Abstract
The advent of bone marrow transplant has opened doors to a different approach and offered a new treatment modality for various hematopoietic stem-cell-related disorders. Since the first bone marrow transplant in 1957, there has been significant progress in managing patients who undergo bone marrow transplants. Plasma-cell disorders, lymphoproliferative disorders, and myelodysplastic syndrome are the most common indications for hematopoietic stem-cell transplant. Despite the advances, invasive fungal infections remain a significant cause of morbidity and mortality in this high-risk population. The overall incidence of invasive fungal infection in patients with hematopoietic stem-cell transplant is around 4%, but the mortality in patients with allogeneic stem-cell transplant is as high as 13% in one study. Type of stem-cell transplant, conditioning regimen, and development of graft-versus-host disease are some of the risk factors that impact the risk and outcomes in patients with invasive fungal infections. Aspergillus and candida remain the two most common organisms causing invasive fungal infections. Molecular diagnostic methods have replaced some traditional methods due to their simplicity of use and rapid turnaround time. Primary prophylaxis has undoubtedly shown to improve outcomes even though breakthrough infection rates remain high. The directed treatment has seen a significant shift from amphotericin B to itraconazole, voriconazole, and echinocandins, which have shown better efficacy and fewer adverse effects. In this comprehensive review, we aim to detail epidemiology, risk factors, diagnosis, and management, including prophylaxis, empiric and directed management of invasive fungal infections in patients with hematopoietic stem-cell transplant.
Keywords: anti-fungal, bone marrow transplant, fungal infections, fungal prophylaxis, stem cell transplant
Introduction
Since the first bone marrow transplant in 1957, there has been significant progress in managing patients who undergo bone marrow transplants. The Center for International Blood and Marrow Transplant Research (CIBMTR) recorded about 8000 allogeneic and 14,000 autologous bone marrow transplants in the United States (US) in 2018. Their data showed that the most common indications for autologous bone marrow transplants are myeloma and lymphomas. In contrast, most allogeneic stem-cell transplants were indicated for acute myelocytic leukemia (AML) and myelodysplastic syndrome (MDS).1 Largely, in 2018, the number of autologous hematopoietic stem-cell transplants (HSCTs) decreased by 5%, whereas allogeneic HSCT increased by 1% when compared with 2017. Interestingly, there is a continuous increase in HSCT among older adults age >70 years.1 Other common indications include aplastic anemia, thalassemia, and severe combined immunodeficiency.
Randomized control trials have suggested benefits for HSCT, including longer progression-free survival, as well as interval freedom from treatment failure.2,3 While this appears promising, common complications associated with HSCT include graft-versus-host disease (GVHD), infection, graft rejections, and organ failure. Beside the progression of primary disease, infections and GVHD are predominantly responsible for negatively affecting HSCT outcomes.
The HSCT treatment period has been divided into three phases: the pre-engraftment period, early post-engraftment period, and late post-engraftment period. Early in the course of transplant, infection accounts for about a third of deaths.1 The microbiology of infections during these periods is based on the underlying defect in the immune system and host risk factors. Common early infections include bacterial infections, including Gram-negative bacilli acquired from the gastrointestinal (GI) tract during the transplantation process and Gram-positive cocci from indwelling access sites. According to one study, while 38% of the infections were viral, about a quarter of the infections occurring in HSCT were fungal, with bacterial infections accounting for a third.4
This review addresses fungal infections in HSCT, including the epidemiology of fungal infections beside candida and aspergillus. We address risk factors predisposing to fungal infections based on the type of HSCT, the time since HSCT, and the presence of GVHD. We compare different options for diagnosing fungal infections, and finally, we review studies addressing prophylaxis and management for fungal infections.
Epidemiology
Invasive fungal infections (IFIs) cause significant morbidity and mortality in high-risk populations such as those with HSCT. The establishment of the TRANSNET in 2001 was helpful in better understanding of epidemiology of IFI on a large scale. This led to the establishment of the Transplant-Associated Infections Surveillance Network (TRANSNET) in 2001, consisting of 23 US academic transplant centers that perform HSCTs and solid organ transplant, administered by the Centers for Disease Control and Prevention (CDC) and coordinated by the University of Alabama at Birmingham. Through TRANSNET, a prospective surveillance study was conducted from 2001 to 2006 on IFI in solid organ and HSCT recipients that revealed essential data on epidemiological trends and the burden of IFI in the US.5 In addition, several other similar studies are now available, aiming to identify the incidence and outcomes of IFI in this population. The SEIFEM-B-2004 study was a major retrospective cohort study undertaken from 1999 to 2003 in 11 academic hospitals in Italy involving HSCT patients who developed IFI.6 Another major study was a multicenter, prospective, observational study performed on the Prospective Antifungal Therapy (PATH) Alliance registry during the period July 2004 through September 2007.7
Based on the TRANSNET data, the incidence of IFI in HSCT patients overall is 3.4%. The SEIFEM study showed a similar incidence rate of 3.7%, where 49% were proven infections and the rest were probable. Both the studies found invasive aspergillosis (IA) to be the most common IFI encountered in this population, followed by invasive candidiasis (IC), other unspecified molds, and finally, zygomycetes. Pneumocystis, endemic fungal infection, and cryptococcus infections were rare. The IFI-attributed rate from the SEIFEM study was 2.4%, with the mortality rate from aspergillosis significantly higher than that reported for candida infection. Epidemiology and risk factors of IFI in HSCT patients are presented in Table 1.
Table 1.
Epidemiology, risk factors, clinical features, and diagnostic tests for invasive fungal infections in patients with hematopoietic stem-cell transplant.5–7.
| Organism | Major species | Epidemiology | Risk factors | Clinical features | Diagnosis | Special comments |
|---|---|---|---|---|---|---|
| Aspergillus8–10 | Aspergillus fumigatus, Aspergillus niger, Aspergillus terreus, Aspergillus flavus | Incidence of IA was 6.7% in allogeneic HSCT and 0.4% in
autologous HSCT According to the SEIFEM study, overall mortality due to this species was 4.9% According to the TRANSNET study, the cumulative incidences at 6 months and 1 month were 1.3% and 1.6%, respectively A. fumigatus was the most common species isolated, whereas infections due to A. flavus, A. niger, and A. terreus were less common Most cases of aspergillosis (78%) were invasive pulmonary aspergillosis The 1-year survival was 25.4% for patients with invasive aspergillosis |
Neutropenia post myeloablative regimen, acute and chronic graft-versus-host disease | Rhino-cerebral aspergillosis (facial swelling, nasal discharge, neurological deficits), pulmonary aspergillosis, cutaneous aspergillosis (ulcers, eschars), disseminated, gastrointestinal | Histopathology, culture (various body fluids), galactomannan assay, β-D-glucan assay, lateral flow device antigen detection, aspergillus PCR (can be used in combination with galactomannan) |
A. fumigatus is the most frequent species
causing IA This is attributed to virulence factors associated with this species A. terreus has been associated with amphotericin B resistance, a lower response rate to treatment, and a higher mortality rate |
| Candida11–13 | Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, Candida krusei, Candida guilliermondii | Incidence of IC was 1.1% and 0.8% in the allogeneic and
autologous transplant setting, respectively, according to the
SEIFEM study According to the TRANSNET study, the 6-month and 12-month cumulative incidences for IC were 1% and 1.1%, respectively, and the 1-year survival in patients with IC was 33.6% PATH registry showed Candida species caused 25% of the IFDs in the HSCT population Overall, non-albicans Candida species accounted for 75.8% of the isolates, and C. glabrata was the most common species (43.5%) Similar results were seen in the TRANSNET study |
Neutropenia, mucositis, chronic indwelling catheters, invasive procedures | Bloodstream infections, chronic hepatosplenic candidiasis, endocarditis, osteomyelitis, endophthalmitis, and urinary tract infections | Blood cultures (gold standard), β-D-glucan test, C. albicans germ-tube antibody assay, candida PCR | Second most common cause of IFI in HSCT patients The routine use of azoles for prophylaxis has contributed to the decreased incidence of IC in this population and increased frequency of non-albicans candida infection C. krusei is innately resistant to azoles, whereas C. glabrata can acquire azole resistance |
| Fusarium14–16 | Fusarium solani, Fusarium oxysporum, Fusarium chlamydosporum and Fusarium moniliforme | The incidence of fusariosis was extremely low in the TRANSNET
and SEIFEM studies According to TRANSNET data, the 1-year survival among the HSCT cohort was the lowest for patients with fusarium infections (6.3%) |
Allogeneic HSCT, acute or chronic graft-versus-host disease | Disseminated infection, cutaneous nodules, pneumonia | Culture of various body fluids, histopathology (however, difficult to distinguish from aspergillosis) | Some Fusarium species can produce mycotoxins |
| Scedosporium14–16 | Scedosporium apiospermum, Scedosporium prolificans | In a retrospective review of 80 scedosporium cases consisting of 22 HSCT recipients, the overall mortality was 68%, with 61.5% for patients with S. apiospermum infection and 77.8% for patients with S. prolificans infection | Prolonged neutropenia, lymphopenia, steroid therapy, allogeneic HSCT, and prophylactic antifungal therapy can have a selective effect on this organism’s growth | Colonization, localized invasive diseases of bones, joint, mycetoma, disseminated disease | Histopathology, body fluid cultures | Have been known to emerge in patients receiving amphotericin B, fluconazole, or itraconazole |
| Zygomycetes11,17 | Mucor, Rhizopus, Rhizomucor, and Absidia species | Composes 8% of IFI in the TRANSNET study and 7% in the PATH
study Per the TRANSNET study, the 6-month and 12-month cumulative incidence of zygomycosis were 0.2 and 0.3, respectively The 1-year survival was 28% (slightly higher compared with aspergillosis and fusariosis) |
Neutropenia, refractory GVHD, steroid therapy and prophylactic antifungal therapy can have a selective effect on the growth of this organism, diabetic ketoacidosis | Classically rhino-cerebral or pulmonary disease, but cutaneous, gastrointestinal, and disseminated infections also occur | Histopathology is the gold standard, blood cultures (poor sensitivity) | |
| Pneumocystis18,19 | Pneumocystis jiroveci | Large retrospective analyses reported an incidence rate between 0% and 2.5% in allogeneic HSCT recipients and 1.4% in autologous HSCT recipients | Lymphopenia, greater HLA mismatch, chronic immunosuppression, patients not on or non-compliant to prophylaxis | Pulmonary involvement | Histopathology with methenamine silver stain, bronchoalveolar lavage, PCR | |
| Cryptococcus11,20 | Cryptococcus neoformans, Cryptococcus gatti, Cryptococcus albidus, Cryptococcus terreus, Cryptococcus laurentii, Cryptococcus adeliensis | Significantly rarer among HSCT recipients, representing <1% of the total IFI. The overall mortality of cryptococcosis in the non-HIV population is 30% | Allogeneic HSCT, immunosuppression | Central nervous system involvement followed by fungemia, disseminated infection, pulmonary cryptococcosis, cerebellitis, and diarrhea | Lumbar puncture, rapid cryptococcal antigen assay on body fluids, body fluid culture | |
| Endemic fungi11,21,22 | Histoplasma capsulatum, Blastomyces dermatidis, and Coccidioides immitis | According to a recent literature review, histoplasma has been shown to occur between 5 weeks and 18 months post-transplantation. It is associated with a high mortality | Present in the soil in certain geographic regions | inhalation of conidia leads to disseminated infections | Blood cultures, serum antigen-antibody tests | These are rarely encountered in HSCT recipients, even in endemic areas |
GVHD, graft-versus-host disease; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; HSCT, hematopoietic stem-cell transplant; IA, invasive aspergillosis; IC, invasive candidiasis; IFI, invasive fungal infections; PCR, polymerase chain reaction.
