegend), and incubated for 20 to 30 minutes in the dark. After the incubation, the cells were then resuspended in flow wash/stain buffer (Biosciences) and centrifuged. Pellets were collected in PBS (Invitrogen) and stained first with Human Fc Block (Biosciences) for 10 minutes and then using selected antibodies for 30 minutes in the dark. To define mesenchymal stem cells (MSC), we sorted for CD90+, CD105+, CD44+, and CD31–, CD34–, and CD45– markers. Cells were compared with unstained cells as a negative control. Samples were acquired via flow cytometric analysis (BD LSRFortessa), and data were further analyzed using FlowJo software (Version 10.6.2; Tree Star Inc).
In Vitro Multilineage Differentiation
The multilineage potential of the TDPCs was tested in vitro via osteogenic, adipogenic, and chondrogenic culture. To evaluate for trilineage differentiation, the treated cells, after a specific time period, were stained. The supernatants and the micropellets were collected before cytochemical staining in order to perform a separate enzyme-linked immunosorbent assay (ELISA) analysis. Samples were also stained with hematoxylin and eosin (H&E) to evaluate the morphology of the cells. Each sample was compared with unstimulated cells as a negative control. After the second passage, cells were harvested using trypsin-EDTA and seeded at 3000 cells/cm2 in a different 6-well plate, and then they were cultured in adipogenic and osteogenic media for up to 3 weeks. For chondrogenesis, cells were collected after the second passage, and micropellets of 1 × 106 cells were created in 15-mL conicals with centrifugation for 5 minutes at 400g. The supernatant was aspirated, and the pellet was partially suspended in 100 µL of culture media. From this suspension, we removed droplets of 6 µL each and cultured them in 40 µL of chondrogenic induction media up to 3 weeks to preserve the micropellet for the cytochemical assay. In parallel, micropellets with a cell density of approximately 1 × 106 viable cells per pellet were placed in 15-mL conicals using the same technique. The supernatant was aspirated, but the pellet was cultured in 4 mL of induction media in the 15-mL conicals for 21 days. After 21 days, the micropellets from the 15-mL conicals were collected, and the cells were lysed for the quantitative analysis.
Adipogenesis
To determine adipogenesis, TDPCs were collected and cultured using the StemPro Adipogenesis Differentiation Kit (Gibco) for up to 2 weeks and stained with Oil Red O to visualize the accumulation of lipid vacuoli after embedding with 4% paraformaldehyde. To quantify adiponectin secreted in the culture medium of adipo-induced cells, we used a standard adiponectin ELISA (Abcam). This assay uses an antibody specific for human adiponectin coated on a 96-well plate. Standards and samples are pipetted into the wells, and adiponectin present in a sample is bound to the wells via the immobilized antibody. The color was developed in proportion to the amount of adiponectin bound and measured at 450 nm using a spectrophotometer (SpectraMax M5; Molecular Devices).
Osteogenesis
To diagnose osteogenesis, cells were cultured using the StemPro Osteogenesis Differentiation Kit (Gibco), and on day 21, osteogenic differentiation of TDPCs was visualized using the intensity of Alizarin Red staining on micros-copy. In addition, the alkaline phosphatase (ALP) activity was quantified using ELISA (Alkaline Phosphatase Assay Kit; Colorimetric Inc) in the cell culture supernatants. This kit uses p-nitrophenyl phosphate as a phosphatase substrate that turns color when dephosphorylated via ALP. The optical density was measured at 405 nm using a spectrophotometer (SpectraMax M5).
Chondrogenesis
To diagnose chondrogenesis, micropellets of TDPCs in 6-well plates were created and then cultured using a chondrogenesis differentiation kit (StemPro). For analysis, the pellets in the 6-well plates were embedded in 4% paraformaldehyde and then stained with Alcian Blue to detect the presence of acidic polysaccharides such as glycosaminoglycans in cartilage cells. For the quantitative assay, the micro cell pellets were lysed, and the presence of SRY-box transcription factor 9 (SOX9) was detected using ELISA. The ELISA uses an affinity tag–labeled capture antibody and a reporter-conjugated detector antibody that immunocaptures the sample analyte in solution. Signal was generated proportionally to the amount of bound analyte, and the intensity was measured at 450 nm using a spectrophotometer (SpectraMax M5).
