Abstract
Background
Sites of entry for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are highly expressed in nasal epithelial cells; however, little is known about the impact of intranasal corticosteroids (INCS) on coronavirus disease 2019 (COVID-19)-related outcomes.
Objective
To determine the association between baseline INCS use and COVID-19-related outcomes.
Methods
Using the Cleveland Clinic COVID-19 Research Registry, we performed a propensity score matching for treatment with INCS before SARS-CoV-2 infection (April 1, 2020, to March 31, 2021). Of the 82,096 individuals who tested positive, 72,147 met inclusion criteria. Our endpoints included the need for hospitalization, admission to the intensive care unit (ICU), or in-hospital mortality.
Results
Of the 12,608 (17.5%) who were hospitalized, 2935 (4.1%) required ICU admission and 1880 (2.6%) died during hospitalization. A significant proportion (n = 10,187; 14.1%) were using INCS before SARS-CoV-2 infection. Compared with nonusers, INCS users demonstrated lower risk for hospitalization (adjusted odds ratio [OR] [95% confidence interval (CI)]: 0.78 [0.72; 0.85]), ICU admission (adjusted OR [95% CI]: 0.77 [0.65; 0.92]), and in-hospital mortality (adjusted OR [95% CI]: 0.76 [0.61; 0.94]). These findings were replicated in sensitivity analyses where patients on inhaled corticosteroids and those with allergic rhinitis were excluded. The beneficial effect of INCS was significant after adjustment for baseline blood eosinophil count (measured before SARS-CoV-2 testing) in a subset of 30,289 individuals.
Conclusion
INCS therapy is associated with a lower risk for COVID-19-related hospitalization, ICU admission, or death. Future randomized control trials are needed to determine if INCS reduces the risk for severe outcomes related to COVID-19.
Key words: SARS-CoV-2, COVID-19, Intranasal corticosteroids, Asthma, Eosinophilia
Abbreviations used: ACE2, Angiotensin-converting enzyme 2; AEC, Absolute eosinophil count; BMI, Body mass index; CCCRR, Cleveland Clinic COVID-19 Research Registry; CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; EHR, Electronic health records; iCS, Inhaled corticosteroids; ICU, Intensive care unit; INCS, Intranasal corticosteroids; IQR, Interquartile range; MICE, Multivariate Imputation by Chained Equations; OR, Odds ratio; PS, Propensity score; RCT, Randomized controlled trial; S, Spike; SARP, Severe asthma research program; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TMPRSS2, Transmembrane serine protease 2
What is already known about this topic? Severe acute respiratory syndrome coronavirus 2 sites of entry are highly expressed in nasal epithelial cells.
What does this article add to our knowledge? Intranasal corticosteroid (INCS) therapy is associated with a lower risk for coronavirus disease 2019 (COVID-19)-related hospitalization, admission to the intensive care unit, and in-hospital mortality.
How does this study impact current management guidelines? Although our findings suggest a potential beneficial role for INCS use, randomized control trials are needed to determine if INCS reduces the risk for severe outcomes related to COVID-19.
The global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused worldwide suffering and death of unprecedented magnitude. The virus consists of a single strand of RNA enveloped in a protein coat with spike proteins on the surface and is transmitted through aerosol droplets. A remarkable feature of coronavirus disease 2019 (COVID-19) is its heterogeneous presentation and outcomes, with the highest mortality rates and disease severity seen in those with certain risk factors including older age, male sex, hypertension, obesity, and diabetes mellitus.1
In both symptomatic and asymptomatic patients, higher viral loads are detected by nasal swabs compared with throat swabs, which suggests that nasal epithelium is a major portal for viral entry and transmission.2 This pathogenicity has been demonstrated in other viral infections, including influenza.3 , 4 In COVID-19, entry into epithelial cells of the respiratory tract by SARS-CoV-2 requires the angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2). SARS-CoV-2 spike (S) protein engages ACE2 as the entry receptor and employs the cellular serine protease TMPRSS2 for S protein priming.5 Expression of ACE2 varies significantly across the respiratory system, with the highest expression in the nose, followed by reductions in expression in the lower respiratory tract. This is mirrored by a significant decline in SARS-CoV-2 viral epithelial cell infection in the lower respiratory tract compared with the upper respiratory tract.6 These findings suggest that nasal epithelial cells may be the major portal of entry for SARS-CoV-2 and that upregulation of the ACE2 receptor in this location increases COVID-19 disease susceptibility and severity.
The nasal expression of ACE2 also depends on age, with lower levels in children as compared with adults. Hence, the heterogeneous presentation of COVID-19 with increased risk and severity in the elderly population could be due to differential ACE2 expression in the respiratory epithelium of adults compared with children.7 This process may also be influenced by allergic airway inflammation. IL-13 was found to modulate ACE2 and TMPRSS2 gene expression in airway epithelial cells obtained from individuals with asthma and atopy. Similarly, eosinophilia (defined by blood absolute eosinophil count [AEC] > 0.15 × 103 cells/μL) was associated with a lower hospitalization risk in a small series of 317 individuals with asthma and COVID-19.8 However, the beneficial effect of eosinophilia in that study could have been confounded by the use of inhaled corticosteroids (iCS). In fact, the use of iCS in a subset of individuals with asthma enrolled in the National Institutes of Health - National Heart, Lung and Blood Institute Severe Asthma Research Program (SARP) was associated with lower expression of ACE2 and TMPRSS2 in sputum cells, whereas the expression levels of both genes did not differ significantly in lower airway epithelial cells from SARP asthmatics or between individuals with and without asthma.9 , 10 Lower expression of ACE2 and TMPRSS2 was also found in airway epithelial cells obtained by bronchoscopy from individuals with chronic obstructive pulmonary disease (COPD) treated with iCS.11 Most importantly, a recent phase II open-label randomized clinical trial (RCT) of 146 individuals showed that early administration of inhaled budesonide reduced the median time to recovery after early COVID-19 by 1 day as well as overall health care utilization.12
To date, the role of intranasal corticosteroids (INCS) in COVID-19 has not been determined. On the basis of the cumulative findings and the fact that ACE2 expression is highest in the nasal mucosa, we hypothesized that, by suppressing viral load and receptor expression, INCS use is protective against hospitalizations, admission to the intensive care unit (ICU), and death due to severe COVID-19.
