Abstract
YY-20394, an oral phosphatidylinositol 3-kinase delta (PI3Kδ) inhibitor, was investigated in a first-in-human study of patients with relapsed or refractory B-cell malignancies. During dose escalation, 25 patients received 20–200 mg of YY-20394 daily. The primary outcome measures were tolerability and dose-limiting toxicity (DLT). The secondary outcomes were pharmacokinetic parameters, progression-free survival (PFS) and the objective response rate (ORR). Since no patients experienced DLT, the maximum tolerated dose (MTD) was not reached. The majority (≥ 5%) of drug-related adverse events were ≥ grade III, being neutropenia (44.0%), pneumonia (16.0%), hyperuricemia (12.0%), lymphocythemia (8.0%), leukopenia (8.0%) and pneumonitis (8.0%). The overall ORR was 64.0% (95% confidence interval (CI): 45.2, 82.8%) including 5 patients with complete remission (CR), 11 with partial remission (PR), 2 with stable disease (SD) and 7 with progressive disease (PD), while the disease control rate (DCR) was 72.0% (95% CI: 54.4, 89.6%). The ORR of 10 patients with follicular lymphoma was 90%. The median PFS time was 255 days. One PR patient with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who received 40 mg q.d. had a durable response of around 36 months. The median PFS time of 10 patients with follicular lymphoma was 300 days. A recommended phase 2 dose of 80 mg q.d. was established. Considering that YY-20394 was well-tolerated with promising preliminary efficacy, further development is warranted.
Trial registration clinicaltrials.gov, NCT03757000, retrospectively registered, November 28, 2018, https://clinicaltrials.gov/ct2/show/NCT03757000?term=NCT03757000&draw=2&rank=1.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-021-01140-z.
Keywords: Linperlisib, PI3Kδ inhibitor, Dose-limiting toxicity, Non-Hodgkin’s lymphoma, Pharmacokinetics
To the editor
The selective PIP2 3-kinase δ (PI3Kδ) inhibitor idelalisib in combination with rituximab [1–3] and the PI3K-δ/γ inhibitor duvelisib (IPI-145) [4] have been approved to treat B-cell malignancies. In addition, a selective PI3Kδ inhibitor parsaclisib (INCB050465) is undergoing phase 2 trials [5, 6]. We report the first-in-human clinical investigation of YY-20394, a novel PI3Kδ-selective inhibitor, in a dose escalation study in patients with relapsed or refractory B-cell malignancies to evaluate its safety, pharmacokinetic (PK) parameters and efficacy.
YY-20394 [N-[5-[6-fluoro-8-[[4-(1-hydroxy-1-methylethyl)-1-piperidinyl]methyl]-2-(4-morpholinyl)-4-quinazolinyl]-2-methoxy-3-pyridinyl]-methanesulfonamide] is structurally different from idelalisib, and is a potent PI3Kδ inhibitor (IC50: 4.6 nM) with less activity against PI3Kγ giving a kinase inhibition profile that is more PI3Kδ-selective by nearly 2 orders of magnitude (Additional file 1: Table S1).
Patients ≥ 18 years old with refractory or relapsed B-cell malignancies were enrolled from November, 2017 to completion of the trial in November, 2019. Inclusion and exclusion criteria are listed in Additional file 2 and baseline patient characteristics in Additional file 3: Table S2.
Of the 27 enrolled patients, 25 were evaluable including 10 follicular lymphoma (FL), 4 mantle cell lymphomas (MCL), 4 chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), 2 diffuse large B-cell lymphoma (DLBCL), 3 DLBCL/FL, 1 marginal zone lymphoma (MZL) and 1 lymphatic plasma cell lymphoma (LPL) patients. During dose escalation, patients received YY-20394 tablets q.d. at dosages of 20, 40, 80, 140 or 200 mg. The maximum tolerated dose (MTD), dose escalation phase and dose-limiting toxicity (DLT) as well as hematological toxicity classifications are described in Additional file 4. The primary endpoints were safety, tolerability and the MTD of YY-20394. Secondary endpoints were PK parameters and efficacy. Response criteria followed the revised International Research Working Group (IRWG) for non-Hodgkin lymphomas (NHL) [7], and the International Working Group on Chronic Lymphocytic Leukemia (IWCLL) criteria for CLL [8]. Efficacy determinations were the objective response rate (ORR), disease control rate (DCR), complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD) and progression-free survival (PFS).
