Abstract
Biomaterials, once inserted in the oral cavity, become immediately covered by a layer of adsorbed proteins that consists mostly of salivary proteins but also of plasma proteins if the biomaterial is placed close to the gingival margin or if it becomes implanted into tissue and bone. It is often this protein layer, rather than the pristine biomaterial surface, that is subsequently encountered by colonizing bacteria or attaching tissue cells. Thus, to study this important initial protein adsorption from human saliva and serum and how it might be influenced through chemical modification of the biomaterial surface, we have measured the amount of protein adsorbed and analyzed the composition of the adsorbed protein layer using gel electrophoresis and western blotting. Here, we have developed an in vitro model system based on silica surfaces, chemically modified with 7 silane-based self-assembled monolayers that span a broad range of physicochemical properties, from hydrophilic to hydrophobic surfaces (water contact angles from 15° to 115°), low to high surface free energy (12 to 57 mN/m), and negative to positive surface charge (zeta potentials from –120 to +40 mV at physiologic pH). We found that the chemical surface functionalities exerted a substantial effect on the total amounts of proteins adsorbed; however, no linear correlation of the adsorbed amounts with the physicochemical surface parameters was observed. Only the adsorption behavior of a few singular protein components, from which physicochemical data are available, seems to follow physicochemical expectations. Examples are albumin in serum and lysozyme in saliva; in both, adsorption was favored on countercharged surfaces. We conclude from these findings that in complex biofluids such as saliva and serum, adsorption behavior is dominated by the overall protein-binding capacity of the surface rather than by specific physicochemical interactions of single protein entities with the surface.
Keywords: biomaterials, surface properties, self-assembled monolayers, pellicle, protein amount, protein composition
Introduction
Any surface that comes into contact with a protein-containing solution will become rapidly coated with a film of adsorbed proteins, which frequently determines further biological processes, such as the attachment of microorganisms, plant, or animal cells (Wilson et al. 2005; Banerjee et al. 2011; Sterzenbach et al. 2020). In many instances, this is an undesired process called biofouling, causing negative effects in the marine and food industry, bioanalytics, and, most important, the medical field (Norde 2008; Banerjee et al. 2011). Within the medical discipline, dentistry uses a great variety of biomaterials. In the mouth, these materials become exposed to the proteins of saliva; in the case of dental implants, to blood plasma; or at the gingival margin, to a mixture of both biofluids. Exposure to saliva leads to the formation of the so-called salivary pellicle, a thin acellular film consisting mostly of salivary proteins and glycoproteins that have adsorbed to the biomaterial surface and serve as substrate for subsequent microbial colonization (Siqueira et al. 2012; Lindh et al. 2014). Exposure to serum on the other side, especially extracellular adhesion proteins, influences cell adhesion and thus tissue integration of an implant (Wilson et al. 2005).
It is therefore important to study the composition of this adsorbed protein layer and to determine how it can be modulated through chemical modification of the biomaterial surface such that undesired negative outcomes can be prevented. Numerous studies have investigated the adsorption of proteins from purified protein solutions, finding that their behavior depends on the properties of the protein, characteristics of the material surface, and environmental conditions (Michiardi et al. 2007; Patil et al. 2007; Norde 2008; Guo et al. 2016). However, less is known about the adsorption behavior of complex biofluids. Most studies there focus mainly on the quantification of protein adsorption (Lindh et al. 1999; Comelles et al. 2010). Valuable investigations of qualitative protein adsorption have been performed on hydroxyapatite as a model for tooth enamel (Heller et al. 2017) and on actual clinical biomaterials either in situ or in vitro (Kohavi et al. 1995; Lee et al. 2001; Fischer and Aparicio 2021). Due to the complex influence of the poorly controllable surface properties of those materials in terms of surface roughness, hydrophilicity, and charge, it is not possible to reliably predict the impact of single surface properties on protein adsorption. A limited number of studies have used model systems but were restricted to only a few surface functionalizations (Aroonsang et al. 2014; Gibbins et al. 2014; Visalakshan et al. 2019). In vivo studies also suffer from a variety of disadvantages, such as interindividual variability in environmental conditions and saliva composition (Schipper et al. 2007), which complicate comparing the results for different surfaces. Besides, small amounts of adsorbed proteins require elaborate sample preparation before analysis (Kohavi et al. 1995).
To take a step back and study the influence of basic physicochemical parameters on adsorption of proteins from complex biofluids, we chose a model system in which we were able to control environmental conditions and ensure an invariant composition of the protein solutions such that results obtained for different surface modifications can be compared. Silica beads were chosen as model substrates due to their high specific surface area, resulting in sufficient amounts of proteins conducive to analytic tests. Silica allowed the functionalization with silane-based self-assembled monolayers (SAMs) of varying functional groups. This way, we could obtain stable coatings with reproducible surface properties that cover a broad range of surface hydrophilicities, surface free energies (SFEs), and zeta potentials, allowing us to test the influence of those characteristics on quantitative and qualitative biofluid protein adsorption in detail. Ample amounts of adsorbed proteins allowed us to study protein layer composition and identify its components by SDS-PAGE and immunoblot analysis. Taken together, our model system enables us to fill an important gap in knowledge by comprehensively investigating the qualitative adsorption of proteins from complex biofluids on a variety of surfaces with precisely tailored and well-characterized physicochemical properties.
Materials and Methods
Materials
As model surfaces for x-ray photoelectron spectroscopy (XPS), static contact angle, as well as streaming current measurements, single-side polished silicon wafers (n-type, phosphor doped; thickness, 650 to 700 µm; resistivity, 5 to 25 Ω·cm) were obtained from Si-Mat Silicon Materials and cut into specimens (10 × 10 mm) by Disco Hi-Tec Europe GmbH. For infrared spectroscopy, monodisperse silica spheres (diameter, 1 µm) were obtained from Micromod Partikeltechnologie GmbH. Protein adsorption experiments were performed with silica beads (diameter, 250 to 315 µm; specific surface area, 0.19 m2/g) obtained from Brace GmbH. All silanes were purchased from abcr GmbH; solvents, probe liquids, and further reagents were obtained from Sigma-Aldrich Chemie GmbH.