Methods
Subjects
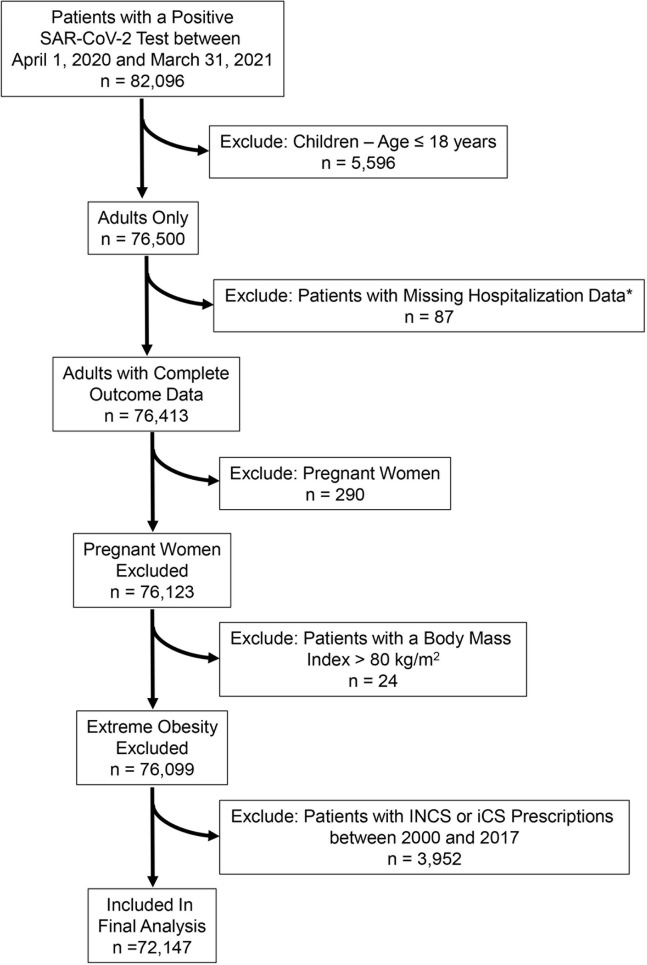
Data on 82,096 individuals included in the Cleveland Clinic COVID-19 Research Registry (CCCRR)13 , 14 who had a positive test for SARS-CoV-2 at the Cleveland Clinic Healthcare System (CCHS) between April 1, 2020, and March 31, 2021, were used for analysis (see Appendix E1 in this article’s Online Repository at www.jaci-inpractice.org). We limited our analysis to individuals 18 years and older (n = 76,500) because children with SARS-CoV-2 infection usually manifest a mild disease or are asymptomatic.15 We also excluded 290 pregnant women, 87 patients with missing hospitalization data, and 24 patients with a body mass index (BMI) greater than 80 kg/m2. Among the remaining 76,099 individuals, we excluded 3952 patients who received a prescription for INCS or iCS before 2018 to exclude bias related to remote INCS or iCS therapy. Among the remaining cohort, 10,187 patients who had a prescription for INCS before the diagnosis of COVID-19, but not before 2018 (defined as INCS users), were compared with the 61,960 who never had an INCS prescription on records (defined as nonusers). Of the initial sample size of 82,096, only 72,147 met our inclusion criteria (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org).16 , 17
Figure E1.
Flow chart of patients in final analysis. iCS, Intranasal corticosteroids; INCS, intranasal corticosteroids; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Study outcomes and group definition
The primary outcome was COVID-19-related hospitalizations. We also studied the following secondary outcomes: the rate of ICU admission and mortality during index hospitalization. We also performed 3 sensitivity analyses. First, sensitivity analysis of 67,242 patients not treated with iCS differentiated the effect of INCS from the potential beneficial effect of iCS or comorbid asthma with eosinophilia previously reported.8 , 12 Sensitivity analyses of 30,289 individuals with baseline blood AEC measurements allowed us to determine whether the effect of INCS on COViD-19 hospitalization was confounded by eosinophil count, which has been proposed to be a protective factor in asthma.8 Third, sensitivity analysis of 65,767 individuals, who did not have a documented diagnosis of allergic rhinitis in their electronic health records (EHR), allowed us to further evaluate the effect of INCS in nonallergic rhinitis. Except for our third sensitivity analysis, we adjusted for allergic rhinitis in all our models to account for this important confounding factor. Of note, we did not consider new prescriptions for either INCS or iCS ordered after the date of the SARS-CoV-2 test result.
Statistical methods
Baseline data are presented as counts with percentages for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Two group comparisons of continuous non-normally distributed variables were performed using the Wilcoxon rank sum test. Categorical variables were compared using a χ2 test.
Definition of subgroups for sensitivity analysis
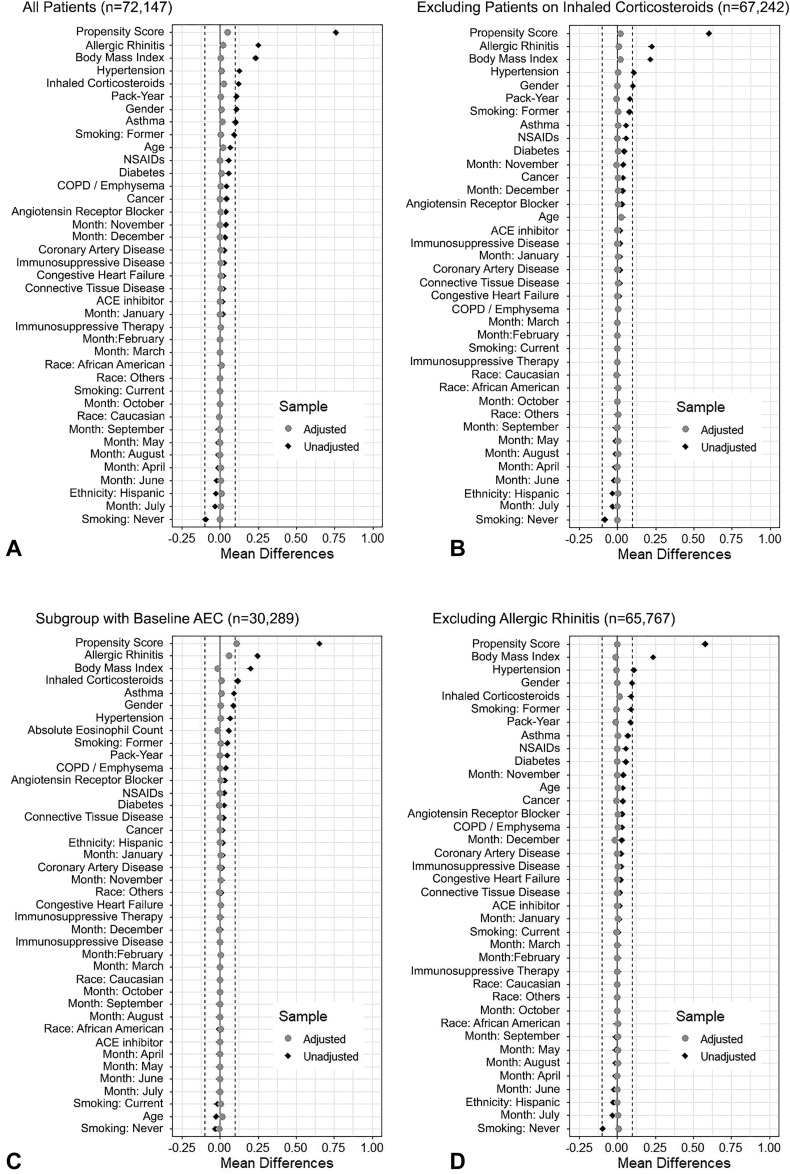
To account for observed covariate differences between INCS users and nonusers before matching (Table I ), we used propensity scores (PSs) to assemble a matched cohort in which both groups would be balanced on key clinical characteristics.18 , 19 PSs are the conditional probability of receiving an exposure (eg, treated with INCS) given a set of measured clinical characteristics, and are estimated for each patient using a nonparsimonious multivariable logistic regression model that measures the relationship between INCS therapy (dependent variable) and clinically relevant characteristics (covariates).20, 21, 22, 23 Such covariates include age, sex, race, ethnicity, BMI, smoking (nonsmokers vs ex-smokers and current smokers), number of pack-years in smokers, diabetes, hypertension, coronary artery disease, congestive heart failure, allergic rhinitis, asthma, COPD, cancer, medications such as nonsteroidal anti-inflammatory drugs, iCS, angiotensin II receptor blockers, ACE inhibitors, and immunosuppressive therapies that include chronic systemic corticosteroid therapy (see Appendix E2 in this article’s Online Repository at www.jaci-inpractice.org), immunosuppressive diseases (adapted from the Agency of Healthcare Research and Quality definition of immunocompromised state diagnosis),24 and the month of testing (see Figure E2 in this article’s Online Repository at www.jaci-inpractice.org). The latter was included to avoid the chronological bias introduced by changes in SARS-CoV-2 testing policies, therapies, and management protocols, and improvements in mortality.25 , 26
