Abstract
Background
We aimed to study the impact of diabetes background on COVID-19 progression from swab testing to health outcomes in type 2 diabetes (T2DM).
Methods
From the database of the diabetes units of Piedmont-Italy we extracted records of T2DM patients, which were linked with the swab-testing-database, and the database of hospital discharges. Five outcomes (PCR testing, PCR testing positivity, hospitalization, Intensive Care Unit (ICU), death) were evaluated using robust Poisson models.
Results
Among 125,021 T2DM patients, 1882 had a positive PCR test. Of these patients, 49.4% were hospitalized within 30 days, 11.8% were admitted to an ICU, and 27.1% died. Greater probability of death was associated with age, male sex, liver and renal impairment, Hba1c above 8%, and former smoking. Hospitalization and ICU admission were mainly affected by age, male sex, hypertension, and metabolic control. Notably, ICU admissions were reduced in very elderly people. No outcomes were associated with educational level.
Conclusions
Hospitalization and ICU admission are heavily affected by age and local triage policy. A key finding was that men who were > 75 years old and poorly compensated were highly vulnerable patients. Renal and/or hepatic impairment are additional factors. This information may be useful for addressing intervention priorities.
Keywords: Type 2 diabetes, SARS-CoV-2, COVID-19, Mortality, Hospitalization, ICU admission
1. Introduction
To date, over 3.7 million cases of coronavirus disease 2019 (COVID-19) and over 114,000 related deaths have been reported in Italy [1]. Northern Italy was the first European area to be affected by the COVID-19 pandemic, and suffered the highest death toll during the so-called “first wave” [2], [3].
Almost all studies have shown that poor prognosis is strongly predicted by older age and certain chronic medical conditions, with type 2 diabetes (T2DM), obesity, hypertension, and cardiovascular disease appearing to have the greatest impacts on COVID-19 progression [4], [5]. Due to the syndromic nature of diabetes, it is likely that multiple factors affect the association between diabetes and worse prognosis. Frequent comorbidities and complications, organ damage, and a pro-inflammatory and pro-coagulative state all probably contribute to the risk of worse outcomes [5], [6].
Current information has been extracted from in-hospital records and/or administrative data relating to type 2 subjects, but there is scarce information available regarding the history and characteristics of patients with diabetes during the period preceding the occurrence of SARS-CoV-2 infection.
Recent reports suggest the possibility that other factors, beside diabetes, may influence the course of COVID-19 infections starting from the first positive swab testing, including the type of treatment administered [7], the coexistence of chronic pulmonary disease [8], as well as the socioeconomic status of the patient [6]. These gaps in our knowledge warrant investigation on the grounds that physicians need a clear picture of how outcomes may be impacted by previous comorbidities, treatments, and quality of metabolic status [9].
To improve our knowledge of this matter, in the present study we linked the data from a large electronic medical record database (containing 14 years of clinical information from a regional diabetes unit network) with data from the regional hospital discharge database, and information derived from the regional PCR testing database. Our main objective was to analyse this population of T2DM patients, to determine the influence of diabetes background on COVID-19 progression from positive swab testing to several health outcomes.
2. Materials and methods
2.1. Study population and selection criteria
The study population was the cohort of patients cared for by the regional network of 19 diabetes care units in Piedmont (4,400,000 inhabitants in northwest Italy). This large diabetes database (Diabetes database) collects demographic and clinical data recorded by diabetologists during patients’ medical examinations. From the Diabetes database, we extracted the data of all patients diagnosed with T2DM who were alive on 21 February 2020, when the first death from COVID-19 was recorded in Italy.
From the start of the COVID-19 epidemic, a surveillance system has been implemented to collect data from all residents undergoing reverse transcriptase-polymerase chain reaction (PCR testing) for SARS-CoV-2. This archive was also pseudo-anonymized and linked to the Diabetes database.
To comply with privacy law, personal patient data are pseudo-anonymized and all the databases are enriched with a unique anonymous identifier, encrypted to protect patient privacy. DBB and data from the COVID-19 surveillance system were thus linked together, and further linked with the regional hospital-discharge database (Hospital discharge database), as well as to the regional registry office. In this way, we were able to enrich the clinical characteristics of the patient records held in the Diabetes database, particularly with regards to comorbidities, and to follow-up each patient in terms of hospitalization and mortality.
Diabetes database
2.2. Outcomes
We considered five separate outcomes that summarize the patients’ disease course during the first wave of the epidemic. We obtained information about testing for SARS-CoV-2 (outcome 1) and positive testing (outcome 2) from the surveillance system. To exclude hospital-acquired COVID-19, we excluded patients who tested positive more than three days after hospital admission. Hospitalization within 30 days after testing positive (outcome 3) was determined from record linkage with the Hospital discharge database. Among these patients, we identified those who were admitted to an intensive care unit (ICU) (outcome 4), based on whether the Hospital discharge database showed evidence of admission to an ICU or an ICD9-CM code referring to mechanical ventilation. Finally, we determined 30-day mortality after testing positive (outcome 5) by record linkage with the registry office.
2.3. Clinical characteristics and comorbidities
Based on the data contained in the Diabetes database, enriched with data from the HD, we categorized the patients according to the socio-demographics, clinical characteristics, and comorbidities that were present on 21 February 2021. Age was categorized into 10-year age intervals: <55, 55–64, 65–74, 75–84, and >84 years old. Individual educational level, obtained by record linkage with the last national census, was available for 97.7% of patients, and was classified as high (university/high school), medium (middle school), low (primary school/no formal education), or missing. Smoking history was only available for 60.4% patients, and was classified as current smoker, past smoker, never smoked, or missing. Body-mass index (BMI, weight in kg divided by height in meters squared) was stratified as follows: <25 (normal), 25–29.99 (overweight), ≥30 (obese), or missing. If more than one measurement had been recorded, we utilized the measurement closest to the date of PCR testing.