Biofluids
Saliva was obtained from consenting donors via a procedure approved by the University at Buffalo Human Subjects Institutional Review Board (030-505616). For the quantification of protein adsorption, mechanically stimulated whole saliva was obtained from 4 individuals via paraffin wax chewing and expectoration into a polypropylene vial. Samples were cooled on ice and sequentially filtered with low–protein binding syringe filters of decreasing pore size (5 to 0.2 µm; Acrodisc with Supor polyethersulfone membrane). Finally, saliva was pooled, aliquoted, and stored at −18 °C.
To determine qualitative protein adsorption, saliva was obtained from donors without stimulus. Collected saliva was centrifuged at 7,000 rpm for 15 min at 4 °C, and the clarified supernatant was used for adsorption experiments.
Human AB serum was obtained from Valley Biomedical. Aliquots were stored at −30 °C until use for adsorption experiments.
Substrate Functionalization and Analysis of Physicochemical Properties
Synthesis routes to obtain surface coatings with 7 terminal functions, as well as all methods to verify the synthesis success and characterize the physicochemical properties, are summarized in the Appendix.
Quantification of Adsorbed Proteins
The bicinchoninic acid assay was used to determine the protein concentration in the investigated biofluids (Sigma-Aldrich Chemie GmbH). A total protein content of 0.91 mg/mL (0.86 to 0.96; median [interquartile range]) was determined for pooled saliva. The protein concentration of saliva from individual donors (n = 9) used for gel electrophoresis was 1.08 mg/mL (0.90 to 1.30). A protein content of 48.3 mg/mL (45.1 to 55.2) was obtained for serum. The bicinchoninic acid assay was also applied to quantify the amount of adsorbed proteins. In the first way, we determined the protein amount directly on the silica beads after adsorption at 25 °C using a procedure that we previously published (Müller et al. 2006; Eichler et al. 2011; Staehlke et al. 2019; see Appendix). In the second way, protein adsorption was performed at 37 °C to mimic the ambient conditions in the oral cavity, and the protein amount was determined after detachment from the surfaces by heating the beads in anionic surfactant solution (SDS; see Appendix).
Gel Electrophoresis, Staining of Proteins and Glycans, and Immunoblotting
SDS-PAGE and immunoblotting were performed following standard procedures recently described by our group (Thamadilok et al. 2020). Details and antibodies are provided in the Appendix.
Results
Verification of Successful Surface Modification
The chemical structures of the silane-based SAMs carrying different terminal functions are depicted in Figure 1A. Five modifications have been developed and characterized during studies of our group (Müller et al. 2006; Schweikl et al. 2007; Katzur et al. 2012), and the modifications with SO3H and Py were newly developed and are used here for the first time. Successful surface functionalization could be proven by XPS, infrared spectroscopy, and water contact angle measurements.
Figure 1.
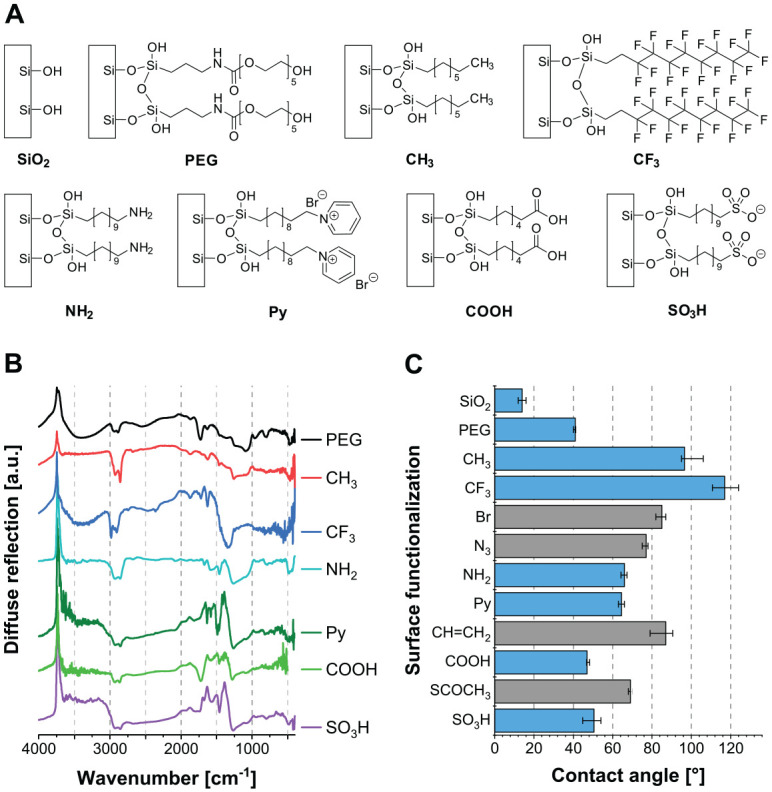
Realized surface coatings. (A) Overview of the examined self-assembled monolayers with different functional groups. The modifications NH2, pyridinium (Py), and SO3H are synthesized via a bromine monolayer (Br), which is substituted by azide (N3) and reduced to NH2, substituted by Py or thioacetate (SCOCH3), and oxidized to SO3H. Carboxylic acid groups (COOH) are generated via oxidation of a monolayer with terminal vinylic groups (CH=CH2). (B) Infrared spectra of SAMs recorded via DRIFT spectroscopy. (C) Water contact angles of final modifications (blue) as well as their intermediate SAMs (gray). Median ± interquartile range (n ≥ 8). SAM, self-assembled monolayer.
Through XPS analysis, successful surface modification is confirmed by a decrease in the silicon content and an increase in the carbon content as compared with uncoated SiO2. Furthermore, the incorporation of new elements, such as nitrogen, sulfur, bromine, and fluorine, are characteristic for the introduction of specific chemical functions.