Table I.
Clinical characteristics of all individuals in the Cleveland Clinic COVID-19 Research Registry with a positive SARS-CoV-2 test stratified by intranasal corticosteroid use
| Variables | No intranasal corticosteroids | Intranasal corticosteroids | P |
|---|---|---|---|
| n | 61,960 | 10,187 | |
| Demographics | |||
| Age (y) | 50.33 [34.02, 65.11] | 52.07 [38.17, 64.71] | <.001 |
| Female sex | 33,039 (53.3) | 6521 (64.0) | <.001 |
| Body mass index (kg/m2) | 29.03 [25.02, 34.06] | 30.68 [26.39, 36.27] | <.001 |
| Race | .303 | ||
| African American | 11,121 (20.3) | 1967 (20.2) | |
| Caucasian | 40,878 (74.6) | 7226 (74.3) | |
| Others | 2775 (5.1) | 529 (5.4) | |
| Hispanic ethnicity∗ | 5327 (10.1) | 786 (7.8) | <.001 |
| Smoking history | <.001 | ||
| Never | 45,033 (73.4) | 6531 (64.1) | |
| Current | 4063 (6.6) | 677 (6.6) | |
| Past | 12,233 (19.9) | 2975 (29.2) | |
| No. of pack-years smoking | 12.50 [5.00, 30.00] | 11.50 [4.00, 25.00] | .03 |
| Baseline eosinophil count (×103/μL)† | 0.13 [0.07, 0.21] | 0.14 [0.08, 0.23] | <.001 |
| Comorbidities | |||
| COPD/emphysema | 2726 (4.4) | 867 (8.5) | <.001 |
| Allergic rhinitis | 3282 (5.3) | 3098 (30.4) | <.001 |
| Asthma | 4602 (7.4) | 1790 (17.6) | <.001 |
| Diabetes | 8247 (13.3) | 1930 (18.9) | <.001 |
| Hypertension | 18,113 (29.2) | 4257 (41.8) | <.001 |
| Coronary artery disease | 4382 (7.1) | 995 (9.8) | <.001 |
| Heart failure | 3092 (5.0) | 747 (7.3) | <.001 |
| Cancer history | 5287 (8.5) | 1287 (12.6) | <.001 |
| Connective tissue disease | 1083 (1.7) | 395 (3.9) | <.001 |
| Immunosuppressive disease | 3979 (6.4) | 924 (9.1) | <.001 |
| Medications | |||
| NSAIDs | 7236 (11.7) | 1764 (17.3) | <.001 |
| ACE inhibitors | 4991 (8.1) | 1011 (9.9) | <.001 |
| Angiotensin receptor blockers | 3358 (5.4) | 933 (9.2) | <.001 |
| Inhaled corticosteroids | 3135 (5.1) | 1770 (17.4) | <.001 |
| Immunosuppressive therapy‡ | 378 (0.6) | 121 (1.2) | <.001 |
ACE, Angiotensin-converting enzyme; COPD, chronic obstructive airway disease; COVID-19, coronavirus disease 2019; NSAIDs, nonsteroidal anti-inflammatory drugs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are presented as n (%) for categorical variables and median [interquartile range] for continuous variables.
(%) accounts for missing data.
Baseline absolute eosinophil count was measured 14 days or more before date of the SARS-CoV-2 test, but not before 2018.
Includes chronic systemic corticosteroid therapy.
Figure E2.
Standardized mean differences plots (Love plot) comparing baseline characteristics between patients treated with and without intranasal corticosteroids (INCS) before and after propensity-score matching. Analysis stratified into 4 groups: (A) all patients who met inclusion criteria; (B) patients who did not have an inhaled corticosteroid prescription on file; (C) patients on whom a blood eosinophil count measurement was obtained at least 14 days before the date of a positive SARS-CoV-2 test, but not before 2018; (D) patients who were never diagnosed with “allergic rhinitis.” An absolute standardized difference of 0% indicates no residual bias, and values <10% indicate inconsequential bias. ACE, Angiotensin-converting enzyme; COPD, chronic obstructive pulmonary disease; NSAIDs, nonsteroidal anti-inflammatory drugs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
To assess the primary (ie, hospitalization) and 2 secondary outcomes (ie, ICU admission and hospital mortality), we used a 1:1 greedy matching without replacement on the linear propensity score to match patients in the following 4 subgroups/cohorts (see Figure E2, A-D in this article’s Online Repository at www.jaci-inpractice.org).