Risk factors
The risk factors for the development of IFI after HSCT depend on the type of HSCT, the presence of acute or chronic GVHD, administration of steroids, the presence of cytomegalovirus (CMV) disease, and antifungal prophylaxis.6
Type of HSCT
Allogeneic HSCT had more negative IFI-associated outcomes compared with autologous HSCT. According to the TRANSNET study, the cumulative incidence of IFI in autologous HSCT was 1.2%. Comparatively, among recipients of allogeneic HSCT, the 12-month cumulative incidence for matched related donor (MRD), an unrelated donor (URD), and mismatched-related donors (MMRs) was 5.8%, 7.7%, and 8.1%, respectively.5 In the SEIFEM study, the mortality rate due to IFI in allogeneic HSCT versus autologous HSCT was 72% versus 35% (p = 0.001), and the incidence and mortality of aspergillosis were higher in allogeneic HSCT than autologous HSCT. There was no difference in incidence and mortality among these groups in invasive candida infection.6 The RISK study, a 2013 multicenter retrospective observational study performed on patients undergoing allogeneic HSCT from six hospitals in Korea, revealed the cumulative incidence of IFI (proven or probable) in allogeneic HSCT to be 15.36% at 1 year, with aspergillus as the most common organism found, followed by candida, cryptococcus, and fusarium.23 Recently, the haploidentical transplant has increased donor availability but also increases the risk of infection, especially when highly active myeloablative regimens are used to reduce GVHD.24 In one retrospective analysis, the cumulative incidence of fungal infections was 24% at day 100, and 28% at 2 years after haploidentical stem-cell transplantation, with post-transplant cyclophosphamide.25 In a study by Omer et al.,26 haploidentical stem-cell transplantation was one of the risk factors identified for the development of IFI [hazard ratio (HR) 3.82; 95% confidence interval (CI) 1.49–9.77; p = 0.005]. In a retrospective study including 381 patients with haploidentical stem-cell transplantation, 20% developed IFI. Invasive aspergillosis was the most common (44%), followed by invasive candidiasis (33%).24
Time since HSCT
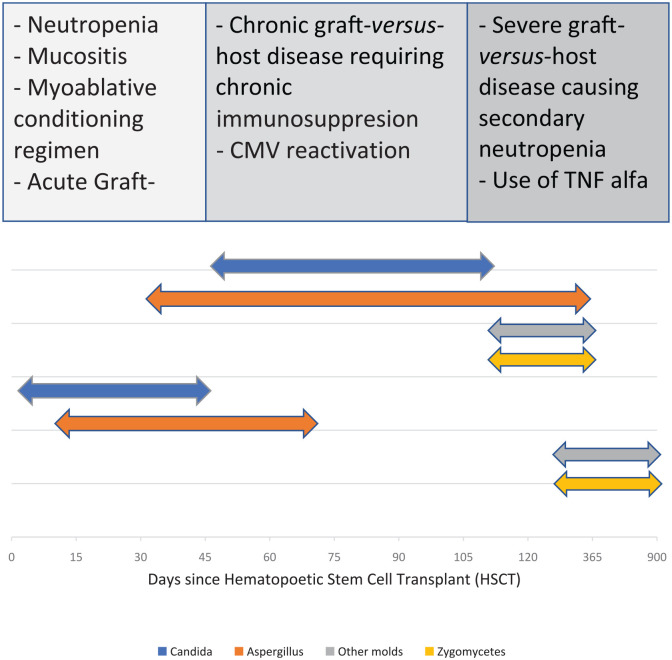
According to the RISK study, risk factors for IFI in allogeneic HSCT patients in the early post-engraftment period (<40 days) were unrelated or related-mismatch HSCT, underlying chronic diseases, and prolonged neutropenia (>3 weeks). In the late post-engraftment period (41–100 days), IFI risks were the prescription of immunosuppressive agents for refractory GVHD and CMV reactivation. Whereas in the very late post-engraftment phase (>100 days), the risk factors were secondary neutropenia, severe GVHD, and use of tumor necrosis factor alpha (TNF-α) inhibitors for the treatment of refractory GVHD.23 A significantly higher mortality was seen in patients with severe GVHD requiring intensive immunosuppressive therapy.27 According to the PATH Alliance registry study, IC tended to occur earlier after autologous HSCT (median 28 days) compared with allogeneic HSCT (median 108 days).7 Early-onset IC is influenced by neutropenia and mucositis, whereas later-onset infection is influenced by GVHD and the presence of chronic indwelling catheters.11 IA tends to occur more frequently, and interval from transplant to diagnosis did not appear to be a significant risk factor for IA. Late IA is associated with an increased fungal burden due to delay in diagnosis and immunosuppression. IFI due to zygomycetes and other molds occurred late after HSCT (median interval, 173 days) and tended to occur later in autologous HSCT recipients than in allogeneic HSCT recipients (median interval, 162 days).7
Conditioning regimen
A conditioning regimen usually consists of myeloablative therapy with or without total body irradiation. It is used to either treat underlying cancer or suppress the recipient’s immune system before HSCT. Intensive regimens are associated with prolonged neutropenia increasing susceptibility to infections. Incidence of mold infection was low in patients who underwent only total body irradiation, since it favored engraftment and neutrophil recovery.28 Currently, reduced intensity/non-myeloablative ablative regimens are increasingly used, are associated with shorter neutropenia duration and less mucosal damage, and lead to fewer adverse outcomes and lower transplant-related mortality.29,30
Type of antifungal prophylaxis
Prophylaxis consists of the administration of antifungal agents at the onset of a period of high risk of infection, usually at the start of the conditioning regimen or the beginning of neutropenia in HSCT recipients. Although prophylaxis has improved early mortality, there have been limited epidemiological studies since posaconazole use was widely accepted for primary prophylaxis. Harrison et al.27 reported a single-center, retrospective cohort study that used primary antifungal prophylaxis with posaconazole (49.5%), voriconazole (26.7%), and fluconazole (16.2%) in 43.3% of allogeneic HSCT patients. Despite a fivefold increase in systemic antifungal prophylaxis, the incidence of IFI was not affected at 10.3% of patients at 1 year.
Organisms causing invasive fungal infections (Table 1 and Figure 1):
Figure 1.
Timeline and risk factors of invasive fungal infections.
CMV, cytomegalovirus; TNF, tumor necrosis factor.
Aspergillus
Aspergillus fumigatus is the most frequent species causing IFI.5,6 This is attributed to virulence factors associated with this species; ongoing research of these complexities may help in the development of new rapid diagnostic tests and antifungal agents.8 Other species that are often detected are Aspergillus niger, Aspergillus terreus, and Aspergillus flavus. Aspergillus infection commonly presents as invasive pulmonary aspergillosis with subsequent dissemination.9 A. terreus infection has been associated with amphotericin B resistance, a lower response rate to treatment, and a higher mortality rate.10 According to the TRANSNET study, the 1-year survival in patients with IA is 25.4%.5 In recipients of allogeneic HSCT, three periods of increased risk for invasive aspergillosis and other mold diseases occur: first, during neutropenia following the conditioning regimen; second, during acute GVHD; and third, during chronic GVHD. The risk is higher during GVHD than during neutropenia due to the use of chronic immunosuppressive therapy9 (Table 1, Figure 1).