In the infrared spectra (Fig. 1B), the negative band at 3,740 cm−1 is caused by the reduced number of surface silanol groups after silanization. In addition, bands in the range of 3,000 to 2,800 cm−1 can be attributed to C–H stretching vibrations of the CH2 units of the SAM backbone. For Py, several bands characteristic for pyridinium can be detected in the range of 1,635 to 1,465 cm−1, while for NH2, characteristic bands of N−H stretching vibrations are found at 1,585 and 1,460 cm−1. The presence of COOH groups was confirmed by the appearance of the C=O stretching vibration at 1,725 cm−1.
The results of the water contact angle measurements (Fig. 1C) show that silanization increased surface hydrophobicity in comparison with SiO2, which correlated well with the new terminal functional group. Nonpolar functions such as CH3 and CF3 resulted in high water contact angles, and polar and ionizable functions in low contact angles. Additionally, the hydrophilicity of final modifications of multiple-step reactions differed significantly from the results obtained for the previous intermediate SAMs and agreed well with our previously published and other literature data (Liu et al. 2002; Shyue et al. 2004; Janssen et al. 2006; Müller et al. 2006; Müller et al. 2007; Schweikl et al. 2007; Müller et al. 2009; Katzur et al. 2012).
Physicochemical Properties of the Modified Surfaces
The SFEs for the different surface coatings—as calculated from static contact angle measurements with water, formamide, and diiodomethane—vary in a broad range (Fig. 2A), from low SFEs of ~25 and ~12 mN/m for CH3 and CF3, respectively, to the highest total SFE for SiO2 (~56 mN/m). The coatings with acidic groups (COOH and SO3H) and SiO2 possessed the largest polar components (13.6 to 15 mN/m). These results are in good accordance with our previously published and other literature data (Janssen et al. 2006; Katzur et al. 2012).
Figure 2.
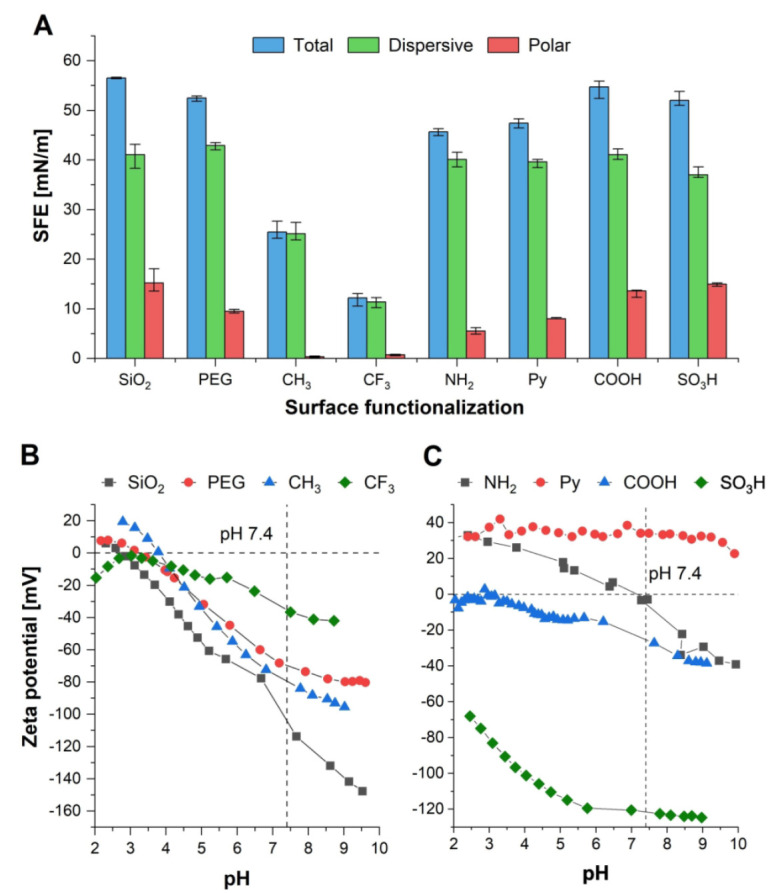
Physicochemical properties of surface coatings. (A) Surface free energy (SFE) as well as its polar and dispersive component of self-assembled monolayers with different functional groups, calculated from static contact angles with water, formamide, and diiodomethane according to the Lifshitz-van der Waals/acid-base approach. Median ± interquartile range (n ≥ 8). (B, C) Zeta potential of functionalized substrates, obtained from streaming current measurements.
The isoelectric points (IEPs) and zeta potentials (at pH 7.4) of the surface coatings were obtained from streaming current measurements (Fig. 2B, C). Results have been published in part, and extracted values are provided in the Appendix (Eichler et al. 2011; Katzur et al. 2012). An IEP of 2.8 and a strongly negative zeta potential under physiologic conditions (<100 mV) were observed for SiO2 in accordance with literature data (Shyue et al. 2004). The surface modifications without ionizable groups (PEG, CH3, and CF3) are characterized by their similar IEPs in the range pH 3 to 4 and their negative zeta potential at pH 7.4, similar to literature data (Chan et al. 2003; Shyue et al. 2004). When compared with these coatings, the pH-dependent protonation behavior of the primary amine or carboxylic acid groups in the modifications NH2 and COOH causes a shift in the IEP. However, for COOH, the zeta potential approaches 0 mV at pH 3, and the IEP of NH2 is shifted to pH 7.1, leading to an almost electrically neutral coating at pH 7.4. In contrast to that, strongly charged surfaces without an IEP in the measured pH range are obtained for Py (~+35 mV) and SO3H (<−60 mV) as previously reported (Shyue et al. 2004).
Quantitative Protein Adsorption
The amounts of adsorbed proteins were quantified on the surfaces as well as from the eluates after protein desorption (Fig. 3). Preliminary experiments showed that maximum protein adsorption occurred after 1 h for saliva and after 10 min for the much higher concentrated serum (Appendix Fig. 6).
Figure 3.