-
1.
The cohort of all individuals who met inclusion criteria (n = 72,147). We matched 10,187 INCS users with an equal number of individuals not receiving INCS.
-
2.
Excluding patients treated with iCS before SARS-CoV-2 testing (n = 67,252). In this subgroup, 8417 INCS users were matched with 8417 untreated individuals.
-
3.
The subgroup of patients who had an AEC measured 14 days or more before SARS-CoV-2 testing but not before 2018 (n = 30,289). In this subgroup, 6811 INCS users were matched with 6811 untreated individuals.
-
4.
Excluding patients diagnosed with allergic rhinitis before SARS-CoV-2 testing (n = 65,767). In this subgroup, 7089 INCS users were matched with 7089 untreated individuals.
In all 4 subgroups, outcomes were compared with INCS therapy using a conditional logistic regression on the matched sample. All covariates included in the PS models were missing fewer than 15% of subjects. Multiple imputation (5 imputations) was carried out using the Multivariate Imputation by Chained Equations (MICE) package.27 Separate results from the 5 imputed datasets were pooled using the Rubin rule to obtain the final results. MICE replaces each missing value by a plausible value drawn from a distribution specifically designed for each missing data point. Between-group balance for each of the 25 clinical characteristics was assessed using standardized mean differences, and the results were presented as a Love plot (Figure E2, available in this article’s Online Repository at www.jaci-inpractice.org).19 , 28 We also repeated all analyses using the original complete nonimputed data (ie, excluding individuals with missing data). All statistical analyses were conducted with R, version 4.0.5 (R Project for Statistical Computing, Vienna, Austria).
Results
Demographics and clinical characteristics in the entire registry
Of the 82,096 patients in the CCCRR who had a positive SARS-CoV-2 test result between April 1, 2020, and March 31, 2021, 72,147 were included in this study after exclusion criteria were applied (see Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org). Of the 72,147 individuals who met inclusion criteria, 10,187 (14.1%) were prescribed INCS since January 1, 2018, but not after the date of SARS-CoV-2 testing (ie, INCS users) (Table I). Overall, 12,608 (17.5%) patients with a positive SARS-CoV-2 test required hospitalization, 2935 (4.1%) were admitted to the ICU, and 1880 (2.6%) died during hospitalization (Table II ).
Table II.
Clinical presentation and outcomes of all individuals in the Cleveland Clinic COVID-19 Research Registry with a positive SARS-CoV-2 test stratified by intranasal corticosteroid use
| Variables | No intranasal corticosteroids | Intranasal corticosteroids | P∗ |
|---|---|---|---|
| n | 61,960 | 10,187 | |
| Clinical presentation | |||
| Cough | 28,237 (45.6) | 5557 (54.5) | <.001 |
| Fever | 15,829 (25.5) | 3349 (32.9) | <.001 |
| Fatigue | 20,938 (33.8) | 4067 (39.9) | <.001 |
| Sputum production | 3332 (5.4) | 863 (8.5) | <.001 |
| Flu-like symptoms | 17,526 (28.3) | 4061 (39.9) | <.001 |
| Dyspnea | 14,817 (23.9) | 3090 (30.3) | <.001 |
| Diarrhea | 4392 (7.1) | 1007 (9.9) | <.001 |
| Loss of appetite | 4339 (7.0) | 854 (8.4) | <.001 |
| Vomiting | 7518 (12.1) | 1490 (14.6) | <.001 |
| Outcomes | |||
| Hospitalization | 10,932 (17.6) | 1676 (16.5) | .003 |
| Admission to the intensive care unit | 2559 (4.1) | 376 (3.7) | .04 |
| Hospital mortality | 1649 (2.7) | 231 (2.3) | .023 |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are presented as n (%).
Unadjusted analysis comparing intranasal corticosteroid users to nonusers.
Patient demographics and clinical characteristics varied considerably between INCS users and nonusers (Table I). The median age of INCS users was 52.07 years (IQR, 38.17, 64.71), and 6521 (64%) were female. Nonusers had a median age of 50.33 years (IQR, 34.02, 65.11), and 33,039 (53.3%) were female. INCS users were also less likely to be of Hispanic ethnicity (7.8% vs 10.1%, P < .001) and had higher BMI compared with nonusers. A greater proportion of INCS users had medical comorbidities, and 4905 (6.8%) of all patients who met inclusion criteria used iCS (Table I). Significant differences also existed in baseline AEC between INCS users (median [IQR] = 0.14 [0.08, 0.23] × 103 cells/μL) and nonusers (median [IQR] = 0.13 [0.07, 0.21] × 103 cells/μL) (P < .001).
INCS and COVID-19-related clinical presentation and outcomes
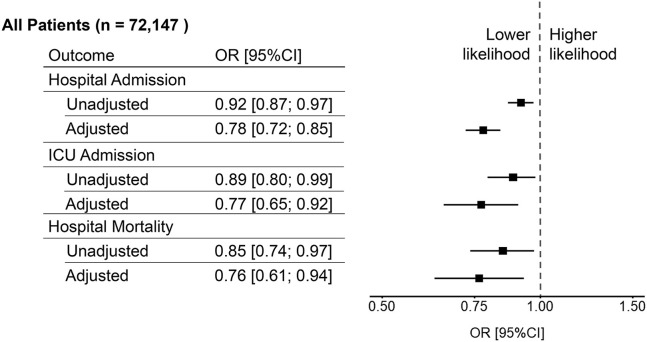
In parallel with the higher rate of comorbidities and medication use, INCS users reported more symptoms related to COVID-19 than nonusers (Table II). In contrast, INCS users had lower rates of hospitalization (16.5% vs 17.6%, P = .003), ICU admission (3.7% vs 4.1%, P = .04), and in-hospital mortality (2.3% vs 2.7%, P < .001) than nonusers (Table II). However, the risk for hospitalization, ICU admission, and hospital mortality among INCS users was confounded by several COVID-19 risk factors for poor outcome (Table I). Adjusted analysis with PS matching using matched data from the whole cohort of patients who met inclusion criteria (n = 72,147) showed that INCS therapy was associated with lower risk for hospitalization (adjusted odds ratio [OR] [95% confidence interval (CI)] = 0.78 [0.72; 0.85]), ICU admission (adjusted OR [95% CI] = 0.77 [0.65; 0.92]), and hospital mortality (adjusted OR [95% CI] = 0.76 [0.61; 0.94]) (Figure 1 ).