Candida
Candida species are the second most common cause of IFI in HSCT patients.12 The epidemiology of candida infection has changed significantly in the past 2 decades after several randomized controlled trials showed that fluconazole significantly decreased the incidence of candidiasis, followed by the subsequent widespread use of fluconazole prophylaxis.13 Candida albicans had historically been the most common pathogen; however, the routine use of azoles for prophylaxis has contributed to an overall decreased incidence of IC and an increased frequency of non-albicans candida infection.11 Non-albicans candida accounted for almost 70% of all candida infections in the TRANSNET study. The major non-albicans species were Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei, with C. glabrata now emerging as an important pathogen. According to the TRANSNET study, the 1-year survival in patients with IC is 33.6%, slightly higher than in other IFI.5
Other molds
These include over 30 non-aspergillus hyalohyphomycetes, including Acremonium, Fusarium, Scedosporium, and Paecilomyces species. Infection with these organisms usually follows environmental exposure.14 During their study period, SEIFEM study reported 0.11% of fungal infections resulting from Fusarium species with a mortality of 53%. Scedosporiosis causes a wide range of clinical diseases, from transient colonization of the respiratory tract to invasive localized and disseminated diseases in immunosuppressed patients and involves two medically important species: Scedosporium apiospermum and Scedosporium prolificans. They have been known to proliferate in patients receiving amphotericin B, fluconazole, or itraconazole.15,16 In a retrospective case series of 80 scedosporium patients which included 22 HSCT recipients, the overall mortality was found to be 68%, with 61.5% for patients having S. apiospermum infection and 77.8% for patients having S. prolificans infection.16
Zygomycetes
Zygomycosis represented 8% of IFI in the TRANSNET Study, and 7% in the PATH study.5,7 According to the TRANSNET study, zygomycosis incidence increased from 1.7 to 6.2 per 1000 from 2001 to 2004. It is a devastatingly invasive disease with mortality of 60–80%.11 The increased incidence of zygomycosis reflects the increase in predisposing factors, such as higher numbers of patients undergoing HSCT and immunosuppressive chemotherapy, with the effectiveness of antifungal therapy against common species correspondingly increasing the emergence of such rare pathogens.16
Pneumocystis
Historically, pneumocystis pneumonia (PCP) was considered a severe life-threatening infection, especially in patients with acute lymphoblastic leukemia, and HSCT recipients. The incidence of PCP, however, dropped after the introduction of prophylaxis. Currently, only HSCT patients not on or non-compliant to prophylaxis develop PCP. Large retrospective analyses have reported an incidence rate between 0% and 2.5% in allogeneic HSCT recipients and 1.4% in autologous HSCT recipients.18 HSCT recipients who develop PCP typically have acute or chronic graft-versus-host disease and are receiving corticosteroids or other immunosuppressive drugs more than 6 months after transplant. A large retrospective analysis reported an incidence rate between 0% and 2.5% in allogeneic HSCT recipients, and 1.4% in autologous HSCT recipients.19
Cryptococcus
Cryptococcus neoformans and Cryptococcus gatti are the main pathogenic species in this genus. Due to effective antifungal prophylaxis, these organisms are significantly rarer among HSCT recipients, representing <1% of the total IFI in this population.5,11 The timing of cryptococcosis in HSCT recipients is not well defined. Types of infection in these patients include meningitis, fungemia, and pneumonia. The overall mortality of cryptococcosis in the non-human immunodeficiency virus (HIV) population is 30%.11
Endemic fungi
This class includes Histoplasma capsulatum, Blastomyces dermatidis, and Coccidioides immitis. These organisms are present in the soil in certain geographic regions, and conidia inhalation leads to disseminated infections. They are rarely encountered in HSCT recipients, even in endemic areas. According to a recent literature review, histoplasmosis has been shown to occur between 5 weeks and 18 months post-transplantation and is associated with high mortality.11,21,22
Diagnosis
The rate of pre-mortem diagnosis of IFI is in the range of 12–60%. This indicates the necessity of improved diagnosis.31 Usually, in clinically unstable patients, IFIs are treated empirically, which raises economic burden, and delay in diagnosis is associated with increased mortality. Different terminologies have been used for the diagnosis of IFI, which has now been standardized per European Organization for Research and Treatment of Cancer/National Institute of Allergy and Infectious Diseases Mycoses Study Group to streamline clinical studies.32 A proven diagnosis requires culture or histopathological evidence from a tissue biopsy or normally sterile body fluids. Probable diagnosis requires conducive host factors such as neutropenia, immunosuppression, clinical suspicion through imaging, direct visualization, or clinical exam, and includes cytology or culture evidence of molds or indirect tests like galactomannan (GM) or β-D-glucan. Thus, a host factor, a clinical criterion, and a mycological criterion are necessary. Possible diagnoses are the cases that meet the criteria for a host factor and a clinical criterion but for which mycological criteria are absent. Conventional techniques for clinical diagnosis are limited by slow turnaround time or associated invasive procedures. Newer methods that do not require detection of the organism in culture or tissues are limited by availability and clinical performance. Thus, standardization of diagnostic tests for each clinical scenario is difficult.
Culture-based detection
This remains the gold standard for diagnosis and provides critical data on organism susceptibility. However, it also leads to considerable delays in the initiation of treatment. The time to initiation of antifungal treatment of at least 12 h after the first positive blood culture sample was an independent determinant of hospital mortality in patients with invasive candidiasis [odds ratio (OR) = 2.09; p = 0.018].33 A prospective study in 2000 determined that fungal colonization detected by surveillance fungal cultures lacked positive predictive value for diagnosis of fungal infection in critically ill patients, and had minimal utility.34 The sensitivity and specificity of cultures vary for organisms and type of body fluid. Cerebrospinal fluid (CSF) cultures for cryptococcus are highly sensitive and specific, whereas blood culture sensitivity in invasive candidiasis is only 50–60%, with 95% specificity. The positive predictive value of cultures depends on the prevalence of the infection and, therefore, is much higher in transplant patients and endemic areas.35 A study looking at immunocompromised patients with positive lower respiratory-tract culture for aspergillus showed a positive predictive value of 72% in patients with hematologic malignancy, granulocytopenia, or bone-marrow transplant; 58% in those with a solid-organ transplant or using corticosteroids; and 14% in those with HIV infection.36 Identification of yeast or molds from clinical specimens is exceptionally laborious. Microbiologists have begun using mass spectrometry identification techniques (MALDI-TOF, matrix-assisted laser desorption time of flight mass spectrometry) on specimens for rapid diagnosis.37
Histopathology
This is another gold-standard test, especially in invasive aspergillosis, pneumocystis, and endemic fungal infections (histoplasma, blastomyces, and coccidioides), often used in conjunction with culture. Histopathology can detect an invasion of fungus, as well as a host response. For example, visualization of a biofilm can help direct therapy since this is associated with resistance to antifungal regimens. Histopathology, unfortunately, gives only a descriptive diagnosis; fungal morphology can be non-specific, and it does not allow for the identification of species, which is usually necessary to direct therapy.35
β-D-glucan
Testing to detect the explicitly fungal cell wall component (1,3)-β-D-glucan (BDG) is commonly used as a screening and diagnostic tool in IFI. The Fungitell® assay is currently the only US Food and Drug Administration (FDA)-approved assay.37 BDG is found in several organisms, including Candida species, pneumocystis, and molds such as Aspergillus species and Fusarium species. In a prospective study of HSCT recipients, BDG assays were found to have a specificity of 98% and a negative predictive value of 99%, making this test a useful tool to rule out IFI.38 Similar results were found in a recent meta-analysis in patients with hematological malignancies where two consecutive BDG tests had an excellent specificity (98.9%) but a low sensitivity (49.6%) for the diagnosis of IFI.39 This low sensitivity is likely related to the higher incidence of colonization in the HSCT population and testing on specific populations like hemodialysis patients or those with Gram-negative bacteremia.
Aspergillus galactomannan
GM is another fungal cell-wall biomarker produced by several fungi, including Aspergillus, Penicillium, and Histoplasma species. This assay is reported as an index of optical density (GM index or GMI) and is currently FDA approved for the detection of aspergillus in serum and bronchoalveolar lavage (BAL) specimens. Serum GM testing is now considered the standard of care given strong recommendations for use in immunocompromised patients by several high-quality studies (in patients not on posaconazole prophylaxis).40 In a retrospective cohort study in allogeneic HSCT recipients with invasive pulmonary aspergillosis (IPA), the magnitude of GMI (drawn on clinical suspicion) correlated with higher all-cause mortality, likely representing increased aspergillus burden.41 Another similar study concluded that a GMI screening strategy could lead to lower mortality by early detection of invasive aspergillosis.42
Antigen–antibody detection
Sensitivity of antibody tests may be limited in immunocompromised patients, as there is a lack of antibody response to infections. They also have limited specificity, since they cannot differentiate between the presence of normal flora, colonization, and infections. However, several specific antibody detection tests are available.
C. albicans germ-tube antibody (CAGTA) assay
This test detects antibodies by an indirect immunofluorescence technique. It has a sensitivity of 77–89% and a specificity of 91–100%.35 Lateral-flow devices detect a glycoprotein in the serum and BAL fluid of patients with invasive aspergillosis. It is used in combination with molecular studies.
Cryptococcal antigen test
When performed on CSF, this test has a sensitivity of 97% and a specificity of 93–100%. It can also be used on a blood specimen, but it has a sensitivity of 87%. It has become a point-of-care screening test in the immunocompromised population.
Histoplasma and blastomyces antigen tests
These tests can be performed on urine or serum and have superior sensitivities (83.3–91.8% for histoplasmosis and 92.9% for blastomycosis) than antibody testing. A novel enzyme-linked immunosorbent assay test has been developed with high specificity for B. dermatidis.
Coccidioides antibodies
This is a highly accurate antibody test using complement fixation and tube precipitin antibody detection methods.
Molecular studies
Molecular diagnostic methods, especially polymerase chain reaction (PCR), have increased in popularity and replaced some traditional methods due to simplicity of use and rapid turnaround time. The high sensitivity of PCR allows for early detection of infection when treatment is easier and may prevent clinical manifestations. Since quantifying microbial burden in IFI is vital in terms of management, the development of real-time PCR, which can quantify the amount of DNA in real-time, has been revolutionary. Some technical difficulties associated with PCR diagnosis include cumbersome methods for cell wall lysis to expose DNA, primer standardization, lab-to-lab discrepancies, and sensitivity to contamination.40
Aspergillus polymerase chain reaction (PCR)
There are several recent meta-analyses analyzing the performance of PCR on blood or serum samples for aspergillus detection. One meta-analysis showed the sensitivity and specificity of blood or serum PCR for two consecutive positive samples were 0.75 (95% CI 0.54–0.88) and 0.87 (95% CI 0.78–0.93), respectively, and for a single positive sample were 0.88 (95% CI 0.75–0.94) and 0.75 (95% CI 0.63–0.84), respectively.43 In another meta-analysis that compared the performance of PCR of BAL with a BAL GM test, mean sensitivity and specificity values for diagnosis of proven or probable IPA were 90.2% (77.2–96.1%) and 96.4% (93.3–98.1%), respectively.44 The American Thoracic Society has published a summary of recommendations based on high-quality meta-analyses for diagnosing IPA in severely immunocompromised patients, such as HSCT with high pretest probability. They recommended testing blood or serum for aspergillus using PCR. In patients with high pretest probability, a single positive PCR gives moderate-to-high sensitivity to exclude the disease, and two positive PCRs provide high specificity. In patients with high pretest probability suspected of having IPA, aspergillus PCR testing of BAL is recommended.40 However, these recommendations have been made for adult patients only. There is a significant lack of studies in the pediatric population, as well as a lack of outcome research in PCR diagnostics.