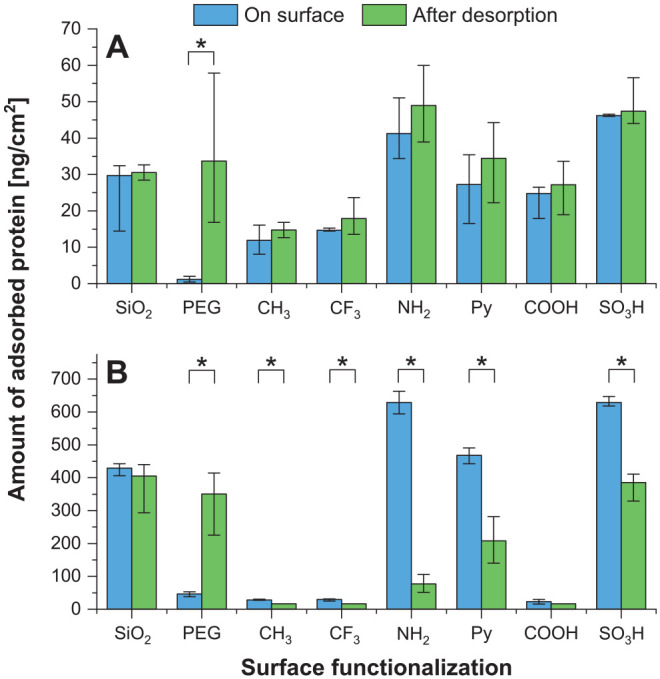
Quantitative protein adsorption. Amounts of adsorbed protein, determined via bicinchoninic acid assay directly on the surface or after protein desorption in the eluates. Median ± interquartile range. Adsorption on functionalized surfaces was performed from (A) whole human saliva or (B) human AB serum. On each surface, differences in the amounts of adsorbed protein obtained with the applied quantification methods were evaluated with the nonparametric Mann-Whitney U test, and statistically significant differences (*P < 0.05) are indicated. Further comparison of the 2 quantification methods as well as time kinetics of protein adsorption from saliva and serum are provided in the Appendix.
For saliva, a statistically significant positive correlation is obtained for the amounts of proteins measured by both methods (excluding PEG: R2 = 0.958, Pearson correlation coefficient r = 0.979, P < 0.01; Appendix Fig. 1). Clearly, the different surface modifications resulted in different amounts of adsorbed proteins, ranging from as low as <20 ng/cm2 for CH3 and CF3 to 40 to 50 ng/cm2 for NH2 and SO3H. However, no linear correlation was obtained between the amounts of adsorbed proteins and hydrophilicity, SFE, or zeta potential (Appendix Figs. 3–5). For PEG, considered a nonfouling surface coating, significant differences were observed between methods of protein measurement. Specifically, small amounts were detected directly on the surface, and considerably larger protein amounts were found after protein desorption.
For serum proteins, larger amounts of adsorbed proteins were measured, ranging from <50 ng/cm2 to ~400 ng/cm2, likely due to the at least 40-fold higher protein concentration of human AB serum when compared with saliva. For CH3, CF3, and COOH, very small amounts of adsorbed proteins were determined with both quantification methods. In contrast, for NH2, Py, and SO3H, the quantification method based on desorption provided considerably lower amounts as compared with measurements directly on the surface. Again, no linear correlation was obtained between the amounts of adsorbed proteins and hydrophilicity, SFE, or zeta potential (Appendix Figs. 3–5). For PEG, a situation similar to the result with salivary proteins was obtained. In terms of the amount of serum protein, no statistically significant correlation was observed between the methods (R2 = 0.215, r = 0.464, P > 0.05; Appendix Fig. 1).
When serum and salivary proteins were compared, data were positively correlated if quantification was performed directly on the surface (R2 = 0.761, r = 0.873, P < 0.01; Appendix Fig. 2). No such correlation could be observed by comparing the protein amounts obtained after protein desorption (R2 = 0.240, r = 0.490, P > 0.05).
Qualitative Protein Adsorption
SDS-PAGE and western blotting revealed a complex pattern of adsorbed proteins that depended on the surface modification and the biofluid analyzed (Figs. 4, 5). Initial time kinetics of adsorption showed that the relative pattern of protein bands did not change substantially between short incubation times (up to 5 min) and longer incubation times (up to 3 h). At time points beyond 3 h, protein degradation became apparent. Optimal band intensity was reached after 1 h for saliva and 10 min for the 40-fold higher concentrated serum (Appendix Fig. 6).
Figure 4.
