Abstract
Objective
To capture the early effects of the coronavirus disease 2019 (COVID-19) pandemic on pediatric clinical research.
Study design
Pediatric clinical research networks from 20 countries and 50 of their affiliated research sites completed two surveys over one month from early May to early June 2020. Networks liaised with their affiliated sites and contributed to the interpretation of results through pan-European group discussions. Based on first detection dates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), countries formed 1 early detecting and 1 late detecting cluster. We tested the hypothesis that this clustering influenced clinical research.
Results
Research sites were first impacted by the pandemic in mid-March 2020 (March 16 ± 10 days, the same date as lockdown initiation; P = .99). From first impact up until early June, site initiation and feasibility analysis processes were affected for >50% of the sites. Staff were redirected to COVID-19 research for 44% of the sites, and 75.5% of sites were involved in pediatric COVID-19 research (only 6.3% reported COVID-19 cases in their other pediatric trials). Mitigation strategies were used differently between the early and late detecting country clusters and between countries with and without a pediatric COVID-19 research taskforce. Positive effects include the development of teleworking capacities.
Conclusions
Through this collaborative effort from pediatric research networks, we found that pediatric trials were affected and conducted with a range of unequally applied mitigations across countries during the pandemic. The global impact might be greater than captured. In a context where clinical research is increasingly multinational, this report reveals the importance of collaboration between national networks.
Keywords: clinical research networks, clinial research sites, COVID-19 pandemic
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
The coronavirus disease 2019 (COVID-19) pandemic has impacted healthcare in many ways. Yet the impact on clinical research1 , 2 is unclear, even more so because different medical specialties might have been affected in different ways. For instance, although there have been reports in oncology3, 4, 5(including pediatric oncology6 , 7), there have been none of comparable extent in the broad field of pediatrics, where recent work includes calls to mitigate the impact on ongoing and future trials.8 , 9 This is perhaps because children are less directly impacted by COVID-19. Nonetheless, the organization of pediatric clinical research has been greatly affected by the pandemic. European pediatric clinical research national networks (Table I; available at www.jpeds.com) are part of conect4children, a pan-European network aimed at facilitating the development of pediatric therapies (https://conect4children.org/).10 , 11
Like other organizations, national networks were facing the unknown when severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected. This report is a joint effort from 19 European national networks and 2 Canadian networks to assess the impact on pediatric clinical research. This effort to capture and give meaning to information is a learning process for the global pediatric clinical research network and contributes to shaping its organization.
This study aimed to assess the early impact of the SARS-CoV-2 pandemic on the conduct and organization of pediatric clinical research on a multinational scale. Data were gathered from research centers across Europe and Canada and analyzed and interpreted collaboratively to comprehensively depict the situation. After observing that the countries formed 2 clusters differing in the timing of the pandemic and potentially in national attitudes to managing it, we tested the hypothesis that clinical research was more impacted where the virus had been present for longer. We also sought further evidence of inconsistency among countries in the mitigation strategies used to ensure trial continuity.
Methods
This study was conducted in 20 countries. Information on the national networks involved (name and number of affiliated research sites) is provided in Table I. A data collection phase using surveys was followed by an interpretation phase in which the data were analyzed and reviewed collaboratively by the national networks to provide a global perspective.
Surveys at the National Network and Research Site Levels
Two web-based surveys (Appendix 2; available at www.jpeds.com) were designed by the French pediatric clinical research network (PEDSTART) (Table I) and administered in Google Forms (Google LLC). A first survey was developed and used to identify at the national and national network level (1) the composition of the national networks in terms of the number of affiliated research centers; (2) how countries were currently affected by the pandemic and the measures undertaken at the national, regional, and network levels; and (3) the impact on pediatric clinical research networks' activities. A second site-focused web-based survey then had to be disseminated by e-mail from the networks to their affiliated research sites. This latter survey was used to evaluate directly the impact of the pandemic on pediatric clinical research at the research site level.
After being pilot tested, the surveys were disseminated by e-mail to the national networks on May 5, 2020. The national networks and associated research sites initially had 10 days (May 5 to May 15, 2020) to complete the online surveys. More time was then allotted to maximize the number of respondents, resulting in the last submission of the site survey on June 11. (The average date of site survey completion was May 15 ± 9 days). No answers were mandatory except contact details. Respondents could modify their responses by completing the questionnaire again and sending an e-mail notification.