Patients were classified into four groups of hypoglycaemic treatment: innovative drugs (incretins and/or SGLT-2 inhibitors), insulin, oral antidiabetic drugs, and diet. Patients taking both insulin and oral antidiabetic drugs were assigned to the insulin group. Patients taking both innovative drugs and any other kind of antidiabetic drug were assigned to the “innovative drugs” group. The duration of diabetes was stratified as follows: <2, 2–3.9, 4–6.9, and ≥7 years. HbA1c level was only considered if recorded during 2018 or 2019 (available for 83.1% of patients), and was stratified into four categories: <7%, 7–7.9%, 8–8.9%, and ≥9%. If more than one measurement was recorded, we used the one closest to the date of PCR testing.
Information regarding comorbidities was retrieved either from the Diabetes database, or from hospitalizations that occurred between 2015–2019. Table 1 contains the ICD9-CM codes used to select comorbidities in the hospital-discharge database.
Table 1.
Sources and criteria used for selecting comorbidity groups.
| From hospital discharge or diabetes database |
From diabetes database only | ||
|---|---|---|---|
| ICD IX CM code (diagnosis) | ICD IX CM (procedure) | ||
| Cirrhosis/chronic hepatitis | 5714–5719 | ||
| Neuropathy | 2506, 337, 354, 355, 3572, 3574, 3581, 5363, 7135 | ||
| Retinopathy | 362, 369, 2505 | 1435 | |
| Coronary heart disease | 410–414 | 0066, 3606, 3607, 361, 362 | |
| Cerebrovascular disease | 430–438 | ||
| Heart failure | 39891, 402, 40401, 40403, 40411, 40413, 40491, 40493, 425, 428, 78,551 | 3751 | |
| Hypertension | 401–405 | ATC C02 (antihypertensive treatment), or PAD > 90, PAS > 140 | |
| Cancer (in the last two years) | 140–239 | ||
| Dialysis | 3995, 5498 | ||
| Diabetic foot/limb amputation/PAD | 2507, 4402, 4439, 44381, 44422, 6811, 6826, 6827, 7071, 7854, 73007–73017, 99,674 | 3925, 3950, 8411–8419, 8663–8666 | |
| Nephropathy | 2504, 58181, 58381, 585, 586, 6393 | EGfr < 60, albumin excretion rate > 20, albumin/creatinine ratio > 2.5 and male, albumin/creatinine ratio > 3.5 and female, microalbuminuria > 30 | |
| COPD | main diagnosis at discharge 490–492, 494, 496 or main diagnosis 51881–51884, 7860, 7862, 7864 and secondary diagnosis 490–492, 494, 496 | Exemption from co-payment due to COPD or drug prescriptions with the ATC codes: R03A, R03CC02–R03CC04, R03CK, R03BB01, R03BB02, R03BB04, R03DA01, R03DA04, R03DA05, R03DA08, R03DA11, R03DA49; at least four different prescriptions during 2018–2019 (excluding patients with asthma) | |
COPD: Chronic obstructive pulmonary disease.
PAD: Phriferal artery disease.
2.4. Statistical analysis
The proportions (i.e. the crude prevalences) of the variables, by each of the five outcomes, were calculated, and the differences in baseline characteristics were evaluated using the X2 test. To investigate the relationship between the outcomes and the explicative variables, we used robust Poisson models, and the results are presented as prevalence ratios (PRs), which are a better estimate of the relative risk when the prevalence of the outcome is high, with 95% confidence intervals [10]. All analyses were performed using SAS version 9.4.
3. Results
At the beginning of the COVID-19 epidemic, 150,392 T2DM patients were cared for by the network of diabetes clinics of Piedmont. Fig. 1 shows the paths that these patients followed from 21 February 2020 to 31 May 2020, with regards to their clinical history related to COVID-19. Among these patients, 7.5% received PCR testing, and 1.56% were positive for COVID-19 (20.9% of those tested). Within 30 days of a positive test, 49.4% of patients were hospitalized, 11.8% were admitted to an ICU, and 27.1% died. Of these deaths, 67.8% occurred in a hospital. Fig. 1 also shows the numbers and percentages of the population who had at least one HbA1c measurement recorded in 2018 or 2019, which represents the cohort analysed in the current study.
Fig. 1.
Study profile. Bold text indicates study population having at least one HbA1c measurement from 2018 or 2019.
3.1. Study population and PCR testing
Table 2 presents the characteristics of the study population, the crude prevalence rates of patients tested for COVID-19 and those who tested positive for the virus. We identified 125,021 T2DM patients who were eligible for the study, of whom, over half were male, 73% were over 65 years old, and 42% had a low education level. Only 11% were current smokers, and 75% were overweight or obese. With regards to their history of diabetes, nearly half of the population had a disease duration exceeding 7 years, 44% were treated with oral hypoglycaemic drugs and a quarter with insulin. Half had normal HbA1c, while 21% had an HbA1c level of >8%. The most frequently reported comorbidity was hypertension (86%), half of the population had some renal impairment (including dialysis), and about 17% had some micro or macro vascular complication.
Table 2.