The safety evaluation of YY-20394 included adverse events (AEs) and serious AEs (SAEs) by standard categorizations. All 25 patients had at ≥ 1 AE. Thirteen (52.0%) and 9 (36.0%) patients experienced SAEs and drug-related AEs. The drug-related AEs that occurred in ≥ 20% of patients were neutropenia (68.0%), leukopenia (44.0%), elevated lactate dehydrogenase (44.0%), elevated α-hydroxybutyrate dehydrogenase (24.0%), thrombocytopenia (20.0%) and hyperuricemia (20.0%). Those that occurred in ≥ 5% of patients are also listed (Table 1). Among 32 ≥ grade III AEs, most were grade III; 3 cases of grade IV hyperuricemia and 4 grade IV neutropenia, but no grade V AEs occurred. Overall, YY-20394 had a manageable safety profile. It is noteworthy that unlike other PI3K inhibitors, the incidence of diarrhea, colitis, and hepatotoxicities [9] was very low.
Table 1.
Drug-related adverse events occurring in ≥ 5% of evaluable patients at grade III or greater
| Drug-related adverse events categorized by SOC and PT | Number of patients with grade I/II at > 5% incidence | Number of patients with ≥ grade III |
|---|---|---|
| Hematological | ||
| Neutropenia | 17 (68.0) | 11 (44.0) |
| Leukopenia | 11 (44.0) | 2 (8.0) |
| Thrombocytopenia | 5 (20.0) | 1 (4.0) |
| Lymphocythemia | 3 (12.0) | 2 (8.0) |
| Anemia | 3 (12.0) | 0 |
| Leukocytosis | 2 (8.0) | 0 |
| Non-hematological | ||
| Elevated serum lactate dehydrogenase | 11 (44.0) | 1 (4.0) |
| Elevated serum α-hydroxybutyrate dehydrogenase | 6 (24.0) | 1 (4.0) |
| Hyperuricemia | 5 (20.0) | 3 (12.0) |
| Upper respiratory tract infection | 4 (16.0) | 1 (4.0) |
| Pneumonia | 4 (16.0) | 4 (16.0) |
| Proteinuria | 4 (16.0) | 0 |
| Hyperbilirubinemia | 3 (12.0) | 0 |
| Elevated alanine aminotransferase | 3 (12.0) | 0 |
| Elevated aspartate aminotransferase | 2 (8.0) | 0 |
| Elevated serum alkaline phosphatase | 3 (12.0) | 0 |
| Weight loss | 3 (12.0) | 0 |
| Pneumonitis | 3 (12.0) | 2 (8.0) |
| Weight gain | 2 (8.0) | 1 (4.0) |
| Elevated γ-glutamyltransferase | 2 (8.0) | 0 |
| Elevated bilirubin | 2 (8.0) | 0 |
| Diarrhea | 2 (8.0) | 0 |
| Cough | 2 (8.0) | 0 |
| Oropharyngeal pain | 2 (8.0) | 0 |
| Maculopapule | 2 (8.0) | 0 |
| Fever | 2 (8.0) | 0 |
| Fatigue | 2 (8.0) | 0 |
AE adverse event, SOC system organ class, PT preferred term
After single administrations of YY-20394 (20 to 140 mg), terminal elimination was consistent and in vivo exposure increased proportionally in a dose-dependent manner (Cmax, AUC0-t, AUC0-∞) (Additional file 5: Table S3). Also, the PK parameters after multiple administrations revealed that the exposure of YY-20394 (Cmax, AUC0-t, AUC0-∞) increased with dosage (20 to 200 mg) (Additional file 6: Table S4). The 80 mg dose level produced a serum concentration of YY-20394, corresponding to 90% inhibition of basophil activation in vitro.