Data Analyses and Pan-European Group Discussions
The data gathered during this initiative were curated by the French national network (PEDSTART). Raw data were first presented to the other national networks, and subsequent dedicated pan-European group discussions were organized. Pan-European group discussions with national networks were used to refine data interpretation by considering cultural, semantic, and other differences among countries. These discussions, in groups of approximately 10 national network representatives, allowed for a multinational interpretation of the results.
Dates were converted to number of days elapsed since January 1, 2020, for graphical representation and statistical analyses. Country clusters based on the date on which a first case of SARS-CoV-2 infection was detected were confirmed using Bayesian information criterion and optimal univariate k-means clustering.12 Differences between clusters not related to clinical research are shown in Table II (available at www.jpeds.com). Nonbinary survey questions were collapsed to binary outcomes for statistical analyses. Differences between clusters and between countries with or without a dedicated pediatric COVID-19 research taskforce were analyzed using the t test and the Fisher exact test for proportions. A significance level of 0.05 was used, and numerical data are presented as mean ± SD.
Results
Pediatric Clinical Research National Networks Evaluating the Impact of the Pandemic
The 21 networks involved in this study (Table I) reported coordinating an average of 12 research sites in their countries (median, 10.5; range, 3-25). Values used for descriptive statistics are presented in Table I. Figure 1 , A shows the number of pediatric clinical research sites from each national network that contributed to this study. There were an average of 3 research sites per national network. Figure 1, B shows how many pediatric clinical studies each site was undertaking before the SARS-CoV-2 outbreak.
Figure 1.
Pediatric clinical research sites that contributed to this study. A, Pediatric clinical research sites that contributed to this study, by national network and B, reported numbers of ongoing pediatric clinical studies in which these sites were involved before the outbreak. No sites associated with the following networks contributed to this study: Finland, Germany, Norway, Poland, Switzerland, and the United Kingdom. More than one-half of the sites (58%) were involved in less than 20 pediatric clinical studies, 24% of the sites in 20 to 40 studies, and 18% in more than 40 studies.
Different National Responses as Reported by the Networks
Countries were impacted and responded differently according to their pediatric clinical research networks. Figure 2 , A shows the delay from the first detected case of SARS-CoV-2 infection until the initiation of lockdown for each country. An early detecting cluster (n = 7 countries) detected a first case of SARS-CoV-2 infection significantly earlier than a late detecting cluster (n = 11 countries) (P < .001) (Figure 2, A and Table II). The early detecting country cluster had a longer delay before going into lockdown (P < .001) (Figure 2, A), and clusters did not differ with respect to lockdown initiation date and other metrics not related to pediatric research except population (Table II). Only 6 countries (5 shown in Figure 2 and Czechia) reported that a pediatric COVID-19 taskforce was in place to help catalog current initiatives and predict consequences. Only German, Canadian, Polish, and British networks reported regional differences in the measures taken at this stage of the pandemic. National networks reported that recommendations for pediatric clinical research during the pandemic were issued by national societies for 39% of the countries (7 of 18).
Figure 2.
A, Delay per country from the first detected case of SARS-CoV-2 infection until lockdown initiation as reported by the pediatric clinical research National Networks. Bars start, the day the first case was detected, end the day of lockdown initiation, and are colored according to whether there was a pediatric COVID-19 taskforce put in place by the networks. Countries are sorted according to the length of the bars. No lockdown initiation date was provided by the Swedish network, no data on the first detected case was provided by the Czech network, and the values for Canada are the average for the 2 networks that contributed. Differences between country clusters are reported in Table 2. Ten sites in Figure 1 (20%) located in Austria (2 sites out of 5, 40%), Greece (3 sites out of 5, 60%), Italy (1 site out of 3, 33%), and Sweden (4 sites out of 5, 80%) reported not be in lockdown when they completed the survey. B, Date of first impact on the activity as reported by the pediatric clinical research sites. The box represents the interquartile range (IQR), the dark line represents the median, whiskers extend to 1.5 times the IQR, and notches give an estimate of the 95% CI around the median.
Impacts of the SARS-CoV-2 Pandemic on Pediatric Clinical Research
A total of 50 pediatric clinical research sites (Figure 1) were involved in this study. The moment when the outbreak started affecting each pediatric research site's activity is plotted in Figure 2, B. On average, pediatric research sites were first affected on March 16 ± 10 days (median, March 16; range, February 3 to April 4), the same average date as initiation of lockdown. There was no significant difference between the dates of lockdown initiation reported by the networks and the dates of first impact on the activity reported by the sites (P = .99) (Figure 2). There was no difference between the early detecting and late detecting country clusters (Figure 2, A) in the date of first impact on site activity (P = .30) (Figure 2, B and Table II), not supporting the hypothesis that clinical research was impacted differently in these countries.