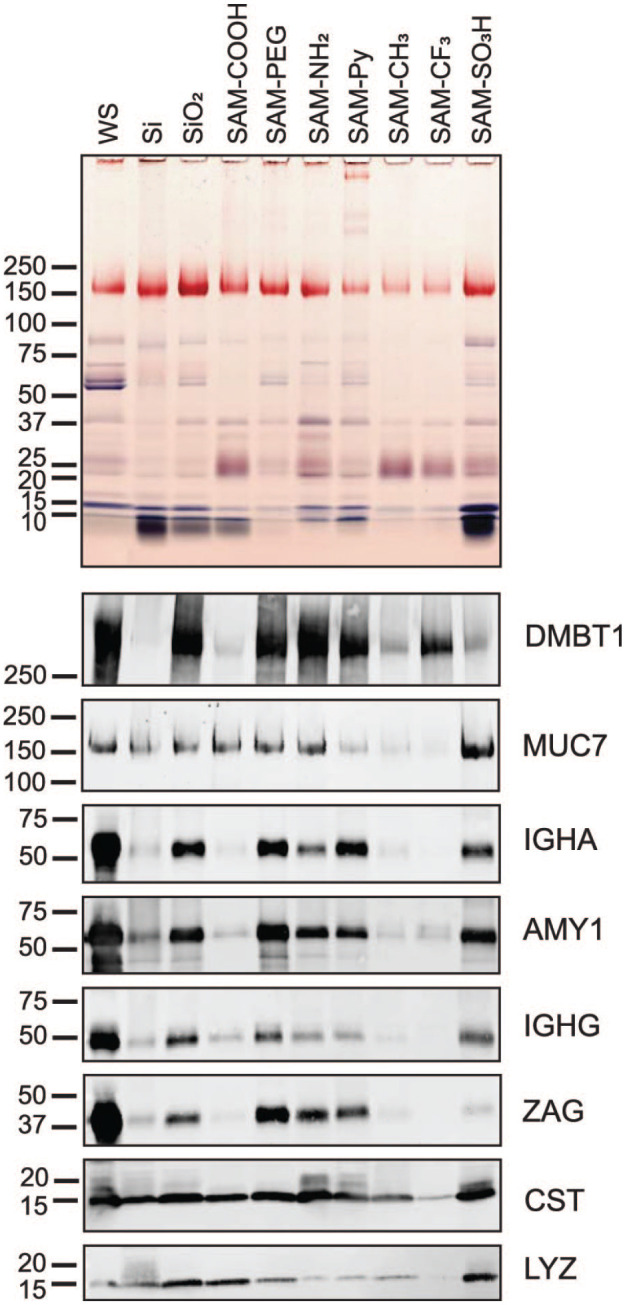
SDS-PAGE and immunoblot analysis of proteins from saliva adsorbed to chemically modified Si beads. Salivary proteins adsorbed to untreated (Si) and oxidized silica beads (SiO2) as well as to beads modified with 7 chemical functionalities were eluted from the silica bead surface by heating in SDS. Optimal time points for complete protein adsorption were obtained in kinetic studies (Appendix Fig. 6). Equal volumes of eluates were separated by SDS-PAGE. The original whole saliva (WS) that was used for adsorption was run for comparison (first lane). Proteins in the gel were stained with Coomassie blue, and glycans on glycoproteins were revealed by periodic acid–Schiff stain (pink bands). Nitrocellulose transfers from gel replicates were probed with antibodies against salivary agglutinin (DMBT1), mucin 7 (MUC7), immunoglobulin A heavy chain (IGHA), salivary α-amylase (AMY1), immunoglobulin G heavy chain Fc portion (IGHG), zinc-α2-glycoprotein (ZAG), cystatins (CST), and lysozyme (LYZ). Bound primary antibodies were detected with Alexa Fluor 488–tagged IgG secondary antisera.
Figure 5.
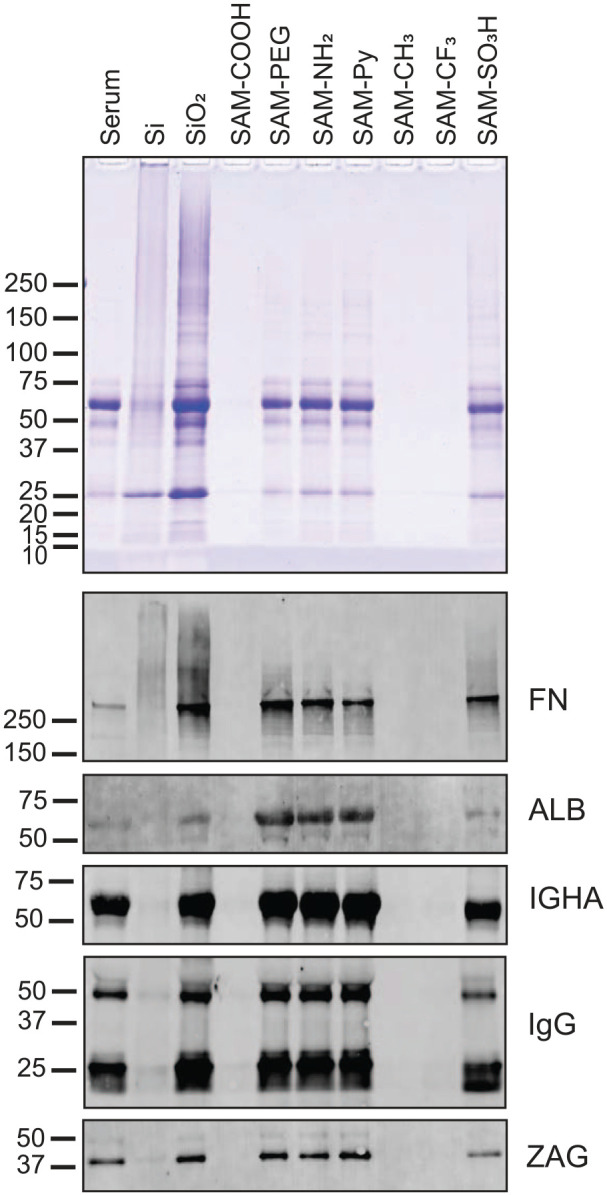
SDS-PAGE and immunoblot analysis of proteins from serum adsorbed to chemically modified Si beads. Serum proteins adsorbed to untreated (Si) and oxidized silica beads (SiO2) as well as to beads modified with 7 chemical functionalities were eluted from the silica bead surface by heating in SDS. Equal volumes of eluates were separated by SDS-PAGE. The original serum that was used for adsorption was run for comparison (first lane). Proteins in the gel were stained with Coomassie blue. Pink bands, generated by the staining of glycoproteins with periodic acid–Schiff stain, were not visible in the gel due to a general lack of densely glycosylated proteins in serum. Nitrocellulose transfers from gel replicates were probed with antibodies against fibronectin (FN), albumin (ALB), immunoglobulin A heavy chain (IGHA), immunoglobulin G F(ab)2 portion (IgG), and zinc-α2-glycoprotein (ZAG). Bound primary antibodies were detected with Alexa Fluor 488–tagged IgG secondary antisera.
Among the salivary proteins identified, lysozyme C stood out because, being a positively charged protein, it adsorbed on the negatively charged SiO2 substrates as well as on the COOH and SO3H modification. However, larger glycoproteins—including the negatively charged salivary agglutinin (DMBT1), mucin 7 (MUC7), and zinc-α2-glycoprotein (ZAG)—exhibited a more complex adsorption pattern: they also bound to the negatively charged SiO2 substrates, the nonfouling PEG, and the positively ionizable NH2 modifications.