Characteristics of the study population and of patients who received PCR testing.
| T2DM population |
PCR tested |
Positive (among tested patients) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | p χ2 | n | % | p χ2 | ||
| Sex | <0.0001 | 0.0045 | |||||||
| Men | 68,760 | 55.0 | 4213 | 6.1 | 957 | 22.7 | |||
| Women | 56,261 | 45.0 | 4573 | 8.1 | 925 | 20.2 | |||
| Age | <0.0001 | <0.0001 | |||||||
| <65 years | 32,275 | 26.2 | 1856 | 5.7 | 300 | 16.2 | |||
| 65–74 years | 39,463 | 31.6 | 1842 | 4.7 | 404 | 21.9 | |||
| 75–84 years | 39,399 | 31.5 | 2954 | 7.5 | 702 | 23.8 | |||
| ≥85 years | 13,384 | 10.7 | 2134 | 15.9 | 476 | 22.3 | |||
| Educational level | <0.0001 | 0.1903 | |||||||
| High | 21,699 | 17.4 | 1356 | 6.3 | 285 | 21.0 | |||
| Medium | 47,109 | 37.7 | 2790 | 5.9 | 565 | 20.3 | |||
| Low | 53,355 | 42.7 | 4468 | 8.4 | 990 | 22.2 | |||
| Missing | 2858 | 2.3 | 172 | 6.0 | 42 | 24.4 | |||
| Smoking habit | <0.0001 | <0.0001 | |||||||
| No | 39,243 | 31.4 | 2603 | 6.6 | 578 | 22.2 | |||
| Yes | 13,935 | 11.2 | 913 | 6.6 | 143 | 15.7 | |||
| Former | 22,340 | 17.9 | 1372 | 6.1 | 329 | 24.0 | |||
| Missing | 49,503 | 39.6 | 3898 | 7.9 | 832 | 21.3 | |||
| Duration of diabetes | 0.2093 | 0.3501 | |||||||
| <2 years | 8113 | 6.5 | 528 | 6.5 | 100 | 18.9 | |||
| 2–4 years | 17,763 | 14.2 | 1262 | 7.1 | 276 | 21.9 | |||
| 4–7 years | 38,121 | 30.5 | 2727 | 7.2 | 605 | 22.2 | |||
| ≥7 years | 61,024 | 48.8 | 4269 | 7.0 | 901 | 21.1 | |||
| Antidiabetic therapy | <0.0001 | 0.8991 | |||||||
| new hypoglycaemic drugs | 26,175 | 20.9 | 1405 | 5.4 | 307 | 21.9 | |||
| Insulin | 31,203 | 25.0 | 3046 | 9.8 | 656 | 21.5 | |||
| oral hypoglycaemic drugs | 55,081 | 44.1 | 2792 | 5.1 | 585 | 21.0 | |||
| no drug therapy | 12,562 | 10.1 | 1543 | 12.3 | 334 | 21.6 | |||
| BMI | <0.0001 | 0.5761 | |||||||
| <25 (underweight/normal) | 27,689 | 22.2 | 2236 | 8.1 | 460 | 20.6 | |||
| 25–29.99 (overweight) | 48,079 | 38.5 | 3006 | 6.3 | 650 | 21.6 | |||
| ≥30 (obese) | 46,060 | 36.8 | 3057 | 6.6 | 659 | 21.6 | |||
| Missing | 3193 | 2.6 | 487 | 15.3 | 113 | 23.2 | |||
| HbA1c | <0.0001 | 0.7136 | |||||||
| <7% | 64,360 | 51.5 | 4513 | 7.0 | 989 | 21.9 | |||
| 7–8% | 34,613 | 27.7 | 2228 | 6.4 | 466 | 20.9 | |||
| 8–9% | 16,150 | 12.9 | 1196 | 7.4 | 251 | 21.0 | |||
| ≥9% | 9898 | 7.9 | 849 | 8.6 | 176 | 20.7 | |||
| Comorbidity groups | |||||||||
| cirrhosis/chronic hepatitis | 5182 | 4.1 | 339 | 6.5 | 0.1623 | 66 | 19.5 | 0.3718 | |
| neuropathy | 11,711 | 9.4 | 993 | 8.5 | <0.0001 | 202 | 20.3 | 0.3793 | |
| retinopathy | 21,290 | 17.0 | 1666 | 7.8 | <0.0001 | 343 | 20.6 | 0.3577 | |
| coronary heart disease | 22,143 | 17.7 | 1958 | 8.8 | <0.0001 | 409 | 20.9 | 0.5153 | |
| cerebrovascular disease | 20,467 | 16.4 | 1957 | 9.6 | <0.0001 | 461 | 23.6 | 0.0090 | |
| heart failure | 13,818 | 11.1 | 1697 | 12.3 | <0.0001 | 353 | 20.8 | 0.4890 | |
| hypertension | 108,477 | 86.8 | 7380 | 6.8 | <0.0001 | 1588 | 21.5 | 0.6110 | |
| COPD | 7563 | 6.1 | 835 | 11.0 | <0.0001 | 175 | 21.0 | 0.7321 | |
| cancer (in the last 2 years) | 6015 | 4.8 | 629 | 10.5 | <0.0001 | 114 | 18.1 | 0.0365 | |
| diabetic foot/limb amputation/PAD | 16,040 | 12.8 | 1628 | 10.2 | <0.0001 | 337 | 20.7 | 0.4326 | |
| Dialysis | 767 | 0.6 | 259 | 33.8 | <0.0001 | 36 | 13.9 | 0.0027 | |
| nephropathy | 61,603 | 49.3 | 5124 | 8.3 | <0.0001 | 1132 | 22.1 | 0.0695 | |
| TOTAL | 125,021 | 100 | 8786 | 7.0 | 1882 | 21.4 | |||
COPD: Chronic obstructive pulmonary disease.
PAD: Peripheral artery disease.
χ2: Chi-square.