Data Analysis
Sample data output generated from flow cytometry was analyzed using FlowJo software. Gating was applied to exclude debris, multicellular aggregates, and dead cells by selecting the central live population of single cells with highly similar morphologic and cytometeric characteristics. Isotype control readings were used against fluorescence readings to determine fluorescence shift. Compensation was conducted using FlowJo software using single-stain analysis readings of monoclonal antibodies. Characterization of TDPCs is based on the expression of MSC-associated surface markers (CD90, CD105, CD44) and non-MSC surface markers (CD31, CD34, CD45). Positive and negative percentages from different samples were combined to obtain a mean ± standard deviation for positive and negative markers. All experiments were performed in duplicate, and TDPC differentiation was performed using cells from each donor.
Statistical Analysis
To evaluate the differences in the presence of adiponectin, ALP, and SOX9 in comparison with the negative control, a 2-tailed Student t test was performed. Data were analyzed using R Foundation for Statistical Computing (version 4.0.0; R Development Core Team). P values <.05 were considered to be statistically significant. All experiments were performed in duplicate. These differentiations were performed using cells from different donors.
Results
Cells Isolation and Expansion
Subcultured TDPCs were cultured onto tissue-treated culture flasks for several passages and adhered to the plastic flask when maintained in standard culture conditions. These cells demonstrated a fibroblastic-like appearance and exhibited distinct colony formation. The TDPCs showed constant proliferation during the expansion and continued to be adherent with a spindle-like cell morphology appearance typical of MSCs (Figure 1), satisfying the first criterion described from the ISCT.12
Figure 1.
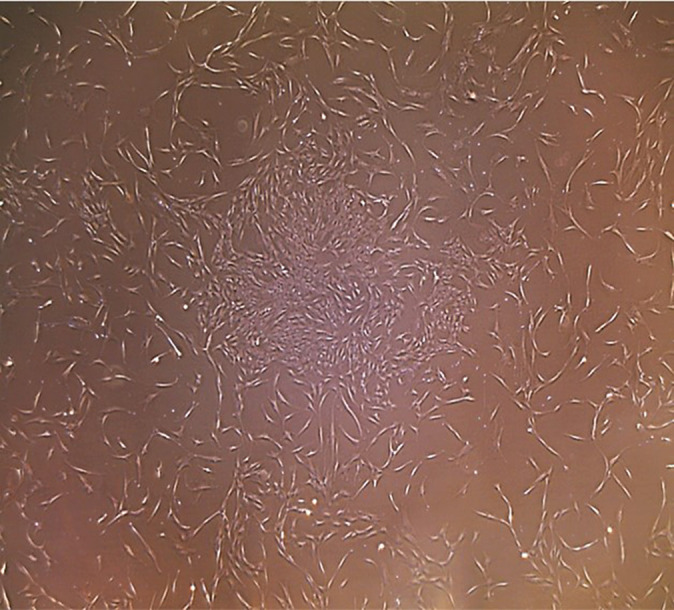
Light microscopy (4×) image demonstrating proliferation and colony formation of spindle-like tendon-derived stem cells.
Expression of MSC Surface Markers
The other minimum criterion for the identification of MSCs is the presence of MSC-associated surface markers, such as CD90, CD105, and CD44, and simultaneous low expression or the absence of non–progenitor cell–associated surface markers, such as CD34, CD31, and CD45. TDPCs demonstrated near-uniform expression of CD90, CD105, and CD44, with minimal expression of CD34, CD31, and CD45 (Figure 2). These results demonstrated that the TDPCs satisfied the surface antigen expression criteria of the ISCT.12
Figure 2.