For serum, mainly 2 groups of surface coatings can be differentiated: protein-repelling ones (CH3, CF3, and COOH) and protein-adhesive ones (SiO2, PEG, NH2, Py, and SO3H). Among the protein-adhesive surfaces, no large differences were seen in the overall pattern of adsorbed proteins. Albumin, the most abundant protein in serum and carrying a slightly negative charge, adsorbed preferentially on PEG and the positively ionizable functions NH2 and Py. Fibronectin, which plays an important role in cell adhesion and is slightly negatively charged, also adsorbed on SiO2 substrates as well as PEG, NH2, and Py, but in contrast to albumin on the negatively charged SO3H modification.
Discussion
We could demonstrate that our silica-based model system can serve as a platform to study the basic principles of protein adsorption from complex biofluids on a broad array of surfaces with precisely tailored physicochemical properties under controllable environmental conditions. We could show here that quantitative adsorption of proteins from complex physiologic fluids is mainly determined by the chemical surface modification. However, no direct correlation was observed between the adsorbed protein amounts and particular physicochemical surface properties, such as hydrophilicity, SFE, and zeta potential. Our data, obtained with saliva and serum, are thus not fully in agreement with postulated principles of protein adsorption deduced from studies with solutions containing only 1 type of protein (Wertz and Santore 2001; Michiardi et al. 2007; Patil et al. 2007; Norde 2008; Guo et al. 2016). Yet, even in these simplified experimental systems, conflicting results have been obtained. One such example is the adsorption of proteins to hydrophobic surfaces (Wertz and Santore 2001; Norde 2008). These discrepancies among results suggest that protein adsorption occurs as a phenomenon influenced by a multitude of factors, much more complex than previously assumed.
While in our study no overall correlation of quantitative protein adsorption with the measured surface characteristics could be delineated, certain observations agree with what has been reported on the adsorption behavior of complex protein solutions. Regarding the effect of surface wettability, the lowest protein adsorption was measured in the present study for the most hydrophobic coatings CH3 and CF3, agreeing with our results (Müller et al. 2007) and those of Wertz and Santore (2001) but contradicting the study of Norde (2008). With respect to the effect of SFE, a report studying the protein adsorption from fetal bovine serum found the lowest amounts of proteins adsorbed on surfaces with the highest SFE, while the highest amounts were observed on surfaces with medium SFE, but overall no clear correlation became apparent (Comelles et al. 2010). As far as the zeta potential is concerned, we found less serum protein on modifications carrying moderately negative surface charges, an observation that had been made (El-Ghannam et al. 2001). One particular puzzling finding was the difference in protein amounts adsorbed on the PEG-coated surface when measured directly on the surface versus when measured in the eluate after desorption from the surface. A possible explanation could be based on the different temperatures for the 2 protein measurements during the adsorption process. Enhanced protein adsorption at elevated temperatures would be in accordance with research showing a loss of PEG’s protein-repelling behavior with increasing temperature (Leckband et al. 1999; Efremova et al. 2001). All these partially conflicting reports and observations illustrate the complexity of protein adsorption and dependence from multiple factors in addition to the physicochemical properties of the surface.
Regarding qualitative protein adsorption, 2 main findings can be stated. First, protein adsorption from saliva appears to be more complex than adsorption from serum. Large variations in adsorption among the surface modifications can be observed for salivary proteins. In contrast to that, adsorption of serum proteins is more uniform and seems to be largely influenced by the abundance of the protein in the biofluid rather than by the widely varying surface properties studied. Thus, adsorption of the majority of the examined proteins cannot directly be linked to the physicochemical properties of the surface modifications. Second, the adsorption pattern of a few proteins, with the positively charged lysozyme (IEP, 11.1) as the most prominent example, is nevertheless governed by characteristics of the surface coating despite the complexity of the biofluid composition (Norde and Lyklema 1991). Accumulation of this protein on negatively charged surfaces is therefore clearly ruled by attractive electrostatic interactions between the surface and the protein. This rule also accounts for albumin (IEP, 5.5 to 5.7) from serum (Walz et al. 2006) in that, according to the immunoblot, it seems to be accumulated on positively charged surfaces more than on surfaces carrying negative charges. When compared with lysozyme, however, the charge-dependent adsorption behavior is significantly less pronounced than for albumin, as it can also adsorb on likewise charged hydrophilic surfaces due to its low internal stability, being a soft protein (Norde and Lyklema 1991; Norde 2008).
Adsorption of many other proteins from these complex biofluids, examined in this study, did not directly and predictably follow physicochemical expectations. In saliva, the glycoproteins MUC7 and ZAG are negatively charged due to extensive glycosylation and sialylation (IEP, 3.1 or 5.2 to 5.4, respectively; Walz et al. 2006). Nevertheless, they adsorb not only to modifications with positively charged groups (NH2, Py) but also to SiO2 and PEG surfaces. The tendency of mucins to adsorb more on hydrophilic surfaces has been reported by Aroonsang et al. (2014). The adsorption behavior of, for example, amylase and immunoglobulins did not follow strict electrostatic interactions as well. Taken together, these observations can possibly be explained by the unique and complex interactions of salivary proteins that involve salivary micelles (Soares et al. 2004). Those globular complexes are characterized by an accumulation of MUC7, lactoferrin, and immunoglobulin A (IgA), whereas lysozyme mainly occurs as a single component (Soares et al. 2004). Micelles possibly adsorb via a multitude of physicochemical interactions and may explain the simultaneous adsorption of diverse saliva proteins.
