Abstract
Previous studies have found critically ill patients with COVID-19 to have an increased risk of thromboembolic complications. In this case report of two patients admitted with symptomatic COVID-19, both patients developed pulmonary embolism within a few days after hospital discharge. Both patients received thromboprophylaxis and had an increasing fibrin D-dimer during their hospital stay. Continued thromboprophylaxis after hospital discharge may be indicated for patients with COVID-19, especially for patients at high risk of thrombosis with elevated levels of fibrin D-dimer.
Keywords: Pulmonary circulation and disease, hypertension, cardiology, deep vein thrombosis, anticoagulants, stroke treatment – medical, thrombosis risk factors
Introduction
Recent studies show that critically ill patients with COVID-19 have a high risk of developing venous thromboembolism (VTE) and pulmonary embolism (PE) due to a heavy immune response (hemostatic derangement), immobility, and mechanical ventilation at the ICU.1 Thromboprophylaxis with low-weight molecular heparin (LMWH) is recommended as routine treatment for patients with symptomatic COVID-19 infection in Denmark.2 Prophylactic treatment after hospital discharge is not recommended due to limited evidence of benefits.1
We present two patients who were hospitalized with COVID-19, received thromboprophylaxis during their hospital stay, and later developed PE after hospital discharge.
Case I (15th of November to 30th of November)
A 61-year-old man was admitted with shortness of breath, vomiting, red spots on his upper torso, and confirmed COVID-19 illness. His blood tests on the day of admission showed lymphopenia (0,48 x 109/L) and elevated ferritin (505 µg/L). The patient required oxygen (up to 5 L/min) and had elevated measurements of fibrin D-dimer (Table 1). A chest x-ray showed bilateral pulmonary infiltrates. Treatment with LMWH (5000 IU per day), dexamethasone (6 mg per day), and mobilization by a physiotherapist was initiated in line with the regional guidelines in Denmark. The patient’s dyspnea improved over the following days, although desaturation remained during physical activity. He was discharged after one week without the need for oxygen therapy and without thromboprophylaxis. The fibrin D-dimer increased during hospitalization (Table 1).
Table 1.
Fibrin d-dimer levels during hospitalization.
|
Patient I |
Patient II |
||
|---|---|---|---|
| Day of hospitalization | Value (mg/L) | Day of hospitalization | Value (mg/L) |
| 1 | 1.3 | 3 | 0.9 |
| 2 | 3.2 | 4 | 1.1 |
| 3 | 2.3 | 5 | 1.3 |
| 4 | 3.9 | 6 | 1.5 |
| 5 | 3.6 | 7 | 2.2 |
| 6 (discharged) | 4.7 | 8 | 1.2 |
| 9 (readmitted) | 9.8 | 9 | 1.0 |
| 11 | 4.2 | 10 | 1.6 |
| 14 | 3.0 | 12 | 1.7 |
| 15 | 2.7 | 13-14 | 1.1 |
| 16 | 1.6 | ||
| 17 | 1.9 | ||
| 18 | 2.2 | ||
| 19 (discharged) | 2.2 | ||
| 23 (readmitted) | >20 | ||
| 24 | 18.7 | ||
| 25 | 11.1 | ||
| 26 | 11.1 | ||
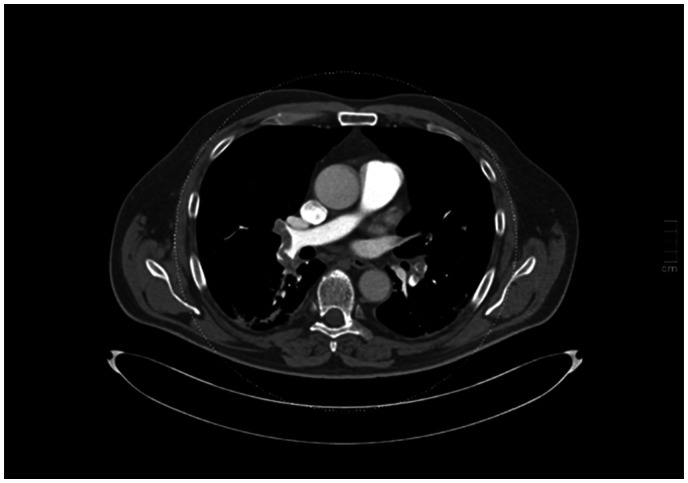
He was readmitted after three days with a sudden onset of severe dyspnea and chest pain. Blood tests showed fibrin D-dimer of 9.8 mg/L and a troponin of 1056 ng/L. The ECG was without any sign of ischemia. An echocardiography showed a D-shaped right ventricle and elevated pulmonary pressure suggesting thromboembolism. A CT pulmonary angiogram (CTPA) was performed upon admission showing bilateral PE and sporadic infiltrates (Figure 1). The patient was treated with LMWH for four days and then switched to direct oral anticoagulants (DOAC). He was discharged with no need for oxygen therapy after eight days of hospitalization.