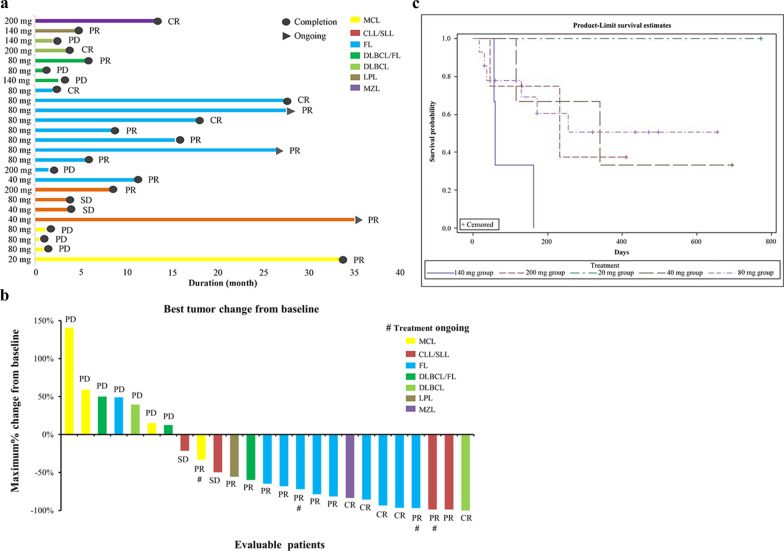
YY-20394 treatment produced an overall 64.0% ORR (16/25) (95%confidence interval (CI): 45.2, 82.8%) and a 72.0% DCR (18/25) (95%CI: 54.4, 89.6%) in B-cell malignancies, including 5 CR, 11 PR, 2 SD and 7 PD cases. Notably, in the FL patients, a 90% ORR (9/10) (95%CI: 71.4, 100.0) and 90% DCR (9/10) (95%CI: 71.4, 100.0) were found (Fig. 1a), with 3 CR (80 mg), 6 PR (1/40 mg and 5/80 mg) and 1 PD (200 mg) (Fig. 1b), with a median PFS time of 300 days. The median PFS was 255 days when all evaluable patients data were combined, with the longest treatment duration being 36 months (40 mg, CLL/ SLL patient) (Fig. 1c).
Fig. 1.
Efficacy evaluation of YY-20394 treatments in the dose escalation study of B-cell malignancies. a Overall efficacy chart of YY-20394. b Waterfall plot of overall tumor changes from baseline #indicates transient staging with ongoing treatment at the end of the study period. c PFS curve (days) in the 5 patient dosing groups. Note: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CR, complete remission; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LPL, Lymphatic plasma cell lymphoma; MCL, mantle cell lymphoma; MZL marginal zone lymphoma; PD, progressive disease; PR, partial remission; SD, stable disease
From the combination of safety, PK, ORR and DOR data, the recommended phase 2 dose for YY-20394 monotherapy was established at 80 mg q.d.
With its excellent efficacy and tolerability in aggressive lymphomas the clinical development of YY-20394 as a novel treatment for relapsed or refractory hematological malignancies is warranted.
Supplementary information
Additional file 1.Table S1: YY-20394 is highly selective in targeting PI3Kδ.
Additional file 2. Inclusion and exclusion criteria.
Additional file 3.Table S2: Basic characteristics of patients in each group.
Additional file 4. The methods and definition of MTD, dose escalation phase and DLT as well as hematological toxicity.
Additional file 5.Table S3: Mean pharmacokinetic parameters of patients after a single dose in each dosage group.
Additional file 6.Table S4: Mean pharmacokinetic parameters of patients after multiple administrations in each dosage group.
Acknowledgements
We thank Shanghai Yingli Pharmaceutical Co., Ltd. for sponsoring this study, and providing medical writing and editorial assistance, and Dr. Jia He and colleagues from the Department of Health Statistics, Second Military Medical University. We also thank the patients enrolled in this trial.