Candida PCR
Similar studies were performed for assessing the utility of diagnostic PCR in patients suspected of invasive candidiasis. In one meta-analysis of 54 studies that included almost 5000 patients tested by blood-based PCR, pooled sensitivity and specificity for proven or probable invasive candidiasis versus at-risk controls were 0.95 (CI, 0.88–0.98) and 0.92 (CI 0.88–0.95), respectively.45 Recently, there has been an innovative contribution to invasive candidiasis diagnostics in the form of targeted molecular direct detection using an FDA-approved candida nucleic acid test. It combines targeted PCR with T2 magnetic resonance to detect the five most common Candida species (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei) directly from blood specimens. The overall specificity was 99.4%, and sensitivity was 91.1% in the multicenter DIRECT trial.46
Aspergillus PCR + galactomannan
An emerging strategy is to combine molecular and antigen testing to improve clinical utility. This was assessed in a recent meta-analysis of high-risk hematological patients, which found that the highest sensitivity (99%) was achieved when at least one positive GM or PCR was used to define a positive episode. The absence of any positive test result had a negative predictive value of 100%, averting unnecessary antifungal exposure.47
PCR + BDG assay
A similar approach was made for the diagnosis of Pneumocystis jirovecii in a large HSCT population with clinical suspicion of P. jirovecii pneumonia (PJP). This retrospective cohort study used PJP PCR on bronchoscopy samples and serum BDG testing. In patients with a positive BAL PCR result, a positive BDG resulted in 100% specificity with a 100% positive predictive value.48
Next-generation sequencing techniques
This includes sequencing of cell-free DNA (cfDNA) circulating in the bloodstream to identify non-human sequences, and compare them with known genomic databases of bacterial, viral, and fungal pathogens. This is a newer technique and its applicability in detection of IFI in HSCT patient is still under review.35,49
Management
Early recognition and early initiation of treatment of invasive fungal infection may improve patient outcomes. Effective management strategies include prophylaxis (primary and secondary), empiric antifungal treatment, and directed treatment of established fungal infections. An increased incidence of fungal infections in bone marrow transplant patients has been seen over time.50 Given the high mortality related to invasive fungal infection, prophylaxis and empiric therapy should not be delayed while awaiting identification of the pathogen.51
Primary prophylaxis
Table 2 lists major trials of primary prophylaxis agents. In the late 1900s, several trials explored the benefits of prophylactic antifungal regimens including nystatin and ketoconazole.52–55 These agents are seldom used now. A landmark trial by Goodman et al.56 showed reduction in the incidence of superficial and systemic fungal infections with 400 mg of fluconazole without any difference in 90-day mortality. Subsequently, Slavin et al.13 reported a statistically significant difference in mortality at 110 days with 400 mg daily prophylactic fluconazole versus placebo. These two studies established the use of fluconazole in bone marrow transplants as the standard of care at the end of the 20th century. Long-term follow up by Marr et al.57 showed survival benefit in the fluconazole group compared with placebo. However, this benefit was statistically significant only in the allogeneic transplant patient subgroup.
Table 2.
Recent trials of primary prophylaxis in invasive fungal infections.
| Author | Drug | Number of patients | Results |
|---|---|---|---|
| Marr et al.58 | Itraconazole versus fluconazole | 300, all allogeneic | Statistically significant difference in the incidence of invasive fungal infections, especially mold infections, but a significantly higher incidence of hepatotoxicity and GI side effects |
| Van Burik et al.59 | Micafungin versus fluconazole | 882: 476 allogeneic | Statistically higher incidence of the absence of proven or probable systemic fungal infections with micafungin compared with fluconazole, similar adverse events in both groups |
| Ullmann et al.60 | Posaconazole versus fluconazole | 600 patients, all with GVHD | No significant difference in proven or probable systemic fungal infections, but the posaconazole group had a statistically significant lower incidence of aspergillosis, similar adverse events, but trends better towards posaconazole |
| Wingard et al.61 | Voriconazole versus fluconazole | 600 allogeneic patients | No significant difference in fungal-free survival and incidence of fungal infections between the two drugs, similar adverse events |
GI, gastrointestinal; GVHD, graft-versus-host disease.
Consequently, itraconazole was introduced as a potential prophylactic with some evidence that itraconazole may be a superior antifungal for prophylaxis than fluconazole.62,63 However, there also was concern regarding its GI side effects.63 In 2004, Marr published a prospective comparative study between fluconazole and itraconazole, which reiterated the abovementioned aspects, including reduced IFI with itraconazole compared with fluconazole.58 The study showed no difference in the incidence of candidemia between the two groups. However, fluconazole prophylaxis was recommended, since there was no difference in fungal-free survival between the two groups, but increased side effects associated with itraconazole, especially in the GI tract, leading to frequent discontinuation of the drug. Fluconazole and voriconazole had similar fungal-free survival and overall survival in patients with allogeneic HSCT.61
With the introduction of echinocandins, the prophylactic potential of micafungin was studied, given its anti-mold effects. There was some concern that fluconazole was not as effective against mold species as it was against yeast species, based on a single-center autopsy study in pediatric patients, which defined fluconazole prophylaxis as a minimum of five consecutive doses of fluconazole. In approximately 300 autopsies, they reported a decreased incidence of candida but increased incidence of aspergillus via histopathology or culture.64 In 2004, a randomized controlled trial compared micafungin with fluconazole as prophylactic medication in bone marrow transplant patients with a non-inferiority margin of 10%. The study reported micafungin as superior to fluconazole with a statistically significantly higher success rate, the primary outcome defined as the absence of proven, suspected, or probable IFI. While there was no difference in mortality, candidemia, or colonization rates reported in the two groups, the study reported a non-significant but higher incidence of aspergillosis in the fluconazole group (seven breakthrough aspergillus infections) compared with micafungin (one breakthrough aspergillus infection). There were no significant differences in the adverse effect profiles of the two drugs, which involved approximately 400 patients in each group.59 This has provided a different drug-class option for fungal prophylaxis.
A study was published in the New England Journal of Medicine in 2007 that compared the newer drug posaconazole with fluconazole in a randomized trial, based on evidence of better in vitro efficacy of posaconazole against aspergillus. The study was conducted in patients who had acute GVHD grade 2–4 or chronic GVHD. Approximately 300 patients in each GVHD category were randomized, and no differences were found in the total incidence of fungal infections. More importantly, the study concluded that posaconazole administration was superior in reducing the incidence of aspergillosis and resulted in statistically significantly fewer deaths related to fungal infections, raising the possibility of improved protection with posaconazole in GVHD patients.60 The side-effect profiles were similar for the two drugs.
Other medications evaluated for prophylaxis have included liposomal amphotericin B and voriconazole.65,66 Once-weekly liposomal amphotericin was evaluated from a safety standpoint, as it is not affected by GI absorption variability, which is a particular concern in GVHD patients. Although it was known to have effective anti-mold activity, studies found it to be frequently discontinued in SCT patients due to infusion-related adverse events.67,68 In one study, voriconazole-treated patients trended towards fewer IFI and aspergillus infections than those treated with fluconazole, but the difference remained statistically insignificant.63
Over time, primary prophylaxis has developed for IFI in the bone marrow transplant population (Table 2). Fluconazole has demonstrated prophylaxis against candida infection, while newer azoles and echinocandins show trends towards more anti-mold activity, which fluconazole lacked, providing better options for primary prophylaxis. Recently, robust data for voriconazole and posaconazole have made them favorable options for prophylaxis against IFI in bone marrow transplant patients.
Secondary prophylaxis
While there have been considerable advances in primary prophylaxis, the IFI relapse rate remains at 30–40%.69 Hence, secondary, or continued prophylaxis is an area that remains to be satisfactorily addressed. A small case series reported outcomes of 11 patients who had undergone HSCT with voriconazole as secondary prophylaxis. None of them went on to develop IFI during the chemotherapy-induced neutropenia phase.70 The best evidence for secondary prophylaxis came from a prospective study in 45 patients, of which 31 had aspergillosis, and 5 had candidiasis. Voriconazole was used for prophylaxis, and at the end of 12 months, the cumulative incidence of IFI was 6% compared with 30% previously reported otherwise.71 Both studies reported hepatotoxicity as an adverse event. Although neither was a randomized trial and both were conducted in a small number of patients, they provide some evidence to consider for secondary prophylaxis in patients, especially given the known relapse rates and the significant morbidity and mortality burden. However, the optimum antifungal, dose, and duration remain to be addressed.
Empiric treatment
In patients with a neutropenic fever after HSCT, empiric antifungal therapy is considered after 3–5 days of broad-spectrum antibiotics and persistent or recurrent fever,72 although neutrophil recovery generally occurs quickly in this subset of patients. Liposomal amphotericin B, which is less toxic and similar in efficacy to amphotericin B, is used for empiric therapy for IFI.73,74 Azoles and echinocandins are favorable for empiric treatment as well, except for fluconazole as it is frequently used in prophylaxis.75 Boogaerts et al.76 compared itraconazole and amphotericin B for empiric antifungal therapy in patients with persistent neutropenic fever. Both drugs had similar efficacy, but itraconazole had significantly fewer adverse events or withdrawal of therapy, and more renal toxicity was seen in the amphotericin group. Similar safety outcomes were seen in a more recent study, where the itraconazole group demonstrated a higher success rate than the amphotericin B group.77 Caspofungin had similar efficacy and less adverse events compared with liposomal amphotericin B when studied in a randomized controlled trial involving over 1000 patients.78 Voriconazole may not be a preferred agent for empiric treatment as it resulted in a lower success rate when compared with liposomal amphotericin B, even though voriconazole had fewer infusion-related adverse effects and less renal toxicity.79
Pre-emptive treatment
It has long been debated whether using fever as the sole criterion for initiation of antimicrobial treatment is appropriate, especially with the advent of imaging and biomarker assays. It was argued that reserving antifungal treatment for patients with strong evidence of infection in clinical evaluation and non-invasive tests may help bring down cost and drug toxicity, and thereby may have effect on overall mortality. Cordonnier et al.80 conducted a randomized clinical trial to look for survival benefit in empirical versus pre-emptive treatment and concluded that although there was no difference in mortality or nephrotoxicity, pre-emptive treatment was more cost-effective. Thereafter, several randomized control trails and meta-analyses have been published comparing empirical versus pre-emptive use of antimicrobials in high-risk neutropenic patients and although there was no survival benefit, there is no strong evidence supporting a significant difference in cost effectiveness as well, and therefore, guidelines advise caution in using a pre-emptive approach, due to lack of strong evidence.81–84
Recently, there has been a shift from empirical to pre-emptive treatment strategies in clinically stable patients to support resource reduction and a diagnostic-driven approach which can be seen in newer updated guidelines.85,86
Directed treatment
Historically, amphotericin B has been the gold standard for the treatment of IFI. However, infusion-related adverse effects and renal toxicity limit its use, and newer antifungal agents are preferred. Tables 3 and 4 detail the major clinical trials of various antifungal agents used for the treatment of invasive fungal infections.51,75,87–110 For invasive aspergillosis, voriconazole and isavuconazole are the preferred agents with the highest quality of evidence. Voriconazole requires monitoring of serum levels.86,111 Caspofungin and itraconazole have been used, but their efficacy is unproven as data is limited. Liposomal amphotericin B remains efficacious and is used as salvage therapy.112,113 For invasive candidiasis in hematological patients, echinocandins and liposomal amphotericin B are preferred, as is voriconazole, as there is increased innate resistance to fluconazole.111,114 Mucormycosis carries high mortality, ranging from 40% to 80%.115 Management of mucormycosis is challenging and involves a multidisciplinary approach among infectious disease, oncology, and surgical specialists. Liposomal amphotericin B with early surgery is recommended. Posaconazole has shown similar efficacy to liposomal amphotericin B.111,115 Table 5 summarizes suggestive treatment of IFI.
Table 3.