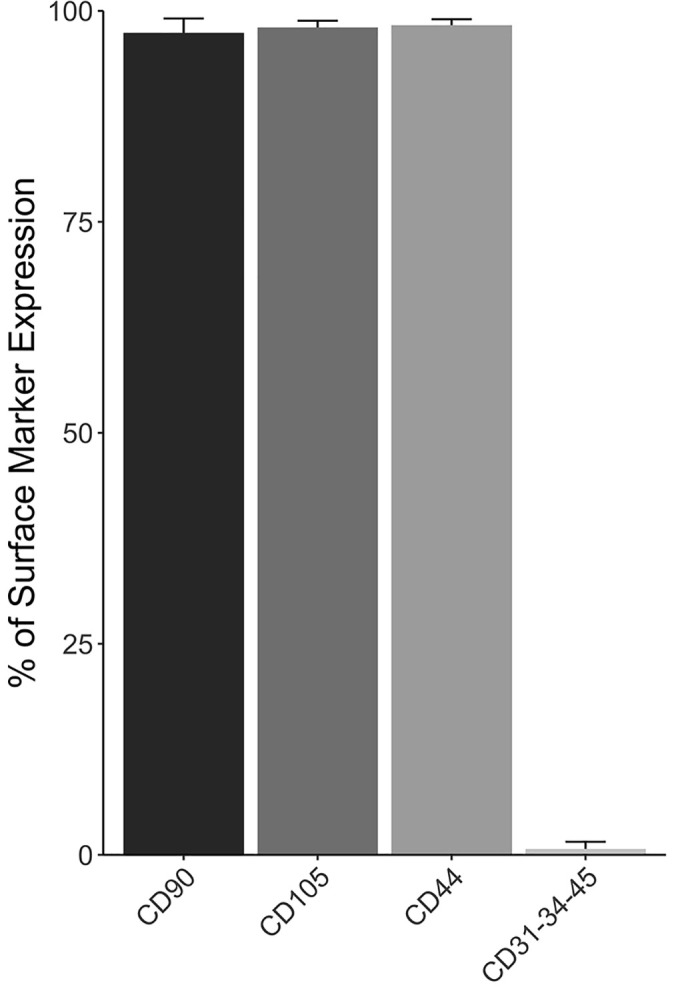
Percentage of tendon-derived progenitor cell surface immunophenotype at second passage. Data are presented as mean ± SD.
In Vitro Multilineage Differentiation Assay
To determine whether our isolated TDPCs were capable of adipogenic, osteogenic, and chondrogenic differentiation, cytochemical staining and ELISA were performed.
Adipogenesis
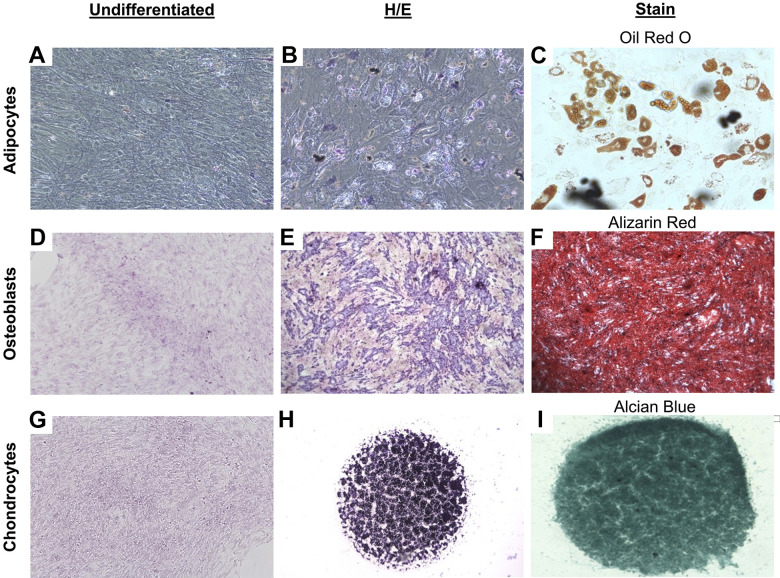
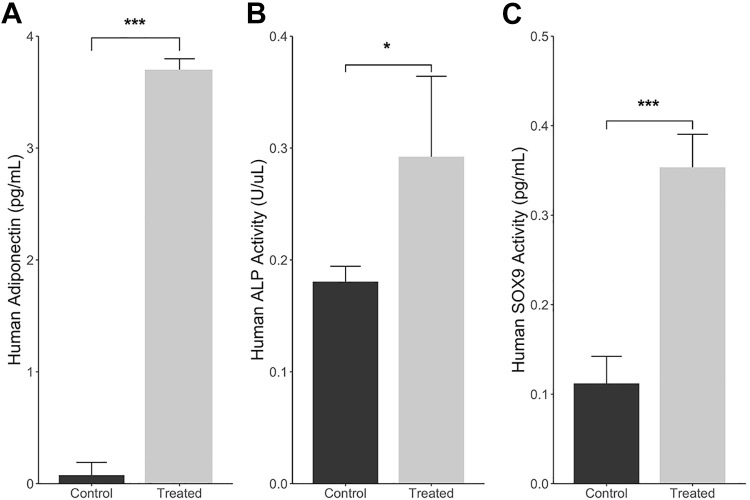
Cells demonstrated many intracellular lipid vacuoles typical of adipocytes after staining with Oil Red O (Figure 3C). Furthermore, H&E staining demonstrated that TDPCs changed in morphology from spindle-shaped to larger, more rounded cells (Figure 3, A and B). Analysis using ELISA revealed significantly increased expression of adiponectin as compared with the untreated TDPCs (P < .001) (Figure 4A).
Figure 3.
Multilineage differentiation potential of tendon-derived progenitor cells at second passage. (A) Negative control for adipogenesis (10×); cell differentiation into adipocytes stained with (B) hematoxylin and eosin (H&E) and (C) Oil Red O (20×). (D) Negative control for osteogenesis (10×); cell differentiation into osteoblasts stained with (E) H&E and (F) Alizarin Red (2×). (G) Negative control for chondrogenesis (2×); cell differentiation into chondrocytes stained with (H) H&E and (I) Alcian Blue (2×).
Figure 4.
Enzyme-linked immunosorbent assay analysis of multilineage differentiation potential of tendon-derived progenitor cells. (A) Adiponectin concentration in cellular supernatant after induction of adipogenesis. (B) Alkaline phosphatase (ALP) concentration in cellular supernatant after induction of osteogenesis. (C) SRY-box transcription factor 9 (SOX9) concentration in cellular supernatant after induction of chondrogenesis. Data are presented as mean ± SD. *P < .05; *** P < .01.
Osteogenesis