Abbreviations
- AEs
Adverse events
- CI
Confidence interval
- CLL
Chronic lymphocytic leukemia
- CR
Complete remission
- DCR
Disease control rate
- DLBCL
Diffuse large B-cell lymphomas
- DLT
Dose-limiting toxicity
- FL
Follicular lymphoma
- IRWG
International Research Working Group
- IWCLL
International Working Group on Chronic Lymphocytic Leukemia
- MTD
Maximum tolerated dose
- NHL
Non-Hodgkin lymphomas
- ORR
Objective response rate
- PD
Progressive disease
- PFS
Progression free survival
- PI3Kδ
Phosphatidylinositol 3-kinase delta
- PK
Pharmacokinetic
- PR
Partial remission
- SAEs
Serious AEs
- SD
Stable disease
- SLL
Small lymphocytic lymphoma
Authors' contributions
JQ, YS, HB and LQ were responsible for the conception and design of the study. BJ, ZJL, MT, LP and ZLL were responsible for acquisition and analysis of data. JQ, ZX and LQ contributed statistical analysis. BJ and ZLL drafted the manuscript. JQ, HB and LQ revised and commented on the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Shanghai Yingli Pharmaceutical Co., Ltd., and the Biomedical Science and Technology Support Program of Shanghai Science and Technology Commission [grant No. 18431904700].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Institutional Review Boards of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Affiliated Cancer Hospital of Peking University involved gave approval for the study protocols and all enrolled patients gave their consent.
Consent for publication
Not applicable.
Competing interests
Prof. Lugui Qiu and Prof. Junyuan Qi received research grants from Shanghai Yingli Pharmaceutical Co., Ltd.; Prof. Bo Jiang, Prof. Lugui Qiu, Prof. Junyuan Qi, Prof. Yuqin Song, Prof. Meifeng Tu, Prof. Lingyan Ping, Prof. Zengjun Li received consulting fees from Shanghai Yingli Pharmaceutical Co., Ltd; Dr. Zusheng Xu is a shareholder of Shanghai Yingli Pharmaceutical Co., Ltd; Dr. Hanying Bao and Mr. Zongliang Liu are employees of Shanghai Yingli Pharmaceutical Co., Ltd.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Junyuan Qi, Email: qijy@ihcams.ac.cn.
Lugui Qiu, Email: qiulg@ihcams.ac.cn.
References
- 1.Bird ST, Tian F, Flowers N, Przepiorka D, Wang R, Jung TH, et al. Idelalisib for treatment of relapsed follicular lymphoma and chronic lymphocytic leukemia: a comparison of treatment outcomes in clinical trial participants vs medicare beneficiaries. JAMA Oncol. 2020;6(2):248–254. doi: 10.1001/jamaoncol.2019.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128(3):331–336. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger JA, Okkenhaug K. Haematological cancer: idelalisib-targeting PI3Kδ in patients with B-cell malignancies. Nat Rev Clin Oncol. 2014;11(4):184–186. doi: 10.1038/nrclinonc.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flinn IW, O’Brien S, Kahl B, Patel M, Oki Y, Foss FF, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ, γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877–887. doi: 10.1182/blood-2017-05-786566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman M, Belada D, Casasnovas RO, Gressin R, Lee HP, Mehta A, et al. Phase 2 study of parsaclisib (INCB050465), a highly selective, next-generation PI3Kδ inhibitor, in relapsed or refractory diffuse large B-cell lymphoma (CITADEL-202) Leuk Lymphoma. 2021;62(2):368–376. doi: 10.1080/10428194.2020.1832660. [DOI] [PubMed] [Google Scholar]
- 6.Shin N, Stubbs M, Koblish H, Yue EW, Soloviev M, Douty B, et al. Parsaclisib is a next-generation phosphoinositide 3-kinase δ inhibitor with reduced hepatotoxicity and potent antitumor and immunomodulatory activities in models of B-cell malignancy. J Pharmacol Exp Ther. 2020;374(1):211. doi: 10.1124/jpet.120.265538. [DOI] [PubMed] [Google Scholar]
- 7.Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017) Ann Oncol. 2017;28(7):1436–1447. doi: 10.1093/annonc/mdx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 9.Hanlon A, Brander DM. Managing toxicities of phosphatidylinositol-3-kinase (PI3K) inhibitors. Hematology Am Soc Hematol Educ Program. 2020;2020(1):346–356. doi: 10.1182/hematology.2020000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1.Table S1: YY-20394 is highly selective in targeting PI3Kδ.
Additional file 2. Inclusion and exclusion criteria.
Additional file 3.Table S2: Basic characteristics of patients in each group.
Additional file 4. The methods and definition of MTD, dose escalation phase and DLT as well as hematological toxicity.
Additional file 5.Table S3: Mean pharmacokinetic parameters of patients after a single dose in each dosage group.
Additional file 6.Table S4: Mean pharmacokinetic parameters of patients after multiple administrations in each dosage group.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.