Testing for COVID-19 was more frequent among women, older people, less educated people, patients who did not take any hypoglycaemic drugs, patients with higher HbA1c levels, and patients of normal weight. Most patients with comorbidities exhibited above average use of PCR testing, ranging from 8% among those with retinopathy to 33% among patients on dialysis. The multivariate model (Table 3 ) revealed that PCR testing was more common among women and people at higher risk of serious consequences in the event of COVID-19. Overall, PCR test positivity only slightly differed among the tested patient groups. Notably, the prevalence of infection increased with age, particularly in patients over 65 years old, and in former smokers or non-smokers. The multivariate model (Table 3) showed that age was the strongest determinant of COVID-19 infection, while positivity rates were lower among women, smokers, and patients on dialysis or with cancer.
Table 3.
PCR testing: multivariate analysis.
| PCR tested |
At least one positive test (among tested patients) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | p value | PR | 95% CI | p value | ||||
| Sex | |||||||||
| men | 1 | 1 | |||||||
| women | 1.26 | 1.21 | 1.32 | <0.0001 | 0.85 | 0.78 | 0.93 | 0.0004 | |
| Age | |||||||||
| <65 years | 1 | 1 | |||||||
| 65–74 years | 0.76 | 0.72 | 0.82 | <0.0001 | 1.34 | 1.17 | 1.55 | <0.0001 | |
| 75–84 years | 1.11 | 1.04 | 1.18 | 0.0017 | 1.45 | 1.26 | 1.67 | <0.0001 | |
| ≥85 years | 2.07 | 1.93 | 2.22 | <0.0001 | 1.38 | 1.19 | 1.61 | <0.0001 | |
| Educational level | |||||||||
| high | 1 | 1 | |||||||
| medium | 0.92 | 0.87 | 0.98 | 0.0121 | 0.96 | 0.85 | 1.09 | 0.5281 | |
| low | 0.98 | 0.93 | 1.05 | 0.6075 | 0.96 | 0.84 | 1.09 | 0.4927 | |
| missing | 0.96 | 0.82 | 1.11 | 0.5684 | 1.16 | 0.88 | 1.54 | 0.2927 | |
| Smoking habit | |||||||||
| no | 1 | 1 | |||||||
| yes | 1.22 | 1.13 | 1.31 | <0.0001 | 0.74 | 0.62 | 0.87 | 0.0005 | |
| former | 1.05 | 0.99 | 1.12 | 0.1295 | 1.02 | 0.90 | 1.16 | 0.7590 | |
| missing | 1.18 | 1.12 | 1.24 | <0.0001 | 0.94 | 0.85 | 1.03 | 0.1715 | |
| BMI | |||||||||
| <25 (underweight/normal) | 1 | 1 | |||||||
| 25–29.99 (overweight) | 0.87 | 0.83 | 0.92 | <0.0001 | 1.07 | 0.96 | 1.19 | 0.2312 | |
| ≥30 (obese) | 0.94 | 0.89 | 0.99 | 0.0193 | 1.12 | 1.00 | 1.25 | 0.0431 | |
| missing | 1.48 | 1.35 | 1.63 | <0.0001 | 1.13 | 0.93 | 1.38 | 0.2035 | |
| Duration of diabetes | |||||||||
| <2 years | 1 | 1 | |||||||
| 2–4 years | 1.10 | 0.99 | 1.21 | 0.0668 | 1.14 | 0.93 | 1.41 | 0.2045 | |
| 4–7 years | 1.12 | 1.02 | 1.22 | 0.0197 | 1.16 | 0.95 | 1.41 | 0.1434 | |
| ≥7 years | 1.04 | 0.95 | 1.14 | 0.3708 | 1.09 | 0.89 | 1.33 | 0.3875 | |
| Antidiabetic therapy | |||||||||
| new hypoglycaemic drugs | 1 | 1 | |||||||
| insulin | 1.49 | 1.40 | 1.59 | <0.0001 | 0.99 | 0.88 | 1.12 | 0.8888 | |
| Oral hypoglycaemic drugs | 0.98 | 0.92 | 1.05 | 0.6220 | 0.94 | 0.83 | 1.07 | 0.3563 | |
| no drug therapy | 2.00 | 1.86 | 2.15 | <0.0001 | 0.95 | 0.83 | 1.10 | 0.5055 | |
| HbA1c | |||||||||
| <7% | 1 | 1 | |||||||
| 7–8% | 0.95 | 0.90 | 1.00 | 0.0337 | 0.95 | 0.86 | 1.05 | 0.2827 | |
| 8–9% | 0.99 | 0.93 | 1.06 | 0.8477 | 0.95 | 0.84 | 1.08 | 0.4352 | |
| ≥9% | 1.11 | 1.03 | 1.20 | 0.0067 | 0.97 | 0.83 | 1.13 | 0.6813 | |
| Comorbidity groups** | |||||||||
| cirrhosis/chronic hepatitis | 1.04 | 0.94 | 1.16 | 0.4152 | 0.91 | 0.73 | 1.13 | 0.3999 | |
| neuropathy | 0.99 | 0.92 | 1.06 | 0.7465 | 0.95 | 0.82 | 1.09 | 0.4711 | |
| retinopathy | 1.01 | 0.95 | 1.06 | 0.8122 | 0.94 | 0.84 | 1.05 | 0.2598 | |
| coronary heart disease | 1.07 | 1.01 | 1.13 | 0.0142 | 0.92 | 0.83 | 1.02 | 0.1264 | |
| cerebrovascular disease | 1.27 | 1.21 | 1.33 | <0.0001 | 1.11 | 1.01 | 1.22 | 0.0369 | |
| heart failure | 1.37 | 1.29 | 1.44 | <0.0001 | 0.94 | 0.85 | 1.05 | 0.3087 | |
| hypertension | 0.78 | 0.74 | 0.83 | <0.0001 | 1.01 | 0.89 | 1.14 | 0.9043 | |
| COPD | 1.28 | 1.19 | 1.37 | <0.0001 | 0.95 | 0.82 | 1.09 | 0.4488 | |
| cancer (in the last 2 years) | 1.43 | 1.32 | 1.54 | <0.0001 | 0.83 | 0.70 | 0.99 | 0.0355 | |
| diabetic foot/limb amputation/PAD | 1.29 | 1.21 | 1.37 | <0.0001 | 0.98 | 0.87 | 1.11 | 0.7603 | |
| dialysis | 3.32 | 2.98 | 3.69 | <0.0001 | 0.65 | 0.47 | 0.89 | 0.0069 | |
| nephropathy | 1.16 | 1.11 | 1.21 | <0.0001 | 1.05 | 0.96 | 1.15 | 0.2851 | |
* Within 30 days after testing positive on PCR test.