Alizarin Red staining demonstrated the abundant formation of calcium on the differentiated TDPCs, which was not observed in the undifferentiated cells (Figure 3, D and F). The H&E staining also demonstrated a rounded cell morphology, different from that of undifferentiated TDPCs (Figure 3E). The quantitative analysis showed significantly higher ALP expression in the induced cells compared with the noninduced control group (P = .035) (Figure 4B).
Chondrogenesis
Culture in chondrogenic medium resulted in the production of glycoproteins of the cartilage matrix as seen via Alcian Blue staining (Figure 3, G-I). The expression of SOX9 was significantly increased in the cells that underwent chondrogenic culture differentiation versus undifferentiated TDPCs (P < .001) (Figure 4C).
Discussion
The present investigation demonstrated successful isolation of TDPCs from human patellar tendon. These data demonstrate the promise of patellar tendon tissue as a source of TDPCs for use in biologic therapies for the treatment of tendinopathy. Prior investigations have reported the presence of TDPC in human tendon samples, but TDPCs have been predominantly isolated from hamstring tendons. Bi et al,1 who were the first to report on the isolation of TDPCs, utilized pediatric hamstring samples from children undergoing hamstring tenotomy for knee flexion contracture. Ruzzini et al43 and Stanco et al46 were also able to isolate tendon-derived progenitor cells, but they utilized hamstring tendon samples from patients undergoing ACL reconstruction. The use of patellar tendon, which is more superficial in nature, provides a more accessible source of TDPC for the typical patient with tendinopathy who does not require ACL reconstruction. In fact, needle biopsy of the patellar tendon for other indications has been described in multiple publications.35,51,52 Even for those patients with signs and symptoms of patellar tendinopathy, tendon samples could be acquired from the contralateral limb or distally in the patellar tendon away from the typical location of proximal patellar tendinopathy.