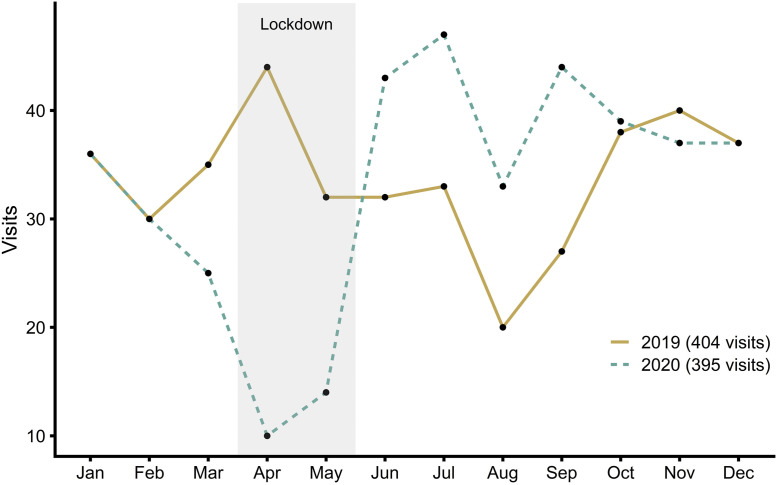
Table III describes COVID-19 research at the various sites, how pediatric clinical research was impacted, and the mitigations used to ensure continuity of clinical trials during the pandemic. There again were no differences in the impacts on clinical research and COVID-19 research at sites between the early detecting and late detecting country clusters and between countries with a taskforce and those without a taskforce. Close to one-half (44%) of the research sites reported that some of their staff were redirected to COVID-19 research projects. Although 75.5% of the sites were involved in pediatric COVID-19 research projects, only 6.3% reported SARS-CoV-2 infections in children participating in non-COVID clinical trials. Sixty-six percent of the sites involved in this study reported that clinical research site initiation visits were cancelled, and 54.3% reported that the clinical trial feasibility analysis process was affected. There were reports of studies being temporarily or permanently discontinued. Fewer clinical research visits were performed during the pandemic at 36.7% of sites, a figure perhaps decreased by the involvement of some sites in COVID-19 projects. Figure 3 shows the documented effect of the pandemic on pediatric clinical research visits at a French site (1 with more than 40 ongoing studies; Figure 1, B). Compared with 2019, there was an abrupt decrease in the number of visits performed during the first 2020 lockdown and an abrupt overshoot afterward (Figure 3), resulting in a similar total annual number of visits performed.
Table III.
COVID-19 research at sites, impact on pediatric clinical research, and mitigation strategies used to ensure continuity of clinical trials
| Variables | All sites, % (n/N) | Clusters |
Taskforce |
||||
|---|---|---|---|---|---|---|---|
| EDC, % (n/N) | LDC, % (n/N) | P | Negative, % (n/N) | Positive, % (n/N) | P | ||
| COVID-19 research at sites | |||||||
| Staff redirection to COVID-19 research | 44 (22/50) | 50 (13/26) | 33 (6/18) | .36 | 45 (15/33) | 41 (7/17) | 1.0 |
| Staff involvement in pediatric projects | 76 (37/49) | 80 (20/25) | 67 (12/18) | .48 | 82 (27/33) | 63 (10/16) | .17 |
| Impact on clinical research | |||||||
| Initiation visit cancellations | 66 (31/47) | 68 (17/25) | 71 (12/17) | 1.0 | 72 (23/32) | 53 (8/15) | .32 |
| Feasibility process impacted | 54 (25/46) | 63 (15/24) | 53 (9/17) | .75 | 56 (18/32) | 50 (7/14) | .75 |
| Less research visits on site | 37 (18/49) | 44 (11/25) | 22 (4/18) | .20 | 39 (13/33) | 31 (5/16) | .75 |
| Mitigation strategies∗ | |||||||
| Visits by phone call | 86 (42/49) (100) | 92 (24/26) | 78 (14/18) | .21 | 88 (28/32) | 82 (14/17) | .68 |
| Visits by home nurse intervention | 22 (11/49) (57) | 27 (7/26) | 11 (2/18) | .27 | 19 (6/32) | 29 (5/17) | .48 |
| Biological safety assessments at the patient's home or a nearby laboratory | 23 (9/39) (36) | 30 (7/23) | 17 (2/12) | .45 | 15 (4/26) | 38 (5/13) | .13 |
| Questionnaires and standard tests by telephone or videoconference | 72 (28/39) (79) | 65 (15/23) | 75 (9/12) | .71 | 77 (20/26) | 62 (8/13) | .45 |
| Sending study drug to the patient's home | 26 (10/39) (29) | 43 (10/23) | 0 (0/12) | <.01 | 15 (4/26) | 46 (6/13) | .06 |
EDC, early detecting cluster; LCD, late detecting cluster.