** “without comorbidity” is the reference group.
COPD: Chronic obstructive pulmonary disease
PAD: Peripheral artery disease
3.2. Hospitalization
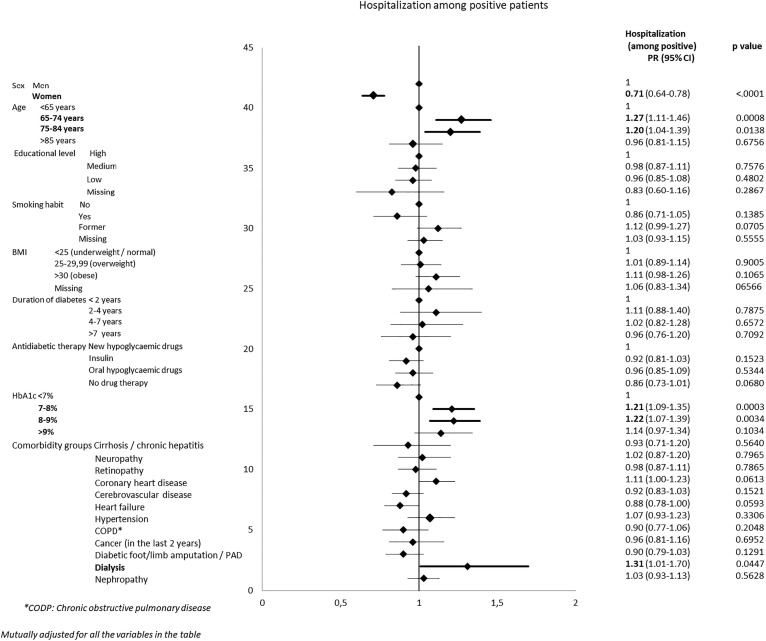
Among the PCR-positive patients, half were admitted to a hospital within 30 days from testing (Table 4 ). The hospitalization rate was higher among men, patients 65–74 years old, former smokers, overweight or obese patients, patients with high HbA1c levels, and patients treated with new hypoglycaemic drugs. Patients on dialysis had the highest risk of hospitalization. On the other hand, very elderly patients and patients with a low education level had much lower chances of hospital admission. The multivariate model largely confirmed the observations in the univariate analysis (Fig. 2 ) Age showed a U-shaped pattern, with people of 65–84 years old having a higher risk of hospitalization. Hospitalization risk was also higher for men, former smokers, and patients with poorly controlled glycemia. In the multivariate model, differences according to educational level disappeared, and dialysis was the only comorbidity that remained significantly associated with hospital admission.
Table 4.
Outcomes within 30 days after a positive PCR test.
| Hospitalization |
ICU |
Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | p value | n | % | p value | n | % | p value | |
| Sex | <0.0001 | <0.0001 | <0.0001 | ||||||
| Male | 580 | 60.6 | 171 | 17.9 | 305 | 31.9 | |||
| Female | 367 | 39.7 | 65 | 7.0 | 207 | 22.4 | |||
| Age | <0.0001 | <0.0001 | <0.0001 | ||||||
| <65 years | 144 | 48.0 | 55 | 18.3 | 22 | 7.3 | |||
| 65–74 years | 250 | 61.9 | 112 | 27.7 | 87 | 21.5 | |||
| 75–84 years | 368 | 52.4 | 62 | 8.8 | 225 | 32.1 | |||
| ≥85 years | 185 | 38.9 | 7 | 1.5 | 178 | 37.4 | |||
| Educational level | 0.0139 | <0.0001 | 0.0054 | ||||||
| High | 163 | 57.2 | 51 | 17.9 | 66 | 23.2 | |||
| Medium | 297 | 52.6 | 85 | 15.0 | 135 | 23.9 | |||
| Low | 468 | 47.3 | 92 | 9.3 | 303 | 30.6 | |||
| Missing | 19 | 45.2 | 8 | 19.0 | 8 | 19.0 | |||
| Smoking habit | <0.0001 | <0.0001 | <0.0001 | ||||||
| No | 265 | 45.8 | 57 | 9.9 | 124 | 21.5 | |||
| yes | 64 | 44.8 | 13 | 9.1 | 29 | 20.3 | |||
| Former | 201 | 61.1 | 69 | 21.0 | 116 | 35.3 | |||
| Missing | 417 | 50.1 | 97 | 11.7 | 243 | 29.2 | |||
| Duration of diabetes | 0.4555 | 0.2827 | 0.1393 | ||||||
| <2 years | 47 | 47.0 | 14 | 14.0 | 20 | 20.0 | |||
| 2–4 years | 148 | 53.6 | 38 | 13.8 | 65 | 23.6 | |||
| 4–7 years | 311 | 51.4 | 85 | 14.0 | 171 | 28.3 | |||
| ≥7 years | 441 | 48.9 | 99 | 11.0 | 256 | 28.4 | |||
| Antidiabetic therapy | <0.0001 | <0.0001 | 0.0013 | ||||||
| new hypoglycaemic drugs | 180 | 58.6 | 60 | 19.5 | 79 | 25.7 | |||
| Insulin | 330 | 50.3 | 72 | 11.0 | 214 | 32.6 | |||
| oral hypoglycaemic drugs | 302 | 51.6 | 81 | 13.8 | 143 | 24.4 | |||
| no drug therapy | 135 | 40.4 | 23 | 6.9 | 76 | 22.8 | |||