In comparison to isolation from hamstring tendons, TDPCs derived from patellar tendon samples have been characterized by fewer studies.22,38 In 1 investigation, Lee et al22 found that hypoxic conditions promoted proliferation of patellar tendon TDPCs but reduced the osteogenic, adipogenic, and chondrogenic differentiation. Further, Qin et al38 utilized human patellar tendon TDPCs to demonstrate that TDPCs also have fibrochondrogenic differentiation potential. Both studies demonstrated that TDPCs had all characteristics of multipotent mesenchymal stromal cells. As such, combined with previous findings, the present investigation further demonstrates that TDPCs can be consistently isolated from the patellar tendon.
Currently, BMSCs and ADSCs provide most progenitor cells for clinical use for a variety of indications,29,47,48 but these cells may not be optimal for the treatment of tendinopathy because they are not “primed” for tendon regeneration and have been shown to be phenotypically distinct from bone marrow–derived progenitor cells.1 TDPCs may be superior because they are isolated from the native tendon source and express higher levels of tenogenic markers, such as tenomodulin and scleraxis (Scx), key regulators of tenocyte differentiation.1,11,23,48 Tan et al48 demonstrated that TDPCs expressed higher levels of tenomodulin, Scx, and type I collagen compared with BMSCs. Additionally, the authors showed that TDPCs proliferate faster and have higher clonogenicity potential than do BMSCs. Further, Youngstrom and colleagues57 evaluated the tenogenesis of TDPCs, BMSCs, and ADSCs in a dynamic bioreactor and found that of those cell types, TDPCs expressed the highest levels of Scx and type I collagen transcripts and resulted in the most mature tendon-like phenotype in vitro.
TDPCs have a multifactorial role in tendon repair. Studies have shown that TDPCs can spontaneously differentiate into tenocytes in vitro without induction medium.49 Platelet-rich plasma supplementation in vitro can also promote TDPC differentiation into tenocytes and enhance in vivo tendon healing in animal models.55,60 Further, mechanical loading also has an effect on TDPC outcome, as Zhang and Wang59 determined that low mechanical stretching promoted tenogenic differentiation of TDPCs, whereas higher loading induced adipogenic, chondrogenic, and osteogenic changes. It has also been shown that prostaglandin E2 decreases TDPC proliferation and induces osteogenic differentiation, leading to increased production of BMP-2. This mechanism may play a role in the pathogenesis of calcific tendinopathy.58 Additionally, another study has reported that despite a higher number of total resident TDPCs in tendinopathic tissue, these TDPCs are unable to differentiate into tenocytes to regulate the extracellular matrix (ECM).5
The difficulty in treating tendinopathy lies in the multifactorial pathogenesis of the disease. The utilization of tendon derived stem cells (TDSC) as a biologic agent may address many of these aberrant pathways. One of the main pathological mechanisms in the development of tendinopathy involves repetitive tendon mechanical overload leading to microscopic collagen fibril failure.41 Given the poor intrinsic healing capacity of tendon, ECM damage may accumulate over time, leading to progressive structural injury and symptomatic disease.28 Healthy tendons are predominantly composed of type I collagen, which gives them their mechanical strength. In tendinopathy, there is increased production of type III collagen, increased vascularity, and deposition of additional ECM proteins, leading to loss of collagen organization.9,32 In addition to mechanical overload, other mechanisms that may propagate the development of tendinopathy include matrix metalloproteinase and tissue inhibitors of metalloproteinase imbalance,40 hypoxia,33 dysregulated apoptosis,17 and neuronal proliferation.10 Given their role as a central regulatory cell within the tendon environment,25 the utilization of TDSCs may mitigate many of these pathological processes. For instance, the use of TDSCs as a biologic therapy may allow for the increased production of type I collagen over type III collagen, addressing a major difference in the composition of the ECM in tendinopathy versus healthy tendon.