Population data were obtained from the World Bank (World Development Indicators, 2020). EDC, early detecting cluster (n = 7 countries); LCD, late detecting cluster (n = 11 countries).
Percentages of countries in Figure 1 are also shown in parentheses for mitigation strategies.
Figure 3.
Effect of the pandemic on pediatric clinical research visits. Total visits per month documented at a French site (Lille University Hospital) in 2019 (solid line; 404 visits in total) and 2020 (dotted line; 395 visits in total). The gray-shaded area indicates the first 2020 lockdown (March 17-May 10).
In this context, mitigation strategies were used by research sites but inconsistently among countries. Some clinical research visits were reported to be replaced by phone calls for 85.7% of the surveyed sites and by home nurse interventions for 22.4% of them (Table III). Other procedures were put in place to ensure continuity of care and safety of enrolled patients and included sending study drug to patients' homes, performing biological safety assessments at patients' homes or at a nearby laboratory, and administering questionnaires and standard tests via telephone or videoconference (Table III). There were no differences between the early detecting and late detecting country clusters and between countries with and those without a taskforce on the mitigation strategies used except for sending study drugs to patients' homes that occurred more often in the early detecting cluster (P < .01) and in countries with a taskforce, with a difference close to statistically significant for the latter (P = .06) (Table III). Takeaway themes from group discussions and open-ended survey questions are presented in Table IV (available at www.jpeds.com), along with other mitigation strategies, concerns of research sites, and potentially positive effects of the pandemic.
Discussion
The present study is a multinational effort by pediatric clinical research national networks from Europe and Canada and their affiliated research sites to capture the acute effect of the outbreak on clinical research efforts. Although the extent of the impact and the measures undertaken have varied widely across countries and research sites, we provide a global overview of how pediatric clinical research was affected and reorganized in the early stage of the outbreak. Direct overload from COVID-19 was limited, but changes in pediatric research activities were substantial, with feasibility and site initiation processes impacted at >50% of the surveyed sites (Table III). This is in line with site initiation visit cancellations reported by the Innovative Therapies for Children with Cancer consortium.6
Twenty countries were involved (Table I). The average participation rate of 25% of national networks–affiliated research sites (Figure 1, A) is encouraging for follow-up studies. The data that we have gathered suggests the different countries were initially impacted at different rates by the pandemic (Figure 2, A and Table II). Previous nonpediatric reports have noted country-specific impacts of the pandemic on clinical research3 , 13; for instance, at the time when a previous survey was administered, there was a smaller effect on enrollment in oncology trials in Asia compared with Europe and the US.3 This reinforces the dynamic nature of the pandemic and demonstrates that these differences between countries at one point in time could be the main caveat of the current analysis. Yet we found no difference on the impact on clinical research between the early detecting and late detecting country clusters and between countries with and those without a taskforce. The 28-day difference in the initiation of lockdown between the early detecting and late detecting country clusters (Table II) could reflect national attitudes toward managing the pandemic. The extent to which this clustering is meaningful, besides reflecting population differences (Table II), is uncertain, given that the alternative of using 1 detected case per million inhabitants14 does not cluster countries in a similar way. Taken together, our data suggest that what impacts clinical research—and what did not differ much here between countries (Figure 2 and Table II)—is the timing of containment measures.
The impact of the pandemic was on average first felt by pediatric clinical research sites (Figure 1) in mid-March 2020 (Figure 2, B). Staff were mobilized to COVID-19 research in some, but not all, sites (Table III). A substantial proportion of surveyed sites (76%) were involved in pediatric COVID-19 research as well. Non–COVID-19 studies that were perturbed and reorganized during the first lockdown were not disturbed much by SARS-CoV-2 infections in children. In parallel, it was reported that the lockdown appreciably reduced pediatric admissions and visits for viral and nonviral infections.15 Main pandemic hurdles for pediatric clinical research concerned the feasibility and site initiation processes for trials that had not yet started and patient visits to research centers for the ongoing trials (Table III). This is corroborated by more granular data from a French site showing patient visit cancellations during the early stage of the pandemic and the lockdown and a subsequent overshoot, with more visits than usual being performed after restrictive measures were lifted (Figure 3). More data to confirm whether this is generalizable are required, but this is in line with the decrease in new subject enrollment in many therapeutic areas in Europe and Asia reported for the same period.13 Sites' involvement in COVID-19 studies might explain why few sites reported performing fewer patient visits onsite (Table III).