Figure 1.
Association of intranasal corticosteroids with COVID-19-related hospital admission, intensive care unit (ICU) admission, and hospital mortality among all patients who had a positive SARS-CoV-2 test and met inclusion criteria. CI, Confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
INCS were also associated with improved COVID-19-related outcomes after additional adjustment to both outpatient and inpatient COVID-19-specific therapies. Among the 72,147 individuals who met inclusion criteria, 247 received outpatient therapy with anti-COVID-19 monoclonal antibodies (ie, casirivimab/imdevimab or bamlanivimab/etesevimab) and 7212 received inpatient therapy with remdesivir and/or systemic corticosteroids. After additional adjustment for COVID-19-specific therapies, INCS were associated with lower risk for hospitalization (adjusted OR [95% CI] = 0.79 [0.73; 0.86]), ICU admission (adjusted OR [95% CI] = 0.77 [0.67; 0.88]), and in-hospital mortality (adjusted OR [95% CI] = 0.76 [0.65; 0.86]).
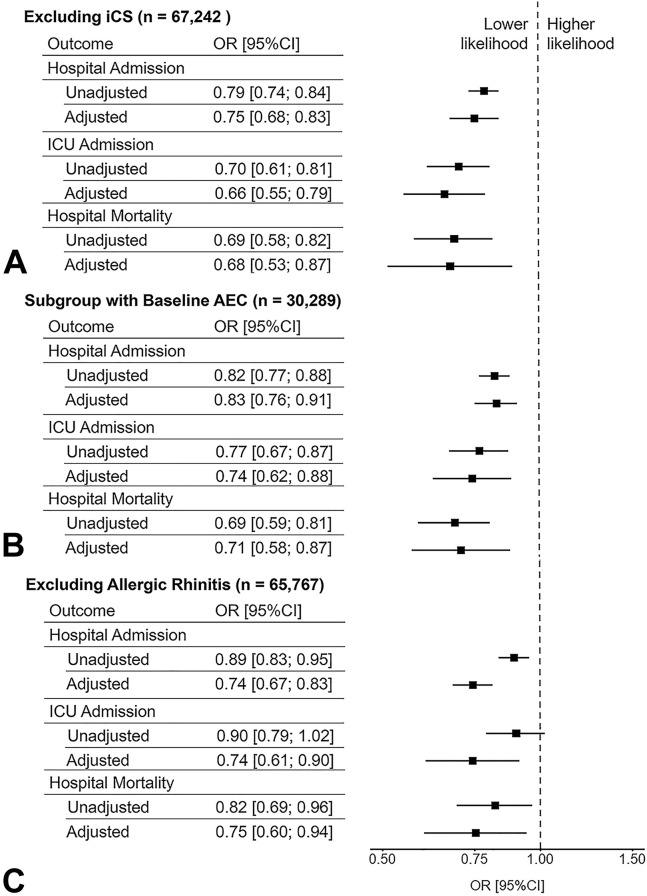
Sensitivity analyses to account for the confounding effect of iCS excluded all individuals who had an iCS prescription on file before the date of SARS-CoV-2 testing resulting in a sample size of 67,242 in which INCS use remained significantly associated with a lower risk for hospitalization (adjusted OR [95% CI] = 0.75 [0.68; 0.83]), ICU admission (adjusted OR [95% CI] = 0.66 [0.55; 0.79]), and in-hospital mortality (adjusted OR [95% CI] = 0.68 [0.53; 0.87]) (Figure 2 ). Of note, iCS therapy before 2018 was one of our exclusion criteria. The clinical characteristics of this subgroup of patients not treated with iCS are listed in Table E1, available in this article’s Online Repository at www.jaci-inpractice.org.
Figure 2.
Sensitivity analyses of the association between intranasal corticosteroids and COVID-19-related hospital admission, intensive care unit (ICU) admission, and hospital mortality among (A) patients who never had an inhaled corticosteroid (iCS) prescription on file, (B) patients on whom a baseline blood absolute eosinophil count (AEC) measurement were available, and (C) patients who were never diagnosed with “allergic rhinitis.” CI, Confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
To account for the potentially protective effect of eosinophilia in those with allergic diseases, we analyzed a subgroup of 30,289 individuals with available baseline blood AEC measurements obtained 14 days before SARS-CoV-2 testing but not before 2018 (see Table E2 in this article’s Online Repository at www.jaci-inpractice.org). In this subgroup, INCS use was also associated with a lower risk for hospitalization (adjusted OR [95% CI] = 0.83 [0.76; 0.91]), ICU admission (adjusted OR [95% CI] = 0.74 [0.62; 0.88]), and in-hospital mortality (adjusted OR [95% CI] = 0.71 [0.58; 0.87]) (Figure 2) in models adjusted for eosinophil count (see Figure E2, C in this article’s Online Repository at www.jaci-inpractice.org). The clinical characteristics of this subgroup of patients with available baseline AEC are listed in Table E2, available in this article’s Online Repository at www.jaci-inpractice.org.
To account for the confounding effect of “allergic rhinitis,” we had a 2-pronged approach. First, we adjusted for “allergic rhinitis” in all our models (see Figure E2, A-C in this article’s Online Repository at www.jaci-inpractice.org). Second, we analyzed a subgroup of 65,767 individuals who did not have the diagnosis “allergic rhinitis” recorded in their EHR (see Table E3 in this article’s Online Repository at www.jaci-inpractice.org). In this subgroup, INCS use was also associated with a lower risk for hospitalization (adjusted OR [95% CI] = 0.74 [0.67; 0.83]), ICU admission (adjusted OR [95% CI] = 0.74 [0.61; 0.90]), and in-hospital mortality (adjusted OR [95% CI] = 0.75 [0.60; 0.94]) (Figure 2).
We repeated all analyses using the original nonimputed data after excluding individuals with missing data (ie, complete cases). Those results were consistent with findings from the main analyses using imputed data (see Table E4 in this article’s Online Repository at www.jaci-inpractice.org).