Given the observed complexity of protein adsorption from biofluids, more research will be needed to delineate rules for predicting the adsorption of certain protein components of interest. For future custom design of material surface coatings for clinical dental applications, it will be of importance to learn more about the adsorption of particular proteins that are known to either foster the attachment of tissue cells or serve as adhesion substrates for colonizing oral bacteria. Since some proteins playing a key role in that context have been described (such as fibronectin from plasma; Wilson et al. 2005) as well as MUC7, DMBT1, IgA, and proline-rich proteins from saliva (Lee et al. 2001; Ligtenberg et al. 2010; Sterzenbach et al. 2020), the next logical experimental step will be to compare biomaterial surface modifications that show substantial differences in the adsorption of such proteins and to study how subsequent attachment of cells or adhesion of bacteria will be influenced by their presence or absence on a given material surface. Equally important will be to get a clearer idea about protein complexes in saliva by determining their molecular structure and studying how they adsorb to surfaces. For clinical dental materials research, SAMs are a valuable tool for in vitro studies as well as for clinical applications. Silane-based SAMs can be designed as functional coatings of passivating metals and oxidic ceramic materials in the interproximal spaces and at the gingival margin of dental restorations or implants where they are not exposed to attritive wear. Fundamental research must be continued for the development of new chemical surface modifications in dentistry.
Author Contributions
J. Lehnfeld, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y. Dukashin, J. Mark, G.D. White, S. Wu, V. Katzur, contributed to data acquisition and analysis, and critically revised the manuscript; R. Müller, S. Ruhl, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211022273 for Saliva and Serum Protein Adsorption on Chemically Modified Silica Surfaces by J. Lehnfeld, Y. Dukashin, J. Mark, G.D. White, S. Wu, V. Katzur, R. Müller and S. Ruhl in Journal of Dental Research
Acknowledgments
The authors wish to thank Jakob Asenbauer and Michael Überreiter for performing static contact angle measurements, Dr. Matthias Kronseder of the physics department of the University of Regensburg for conducting the XPS measurements, and Lubov Neznanova for technical help with the SDS-PAGE–based analysis.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author/authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the DFG (grant MU 2787/1-2 to R. Müller) and National Institute of Dental and Craniofacial Research (NIDCR grant 2R01DE019807 to S. Ruhl). Y. Dukashin, J. Mark, G.D. White, and S. Wu were all supported by summer student research funds provided by the University at Buffalo School of Dental Medicine. J. Lehnfeld thanks the Chemical Industry Fund (Fonds der Chemischen Industrie) of the Chemical Industry Association (Verband der Chemischen Industrie) for the Chemistry Fund Fellowship (Chemiefonds-Stipendium) as well as the Bavarian State Ministry of Science and the Arts for its scholarship (Promotionsabschluss-Stipendium) within the Bavarian Program for the Advancement of Women in Science (Bayerisches Programm zur Realisierung der Chancengleichheit für Frauen in Forschung und Lehre).
ORCID iDs: R. Müller  https://orcid.org/0000-0001-9641-7780
https://orcid.org/0000-0001-9641-7780
References
- Aroonsang W, Sotres J, El-Schich Z, Arnebrant T, Lindh L. 2014. Influence of substratum hydrophobicity on salivary pellicles: organization or composition? Biofouling. 30(9):1123–1132. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Pangule RC, Kane RS. 2011. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater. 23(6):690–718. [DOI] [PubMed] [Google Scholar]
- Chan Y-HM, Schweiss R, Werner C, Grunze M. 2003. Electrokinetic characterization of oligo- and poly(ethylene glycol)-terminated self-assembled monolayers on gold and glass surfaces. Langmuir. 19(18):7380–7385. [Google Scholar]
- Comelles J, Estevez M, Martinez E, Samitier J. 2010. The role of surface energy of technical polymers in serum protein adsorption and MG-63 cells adhesion. Nanomedicine. 6(1):44–51. [DOI] [PubMed] [Google Scholar]
- Efremova NV, Sheth SR, Leckband DE. 2001. Protein-induced changes in poly(ethylene glycol) brushes: molecular weight and temperature dependence. Langmuir. 17(24):7628–7636. [Google Scholar]
- Eichler M, Katzur V, Scheideler L, Haupt M, Geis-Gerstorfer J, Schmalz G, Ruhl S, Müller R, Rupp F. 2011. The impact of dendrimer-grafted modifications to model silicon surfaces on protein adsorption and bacterial adhesion. Biomaterials. 32(35):9168–9179. [DOI] [PubMed] [Google Scholar]
- El-Ghannam A, Hamazawy E, Yehia A. 2001. Effect of thermal treatment on bioactive glass microstructure, corrosion behavior, zeta potential, and protein adsorption. J Biomed Mater Res. 55(3):387–395. [DOI] [PubMed] [Google Scholar]
- Fischer NG, Aparicio C. 2021. The salivary pellicle on dental biomaterials. Colloids Surf B Biointerfaces. 200:111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbins HL, Yakubov GE, Proctor GB, Wilson S, Carpenter GH. 2014. What interactions drive the salivary mucosal pellicle formation? Colloids Surf B Biointerfaces. 120(100):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Zhu X, Li M, Shi L, Ong JL, Jańczewski D, Neoh KG. 2016. Parallel control over surface charge and wettability using polyelectrolyte architecture: effect on protein adsorption and cell adhesion. ACS Appl Mater Interfaces. 8(44):30552–30563. [DOI] [PubMed] [Google Scholar]
- Heller D, Helmerhorst EJ, Oppenheim FG. 2017. Saliva and serum protein exchange at the tooth enamel surface. J Dent Res. 96(4):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D, De Palma R, Verlaak S, Heremans P, Dehaen W. 2006. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Films. 515(4):1433–1438. [Google Scholar]