Despite these varied mechanisms, it has become increasingly evident that a central component of tendinopathy is a chronic inflammatory response. Multiple studies have shown that inflammation is present in early tendinopathy,16,30–32 and current evidence supports the complex interplay between infiltrating immune cells, activation of resident stromal cells, and the innate immune system in response to tissue damage.32 Additionally, recent reviews have demonstrated that the immune response is a major player in both tendon healing and dysregulation.6,7 Tenocytes are the most abundant cell type in tendon, and in the setting of mechanical overload, these cells can produce altered ECMs and inflammatory cytokines, such as interleukin (IL)-1β,44 IL-6,34 and IL-17A,30 that continue to promote the inflammatory conditions in tendinopathy. In addition to tenocyte differentiation, TDPCs can play a role in modulating inflammation in tendinopathy. TDPCs have been shown to secrete anti-inflammatory cytokines, such as IL-10, when delivered into animal models of tendon injury.45,49 Further, recent studies have demonstrated that exosomes derived from TDPCs may also play a role in controlling inflammation in tendinopathy and regulating the tendon ECM.53,62 Zhang et al62 found that animal models of Achilles tendon injury treated using TDPC exosomes demonstrated decreased expression of IL-6 and CCR7, a marker for M1 pro-inflammatory macrophages, and increased expression of IL-10. Additionally, TDPCs combined with their exosomes upregulated tissue inhibitors of metalloproteinase-1 and type I collagen.
There were some limitations to the current study. As this was an in vitro study, the multilineage differentiation potential and clonogenicity exhibited by TDPCs in culture may not be representative of their capabilities in vivo. This study also included a relatively small sample size with tendon samples collected from 2 patients, which may limit the reliability and external validity of our results. Further, we could not determine if the amount and quality of TDPCs changed with increased age. Additionally, samples were only harvested from patients with healthy tendons, even though future TDPC treatment would target patients with tendinopathy. As such, TDPCs harvested from tendinopathic samples may have different characteristics. Finally, this investigation did not study interactions between other cell types and TDPCs. There could be synergistic interactions between TDPCs and tenocytes or MSCs derived from other sources.
Conclusion
Tendon-derived cells that exhibit characteristics of multipotent progenitor cells can be successfully isolated from human patellar tendon samples, offering an easily accessible source of cells for tendinopathy treatment and tendon regeneration.
Footnotes
Final revision submitted January 21, 2021; accepted February 24, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: G.D.A. has received royalties from Orthofix Medical and education payments from Evolution Surgical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Stanford University (eProtocol No. 56522).
References
- 1.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. [DOI] [PubMed] [Google Scholar]
- 2.Butler DL, Gooch C, Kinneberg KRC, et al. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nat Protoc. 2010;5(5):849–863. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198–1211. [DOI] [PubMed] [Google Scholar]
- 5.Chang W, Callan KT, Dragoo JL. The behavior of tendon progenitor cells from tendinopathic tendons: implications for treatment. Tissue Eng Part A. 2020;26(1-2):38–46. [DOI] [PubMed] [Google Scholar]
- 6.Chisari E, Rehak L, Khan WS, Maffulli N. The role of the immune system in tendon healing: a systematic review. Br Med Bull. 2020;133(1):49–64. [DOI] [PubMed] [Google Scholar]
- 7.Chisari E, Rehak L, Khan WS, Maffulli N. Tendon healing in presence of chronic low-level inflammation: a systematic review. Br Med Bull. 2019;132(1):97–116. [DOI] [PubMed] [Google Scholar]
- 8.Cook JL, Rio E, Purdam CR, Docking SI. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? Br J Sports Med. 2016;50(19):1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean BJF, Dakin SG, Millar NL, Carr AJ. Review: emerging concepts in the pathogenesis of tendinopathy. Surgeon. 2017;15(6):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean BJF, Franklin SL, Carr AJ. The peripheral neuronal phenotype is important in the pathogenesis of painful human tendinopathy: a systematic review. Clin Orthop Relat Res. 2013;471(9):3036–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 13.Durgam SS, Stewart MC. Tendon-derived progenitor cells: in vitro characterization and clinical applications for tendon repair. J Stem Cell Res Med. 2016;1(1):8–17. [Google Scholar]