Major clinical trials of antifungal treatment in patients with invasive fungal infections.
| The study, Author | Study type | Study drug 1 | Study drug 2 | Total patients | HSCT patients and special situations | Efficacy outcomes | Safety outcomes |
|---|---|---|---|---|---|---|---|
| Invasive aspergillus | |||||||
| Caillot et al.87 | Prospective, non-randomized, open-label single-agent trial | Itraconazole 200 mg IV every 12 h × 2 days, then once daily for 12 days, then oral 200 mg twice daily from weeks 3 to 14 | Single-drug study | 31 patients | Majority of patients with hematological malignancy, including 6 HSCT patients (3 autologous and 3 allogeneic) | Complete or partial response in 15 patients and stable disease in 6 patients | Well tolerated with no serious adverse events |
| Bowden et al.88 | Double-blind, multicenter, randomized controlled trial | AmB colloidal dispersion (ABCD) 6 mg/kg per day | AmB, 1–1.5 mg/kg per day | 174 patients (88 in group 1 and 86 in group 2) | 38 patients in group 1 and 35 patients in group 2 | Similar in rates of therapeutic response and mortality | Less nephrotoxicity with ABCD but more infusion-related chills and fever as compared with AmB |
| Herbrecht et al.90 | Unblinded, randomized controlled trial | Voriconazole IV two doses of 6 mg/kg of body weight on day 1, then 4 mg/kg twice daily for at least 7 days) followed by 200 mg orally twice daily | AmB deoxycholate IV 1–1.5 mg/kg per day | 277 patients | Allogeneic HSCT (37 in group 1 and 30 in group
2) Autologous HSCT (6 each in both groups) |
Cumulative of complete and partial response 53% in group 1
versus 32% in group 2 12-week survival rate 71% versus 58%; (HR 0.59; 95% CI 0.40–0.88) |
More adverse events in group 2 versus group 1, significant for renal failure, hypokalemia, fever, chills, anaphylaxis, asthenia, or myalgia |
| Herbrecht et al.89 | Prospective, non-randomized phase II single-agent trial | Caspofungin 70 mg loading dose on day 1, then 50 mg/day × 15 days–12 weeks | Single-drug study | 42 patients | 24 patients | 10 patients had complete or partial responses 12 patients had progression of the disease and one patient had stable disease 6- and 12-week survival was 79% and 50%, respectively |
No drug-related serious adverse events or toxicity |
| Cornely et al.91 | Phase II dose-escalation study | Caspofungin IV administered in escalated doses (70 mg, 100 mg, 150 mg, or 200 mg) for a maximum of 28 days | Single-drug study | 46 patients | The majority of patients had hematological malignancies, and many were neutropenic | Favorable treatment response in 70 mg, 100 mg, 150 mg, and 200 mg groups were 44%, 36%, 67%, and 60%, respectively | Mean serious adverse events per patient in the 70 mg, 100 mg, 150 mg, and 200 mg cohorts were 0.2, 1.0, 1.1, and 1.1, respectively |
| Marr et al.92 | Randomized, double-blinded, placebo-controlled trial | Voriconazole IV (6 mg/kg of body weight every 12 h on day 1, then 4 mg/kg every 12 h) × 1 week, then oral 300 mg every 12 h × 5 weeks and IV anidulafungin (200 mg on day 1, then 100 mg every day) × 2–4 weeks | Voriconazole IV (same regimen) and placebo for at least the first two weeks and up to a maximum of 4 weeks | 455 patients | Allogeneic HSCT (44 in group 1 and 42 in group 2) Autologous HSCT (five in group 1 and three in group 2) | Overall mortality 19.5% in group 1 and 27.8% in group 2; difference −8.3, (95% CI −19.0 to 1.5). 12-week mortality 29.3% versus 39.4%; difference 10.1 (95% CI −21.4 to 1.1) | Adverse effects (including hepatotoxicity) were not different |
| SECURE trial, Maertens et al.93 | Phase III, double-blind, randomized controlled trial | Isavuconazole 200 mg, IV three times a day on days 1 and 2, then either IV or orally, once daily | Voriconazole (6 mg/kg IV twice daily on day 1, 4 mg/kg IV twice daily on day 2, then IV 4 mg/kg twice daily or orally 200 mg twice daily from day 3 onwards | 527 patients (263 in group 1 and 264 in group 2) | Allogeneic HSCT (54 in group 1 and 51 in group 2) | No difference in all-cause mortality between the two groups | No difference in treatment-related adverse events Drug-related adverse events higher in group 2 (42% versus 60%, p < 0.001) |
| Invasive candidiasis | |||||||
| Mora-Duarte et al.94 | Blinded, randomized controlled trial | Caspofungin 70 mg loading dose followed by 50 mg/day | AmB 0.6–0.7 mg/kg per day | 239 patients (224 in MITT analysis) |
13% of patients with hematological malignancy and seven patients were status post transplant | Similar treatment success in both groups | Fewer drug-related adverse effects with caspofungin as compared with AmB |
| Rex et al.95 | Multicenter, blinded, randomized controlled trial | Fluconazole 800 mg/day and AmB 0.7 mg/kg per day | Fluconazole 800 mg/day and placebo | 219 patients | 41 patients had cancer, and 5 patients had organ transplantation | Improved overall treatment success and blood clearance with combination therapy | More renal failure with combination therapy and one anaphylaxis in the combination group |
| DiNubile et al.96 | Post-hoc analysis of a double-blind, randomized controlled trial | Caspofungin 70 mg loading dose followed by 50 mg/day | Amphotericin B 0.6–1.0 mg/kg per day | 224 patients in MITT analysis | 33% of patients had active malignancies, a majority of them hematological | Favorable treatment response in 70% of patients in group 1 and 56% in group 2 | |
| Kullberg et al.97 | Multicenter, randomized, non-inferiority study | Voriconazole | AmB followed by fluconazole | 422 patients 370 patients in MITT analysis |
Non-neutropenic patients with candida BSI were included | Both groups were similar in terms of treatment success, time to clear blood cultures | More treatment discontinuation in group 1, although not related to adverse drug events |
| Cornely et al.98 | Post hoc analysis of 2 phase III randomized controlled trials | Trial 1 with micafungin 100 mg/day
versus liposomal AmB 3 mg/kg per
day Trial 2 with micafungin 100 or 150 mg/day versus caspofungin 50 mg/day after 70 mg loading dose |
489 patients in trial 1 and 572 patients in trial 2 | A majority had malignancy, and around 33% of patients were neutropenic | Similar treatment success rates in two trials Micafungin was effective when compared with caspofungin and liposomal AmB |
Similar treatment-related adverse effects across the groups | |
| Andes et al.99 | A patient-level quantitative review of 7 randomized controlled trials | Fluconazole 400 mg/day versus AmB
0.5–0.6 mg/kg per day Caspofungin 50 mg/day versus AmB 0.6–0.7 mg/kg per day Fluconazole 800 mg/day versus AmB 0.6–0.7 mg/kg per day and fluconazole Voriconazole versus AmB followed by fluconazole Anidulafungin versus fluconazole Micafungin versus liposomal AmB Micafungin and fluconazole versus caspofungin and fluconazole |
1915 patients | All patients with candida BSI 4.8% of patients with transplantation |
Treatment with echinocandin antifungal associated with decreased mortality (OR 0.65; 95% CI 0.45–0.94; p = 0.02) | NA | |
| Vazquez et al.100 | Open label, a non-comparative study evaluating IV to oral step-down treatment strategy | Anidulafungin IV 200 mg loading dose, then 100 mg
daily Transition to an oral azole-based regimen |
Continue IV anidulafungin | 282 patients enrolled and 250 in MITT analysis | All patients had candida BSI Severe neutropenia in 11 patients | Similar treatment response rate in both groups | Adverse effects were similar in both groups |
| Fernandez-Ruiz et al.101 | A propensity score analysis from the CANDIPOP surveillance program | Echinocandin-based regimen | Azole-based regimen | 752 episodes of candida BSI 194 patients |
4 patients with HSCT | No difference in clinical failure, persistent candidemia; 30-day all-cause mortality in groups 1 and 2 was 22% and 27%, respectively | NA Initial antifungal therapy type did not relate to poor prognosis |
| The ACTIVE trial, Kullberg et al.102 | Phase III, double-blind, multicenter, non-inferiority, randomized controlled trial | Isavuconazole IV 200 mg three times a day on days 1 and 2, then 200 mg once daily, then switched to oral after 10 days without neutropenia | Caspofungin IV 70 mg on day 1, then 50 mg once daily
Switched to oral voriconazole at 400 mg twice daily on day 1, then 200 mg twice daily after 10 days without neutropenia |
450 patients (221 in group 1 and 219 in group 2) | Immunocompromised patients with invasive candidiasis and or candidemia | Overall positive response in 60% of patients in group 1 and 71%
in group 2 Day 56 mortality similar Time to blood clearance similar in the two groups |
Safety outcomes were similar in the two groups |
| Mucormycosis | |||||||
| Lanternier et al.103 | Prospective pilot study | Liposomal AmB, 10 mg/kg per day | Single-agent study High-dose AmB was combined with surgery if needed |
40 patients (33 patients MITT analysis) | 18 patients with hematological malignancy 5 patients with HSCT |
A 12-week response rate of 45% Overall mortality at week 24 was 53% |
Renal failure and hypokalemia were seen in 40% of the patients |
| The VITAL study, Marty et al.104 | A single-arm, open-label, multicenter, case-control study | Isavuconazole IV 200 mg three times daily × six doses, followed by 200 mg once daily | AmB in matched controls | 46 patients | 22 patients with hematological malignancy and 13 patients with allogeneic HSCT | 42-day all-cause mortality similar between the two groups | Isavuconazole is well tolerated, with gastrointestinal side effects being the most common |
| The MoveOn study, Salmanton-Garcia et al.105 | Case-matched analysis | POSnew and AmB + POSnew | AmB matched controls | 23 patients | Majority with hematological/oncological malignancy | POSnew more effective at day 42 response and lower mortality | POSnew well tolerated |
AmB, amphotericin-B; CI, confidence interval; BSI, bloodstream infection; HR, hazard ratio; HSCT, hematopoietic stem-cell transplant; IV, intravenous; MITT, modified intention-to-treat analysis; NA, not available; OR, odds ratio; POS, posaconazole; POSnew, first-line posaconazole new formulation.
Table 4.