Nonetheless, consistent with some special considerations formulated elsewhere,13 research sites applied mitigation strategies to ensure continuity of clinical trials but heterogeneously among countries (Tables III and IV). We found that the early detecting country cluster and countries with a pediatric COVID-19 taskforce were more prone to sending study drugs to the patients' homes. Given that the early detecting country cluster was ∼3 times more populous (Table II), perhaps this mitigation strategy is more conceivable in larger countries. Understanding the underlying reason could help broaden its future use. Although mitigation ensured the continuity of clinical trials during the pandemic, questions also could be raised about the delivery of medicinal products to patients' homes and about the impact of visits performed remotely (as opposed to face-to-face) on the quality of clinical research. It could be argued that the latter are less reliable for gathering some types of data.
Owing to less travel, teleworking and teleconferencing decreased transportation and maintenance costs and reduced the environmental impact. Meetings were easier to organize. However, the involvement of network stakeholders is difficult to maintain by teleconference. It is also harder for newcomers to find their place (Table IV), and teleconferences do not allow for potentially important networking and exchange of ideas as in face-to-face meetings.
We acknowledge some limitations of this study, including the small sample size in terms of number of research sites within some national networks and differences among countries at the time of the survey, as well as potential semantic and cultural differences affecting interpretation of survey questions. The involvement of pediatric clinical research national networks (Table I) should have helped minimize the impact of the latter. The participation rate was lowered by national networks for which no affiliated site contributed (networks in Switzerland, Finland, Germany, and Norway). Networks in the United Kingdom and Poland were excluded from the calculation because they did not disseminate the survey to their affiliated sites. The British network decided to not distribute the survey considering the level of COVID-19 research that was ongoing at the time of the survey and the pressure on the sites to deliver key COVID-19 research. Canada was also excluded because there is no official national network there. Two networks from Canada contributed to the current study, compared with 1 network in European countries (Table I); however, only 1 research site from Canada was involved (Figure 1, A). The assumption that each site survey respondent represents what national networks consider a distinct research site also could bias our estimate of the participation rate. Compared with lockdown initiation dates reported elsewhere,14 dates reported by the national networks differ slightly for some countries.
COVID-19 has brought unity across and within countries in pediatric clinical research. We have shared practices, which has allowed us to pinpoint potential areas of improvement. With this study, we show how pediatric clinical trials were impacted and reorganized in the early stage of the outbreak, with the major finding that a range of mitigation strategies was used, but these strategies were applied inconsistently in different countries. This crisis allowed us to develop and reflect on different ways of working. Above all, in a context in which clinical research is increasingly multinational, with both academic and industrial stakeholders, this work shows the value of collaboration among national networks.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Network of National Networks Study Group:
Ruth Ladenstein, Andrea Mikolasek, Daphné Christiaens, Eva Degraeuwe, Johan Vande Walle, Lieve Nuytinck, Elise Mok, Jonathon L. Maguire, Thierry Lacaze-Masmonteil, Pavla Pokorna, Pernille Skovby, Heli Rajasaar, Jaana Kallio, Pirkko Lepola, Christele Gras-Le Guen, Frédéric Gottrand, Florentia Kaguelidou, Hugues Chevassus, Isabelle Pin, Jérémie Rouger-Gaudichon, Maya Patel, Eva Neumann, Matthias Schwab, Elias Losifidis, Emmanuel Roilides, Máiréad Murray, Federica La Neve, Francesca Rocchi, Sigrun Margrethe Hjelle, Thomas Halvorsen, Marek Migdał, Aleksander Wiśniewski, Inês Zimbarra Cabrita, Rita Carilho Torrão, Tiago Martins, Cristina Serén Trasorras, Federico Martinón-Torres, Anders Rane, Estelle Naumburg, Klara M. Posfay-Barbe, Manuel Diezi, Paolo Paioni, Fenna Mahler, Saskia N. de Wildt, Tesa Van der Geest, and Karen Wilding
Supplementary Data
Appendix 1. Additional Members of the Network of National Networks (NNN) Study Group
Austria
Ruth Ladenstein, MD, PhD, OKIDS GmbH, Vienna, Austria.