- 14.Elgaz S, Bonig H, Bader P. Mesenchymal stromal cells for osteonecrosis. J Transl Med. 2020;18(1):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648–657. [DOI] [PubMed] [Google Scholar]
- 16.Hall KE, Sarkissian EJ, Sharpe O, Robinson WH, Abrams GD. Identification of differentially expressed micro-RNA in rotator cuff tendinopathy. Muscles Ligaments Tendons J. 2018;8(1):8–14. [Google Scholar]
- 17.Jelinsky SA, Rodeo SA, Li J, Gulotta LV, Archambault JM, Seeherman HJ. Regulation of gene expression in human tendinopathy. BMC Musculoskelet Disord. 2011;12(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabir W, Di Bella C, Jo I, Gould D, Choong PFM. Human stem cell based tissue engineering for in vivo cartilage repair: a systematic review. Tissue Eng Part B Rev. 2021;27(1):74–93. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakidis T, Iosifidis M, Michalopoulos E, Melas I, Stavropoulos-Giokas C, Verdonk R. Good mid-term outcomes after adipose-derived culture-expanded mesenchymal stem cells implantation in knee focal cartilage defects. Knee Surg Sports Traumatol Arthrosc. 2020;28(2):502–508. [DOI] [PubMed] [Google Scholar]
- 20.Lamplot JD, Rodeo SA, Brophy RH. A practical guide for the current use of biologic therapies in sports medicine. Am J Sports Med. 2020;48(2):488–503. [DOI] [PubMed] [Google Scholar]
- 21.Larsson MEH, Käll I, Nilsson-Helander K. Treatment of patellar tendinopathy—a systematic review of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1632–1646. [DOI] [PubMed] [Google Scholar]
- 22.Lee WY, Lui PP, Rui YF. Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng Part A. 2012;18(5-6):484–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lejard V, Brideau G, Blais F, et al. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282(24):17665–17675. [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Sohn J, Shen H, Langhans MT, Tuan RS. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lui PP, Chan KM. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev Rep. 2011;7(4):883–897. [DOI] [PubMed] [Google Scholar]
- 26.Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. J Bone Joint Surg Am. 2010;92(15):2604–2613. [DOI] [PubMed] [Google Scholar]
- 27.Maheshwer B, Polce EM, Paul K, et al. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: a systematic review and meta-analysis. Arthroscopy. 2021;37(1):362–378. [DOI] [PubMed] [Google Scholar]
- 28.Mead MP, Gumucio JP, Awan TM, Mendias CL, Sugg KB. Pathogenesis and management of tendinopathies in sports medicine. Trans Sports Med. 2018;1(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migliorini F, Tingart M, Maffulli N. Progress with stem cell therapies for tendon tissue regeneration. Expert Opin Biol Ther. 2020;20(11):1373–1379. [DOI] [PubMed] [Google Scholar]
- 30.Millar NL, Akbar M, Campbell AL, et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci Rep. 2016;6(1):27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millar NL, Hueber AJ, Reilly JH, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38(10):2085–2091. [DOI] [PubMed] [Google Scholar]
- 32.Millar NL, Murrell GAC, McInnes IB. Inflammatory mechanisms in tendinopathy—towards translation. Nat Rev Rheumatol. 2017;13(2):110–122. [DOI] [PubMed] [Google Scholar]
- 33.Millar NL, Reilly JH, Kerr SC, et al. Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis. 2012;71(2):302–310. [DOI] [PubMed] [Google Scholar]
- 34.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91(3):417–424. [DOI] [PubMed] [Google Scholar]
- 35.Movin T. Tendon tissue sampling. Scand J Med Sci. 2000;10(6):368–371. [DOI] [PubMed] [Google Scholar]
- 36.Ni M, Lui PPY, Rui YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30(4):613–619. [DOI] [PubMed] [Google Scholar]
- 37.Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11(4):223–233. [DOI] [PubMed] [Google Scholar]
- 38.Qin S, Wang W, Liu Z, et al. Fibrochondrogenic differentiation potential of tendon-derived stem/progenitor cells from human patellar tendon. J Orthop Translat. 2020;22:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006;45(5):508–521. [DOI] [PubMed] [Google Scholar]