Characteristics of antifungal agents used in the treatment of invasive fungal infections.51,75,106–110
| Type of antifungal agents | Indication | Dose | Half-life | Renal dosing | Hepatic dosing | Adverse effects | Drug interactions | Other remarks, BA and distribution |
|---|---|---|---|---|---|---|---|---|
| Polyenes | ||||||||
| Amphotericin B deoxycholate | Broad-spectrum activity Second-line therapy for IA, IC, fungal endocarditis, disseminated coccidioidomycosis, disseminated histoplasmosis, fusariosis, zygomycosis, cryptococcus meningitis Various formulations have different uses |
0.3–1.5 mg/kg per day variable per indication | Initial 15–48 h, after multiple doses 15 days | Decrease the dose by 50% if renal dysfunction is present | No adjustments necessary | Fever, chills, chest pain, dyspnea, hypoxia, hypotension or hypertension, tachycardia, hypokalemia, hyperbilirubinemia, nausea/vomiting, electrolyte abnormalities (hypocalcemia, hypomagnesemia, hypophosphatemia), thrombocytopenia, abnormal liver function tests, nephrotoxicity, multiorgan failure, cardiac arrest | Increased nephrotoxicity with cyclosporin, foscarnet, and
aminoglycosides Avoid concurrent use with antineoplastic agents, nephrotoxic agents, and leukocyte transfusions |
Causes direct myocardial toxicity High protein binding (>95%) Poor CSF penetration, 0–38% vitreal penetration, poor urine penetration Adequate brain parenchymal concentration |
| Amphotericin B colloid dispersion (amphotec) | IV 3–5 mg/kg per day | 28 h | No adjustment in mild to moderate, not studied in patients with severe impairment | Not studied | Severe hypoxia One study reported the onset of hypoxia beyond the second day of therapy for 170% of patients111 |
|||
| Amphotericin B lipid complex (Abelcet®) | IV 3–5 mg/kg per day | 173 h | Not studied | Not studied | Severe hypoxia | |||
| Amphotericin B Liposomal (amBisome) | The primary therapy for invasive candidiasis, especially
Candida
glabrata or Candida parapsilosis Alternative therapy for IA First-line therapy for serious IFI in the high-risk population, such as HSCT recipients |
IV 3–6 mg/kg per day | 100–53 h | For AKI no dose adjustment needed as only 5 percent is renal clearance, unlikely to be dialyzed, hence no dose adjustment | No adjustment needed. | Triad of pulmonary toxicity (chest pain, dyspnea, and hypoxia); abdominal, flank, or leg pain; or flushing and urticaria | ||
| Azoles | ||||||||
| Fluconazole | Preferred therapy for IC (including Candida
krusei) for patients who are not critically ill
without prior azole exposure Vaginal, esophageal, and oropharyngeal candidiasis Candida UTI Cryptococcal meningitis and disseminated cryptococcosis Prophylactic therapy for candida |
IV, oral, 800 mg on day 1 and then 400 mg daily | 31 h | Reduce dose by 50% for creatinine clearance of <50 ml/min or
patient on peritoneal dialysis For hemodialysis, administer maintenance dose three times/week after hemodialysis |
none | Reversible alopecia, but otherwise extremely well
tolerated Hepatic toxicity (close monitoring of liver function tests) |
All azoles inhibit CYP450 enzymes Interactions with other drugs that are substrates/inhibitors/inducers of the same CYP450 enzyme systems such as antiretrovirals, immunosuppressants, anticonvulsants, and chemotherapeutic agents Increased risk of bleeding with DOACs Therefore drug–drug interactions should be monitored carefully, and the bleeding should be managed promptly Voriconazole has the greatest risk for drug interaction compared with other azoles |
Oral BA 90%, least affected by gastric pH and
contents Least protein binding Good CSF penetration of >60%, 28–75% vitreal penetration, 90% urine penetration |
| Itraconazole | IA, blastomycosis, histoplasmosis, cryptococcosis. | Oral 200 mg twice daily | 24 h | None | None | Severe nausea and diarrhea A unique triad of hypertension, hypokalemia, and edema Negative inotropic effect leading to decompensated heart failure Hepatic toxicity (close monitoring of liver function tests) Hypokalemia and hypomagnesemia |
Oral BA lowered by higher gastric pH (avoid acid suppressants)
and food consumption >90% protein binding Poor CSF, vitreal, and urine penetration (<10%) |
|
| Voriconazole | First line for IA IC in non-neutropenic patients Prophylactic therapy in HSCT recipients Primary therapy in serious IFI-like Scedosporium species and Fusarium species |
IV: loading dose of 6 mg/kg twice daily on day 1 is followed by
4 mg/kg twice daily Oral: loading dose of 400 mg twice daily on day 1, followed by 200 mg twice daily Trough levels should be checked 1–2 weeks after initiation of therapy, and if low, increase dose to 300 mg twice daily |
6 h | IV formulation not recommended in patients with a creatinine clearance <50 ml/min | Reduce maintenance dose by 50% in patients with mild-to-moderate hepatic impairment | Visual disturbance in the form of photopsia or abnormal vision
in up to 45% of patients (resolves with continued therapy)
Cutaneous phototoxicity Hepatic toxicity (close monitoring of liver function tests) Hypokalemia and hypomagnesemia Rash from mild photosensitivity to Stevens–Johnson syndrome QT prolongation (increased risk of arrhythmias, cardiac arrests, and sudden deaths) |
Oral BA is >90% lowered by food consumption. Moderate protein binding (58%). 60% CSF penetration, 38% vitreal penetration, poor urine penetration |
|
| Posaconazole | Second-line therapy for IA, zygomycosis, and
fusariosis Prophylactic therapy for IFI in HSCT recipients or graft-versus-host disease and in patients with hematologic malignancy with prolonged neutropenia after chemotherapy |
Oral prophylaxis of IFIs: 200 mg three times
daily Refractory IFI: 400 mg is used twice daily The maximum dose is 800 mg daily Trough levels monitoring is gaining importance |
25 h | None | None | Hepatic toxicity (close monitoring of liver function tests) and QT prolongation | Oral BA is higher with a high-fat meal or similar composition
nutritional supplement 99% protein binding Poor CSF, vitreal, and urine penetration |
|
| Echinocandins | ||||||||
| Caspofungin | The primary therapy for invasive candidiasis, especially
C. glabrata or C. parapsilosis Second-line therapy for IA |
IV 70 mg on day 1 followed by 50 mg/day | 30 h | None | Reduce maintenance dose to 35 mg daily in patients with moderate impairment | Histamine-mediated infusion-related syndrome Rarely hepatic toxicity, hematologic toxicity Electrolyte abnormalities (hypokalemia, hypomagnesemia) |
Rifampin, phenytoin, carbamazepine, nelfinavir, efavirenz, and
nevirapine may induce the clearance of caspofungin and therefore
warrant increasing the dose to 70 mg/day There are significant interactions between caspofungin and immunosuppressants (such as cyclosporine and tacrolimus) |
High protein binding Poor CSF, vitreal, and urine penetration |
| Micafungin | Primary therapy for invasive candidiasis, especially
C. glabrata or C. parapsilosis |
IV 100 mg/day for treatment of candidemia and 100–150 mg/day for treatment of IA | 15 h | None | None | Histamine-mediated infusion-related syndrome Rarely hepatic toxicity, hematologic toxicity |
Micafungin increases plasma concentration levels of cyclosporine, sirolimus, and nifedipine | High protein binding Poor CSF, vitreal, and urine penetration |
| Anidulafungin | Primary therapy for invasive candidiasis, especially
C. glabrata or C. parapsilosis |
IV 200 mg on day 1 followed by 100 mg/day | 26 h | None | None | Histamine-mediated infusion-related syndrome | No known drug–drug interaction was seen | High protein binding Poor CSF, vitreal, and urine penetration |
| Flucytosine (pyrimidine analogue) | Cryptococcal meningitis, Candidiasis of CNS, UTI or endophthalmitis | Oral, 50–100 mg/kg per hour in divided doses every 6 h | 2–5 h | In hemodialysis, 25–50 mg/kg per dose every 48–72 h | None | Moderate-to-severe hepatic and hematologic toxicity | Increased toxicity with amphotericin B | 4% protein binding, 75% CSF penetration, 49% vitreal penetration, 90% urine penetration |
AKI, acute kidney injury; BA, bioavailability; CNS, central nervous system; CSF, cerebrospinal fluid; DOACs, direct oral anticoagulants; HSCT, hematopoietic stem-cell transplant; IA, invasive aspergillosis; IC, invasive candidiasis; IFI, invasive fungal infection; IV, intravenous; UTI, urinary tract infection.
Table 5.
| Clinical condition | Preferred treatment | Alternative treatment |
|---|---|---|
| Invasive pulmonary aspergillosis Invasive sinus aspergillosis CNS aspergillosis Cardiac aspergillosis Osteomyelitis or septic arthritis due to aspergillus |
Voriconazole | Liposomal amphotericin B Amphotericin B lipid complex as salvage therapy |
| Invasive candidiasis Candidemia |
Micafungin Anidulafungin Caspofungin |
Liposomal amphotericin B |
| Mucormycosis | Amphotericin B deoxycholate Liposomal amphotericin B Amphotericin B lipid complex Surgery indicated for rhino-orbital-cerebral, soft-tissue and pulmonary infection |
Posaconazole Salvage therapy Posaconazole Lipid amphotericin B + caspofungin Lipid amphotericin B + posaconazole |
CNS, central nervous system.
Persistent fever that does not resolve after 5 days of broad-spectrum antibacterial agents during neutropenia requires extensive reassessment into cause, with due consideration given to IFI. The Infectious Diseases Society of America (IDSA) guideline recommends a set of cultures and diagnostic imaging workup (chest radiograph, chest computed tomography) to rule out fungal infections in patients with neutropenia expected to last >7 days and persistent fever. For persistently febrile patients with pulmonary nodules or nodular pulmonary infiltrates, invasive mold infection should be strongly suspected, and prompt assessment frequently requires bronchoscopy with BAL with cultures, stains, and aspergillus GM antigen testing. The guidelines recommend adding an empiric antifungal agent after 4–7 days in high-risk neutropenic patients in whom total duration of neutropenia exceeds 7 days and who have persistent or recurrent fever without isolation of a clear causative agent. Based on clinical acumen, empirical agents should be considered even earlier in clinically unstable patients. For a patient already receiving anti-mold prophylaxis, switching to a different class of anti-mold antifungal given intravenously should be considered. The choice of agent for empiric antifungal therapy depends upon which fungi are most likely to be causing infection, as well as the toxicity profiles and cost. The treatment and diagnostic algorithms are extensively explained in the IDSA guideline.117,118
Conclusion
Despite advances in the use of HSCT treatment for hematopoietic stem-cell-related disorders, fungal infections continue to cause significant morbidity and mortality. Patient risk factors and the type of conditioning regimen impact the development of IFI. Close initial follow up, early diagnosis, and prophylactic treatment with antifungal agents are important management components. Newer antifungal agents have been developed, which have at least similar efficacy when compared with amphotericin B and a better safety profile. Combination therapy is considered for salvage. Therapeutic drug monitoring and checking for drug interactions is critical. Further research to improve diagnostics and therapeutics is required to improve outcomes in patients with invasive fungal infections.