- Katzur V, Eichler M, Deigele E, Stage C, Karageorgiev P, Geis-Gerstorfer J, Schmalz G, Ruhl S, Rupp F, Müller R. 2012. Surface-immobilized pamam-dendrimers modified with cationic or anionic terminal functions: physicochemical surface properties and conformational changes after application of liquid interface stress. J Colloid Interface Sci. 366(1):179–190. [DOI] [PubMed] [Google Scholar]
- Kohavi D, Klinger A, Steinberg D, Sela MN. 1995. Adsorption of salivary proteins onto prosthetic titanium components. J Prosthet Dent. 74(5):531–534. [DOI] [PubMed] [Google Scholar]
- Leckband D, Sheth S, Halperin A. 1999. Grafted poly(ethylene oxide) brushes as nonfouling surface coatings. J Biomater Sci Polym Ed. 10(10):1125–1147. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kho HS, Lee SW, Yang WS. 2001. Experimental salivary pellicles on the surface of orthodontic materials. Am J Orthod Dentofacial Orthop. 119(1):59–66. [DOI] [PubMed] [Google Scholar]
- Ligtenberg AJ, Karlsson NG, Veerman EC. 2010. Deleted in malignant brain tumors-1 protein (DMBT1): a pattern recognition receptor with multiple binding sites. Int J Mol Sci. 11(12):5212–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh L, Arnebrant T, Isberg PE, Glantz PO. 1999. Concentration dependence of adsorption from human whole resting saliva at solid/liquid interfaces: an ellipsometric study. Biofouling. 14(3):189–196. [Google Scholar]
- Lindh L, Aroonsang W, Sotres J, Arnebrant T. 2014. Salivary pellicles. Monogr Oral Sci. 24:30–39. [DOI] [PubMed] [Google Scholar]
- Liu Q, Ding J, Mante FK, Wunder SL, Baran GR. 2002. The role of surface functional groups in calcium phosphate nucleation on titanium foil: a self-assembled monolayer technique. Biomaterials. 23(15):3103–3111. [DOI] [PubMed] [Google Scholar]
- Michiardi A, Aparicio C, Ratner BD, Planell JA, Gil J. 2007. The influence of surface energy on competitive protein adsorption on oxidized niti surfaces. Biomaterials. 28(4):586–594. [DOI] [PubMed] [Google Scholar]
- Müller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G. 2009. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 30(28):4921–4929. [DOI] [PubMed] [Google Scholar]
- Müller R, Gröger G, Hiller KA, Schmalz G, Ruhl S. 2007. Fluorescence-based bacterial overlay method for simultaneous in situ quantification of surface-attached bacteria. Appl Environ Microbiol. 73(8):2653–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Hiller KA, Schmalz G, Ruhl S. 2006. Chemiluminescence-based detection and comparison of protein amounts adsorbed on differently modified silica surfaces. Anal Biochem. 359(2):194–202. [DOI] [PubMed] [Google Scholar]
- Norde W. 2008. My voyage of discovery to proteins in flatland . . . and beyond. Colloids Surf B Biointerfaces. 61(1):1–9. [DOI] [PubMed] [Google Scholar]
- Norde W, Lyklema J. 1991. Why proteins prefer interfaces. J Biomater Sci Polym Ed. 2(3):183–202. [DOI] [PubMed] [Google Scholar]
- Patil S, Sandberg A, Heckert E, Self W, Seal S. 2007. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 28(31):4600–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper RG, Silletti E, Vingerhoeds MH. 2007. Saliva as research material: biochemical, physicochemical and practical aspects. Arch Oral Biol. 52(12):1114–1135. [DOI] [PubMed] [Google Scholar]
- Schweikl H, Müller R, Englert C, Hiller KA, Kujat R, Nerlich M, Schmalz G. 2007. Proliferation of osteoblasts and fibroblasts on model surfaces of varying roughness and surface chemistry. J Mater Sci Mater Med. 18(10):1895–1905. [DOI] [PubMed] [Google Scholar]
- Shyue JJ, De Guire MR, Nakanishi T, Masuda Y, Koumoto K, Sukenik CN. 2004. Acid-base properties and zeta potentials of self-assembled monolayers obtained via in situ transformations. Langmuir. 20(20):8693–8698. [DOI] [PubMed] [Google Scholar]
- Siqueira WL, Custodio W, McDonald EE. 2012. New insights into the composition and functions of the acquired enamel pellicle. J Dent Res. 91(12):1110–1118. [DOI] [PubMed] [Google Scholar]
- Soares RV, Lin T, Siqueira CC, Bruno LS, Li X, Oppenheim FG, Offner G, Troxler RF. 2004. Salivary micelles: identification of complexes containing MG2, slgA, lactoferrin, amylase, glycosylated proline-rich protein and lysozyme. Arch Oral Biol. 49(5):337–343. [DOI] [PubMed] [Google Scholar]
- Staehlke S, Lehnfeld J, Schneider A, Nebe JB, Müller R. 2019. Terminal chemical functions of polyamidoamine dendrimer surfaces and its impact on bone cell growth. Mater Sci Eng C Mater Biol Appl. 101:190–203. [DOI] [PubMed] [Google Scholar]
- Sterzenbach T, Helbig R, Hannig C, Hannig M. 2020. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin Oral Investig. 24(12):4237–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamadilok S, Choi KS, Ruhl L, Schulte F, Kazim AL, Hardt M, Gokcumen O, Ruhl S. 2020. Human and nonhuman primate lineage-specific footprints in the salivary proteome. Mol Biol Evol. 37(2):395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalakshan RM, MacGregor MN, Sasidharan S, Ghazaryan A, Mierczynska-Vasilev AM, Morsbach S, Mailander V, Landfester K, Hayball JD, Vasilev K. 2019. Biomaterial surface hydrophobicity-mediated serum protein adsorption and immune responses. ACS Appl Mater Interfaces. 11(31):27615–27623. [DOI] [PubMed] [Google Scholar]
- Walz A, Stühler K, Wattenberg A, Hawranke E, Meyer HE, Schmalz G, Blüggel M, Ruhl S. 2006. Proteome analysis of glandular parotid and submandibular-sublingual saliva in comparison to whole human saliva by two-dimensional gel electrophoresis. Proteomics. 6(5):1631–1639. [DOI] [PubMed] [Google Scholar]
- Wertz CF, Santore MM. 2001. Effect of surface hydrophobicity on adsorption and relaxation kinetics of albumin and fibrinogen: single-species and competitive behavior. Langmuir. 17(10):3006–3016. [Google Scholar]
- Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. 2005. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 11(1–2):1–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211022273 for Saliva and Serum Protein Adsorption on Chemically Modified Silica Surfaces by J. Lehnfeld, Y. Dukashin, J. Mark, G.D. White, S. Wu, V. Katzur, R. Müller and S. Ruhl in Journal of Dental Research