- 40.Riley G. Chronic tendon pathology: molecular basis and therapeutic implications. Expert Rev Mol Med. 2005;7(5):1–25. [DOI] [PubMed] [Google Scholar]
- 41.Riley G. The pathogenesis of tendinopathy: a molecular perspective. Rheumatology. 2004;43(2):131–142. [DOI] [PubMed] [Google Scholar]
- 42.Rui YF, Lui PPY, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16(5):1549–1558. [DOI] [PubMed] [Google Scholar]
- 43.Ruzzini L, Abbruzzese F, Rainer A, et al. Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg Sports Traumatol Arthrosc. 2014;22(11):2856–2866. [DOI] [PubMed] [Google Scholar]
- 44.September AV, Nell EM, O’Connell K, et al. A pathway-based approach investigating the genes encoding interleukin-1, interleukin-6 and the interleukin-1 receptor antagonist provides new insight into the genetic susceptibility of Achilles tendinopathy. Br J Sports Med. 2011;45(13):1040–1047. [DOI] [PubMed] [Google Scholar]
- 45.Shen W, Chen J, Yin Z, et al. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell Transplant. 2012;21(5):943–958. [DOI] [PubMed] [Google Scholar]
- 46.Stanco D, Vigano M, Perucca Orfei C, et al. Multidifferentiation potential of human mesenchymal stem cells from adipose tissue and hamstring tendons for musculoskeletal cell-based therapy. Regen Med. 2015;10(6):729–743. [DOI] [PubMed] [Google Scholar]
- 47.Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration. Current status and perspectives. Stem Cells Transl Med. 2012;1(3):237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan Q, Lui PPY, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18(7-8):840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarafder S, Chen E, Jun Y, et al. Tendon stem/progenitor cells regulate inflammation in tendon healing via JNK and STAT3 signaling. FASEB J. 2017;31(9):3991–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teo AQA, Wong KL, Shen L, et al. Equivalent 10-year outcomes after implantation of autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation for chondral defects of the knee. Am J Sports Med. 2019;47(12):2881–2887. [DOI] [PubMed] [Google Scholar]
- 51.Wang A, Breidahl W, Mackie KE, et al. Autologous tenocyte injection for the treatment of severe, chronic resistant lateral epicondylitis: a pilot study. Am J Sports Med. 2013;41(12):2925–2932. [DOI] [PubMed] [Google Scholar]
- 52.Wang A, Mackie K, Breidahl W, Wang T, Zheng MH. Evidence for the durability of autologous tenocyte injection for treatment of chronic resistant lateral epicondylitis: mean 4.5-year clinical follow-up. Am J Sports Med. 2015;43(7):1775–1783. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, He G, Guo Y, et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J Cell Mol Med. 2019;23(8):5475–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber C, Thai V, Neuheuser K, Groover K, Christ O. Efficacy of physical therapy for the treatment of lateral epicondylitis: a meta-analysis. BMC Musculoskelet Disord. 2015;16(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu K, Al-Ani MK, Sun Y, et al. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med. 2017;11(4):1173–1184. [DOI] [PubMed] [Google Scholar]
- 56.Xu Y, Murrell GAC. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466(7):1528–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Youngstrom DW, LaDow JE, Barrett JG. Tenogenesis of bone marrow-, adipose-, and tendon-derived stem cells in a dynamic bioreactor. Connect Tissue Res. 2016;57(6):454–465. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Wang JHC. BMP-2 mediates PGE2-induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res. 2012;30(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Wang JHC. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010;28(5):639–643. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Wang JHC. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38(12):2477–2486. [DOI] [PubMed] [Google Scholar]
- 61.Zhang JY, Fabricant PD, Ishmael CR, Wang JC, Petrigliano FA, Jones KJ. Utilization of platelet-rich plasma for musculoskeletal injuries. Orthop J Sports Med. 2016;4(12):232596711667624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Liu H, Cui Q, et al. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res Ther. 2020;11(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]