Footnotes
Author contributions: All authors made substantial contributions to the conception or design of the work, the acquisition, analysis or interpretation of data for the work, drafted and assisted in critical revisions to work for important intellectual content, provided final approval of the version to be published, and are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics statement: Our study did not require an ethical board approval because it did not contain human or animal trials.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mandeep Singh Rahi  https://orcid.org/0000-0002-4445-5758
https://orcid.org/0000-0002-4445-5758
Contributor Information
Mandeep Singh Rahi, Division of Pulmonary Diseases and Critical Care Medicine, Yale–New Haven Health Bridgeport Hospital, 267 Grant Street, Bridgeport, CT 06610, USA.
Vishal Jindal, Division of Hematology and Oncology, Oakland University–William Beaumont School of Medicine, Royal Oak, MI, USA.
Prachi Pednekar, Department of Internal Medicine, Yale–New Haven Health Bridgeport Hospital, Bridgeport, CT, USA.
Jay Parekh, Department of Internal Medicine, Yale–New Haven Health Bridgeport Hospital, Bridgeport, CT, USA.
Kulothungan Gunasekaran, Division of Pulmonary Diseases and Critical Care Medicine, Yale–New Haven Health Bridgeport Hospital, Bridgeport, CT, USA.
Sorabh Sharma, Department of Internal Medicine, Banner University Medical Center, Tucson, AZ, USA.
Michael Stender, Division of Hematology and Oncology, Oakland University–William Beaumont School of Medicine, Royal Oak, MI, USA.
Ishmael A. Jaiyesimi, Division of Hematology and Oncology, Oakland University–William Beaumont School of Medicine, Royal Oak, MI, USA
References
- 1.D’Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 2020; 26: e177–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014; 371: 895–905. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet 2002; 359: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 4.Styczynski J, Czyzewski K, Wysocki M, et al. Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: a multicentre nationwide study. Clin Microbiol Infect 2016; 22: 179.e171–179.e110. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 2010; 50: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 6.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis 2007; 45: 1161–1170. [DOI] [PubMed] [Google Scholar]
- 7.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter prospective antifungal therapy (PATH) alliance registry. Clin Infect Dis 2009; 48: 265–273. [DOI] [PubMed] [Google Scholar]
- 8.Abad A, Fernandez-Molina JV, Bikandi J, et al. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 2010; 27: 155–182. [DOI] [PubMed] [Google Scholar]
- 9.Segal BH.Aspergillosis. N Engl J Med 2009; 360: 1870–1884. [DOI] [PubMed] [Google Scholar]
- 10.Lass-Florl C.Treatment of infections due to Aspergillus terreus species complex. J Fungi (Basel) 2018; 4: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Person AK, Kontoyiannis DP, Alexander BD.Fungal infections in transplant and oncology patients. Infect Dis Clin North Am 2010; 24: 439–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesaro S, Tridello G, Blijlevens N, et al. Incidence, risk factors, and long-term outcome of acute leukemia patients with early candidemia after allogeneic stem cell transplantation: a study by the acute leukemia and infectious diseases working parties of European Society for Blood and Marrow Transplantation. Clin Infect Dis 2018; 67: 564–572. [DOI] [PubMed] [Google Scholar]
- 13.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation–a prospective, randomized, double-blind study. J Infect Dis 1995; 171: 1545–1552. [DOI] [PubMed] [Google Scholar]
- 14.Cornely OA, Aversa F, Cook P, et al. Evaluating the role of prophylaxis in the management of invasive fungal infections in patients with hematologic malignancy. Eur J Haematol 2011; 87: 289–301. [DOI] [PubMed] [Google Scholar]
- 15.Marques DS, Pinho Vaz C, Branca R, et al. Rhizomucor and scedosporium infection post hematopoietic stem-cell transplant. Case Rep Med 2011; 2011: 830769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husain S, Munoz P, Forrest G, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis 2005; 40: 89–99. [DOI] [PubMed] [Google Scholar]
- 17.Cuenca-Estrella M, Bernal-Martinez L, Isla G, et al. Incidence of zygomycosis in transplant recipients. Clin Microbiol Infect 2009; 15(Suppl. 5): 37–40. [DOI] [PubMed] [Google Scholar]
- 18.Cordonnier C, Cesaro S, Maschmeyer G, et al. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016; 71: 2379–2385. [DOI] [PubMed] [Google Scholar]
- 19.Williams KM, Ahn KW, Chen M, et al. The incidence, mortality and timing of Pneumocystis jiroveci pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant 2016; 51: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firacative C, Carvajal SK, Escandón P, et al. Cryptococcosis in hematopoietic stem cell transplant recipients: a rare presentation warranting recognition. Can J Infect Dis Med Microbiol 2020; 2020: 3713241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glenn TJ, Blair JE, Adams RH.Coccidioidomycosis in hematopoietic stem cell transplant recipients. Med Mycol 2005; 43: 705–710. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan M, Swierzbinski MJ, Maxwell S, et al. Pulmonary histoplasma infection after allogeneic hematopoietic stem cell transplantation: case report and review of the literature. Open Forum Infect Dis 2017; 4: ofx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JK, Cho SY, Yoon SS, et al. Epidemiology and risk factors for invasive fungal diseases among allogeneic hematopoietic stem cell transplant recipients in Korea: results of “RISK” study. Biol Blood Marrow Transplant 2017; 23: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 24.Fayard A, Daguenet E, Blaise D, et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: a study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transplant 2019; 54: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 25.Mohty R, Brissot E, Battipaglia G, et al. Infectious complications after post-transplantation cyclophosphamide and anti-thymocyte globulin-based haploidentical stem cell transplantation. Br J Haematol 2019; 187: e64–e68. [DOI] [PubMed] [Google Scholar]
- 26.Omer AK, Ziakas PD, Anagnostou T, et al. Risk factors for invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a single center experience. Biol Blood Marrow Transplant 2013; 19: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 27.Harrison N, Mitterbauer M, Tobudic S, et al. Incidence and characteristics of invasive fungal diseases in allogeneic hematopoietic stem cell transplant recipients: a retrospective cohort study. BMC Infect Dis 2015; 15: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan J, Wannemuehler KA, Marr KA, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol 2005; 43(Suppl. 1): S49–S58. [DOI] [PubMed] [Google Scholar]
- 29.Wingard JR, Hsu J, Hiemenz JW.Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol Oncol Clin North Am 2011; 25: 101–116. [DOI] [PubMed] [Google Scholar]
- 30.Gyurkocza B, Sandmaier BM.Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 2014; 124: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dignani MC.Epidemiology of invasive fungal diseases on the basis of autopsy reports. F1000Prime Rep 2014; 6: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clin Infect Dis 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrell M, Fraser VJ, Kollef MH.Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005; 49: 3640–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelz RK, Lipsett PA, Swoboda SM, et al. The diagnostic value of fungal surveillance cultures in critically ill patients. Surg Infect (Larchmt) 2000; 1: 273–281. [DOI] [PubMed] [Google Scholar]
- 35.Arvanitis M, Anagnostou T, Fuchs BB, et al. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev 2014; 27: 490–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath JA, Dummer S.The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med 1996; 100: 171–178. [DOI] [PubMed] [Google Scholar]
- 37.Terrero-Salcedo D, Powers-Fletcher MV.Updates in laboratory diagnostics for invasive fungal infections. J Clin Microbiol 2020; 58: e01487-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reischies FM, Prattes J, Woelfler A, et al. Diagnostic performance of 1,3-beta-D-glucan serum screening in patients receiving hematopoietic stem cell transplantation. Transpl Infect Dis 2016; 18: 466–470. [DOI] [PubMed] [Google Scholar]
- 39.Lamoth F, Cruciani M, Mengoli C, et al. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the third European conference on infections in leukemia (ECIL-3). Clin Infect Dis 2012; 54: 633–643. [DOI] [PubMed] [Google Scholar]
- 40.Hage CA, Carmona EM, Epelbaum O, et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019; 200: 535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher CE, Stevens AM, Leisenring W, et al. The serum galactomannan index predicts mortality in hematopoietic stem cell transplant recipients with invasive aspergillosis. Clin Infect Dis 2013; 57: 1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikulska M, Raiola AM, Signori A, et al. Screening with serum galactomannan might be associated with better outcome than symptom-triggered galactomannan testing in allogeneic HSCT recipients with invasive aspergillosis. Clin Infect Dis 2013; 57: 1786–1787. [DOI] [PubMed] [Google Scholar]
- 43.Mengoli C, Cruciani M, Barnes RA, et al. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect Dis 2009; 9: 89–96. [DOI] [PubMed] [Google Scholar]
- 44.Avni T, Levy I, Sprecher H, et al. Diagnostic accuracy of PCR alone compared to galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis: a systematic review. J Clin Microbiol 2012; 50: 3652–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avni T, Leibovici L, Paul M.PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J Clin Microbiol 2011; 49: 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 2015; 60: 892–899. [DOI] [PubMed] [Google Scholar]
- 47.Arvanitis M, Anagnostou T, Mylonakis E.Galactomannan and polymerase chain reaction-based screening for invasive Aspergillosis among high-risk hematology patients: a diagnostic meta-analysis. Clin Infect Dis 2015; 61: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 48.Morjaria S, Frame J, Franco-Garcia A, et al. Clinical performance of (1,3) beta-D glucan for the diagnosis of pneumocystis pneumonia (PCP) in cancer patients tested with PCP polymerase chain reaction. Clin Infect Dis 2019; 69: 1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armstrong AE, Rossoff J, Hollemon D, et al. Cell-free DNA next-generation sequencing successfully detects infectious pathogens in pediatric oncology and hematopoietic stem cell transplant patients at risk for invasive fungal disease. Pediatr Blood Cancer 2019; 66: e27734. [DOI] [PubMed] [Google Scholar]
- 50.Mahfouz T, Anaissie E.Prevention of fungal infections in the immunocompromised host. Curr Opin Investig Drugs 2003; 4: 974–990. [PubMed] [Google Scholar]
- 51.Michallet M, Ito JI.Approaches to the management of invasive fungal infections in hematologic malignancy and hematopoietic cell transplantation. J Clin Oncol 2009; 27: 3398–3409. [DOI] [PubMed] [Google Scholar]