Andrea Mikolasek, OKIDS GmbH, Vienna, Austria.
Belgium
Daphné Christiaens, Clinical Research Coordinator–Scientific Associate, University Hospital Ghent, Safepedrug Clinical Trial Unit, University of Ghent, IMI c4c Project National Hub Belgium, Ghent, Belgium.
Eva Degraeuwe, MD, University of Ghent, Ghent, Belgium.
Johan Vande Walle, MD, PhD, Professor, Safepedrug, Department of Pediatrics, Corneel Heymanslaan UZGent, University of Ghent, Ghent, Belgium.
Lieve Nuytinck, PhD, HIRUZ, University Hospital Ghent, Gent, Belgium.
Canada
Elise Mok, PhD, Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada.
Jonathon L. Maguire, MD, MSc, FRCPC, Lawson Chair in Patient Engagement in Child Nutrition, Professor of Pediatrics and Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, and Pediatrician and Scientist, Li Ka Shing Knowledge Institute, Unity Health Toronto, Toronto, Ontario, Canada.
Thierry Lacaze-Masmonteil, MD, PhD, Scientific Director, Maternal Infant Child and Youth Research Network, Calgary, Alberta, Canada.
Czechia
Pavla Pokorna, MD, PhD, Institute of Pharmacology and Department of Pediatrics and Inherited Metabolic Disorders, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czechia; Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center, Sophia Children's Hospital, Rotterdam, The Netherlands; and Department of Physiology and Pharmacology, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Denmark
Pernille Skovby, Department of Pediatrics, Regional Hospital Unit West Jutland, Herning Hospital, Herning, Denmark.
Estonia
Heli Rajasaar, MD, University of Tartu, Tartu, Estonia.
Finland
Jaana Kallio, MD, PhD, Specialist in Pediatrics and Clinical Pharmacology, Department of Children and Adolescents, Helsinki University Hospital, Helsinki, Finland.
Pirkko Lepola, BSc, MSc, Department of Children and Adolescents, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
France
Christele Gras-Le Guen, MD, PhD, Professor, Clinical Investigation Centre, University Hospital Nantes, Nantes, France.
Frédéric Gottrand, MD, PhD, Professor, Centre Hospitalier Universitaire, Hôpital Jeanne de Flandre, Pôle Enfant, Lille, and Institute for Translational Research in Inflammation, UMR 1286 INSERM, Faculté de Médecine, Université de Lille, Lille, France.
Florentia Kaguelidou, MD, PhD, AP-HP, Hôpital Robert Debré, Centre d'Investigations Cliniques, INSERM CIC1426, Université de Paris, UMR 123, ECEVE, Paris, France.
Hugues Chevassus, PhD, CHU Montpellier, Centre d'Investigation Clinique, INSERM CIC1411, Montpellier, France.
Isabelle Pin, MD, Centre Hopitalier Universitaire de Grenoble, Grenoble, France.
Jérémie Rouger-Gaudichon, MD, PhD, Pediatric Oncohematology, Department of Pediatrics, CHU de Caen, Caen, France.
Maya Patel, MSc, Université de Tours, INSERM, N2C UMR 1069, and French Clinical Research Infrastructure Network–PEDSTART, Tours, France.
Germany
Eva Neumann, Dr Margarete Fischer-Bosch Institute of Clinical Pharmacology, Stuttgart, Germany.
Matthias Schwab, MD, Dr Margarete Fischer-Bosch Institute of Clinical Pharmacology, Stuttgart, and Department of Clinical Pharmacology, University Hospital, Tübingen, Germany.
Greece
Elias Losifidis, MD, PhD, 3rd Department of Pediatrics, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Emmanuel Roilides, MD, PhD, 3rd Department of Pediatrics, Special Unit for Biomedical Research and Education, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Ireland
Máiréad Murray, BSc, HDip, MSc, INFANT, University College Cork, Cork, Ireland.
Italy
Federica La Neve, PhD, Bambino Gesù Children's Hospital, Rome, Italy.
Francesca Rocchi, PharmD, MSc, Bambino Gesù Children's Hospital, Rome, Italy.
Norway
Sigrun Margrethe Hjelle, MSc, PhD, Children and Youth Clinic, Haukeland University Hospital, Bergen, Norway.
Thomas Halvorsen, MD, PhD, Consultant and Professor of Pediatrics, Haukeland University Hospital, Bergen, Norway.
Poland
Marek Migdał, MD, PhD, CEO, Children's Memorial Health Institute, Warsaw, Poland.