Figure 1.
A CT pulmonary angiogram showing the pulmonary embolism at the time of readmission.
Case II (21st of November to 18th of December)
A healthy 87-year-old man presented to the Emergency Department with fever (38,9° C), three days of coughing, and confirmed COVID-19 illness. The patient was treated with remdesivir, dexamethasone and LMWH according to guidelines.3 He was severely hypoxic (saturation of 88% on 5 L/min oxygen) and was treated with high flow oxygen (25 L/min) and Continuous Positive Airway Pressure (CPAP) but remained circulatory stable. The patients’ blood pressure was measured at 167/86 mmHg at the time of admission, and his heart rate never exceeded 100 bpm during hospitalization. Blood tests showed lymphopenia (0,73 x 109/L) as well as increased ferritin (506 µg/L). Antibiotics was given under the suspicion of a urinary tract infection. A chest x-ray showed bilateral infiltrations. The patient received physiotherapy. Elevated D-dimer levels were seen on day 6 (Table 1), and the dose of LMWH was increased to 5000 IU twice per day. He experienced both clinical (overall improvement of performance status) and radiological improvement (reduced infiltrates on chest x-ray) during the following week. On day 17 of the hospital stay, the patient was weaned from oxygen therapy and was discharged on day 19 with a fibrin D-dimer of 2.2 (Table 1).
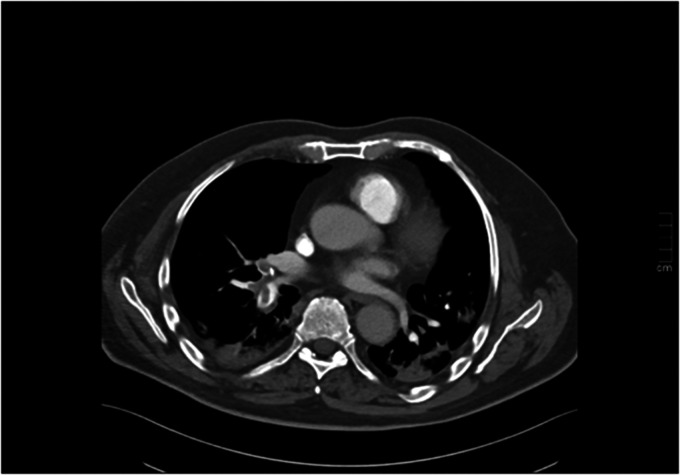
He was readmitted after five days with severe dyspnea, hypoxia, fever, and hypotension. Treatment with LMWH and dexamethasone was initiated upon admission. Blood tests showed fibrin D-dimer of >20 mg/L, but no elevation in troponin. Echocardiography showed a slight increase in pulmonary pressure. A CTPA revealed multiple central PEs (Figure 2). He was switched to DOAC on the 3rd day and discharged after 7 days.
Figure 2.
A CT pulmonary angiogram showing multiple pulmonary embolisms at the time of readmission.