- 52.Levine AS, Siegel SE, Schreiber AD, et al. Protected environments and prophylactic antibiotics. A prospective controlled study of their utility in the therapy of acute leukemia. N Engl J Med 1973; 288: 477–483. [DOI] [PubMed] [Google Scholar]
- 53.Williams C, Whitehouse JM, Lister TA, et al. Oral anticandidal prophylaxis in patients undergoing chemotherapy for acut- leukemia. Med Pediatr Oncol 1977; 3: 275–280. [DOI] [PubMed] [Google Scholar]
- 54.Hann IM, Prentice HG, Corringham R, et al. Ketoconazole versus nystatin plus amphotericin B for fungal prophylaxis in severely immunocompromised patients. Lancet 1982; 1: 826–829. [DOI] [PubMed] [Google Scholar]
- 55.Jones PG, Kauffman CA, McAuliffe LS, et al. Efficacy of ketoconazole v nystatin in prevention of fungal infections in neutropenic patients. Arch Intern Med 1984; 144: 549–551. [PubMed] [Google Scholar]
- 56.Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326: 845–851. [DOI] [PubMed] [Google Scholar]
- 57.Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 2000; 96: 2055–2061. [PubMed] [Google Scholar]
- 58.Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 2004; 103: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 59.Van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 60.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335–347. [DOI] [PubMed] [Google Scholar]
- 61.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116: 5111–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nucci M, Biasoli I, Akiti T, et al. A double-blind, randomized, placebo-controlled trial of itraconazole capsules as antifungal prophylaxis for neutropenic patients. Clin Infect Dis 2000; 30: 300–305. [DOI] [PubMed] [Google Scholar]
- 63.Winston DJ, Maziarz RT, Chandrasekar PH, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med 2003; 138: 705–713. [DOI] [PubMed] [Google Scholar]
- 64.Van Burik JH, Leisenring W, Myerson D, et al. The effect of prophylactic fluconazole on the clinical spectrum of fungal diseases in bone marrow transplant recipients with special attention to hepatic candidiasis. An autopsy study of 355 patients. Medicine (Baltimore) 1998; 77: 246–254. [DOI] [PubMed] [Google Scholar]
- 65.El Cheikh J, Castagna L, Wang L, et al. Once-weekly liposomal amphotericin B for prophylaxis of invasive fungal infection after graft-versus-host disease in allogeneic hematopoietic stem cell transplantation: a comparative retrospective single-center study. Hematol Oncol Stem Cell Ther 2010; 3: 167–173. [DOI] [PubMed] [Google Scholar]
- 66.Chaftari AM, Hachem RY, Ramos E, et al. Comparison of posaconazole versus weekly amphotericin B lipid complex for the prevention of invasive fungal infections in hematopoietic stem-cell transplantation. Transplantation 2012; 94: 302–308. [DOI] [PubMed] [Google Scholar]
- 67.Cordonnier C, Mohty M, Faucher C, et al. Safety of a weekly high dose of liposomal amphotericin B for prophylaxis of invasive fungal infection in immunocompromised patients: PROPHYSOME study. Int J Antimicrob Agents 2008; 31: 135–141. [DOI] [PubMed] [Google Scholar]
- 68.El-Cheikh J, Faucher C, Furst S, et al. High-dose weekly liposomal amphotericin B antifungal prophylaxis following reduced-intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 39: 301–306. [DOI] [PubMed] [Google Scholar]
- 69.Offner F, Cordonnier C, Ljungman P, et al. Impact of previous aspergillosis on the outcome of bone marrow transplantation. Clin Infect Dis 1998; 26: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 70.Cordonnier C, Maury S, Pautas C, et al. Secondary antifungal prophylaxis with voriconazole to adhere to scheduled treatment in leukemic patients and stem cell transplant recipients. Bone Marrow Transplant 2004; 33: 943–948. [DOI] [PubMed] [Google Scholar]
- 71.Cordonnier C, Rovira M, Maertens J, et al. Voriconazole for secondary prophylaxis of invasive fungal infections in allogeneic stem cell transplant recipients: results of the VOSIFI study. Haematologica 2010; 95: 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002; 34: 730–751. [DOI] [PubMed] [Google Scholar]
- 73.Prentice HG, Hann IM, Herbrecht R, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol 1997; 98: 711–718. [DOI] [PubMed] [Google Scholar]
- 74.Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med 1999; 340: 764–771. [DOI] [PubMed] [Google Scholar]
- 75.Asano-Mori Y.Fungal infections after hematopoietic stem cell transplantation. Int J Hematol 2010; 91: 576–587. [DOI] [PubMed] [Google Scholar]
- 76.Boogaerts M, Winston DJ, Bow EJ, et al. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy. A randomized, controlled trial. Ann Intern Med 2001; 135: 412–422. [DOI] [PubMed] [Google Scholar]
- 77.Schuler U, Bammer S, Aulitzky WE, et al. Safety and efficacy of itraconazole compared to amphotericin B as empirical antifungal therapy for neutropenic fever in patients with haematological malignancy. Onkologie 2007; 30: 185–191. [DOI] [PubMed] [Google Scholar]
- 78.Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 2004; 351: 1391–1402. [DOI] [PubMed] [Google Scholar]
- 79.Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 2002; 346: 225–234. [DOI] [PubMed] [Google Scholar]
- 80.Cordonnier C, Pautas C, Maury S, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis 2009; 48: 1042–1051. [DOI] [PubMed] [Google Scholar]
- 81.Fung M, Kim J, Marty FM, et al. Meta-analysis and cost comparison of empirical versus pre-emptive antifungal strategies in hematologic malignancy patients with high-risk febrile neutropenia. PLoS One 2015; 10: e0140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant 2011; 46: 709–718. [DOI] [PubMed] [Google Scholar]
- 83.Slavin MA, Grigg AP, Schwarer AP, et al. A randomized comparison of empiric or pre-emptive antibiotic therapy after hematopoietic stem cell transplantation. Bone Marrow Transplant 2007; 40: 157–163. [DOI] [PubMed] [Google Scholar]
- 84.Walker BS, Schmidt RL, Tantravahi S, et al. Cost-effectiveness of antifungal prophylaxis, preemptive therapy, or empiric treatment following allogeneic hematopoietic stem cell transplant. Transpl Infect Dis 2019; 21: e13148. [DOI] [PubMed] [Google Scholar]
- 85.Ruhnke M, Cornely OA, Schmidt-Hieber M, et al. Treatment of invasive fungal diseases in cancer patients-revised 2019 recommendations of the infectious diseases working party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Mycoses 2020; 63: 653–682. [DOI] [PubMed] [Google Scholar]
- 86.Patterson TF, Thompson GR, III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63: e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caillot D, Bassaris H, McGeer A, et al. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin Infect Dis 2001; 33: e83–e90. [DOI] [PubMed] [Google Scholar]
- 88.Bowden R, Chandrasekar P, White MH, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis 2002; 35: 359–366. [DOI] [PubMed] [Google Scholar]
- 89.Herbrecht R, Maertens J, Baila L, et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 2010; 45: 1227–1233. [DOI] [PubMed] [Google Scholar]
- 90.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347: 408–415. [DOI] [PubMed] [Google Scholar]
- 91.Cornely OA, Vehreschild JJ, Vehreschild MJ, et al. Phase II dose escalation study of caspofungin for invasive aspergillosis. Antimicrob Agents Chemother 2011; 55: 5798–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 2015; 162: 81–89. [DOI] [PubMed] [Google Scholar]
- 93.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387: 760–769. [DOI] [PubMed] [Google Scholar]
- 94.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347: 2020–2029. [DOI] [PubMed] [Google Scholar]
- 95.Rex JH, Pappas PG, Karchmer AW, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis 2003; 36: 1221–1228. [DOI] [PubMed] [Google Scholar]
- 96.DiNubile MJ, Hille D, Sable CA, et al. Invasive candidiasis in cancer patients: observations from a randomized clinical trial. J Infect 2005; 50: 443–449. [DOI] [PubMed] [Google Scholar]
- 97.Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 2005; 366: 1435–1442. [DOI] [PubMed] [Google Scholar]
- 98.Cornely OA, Marty FM, Stucker F, et al. Efficacy and safety of micafungin for treatment of serious candida infections in patients with or without malignant disease. Mycoses 2011; 54: e838–e847. [DOI] [PubMed] [Google Scholar]
- 99.Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54: 1110–1122. [DOI] [PubMed] [Google Scholar]
- 100.Vazquez J, Reboli AC, Pappas PG, et al. Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis 2014; 14: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernández-Ruiz M, Aguado JM, Almirante B, et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis 2014; 58: 1413–1421. [DOI] [PubMed] [Google Scholar]
- 102.Kullberg BJ, Viscoli C, Pappas PG, et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: the ACTIVE trial. Clin Infect Dis 2019; 68: 1981–1989. [DOI] [PubMed] [Google Scholar]
- 103.Lanternier F, Poiree S, Elie C, et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother 2015; 70: 3116–3123. [DOI] [PubMed] [Google Scholar]
- 104.Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016; 16: 828–837. [DOI] [PubMed] [Google Scholar]
- 105.Salmanton-García J, Seidel D, Koehler P, et al. Matched-paired analysis of patients treated for invasive mucormycosis: standard treatment versus posaconazole new formulations (MoveOn). J Antimicrob Chemother 2019; 74: 3315–3327. [DOI] [PubMed] [Google Scholar]
- 106.Ashley ESD, Lewis R, Lewis JS, et al. Pharmacology of systemic antifungal agents. Clinical Infectious Diseases 2006; 43: S28–S39. [Google Scholar]
- 107.Mazzei T, Novelli A.Pharmacological properties of antifungal drugs with a focus on anidulafungin. Drugs 2009; 69(Suppl. 1): 79–90. [DOI] [PubMed] [Google Scholar]
- 108.Wilson DT, Drew RH, Perfect JR.Antifungal therapy for invasive fungal diseases in allogeneic stem cell transplant recipients: an update. Mycopathologia 2009; 168: 313–327. [DOI] [PubMed] [Google Scholar]
- 109.Gunasekaran K, Rajasurya V, Devasahayam J, et al. A review of the incidence diagnosis and treatment of spontaneous hemorrhage in patients treated with direct oral anticoagulants. J Clin Med 2020; 9: 2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang SH, Chou IJ, Yeh YH, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017; 318: 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017; 102: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allen U.Antifungal agents for the treatment of systemic fungal infections in children. Can J Infect Dis Med Microbiol 2010; 21: e116–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med 2011; 183: 96–128. [DOI] [PubMed] [Google Scholar]
- 114.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62: e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019; 19: e405–e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pound MW, Townsend ML, Dimondi V, et al. Overview of treatment options for invasive fungal infections. Med Mycol 2011; 49: 561–580. [DOI] [PubMed] [Google Scholar]
- 117.Villafuerte-Gutierrez P, Villalon L, Losa JE, et al. Treatment of febrile neutropenia and prophylaxis in hematologic malignancies: a critical review and update. Adv Hematol 2014; 2014: 986938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52: e56–e93. [DOI] [PubMed] [Google Scholar]