| BMI | 0.0283 | <0.0001 | 0.4112 | ||||||
| <25 (underweight/normal) | 209 | 45.4 | 32 | 7.0 | 134 | 29.1 | |||
| 25–29.99 (overweight) | 332 | 51.1 | 89 | 13.7 | 169 | 26.0 | |||
| ≥30 (obese) | 355 | 53.9 | 108 | 16.4 | 173 | 26.3 | |||
| Missing | 51 | 45.1 | 7 | 6.2 | 36 | 31.9 | |||
| HbA1c | 0.0002 | 0.0091 | 0.1201 | ||||||
| <7% | 451 | 45.6 | 101 | 10.2 | 249 | 25.2 | |||
| 7–8% | 264 | 56.7 | 68 | 14.6 | 138 | 29.6 | |||
| 8–9% | 140 | 55.8 | 36 | 14.3 | 79 | 31.5 | |||
| ≥9% | 92 | 52.3 | 31 | 17.6 | 46 | 26.1 | |||
| Comorbidity groups | |||||||||
| cirrhosis/chronic hepatitis | 31 | 47.0 | 0.5796 | 10 | 15.2 | 0.5143 | 23 | 34.8 | 0.1555 |
| neuropathy | 99 | 49.0 | 0.6937 | 25 | 12.4 | 0.9408 | 50 | 24.8 | 0.4071 |
| retinopathy | 173 | 50.4 | 0.9613 | 42 | 12.2 | 0.8553 | 104 | 30.3 | 0.1516 |
| coronary heart disease | 233 | 57.0 | 0.0024 | 53 | 13.0 | 0.7726 | 149 | 36.4 | <0.0001 |
| cerebrovascular disease | 216 | 46.9 | 0.0869 | 41 | 8.9 | 0.0065 | 141 | 30.6 | 0.0605 |
| heart failure | 162 | 45.9 | 0.0650 | 36 | 10.2 | 0.1405 | 129 | 36.5 | <0.0001 |
| hypertension | 812 | 51.1 | 0.1004 | 212 | 13.4 | 0.0136 | 448 | 28.2 | 0.0226 |
| COPD | 83 | 47.4 | 0.4220 | 18 | 10.3 | 0.3444 | 58 | 33.1 | 0.0638 |
| cancer (in the last 2 years) | 59 | 51.8 | 0.7518 | 14 | 12.3 | 0.9313 | 41 | 36.0 | 0.0301 |
| diabetic foot/limb amputation/PAD | 160 | 47.5 | 0.2496 | 43 | 12.8 | 0.8930 | 104 | 30.9 | 0.0961 |
| Dialysis | 23 | 63.9 | 0.1001 | 4 | 11.1 | 0.2017* | 15 | 41.7 | 0.0490 |
| Nephropathy | 571 | 50.4 | 0.8958 | 130 | 11.5 | 0.0893 | 363 | 32.1 | <0.0001 |
| TOTAL | 947 | 50.3 | 236 | 12.5 | 512 | 27.2 | |||
* Fisher’s exact test.
COPD: Chronic obstructive pulmonary disease.
PAD: Peripheral artery disease.
Fig. 2.
Forest plots showing adjusted prevalence ratios for Covid-19 related hospitalization in positive patients with type 2 diabetes in Piedmont (Italy).
3.3. ICUxxx
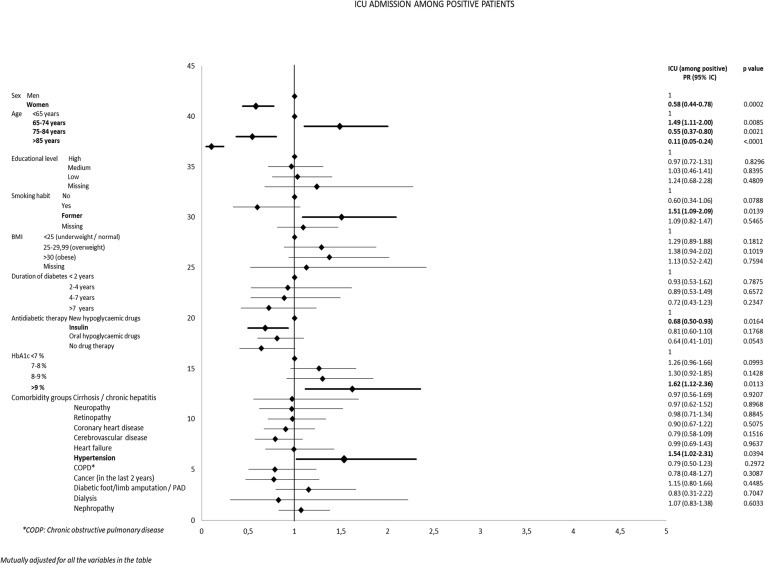
Among the hospitalized patients, one-quarter (12.5% of the positive patients) required ICU admission (Table 4). Similarly to hospitalization, the ICU admission rates were higher in men, former smokers, overweight and obese patients, and patients with high HbA1c levels. ICU admission rates declined sharply among patients over 75 years of age, and were almost negligible among very old patients. Multivariate analysis (Fig. 3 ) confirmed the increased risk of ICU admission for men, as well as the U-shaped pattern in terms of age, with the elderly, and especially very elderly, people almost excluded from ICU care. Women and insulin-treated patients were less likely to be admitted to an ICU, while former smokers and decompensated patients were at increased risk. As regards comorbidities, only hypertension showed a statistically significant association with ICU admission. Educational level was not associated with ICU admission.