Discussion
In this case report of two patients admitted with COVID-19, both patients developed PE within a few days after hospital discharge. Treatment with thromboprophylaxis was used in both patients during the first hospitalization but was discontinued at discharge. Both patients were males, above 60 years of age, and BMI was above average (respectively 27 and 32) and therefore shared some of the characteristics that are known to increase the risk of VTE.4 Both patients received thromboprophylaxis after readmission.
The incidence of PE reported in COVID-19 patients has fluctuated between 1.9% and 8.9%.1 In a recent nationwide Danish study, the incidence of VTE in patients hospitalized with COVID-19 was reported at 4% in patients admitted to the wards and 11% in patients admitted to the ICU.5
Poissy et al. observed that 20.6% of the discharged patients developed PE despite thromboprophylaxis during hospitalization (with a median time of 6 days until the PE diagnosis after discharge).6 Mariatta et al. provided LMWH for 7–14 days after hospital discharge to COVID-19 patients at higher risk of thrombosis (risk factors such as BMI >30, previous VTE, reduced mobility, and active cancer).7 The authors found a reduced mortality of 28 days. The disadvantage of anticoagulant treatment is the potential risk of bleeding, although this risk may not be as high as the risk of thrombosis in patients with COVID-19.1
Fibrin D-dimer was found to increase during hospitalization in both cases (Table 1), which could potentially be an early marker of VTE. Fibrin D-dimer has been shown to have high negative predictive value (NPV), e.g., a negative test may be used to rule out VTE in patients with low pretest probability. For example, a recent systematic review and meta-analysis found an overall pooled incidence rate of PE at 10.5% in patients not admitted to the ICU, a NPV of 95% (95%CI, 89–100), and a positive predictive value of 11% (95%CI, 10, 12) at a D-dimer cutoff value of 500 mcg/L.8
The reported cases should be interpreted as hypothesis generating and in the context of the inherent limitations of this study design, including a lack of generalizability and our purpose was not to establish a causal relationship between COVID-19, thromboprophylaxis, and the risk of VTE.
Since COVID-19 has been shown to increase the risk of coagulopathy and thrombosis, continuing thromboprophylaxis after hospital discharge may be indicated in selected high-risk patients. Clinical trials are ongoing to address this question (ClinicalTrials.Gov, NCT04650087 and NCT04662684).
Acknowledgements
The authors would like to thank Rasmus Gissel for proofreading the article.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Written informed consent was obtained from both participants.
Guarantor: MH.
Contributorship: Mikkel Deutch and Malene Hollingdal were responsible for the data acquisition and drafted the manuscript. All authors interpreted the work and critically revised the manuscript. All authors approved the final manuscript as submitted.
ORCID iD: Mikkel Rodin Deutch https://orcid.org/0000-0001-7959-585X
References
- 1.Bikdeli B, Madhavan MV, Jimenez D, et al.; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75: 2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forebyggelse og behandling af trombose og blødning hos COVID-19 patienter 2020. 4. udgave, www.dsth.dk/pdf/COVID-19-retningslinje-web.pdf (acessed 14 July 2021).
- 3.Jeschke K.Guideline for håndtering af COVID-19 patienter under indlæggelse på sengeafdeling. Denmark: Dansk Lungemedicinsk Selskab, 2021. [Google Scholar]
- 4.Áinle FN, Kevane B.Which patients are at high risk of recurrent venous thromboembolism (deep vein thrombosis and pulmonary embolism)? Hematology Am Soc Hematol Educ Program 2020; 2020: 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalager-Pedersen M, Lund LC, Mariager T, et al. Venous thromboembolism and major bleeding in patients with COVID-19: a nationwide population-based cohort study. Clin Infect Dis. Epub ahead of print 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poissy J, Goutay J, Caplan M, et al.; Lille ICU Haemostasis COVID-19 Group. Pulmonary embolism in patients with COVID-19. Circulation 2020; 142: 184–186. [DOI] [PubMed] [Google Scholar]
- 7.Marietta M, Ageno W, Artoni A, et al. COVID-19 and haemostasis: a position paper from Italian society on thrombosis and haemostasis (SISET). Blood Transfus 2020; 18: 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology 2021; 298: E70–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]