Aleksander Wiśniewski, RN, PhD, Scientific Research and International Cooperation Division, Children's Memorial Health Institute, Warsaw, Poland.
Portugal
Inês Zimbarra Cabrita, MSc, PhD, STAND4Kids–Supporting Pediatric Trials in Portugal, Associação para a Investigação e Desenvolvimento da Faculdade de Medicina, Cardiovascular Centre at Universidade de Lisboa, and Portuguese Red Cross Health School, Lisbon, Portugal.
Rita Carilho Torrão, MSc, PhD, STAND4Kids–Supporting Pediatric Trials in Portugal, Associação para a Investigação e Desenvolvimento da Faculdade de Medicina and Cardiovascular Centre at Universidade de Lisboa, Lisbon, Portugal.
Tiago Martins, RD, MSc, STAND4Kids–Supporting Pediatric Trials in Portugal, Associação para a Investigação e Desenvolvimento da Faculdade de Medicina, Lisbon, Portugal.
Spain
Cristina Serén Trasorras, MSc, Spanish Paediatric Clinical Trials Network, Santiago de Compostela, Spain.
Federico Martinón-Torres, MD, PhD, Associate Professor, Pediatrics Department, Hospital Clínico Universitario de Santiago, Santiago de Compostela, Spain.
Sweden
Anders Rane, MD, PhD, Senior Professor, Division of Clinical Pharmacology, Karolinska Institutet, Stockholm, Sweden.
Estelle Naumburg, MD, PhD, Associate Professor, Division of Clinical Pharmacology, Karolinska Institutet and Institution of Clinical Sciences, Pediatrics, Umeå University, Umeå, Stockholm, Sweden.
Switzerland
Klara M. Posfay-Barbe, MD, MS, Division of General Pediatrics, University Hospitals of Geneva and University of Geneva, Geneva, Switzerland.
Manuel Diezi, MD, Consultant, Pediatric Hemato-Oncology Unit, Department of Pediatrics, Lausanne University Hospital and University of Lausanne, Lausanne, Vaud, Switzerland.
Paolo Paioni, MD, Division of Infectious Diseases and Hospital Epidemiology, University Children's Hospital Zurich, Zurich, Switzerland.
The Netherlands
Fenna Mahler, Drs, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Saskia N. de Wildt, MD, PhD, Department of Pharmacology and Toxicology, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Tesa Van der Geest, PhD, Department of Pharmacology and Toxicology, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
United Kingdom
Karen Wilding, MRes, University of Liverpool, Liverpool, United Kingdom.
Table I.
Pediatric clinical research national networks that contributed to this study
| Country | National network | Affiliated sites |
|---|---|---|
| Austria | OKIDS GmbH | 6∗ |
| Belgium | Safepedrug-BPCRN | 9∗ |
| Canada | TARGet Kids! | 12 |
| Canada | Maternal Infant Child Youth Research Network | 17 |
| Czechia | CUNI | 4∗ |
| Denmark | DanPedMed (Trial Nation) | 16∗ |
| Estonia | University of Tartu (ELAV) | 3∗ |
| Finland | HUS | 10∗ |
| France | PEDSTART | 15∗ |
| Germany | RBMF/GermanNetPaeT | 23∗ |
| Greece | HELPnet | 13∗ |
| Ireland | in4kids | 11∗ |
| Italy | INCIPIT | 12∗ |
| Norway | NorPedMed | 6∗ |
| Poland | Childrens Memorial Health Institute | 10∗ |
| Portugal | STAND4Kids | 10∗ |
| Spain | RECLIP | 25∗ |
| Sweden | SwedPedMed | 8-15∗ |
| Switzerland | SwissPedNet | 9∗ |
| The Netherlands | Pedmed-NL | 17∗ |
| United Kingdom | National Institute for Health Research (NIHR) | 223† |
Values used for descriptive statistics.
The network reported that there are 223 National Health Service trusts that are automatically affiliated with the NIHR.
Table II.
Differences among country clusters
| Variables | EDC | LDC | P value |
|---|---|---|---|
| First detected case | 29/01 ± 3 d | 27/02 ± 2 d | <.001 |
| Delay to lockdown, d | 46 ± 6 | 18 ± 6 | <.001 |
| Lockdown initiation | 15/03 ± 4 d | 16/03 ± 6 d | .52 |
| Population, × 106 inhabitants | 44.6 ± 28.6 | 16.2 ± 19.5 | <.05 |
EDC, early detecting cluster; LCD, late detecting cluster.