Discussion
The principal finding of our study was that INCS use was associated with a lower risk for COVID-19-related hospitalization, ICU admission, and hospital mortality after adjusting for iCS usage, allergic rhinitis, and baseline blood AEC. INCS users also exhibited more symptoms than nonusers. However, this could be confounded by the higher rates of comorbid conditions among users. For example, the higher prevalence of dyspnea and cough among users could simply be related to the increased prevalence of asthma and COPD in this group.
Previous analyses have demonstrated that patients with allergic disorders (eg, allergic rhinitis or asthma) had lower rates of hospital admission and need for mechanical ventilation due to COVID-19, especially when coupled with eosinophilia.8 , 29, 30, 31, 32 However, these studies either failed to correct for the use of iCS or were based on administrative datasets that lacked clinical information such as AEC. Our findings demonstrate that INCS use was associated with a lower risk for hospitalization even after adjustment for baseline AEC, allergic rhinitis, and baseline iCS use. Adjusting for such confounders is crucial, given that iCS usage was also linked to decreased expression of ACE2 and TMPRSS2 in vitro. 9 , 11 , 33 To address this important issue, we adjusted for iCS and allergic rhinitis in the majority of our models and performed 2 separate sensitivity analyses. We also performed a third sensitivity analysis to adjust for the effect of blood eosinophils.
This is the first study to examine the effects of INCS in the context of COVID-19 infections and outcomes. Furthermore, the effect size of the association between INCS and COVID-19-related outcomes is similar to other Food and Drug Administration–approved therapies such as remdesivir and systemic steroids.34 , 35 Our findings are particularly significant, as decreased COVID-19 hospitalizations, ICU admissions, and mortality could alleviate the strain on health care systems with limited resources across the globe, especially in developing countries where there is limited access to vaccines and where mutations in SARS-CoV-2 have emerged.36 If proven effective in future RCTs, the benefits associated with INCS could reduce the need for health care resources including medications, oxygen, supplies, and health care workers. In the United States, INCS does not require a prescription or management by a health care provider. However, while INCS are low cost, accessible, over-the-counter, and well-tolerated, interethnic differences in INCS use might still exist because of socioeconomic differences and access to nasal steroids that could affect COVID-19 outcomes in minorities.
Although the exact biological mechanisms are not well known, recent studies have suggested that corticosteroids might play a beneficial role in COVID-19. For example, ciclesonide and mometasone, 2 inhaled corticosteroids used in human asthma, were found to suppress coronavirus replication in vitro.37 In COPD, inhaled corticosteroids were found to downregulate the SARS-CoV-2 receptor ACE2 through suppression of type I interferon.11 Overall, our knowledge regarding the role corticosteroids in COVID-19 is still very limited, and further research is needed to explore the association between corticosteroids, viral replication, ACE2 gene expression, inflammatory pathway activation, and outcomes.38
Our study has many strengths that are worth mentioning. These include a large sample size, strict and predefined methods for data collection, and a thorough analysis of data pertaining to INCS usage. Factors such as eosinophil counts, comorbidities such as diabetes mellitus, hypertension, and obesity, allergic rhinitis, and use of iCS were considered during statistical modeling. Although future studies are needed including RCTs, our findings demonstrate a significant association between INCS and the decreased disease severity of COVID-19.
Our study has several limitations. This is a cross-sectional study that used data collected from EHR. Thus, it could be subject to bias introduced by other measured and unmeasured confounding variables. One possibility of note is that INCS does not require a prescription from a physician and can be purchased over the counter, which means it is less likely to be documented in the EHR. Furthermore, this study was from a single health care system, and our findings might not reflect the medical management of patients with COVID-19 cared for outside the CCHS. Given that we limited our analysis to in-hospital mortality and to patients with a positive SARS-CoV-2 admitted to the CCHS, our analysis could have missed patients who subsequently died after being discharged to long-term acute care hospitals or after being admitted to hospitals outside the CCHS. By limiting our analyses to individuals with a positive SARS-CoV-2 test, we cannot make any inferences regarding the effect of INCS on the risk of infection with SARS-CoV-2. RCTs are needed to corroborate our findings that suggest that INCS reduces the risk for severe health care outcomes related to COVID-19. Dedicated studies analyzing gene expression patterns of ACE2 and TMPRSS2 before and after INCS administration are also needed.
In summary, our study demonstrates that INCS usage is associated with decreased COVID-19-related hospital admission, ICU admission, and mortality. Our findings demonstrate the need for future studies including RCTs on INCS usage to corroborate our findings and determine whether INCS improves health care–related outcomes due to COVID-19.
Acknowledgments
We thank Bradly Souder from CCF Business Intelligence Department, Laura Peterson, BA, from Lerner Research Institute, and Greg Strnad, MS, from CCF Clinical Outcomes Research Center for their valuable input and help.
J. G. Zein, R. Strauss, and N. Jawhari made substantial contributions to the conception or design of the work; J. G. Zein acquired, analyzed, and interpreted the data for the work; B. Hu supervised data analysis; A. Milinovich extracted data from HER; J. G. Zein, R. Strauss, N. Jawhari, A. H. Attaway, B. Hu, L. Jehi, and V. E. Ortega contributed to drafting the work or revising it critically for important intellectual content; J. G. Zein agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and J. G. Zein, R. Strauss, N. Jawhari, A. H. Attaway, B. Hu, L. Jehi, A. Milinovich, and V. E. Ortega gave the final approval of the version to be published.
Footnotes
This study was funded by the National Institutes of Health - National Heart, Lung, and Blood Institute (K08 HL133381, JGZ), and the National Institute of Neurological Disorders and Stroke (R01 NS097719, LJ).
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Appendix E2. Immunosuppressive Therapy
List of systemic medications used to define immunosuppressive therapy
|
|
|
Table E1.