Fig. 3.
Forest plots showing adjusted prevalence ratios for Covid-19 related ICU admission in positive patients with type 2 diabetes in Piedmont (Italy).
3.4. Mortality
Finally, mortality rates were higher among men, elderly patients, poorly educated people, patients with longer disease duration, insulin-treated patients, and in all patients with comorbidity (particularly nephropathy, cancer, heart failure, or coronary artery disease) (Table 4). The multivariate model (Fig. 4 ) confirmed the causal role of age, while we no longer saw associations with conditions closely related to age, such as educational level, diabetes duration, high BMI, and insulin therapy. Mortality was also significantly increased among men, former smokers, and patients with poor metabolic control. Increased risk of death was also associated with liver disease, cancer, and dialysis or nephropathy.
Fig. 4.
Forest plots showing adjusted prevalence ratios for Covid-19 related mortality in positive patients with type 2 diabetes in Piedmont (Italy).
4. Discussion
The Piedmont region of Italy was severely impacted by the first wave of the COVID-19 pandemic. Here we found that, compared to previously published data from the general population of this region, DMT2 patients exhibited a PCR positivity rate that was nearly three times higher, and that positive cases showed a very high hospital admission rate, and a doubled 30-day case-fatality rate, despite the fact that the median age of the general population died from COVID-19 in Piedmont was 83 years, the same of the DMT2 patients of the present study [3]. These rates are higher than those reported in other countries [11], [12], and indicate that persons with COVID-19 and diabetes have more severe hospital stays, poorer outcomes, and higher resource utilisation [13]. The primary objective of our research was to further examine whether worse health outcomes of COVID-19 infection, starting from a positive swab test, may be associated with specific clinical, social, or anthropometric characteristics, related to the patients’ diabetes history and to the extraordinary situation during the first months of the pandemic.
In our analyses, male gender emerged as being associated with everything that marks worse COVID-19 progress, confirming that the predisposition of males to this infection (as seen in the general population) holds true in cases of diabetes. On the other hand, PCR swab testing was more frequent among women, possibly reflecting the typical increased health awareness of women. Interestingly, women were less likely to be hospitalized or admitted to an ICU, and also had a lower probability of death. This may be considered an indirect confirmation of the finding that female sex confers increased protection against the most unfavourable outcomes of COVID-19 [14].
We also found that age was a powerful predictive factor in terms of mortality, with the highest PR found for patients over 75 years old. However elderly people showed the lowest PR for admission to an ICU. Likely explanations for this finding are that age-based admission triage was performed during the overwhelming influx of patients with respiratory failure in March and April of 2020, along with the higher mortality prior to ICU admission among the elderly.
In contrast to reports from other countries, such as the UK [6], Sweden [15], or the USA [16], we did not find that educational level was associated with any of the evaluated outcomes in our study population. Additionally, we found that current smoking played a neutral role in outcomes, while previous smoking had a negative impact; similar findings have been previously reported [6], [11], whereas other authors [17] have highlighted opposite conclusions. This unexpected finding should not be considered evidence that smoking is protective against COVID-19 infection in patients with diabetes, as some media have reported. It cannot be excluded that the designation “former smoker” acts as a proxy of advanced chronic obstructive lung disease [18].
As expected, the degree of metabolic control affected both hospital and ICU admission, as well as mortality. HbA1c levels of over 7% predisposed patients to more frequent hospital admissions, in agreement with the well-known association between poor glucose control and rate of hospital admissions for all causes beyond the context of COVID-19. It is basic rule of clinical diabetology that high blood glucose levels are a powerful antagonist of immune responses.
In studies that have compared patients with both type 1 and type 2 diabetes versus the general population, BMI is frequently reported as a predictor of SARS-CoV-2 infection and hospitalization [19], [20]. Our present results indicated that BMI was somewhat correlated with greater ICU admissions and mortality in univariate analysis, but not in the multivariate models. Notably, our analysis was performed in a population with type 2 diabetes, and these patients are generally overweight, which may make it difficult to assess the specific contribution of BMI [21].
Our results indicated that cancer, cirrhosis, haemodialysis, and nephropathy—all well-defined conditions characterized by immunodeficiency and debilitation—were associated with higher mortality but not more frequent hospitalization or ICU admission. Since one-third of deaths occurred out of the hospital, it cannot be excluded that these patients were denied hospitalization in favour of patients with a higher life expectancy, given the shortage of hospital beds during the peak days of the epidemic. One novelty in our findings is that liver cirrhosis had a negative impact on mortality. From a clinical perspective, this is a highly plausible result, given the general debilitation and immunodeficiency of these patients. To our knowledge, there is only one prior report of an association between liver disease and COVID-19 [11].
One strength of our study is that we did not limit the study population to hospitalized patients, which would be a potential source of bias [9], but we rather included people with type 2 diabetes who received care within the regional network of diabetes clinics, and who had a COVID-19 diagnosis confirmed by a positive PCR test. The separation of type 2 diabetes from type 1 diabetes may have provided more accurate results. Furthermore, collecting data from both clinical and administrative sources (Hospital Discharges, national census, and population register) enabled us to describe, and adjust for, some patient socio-demographic and clinical characteristics with a fair degree of accuracy. Likewise, we followed the patients’ entire clinical history at the population level (i.e. including out-of-hospital deaths), from the time of PCR testing to the assessed health outcomes, with a rather long-term prognosis. Even if the cause of death was not available from the death certificates, the time window of 30 days from a positive test is commonly used to identify COVID-related deaths [3], [22]. Notably, in Piedmont, 95% of deaths occurred within the first 30 days following diagnosis [3]. With regards to hospitalization, 98.7% had a main or secondary diagnosis at discharge, which was certainly related to COVID-19.