Population data were obtained from the World Bank (World Development Indicators, 2020). EDC, early detecting cluster (n = 7 countries); LCD, late detecting cluster (n = 11 countries).
Table IV.
Takeaway themes from group discussions and open-ended survey questions
| Main themes | Subthemes |
|---|---|
| Network sustainability | Concerns that the absence of face-to-face interactions combined with a high rate of staff turnover would weaken the networks and affect the integration of new members |
| Mitigation strategies at sites | Other mitigation strategies than reported in Table III; blood sampling at home; rescheduling patients visits when clinical activity was lower; delivering more than usual medication to prevent visits to the hospital; using both visits by phone call and home nurse intervention for some protocols |
| Concerns of research sites | Financial issues because COVID-19 studies are prioritized; delays for future and ongoing pediatric clinical research |
| Development during the pandemic | Teleworking, teleconsulting, teleconferencing; communication capabilities; team cohesion and collaboration |
References
- 1.McDermott M.M., Newman A.B. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323:2135–2136. doi: 10.1001/jama.2020.4689. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration Conduct of clinical trials of medical products during the COVID-19 public health emergency: guidance for industry, investigators, and institutional review boards. https://www.fda.gov/media/136238/download Accessed September 7, 2021.
- 3.Upadhaya S., Yu J.X., Oliva C., Hooton M., Hodge J., Hubbard-Lucey V.M. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov. 2020;19:376–377. doi: 10.1038/d41573-020-00093-1. [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse D.M., Harvey R.D., Hurley P., Levit L.A., Kim E.S., Klepin H.D., et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology Survey. JCO Oncol Pract. 2020;16:417–421. doi: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]
- 5.Tolaney S.M., Lydon C.A., Li T., Dai J., Standring A., Legor K.A., et al. The impact of COVID-19 on clinical trial execution at the Dana-Farber Cancer Institute. J Natl Cancer Inst. 2020;(djaa144) doi: 10.1093/jnci/djaa144. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubio-San-Simón A., André N., Cefalo M.G., Aerts I., Castañeda A., Benezech S., et al. Impact of COVID-19 in paediatric early-phase cancer clinical trials in Europe: a report from the Innovative Therapies for Children with Cancer (ITCC) consortium. Eur J Cancer. 2020;141:82–91. doi: 10.1016/j.ejca.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio-San-Simon A., Verdú-Amorós J., Hladun R., Juan-Ribelles A., Molero M., Guerra-García P., et al. Challenges in early-phase clinical trials for childhood cancer during the COVID-19 pandemic: a report from the new agents group of the Spanish Society of Paediatric Haematology and Oncology (SEHOP) Clin Transl Oncol. 2021;23:183–189. doi: 10.1007/s12094-020-02399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flamein F., Gottrand F., Patel M.L., Hankard R. Impact of COVID-19 on pediatric clinical research in France. CMAJ. 2020;192:E589. doi: 10.1503/cmaj.75416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stiles-Shields C., Plevinsky J.M., Psihogios A.M., Holmbeck G.N. Considerations and future directions for conducting clinical research with pediatric populations during the COVID-19 pandemic. J Pediatr Psychol. 2020;45:720–724. doi: 10.1093/jpepsy/jsaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner M.A., Cheng K., de Wildt S., Hildebrand H., Attar S., Rossi P., et al. European research networks to facilitate drug research in children. Br J Clin Pharmacol. 2020 doi: 10.1111/bcp.14545. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner M.A., Hildebrand H., Fernandes R.M., de Wildt S.N., Mahler F., Hankard R., et al. The conect4children (c4c) consortium: potential for improving European clinical research into medicines for children. Pharmaceut Med. 2021;35:71–79. doi: 10.1007/s40290-020-00373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Song M. Ckmeans.1d.dp: optimal κ-means clustering in one dimension by dynamic programming. R J. 2011;3:29–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Sathian B., Asim M., Banerjee I., Pizarro A.B., Roy B., van Teijlingen E.R., et al. Impact of COVID-19 on clinical trials and clinical research: a systematic review. Nepal J Epidemiol. 2020;10:878–887. doi: 10.3126/nje.v10i3.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Born B., Dietrich A.M., Müller G.J. The lockdown effect: a counterfactual for Sweden. Plos One. 2021;16:e0249732. doi: 10.1371/journal.pone.0249732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angoulvant F., Ouldali N., Yang D.D., Filser M., Gajdos V., Rybak A., et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and non-viral infections—a time series analysis. Clin Infect Dis. 2021;72:319–322. doi: 10.1093/cid/ciaa710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.