Clinical characteristics, presentation, and outcomes of a subgroup of patients who did not have a prescription for inhaled corticosteroids (iCS) on file before the date of SARS-CoV-2 testing—analysis stratified by the use of iCS
| Variables | No iCS | iCS | P |
|---|---|---|---|
| n | 58,825 | 8417 | |
| Demographics | |||
| Age (y) | 49.76 [33.58, 64.52] | 50.70 [36.48, 63.68] | <.001 |
| Female sex | 31,128 (52.9) | 5325 (63.3) | <.001 |
| Body Mass Index (kg/m2) | 28.92 [24.98, 33.91] | 30.45 [26.15, 35.87] | <.001 |
| Race | .382 | ||
| African American | 10,463 (20.2) | 1575 (19.7) | |
| Caucasian | 38,652 (74.7) | 5993 (74.9) | |
| Others | 2647 (5.1) | 430 (5.4) | |
| Hispanic ethnicity∗ | 5169 (10.5) | 646 (7.8) | <.001 |
| Smoking history | <.001 | ||
| Never | 43,368 (74.5) | 5581 (66.3) | |
| Current | 3776 (6.5) | 567 (6.7) | |
| Past | 11,057 (19.0) | 2265 (26.9) | |
| Pack-years of smoking | 11.00 [4.00, 26.25] | 10.00 [4.00, 22.00] | .007 |
| Baseline eosinophil count (×103/μL)† | 0.13 [0.07, 0.21] | 0.14 [0.08, 0.22] | <.001 |
| Clinical presentation | |||
| Cough | 26,663 (45.3) | 4516 (53.7) | <.001 |
| Fever | 14,965 (25.4) | 2811 (33.4) | <.001 |
| Fatigue | 19,711 (33.5) | 3364 (40.0) | <.001 |
| Sputum production | 2995 (5.1) | 625 (7.4) | <.001 |
| Flu-like symptoms | 16,521 (28.1) | 3390 (40.3) | <.001 |
| Dyspnea | 13,493 (22.9) | 2296 (27.3) | <.001 |
| Diarrhea | 4109 (7.0) | 808 (9.6) | <.001 |
| Loss of appetite | 4041 (6.9) | 677 (8.0) | <.001 |
| Vomiting | 7078 (12.0) | 1204 (14.3) | <.001 |
| Comorbidities | |||
| COPD/emphysema | 1690 (2.9) | 287 (3.4) | .007 |
| Allergic rhinitis | 2779 (4.7) | 2293 (27.2) | <.001 |
| Asthma | 3488 (5.9) | 991 (11.8) | <.001 |
| Diabetes | 7437 (12.6) | 1443 (17.1) | <.001 |
| Hypertension | 16,466 (28.0) | 3254 (38.7) | <.001 |
| Coronary artery disease | 3831 (6.5) | 689 (8.2) | <.001 |
| Heart failure | 2577 (4.4) | 470 (5.6) | <.001 |
| Cancer history | 4758 (8.1) | 985 (11.7) | <.001 |
| Connective tissue disease | 925 (1.6) | 256 (3.0) | <.001 |
| Immunosuppressive disease | 3480 (5.9) | 646 (7.7) | <.001 |
| Medications | |||
| NSAIDs | 6735 (11.4) | 1439 (17.1) | <.001 |
| ACE inhibitors | 4610 (7.8) | 823 (9.8) | <.001 |
| Angiotensin receptor blockers | 3025 (5.1) | 684 (8.1) | <.001 |
| Immunosuppressive therapy | 300 (0.5) | 61 (0.7) | .015 |
| Outcomes | |||
| Hospitalization | 9893 (16.8) | 1155 (13.7) | <.001 |
| Admission to the intensive care unit | 2257 (3.8) | 230 (2.7) | <.001 |
| Hospital mortality | 1456 (2.5) | 145 (1.7) | <.001 |
ACE, Angiotensin-converting enzyme; COPD, chronic obstructive airway disease; NSAIDs, nonsteroidal anti-inflammatory drugs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are presented as n (%) for categorical variables and median [interquartile range] for continuous variables.
(%) accounts for missing values.
Baseline blood absolute eosinophil count was measured 14 days or more before the date of SARS-CoV-2 testing, but not before 2018.
Table E2.
Clinical characteristics, presentation, and outcomes of patients with available baseline blood absolute eosinophil count∗ measurements stratified by the use of intranasal corticosteroids
| Variables | No intranasal corticosteroids | Intranasal corticosteroids | P |
|---|---|---|---|
| n | 23,478 | 6811 | |
| Demographics | |||
| Age (y) | 55.00 [38.75, 69.22] | 54.69 [41.15, 67.16] | .168 |
| Female sex | 13,428 (57.2) | 4502 (66.1) | <.001 |
| Body mass index (kg/m2) | 29.39 [25.23, 34.80] | 30.90 [26.58, 36.61] | <.001 |
| Race | .077 | ||
| African American | 5011 (22.3) | 1423 (21.8) | |
| Caucasian | 16,214 (72.3) | 4714 (72.2) | |
| Others | 1201 (5.4) | 395 (6.0) | |
| Hispanic ethnicity† | 1680 (7.3) | 612 (9.1) | <.001 |
| Smoking history | <.001 | ||
| Never | 14,960 (64.0) | 4157 (61.1) | |
| Current | 1996 (8.5) | 452 (6.6) | |
| Past | 6431 (27.5) | 2198 (32.3) | |
| Pack-years of smoking | 15.00 [5.00, 30.00] | 12.00 [4.50, 27.00] | .002 |
| Baseline eosinophil count (×103/μL)∗ | 0.13 [0.07, 0.22] | 0.14 [0.08, 0.22] | <.001 |
| Clinical presentation | |||
| Cough | 10,914 (46.5) | 3763 (55.2) | <.001 |
| Fever | 6843 (29.1) | 2212 (32.5) | <.001 |
| Fatigue | 8755 (37.3) | 2770 (40.7) | <.001 |
| Sputum production | 1659 (7.1) | 626 (9.2) | <.001 |
| Flu-like symptoms | 7639 (32.5) | 2723 (40.0) | <.001 |
| Dyspnea | 6409 (27.3) | 2201 (32.3) | <.001 |
| Diarrhea | 2115 (9.0) | 726 (10.7) | <.001 |
| Loss of appetite | 2159 (9.2) | 620 (9.1) | .834 |
| Vomiting | 3456 (14.7) | 1037 (15.2) | .311 |
| Comorbidities | |||
| COPD/emphysema | 1682 (7.2) | 743 (10.9) | <.001 |
| Allergic rhinitis | 1824 (7.8) | 2216 (32.5) | <.001 |
| Asthma | 2312 (9.8) | 1301 (19.1) | <.001 |
| Diabetes | 4692 (20.0) | 1545 (22.7) | <.001 |
| Hypertension | 9972 (42.5) | 3343 (49.1) | <.001 |
| Coronary artery disease | 2662 (11.3) | 850 (12.5) | .01 |
| Heart failure | 2165 (9.2) | 668 (9.8) | .15 |
| Cancer history | 3237 (13.8) | 1062 (15.6) | <.001 |
| Connective tissue disease | 702 (3.0) | 346 (5.1) | <.001 |
| Immunosuppressive disease | 2754 (11.7) | 829 (12.2) | .331 |
| Medications | |||
| NSAIDs | 3755 (16.0) | 1276 (18.7) | <.001 |
| ACE inhibitors | 2647 (11.3) | 730 (10.7) | .207 |
| Angiotensin receptor blockers | 1837 (7.8) | 740 (10.9) | <.001 |
| Inhaled corticosteroids | 2120 (9.0) | 1402 (20.6) | <.001 |
| Immunosuppressive therapy | 255 (1.1) | 107 (1.6) | .001 |
| Outcomes | |||
| Hospitalization | 5387 (22.9) | 1341 (19.7) | <.001 |
| Admission to the intensive care unit | 1303 (5.5) | 294 (4.3) | <.001 |
| Hospital mortality | 943 (4.0) | 191 (2.8) | <.001 |
ACE, Angiotensin-converting enzyme; COPD, chronic obstructive airway disease; NSAIDs, nonsteroidal anti-inflammatory drugs.