Some limitations of this study must also be acknowledged. First, we excluded 17% of patients due to a lack of a recent HbA1c determination. However, the two populations showed very small differences in the prevalence of outcomes (as shown in Fig. 1); thus, it is unlikely that a selection bias was introduced. Secondly, patients receiving care at diabetes clinics are not representative of the full population of diabetes patients. Notably, patients attending clinics are more likely to adhere to clinical guidelines and have more favourable outcomes [23]. This suggests that our results can likely be considered more favourable compared to the health outcomes in the total population with diabetes.
5. Conclusions
Within a population with diabetes, male sex, age of > 75 years, and poor glycemic compensation were factors associated with unfavourable outcomes in COVID-19 patients. Moreover, a patient’s risk may be better characterized based on the presence of renal impairment and/or hepatic disease. Patients’ educational level did not influence hospitalization or ICU admission neither mortality. Our present study clearly demonstrated that among patients with type 2 diabetes, older age is a determinant of poor prognosis after infection, powerful enough to warrant special attention. Efforts should be made to direct vaccination priority and use of innovative treatments, such as monoclonal antibodies, towards this category of patients.
Funding
This study was in part supported by Chaira Medica Association (non-profit organization for the study of endocrine and metabolic disorders), Chieri, Italy, where BT is employee of the Association. The authors received no funding from an external source.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
CBG literature search, study design, data collection, data interpretation, writing.; RP data collection, data analysis; BT literature search, data collection; EN literature search, data collection; MD literature search, data collection, FR literature search, study design, data interpretation; GC data interpretation, writing; RG literature search, study design, data collection, data interpretation, writing.
All authors had full access to the data, approved the final version, and accept the responsibility to submit the study for publication.
References
- 1.L'epidemiologia per la sanità pubblica Istituto Superiore di Sanità. Accessed 6 May 202Available from www.epicentro.iss.it
- 2.Distante C., Piscitelli P., Miani A. Covid-19 outbreak progression in Italian regions: approaching the peak by the end of March in Northern Italy and first week of April in Southern Italy. Int J Environ Res Public Health. 2020;17:3025. doi: 10.3390/ijerph17093025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabiani M, Onder G, Boros S, et al. Il case fatality rate dell’infezione SARS-CoV-2 a livello regionale e attraverso le differenti fasi dell’epidemia in Italia. Versione del 20 gennaio 2021. Roma: Istituto Superiore di Sanità; 2021. (Rapporto ISS COVID-19 n. 1/2021). Accessed 6 May 2021. Available from https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID-19+n.+1_2021.pdf/eef324b0-983d-c257-96fd-e8d430e1ca82?t=1612179039051.
- 4.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 5.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solerte S.B., D’Addio F., Trevisan R., Lovati E., Rossi A., Pastore I., et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43(12):2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung J.M., Niikura M., Yang C.W.T., Sin D.D. COVID-19 and COPD. Eur Respir J. 2020;56(2):2002108. doi: 10.1183/13993003.02108-202010.1183/13993003.02108-2020.Shareable1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvin E., Juraschek S.P. Diabetes epidemiology in the COVID-19 pandemic. Diabetes Care. 2020;43(8):1690–1694. doi: 10.2337/dc20-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deddens J.A., Petersen M.R. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65(7):501–506. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- 11.McGurnaghan S.J., Weir A., Bishop J., et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory J.M., Slaughter J.C., Duffus S.H., Smith T.J., LeStourgeon L.M., Jaser S.S., et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic's impact in Type 1 and Type 2 diabetes. Diabetes Care. 2021;44(2):526–532. doi: 10.2337/dc20-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorda C.B. The role of the care model in modifying prognosis in diabetes. Nutr Metab Cardiovasc Dis. 2013;23(1):11–16. doi: 10.1016/j.numecd.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Maleki Dana P., Sadoughi F., Hallajzadeh J., Asemi Z., Mansournia M.A., Yousefi B., et al. An insight into the sex differences in COVID-19 patients: what are the possible causes? Prehosp Disaster Med. 2020;35(4):438–441. doi: 10.1017/S1049023X20000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drefahl S., Wallace M., Mussino E., Aradhya S., Kolk M., Brandén M., et al. A population-based cohort study of socio-demographic risk factors for COVID-19 deaths in Sweden. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-18926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins R.B., Charles E.J., Mehaffey J.H. Socio-economic status and COVID-19-related cases and fatalities. Public Health. 2020;189:129–134. doi: 10.1016/j.puhe.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO/2019-nCoV/Sci_Brief/Smoking/2020.2.
- 18.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cariou B., Hadjadj S., Wargny M., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonnet A., Chetboun M., Poissy J., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longmore D.K., Miller J.E., Bekkering S., Saner C., Mifsud E., Zhu Y., et al. Diabetes and overweight/obesity are independent, nonadditive risk factors for in-hospital severity of COVID-19: an international, multicenter retrospective meta-analysis. Diabetes Care. 2021;44(6):1281–1290. doi: 10.2337/dc20-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto-Alhambra D, Balló E, Coma E, et al. Filling the gaps in the characterization of the clinical management of COVID-19: 30-day hospital admission and fatality rates in a cohort of 118 150 cases diagnosed in outpatient settings in Spain. Int J Epidemiol 2021;49:1930–1939. 10.1093/ije/dyaa190. [DOI] [PMC free article] [PubMed]
- 23.Giorda C, Picariello R, Nada E, et al. The impact of adherence to screening guidelines and of diabetes clinics referral on morbidity and mortality in diabetes. PLoS One 2012;7:e33839 10.1371/journal.pone.0033839. [DOI] [PMC free article] [PubMed]