Data are presented as n (%) for categorical variables and median [interquartile range] for continuous variables.
Baseline blood absolute eosinophil count was measured 14 days or more before the date of severe acute respiratory syndrome coronavirus 2 testing, but not before 2018.
(%) accounts for missing values.
Table E3.
Clinical characteristics, presentation, and outcomes of patients with a positive SARS-CoV-2 test, excluding all individuals who ever receive a diagnosis of allergic rhinitis
| Variables | No intranasal corticosteroids | Intranasal corticosteroids | P |
|---|---|---|---|
| n | 58,678 | 7089 | |
| Demographics | |||
| Age (y) | 50.35 [33.97, 65.24] | 51.53 [37.24, 64.29] | <.001 |
| Female sex | 31,115 (53.0) | 4453 (62.8) | <.001 |
| Body mass index | 29.01 [25.02, 34.04] | 30.68 [26.31, 36.35] | <.001 |
| Race | .142 | ||
| African American | 10,616 (20.6) | 1347 (20.0) | |
| Caucasian | 38,331 (74.3) | 5002 (74.3) | |
| Others | 2650 (5.1) | 380 (5.6) | |
| Hispanic ethnicity∗ | 5200 (10.6) | 597 (8.6) | <.001 |
| Smoking history | <.001 | ||
| Never | 42,719 (73.6) | 4535 (64.0) | |
| Current | 3871 (6.7) | 512 (7.2) | |
| Past | 11,463 (19.7) | 2038 (28.8) | |
| No. of pack-years of smoking | 13.00 [5.00, 30.00] | 10.75 [4.00, 25.00] | .004 |
| Baseline absolute eosinophil Count† | 0.13 [0.07, 0.21] | 0.14 [0.08, 0.22] | <.001 |
| Clinical presentation | |||
| Cough | 26,547 (45.2) | 3799 (53.6) | <.001 |
| Fever | 14,703 (25.1) | 2309 (32.6) | <.001 |
| Fatigue | 19,676 (33.5) | 2820 (39.8) | <.001 |
| Sputum production | 3142 (5.4) | 590 (8.3) | <.001 |
| Flu-like symptoms | 16,272 (27.7) | 2781 (39.2) | <.001 |
| Dyspnea | 13,955 (23.8) | 2117 (29.9) | <.001 |
| Diarrhea | 4126 (7.0) | 669 (9.4) | <.001 |
| Loss of appetite | 4083 (7.0) | 558 (7.9) | .005 |
| Vomiting | 7081 (12.1) | 1025 (14.5) | <.001 |
| Comorbidities | |||
| COPD/dmphysema | 2528 (4.3) | 527 (7.4) | <.001 |
| Asthma | 3954 (6.7) | 972 (13.7) | <.001 |
| Diabetes | 7804 (13.3) | 1338 (18.9) | <.001 |
| Hypertension | 16,994 (29.0) | 2831 (39.9) | <.001 |
| Coronary artery disease | 4162 (7.1) | 685 (9.7) | <.001 |
| Heart failure | 2949 (5.0) | 505 (7.1) | <.001 |
| Cancer history | 4935 (8.4) | 850 (12.0) | <.001 |
| Connective tissue disease | 993 (1.7) | 247 (3.5) | <.001 |
| Immunosuppressive disease | 3737 (6.4) | 631 (8.9) | <.001 |
| Medications | |||
| NSAIDs | 6784 (11.6) | 1215 (17.1) | <.001 |
| ACE inhibitors | 4708 (8.0) | 685 (9.7) | <.001 |
| Angiotensin receptor blockers | 3129 (5.3) | 606 (8.5) | <.001 |
| Inhaled corticosteroids | 2632 (4.5) | 965 (13.6) | <.001 |
| Immunosuppressive therapy | 355 (0.6) | 77 (1.1) | <.001 |
| Outcomes | |||
| Hospitalized | 10,494 (17.9) | 1152 (16.3) | .001 |
| Intensive care unit admission | 2456 (4.2) | 268 (3.8) | .113 |
| Death | 1587 (2.7) | 158 (2.2) | .021 |
ACE, Angiotensin-converting enzyme; COPD, chronic obstructive airway disease; NSAIDs, nonsteroidal anti-inflammatory drugs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are presented as n (%) for categorical variables and median [interquartile range] for continuous variables.
(%) accounts for missing values.
Baseline blood absolute eosinophil count was measured 14 days or more before the date of SARS-CoV-2 testing, but not before 2018.
Table E4.
Association between intranasal corticosteroids (iCS) use before and after propensity score (PS) matching using complete cases (ie, excluding patients with missing data and without imputation)
| Variables | Outcomes | Total no. of patients | Unadjusted OR [95% CI] | No. of PS matched patients | Adjusted OR [95% CI] |
|---|---|---|---|---|---|
| All patients | |||||
| Hospitalization | 10,366 | 53,718 | 0.85 [0.80; 0.90] | 9538 | 0.75 [0.70; 0.81] |
| ICU admission | 2,575 | 53,718 | 0.76 [0.68; 0.85] | 9538 | 0.74 [0.64; 0.85] |
| Hospital mortality | 1,549 | 53,718 | 0.79 [0.68; 0.91] | 9538 | 0.73 [0.61; 0.88] |
| Excluding iCS | |||||
| Hospitalization | 8,833 | 49,059 | 0.73 [0.68; 0.78] | 7828 | 0.72 [0.66; 0.78] |
| ICU admission | 2,135 | 49,059 | 0.60 [0.52; 0.69] | 7828 | 0.59 [0.50; 0.70] |
| Hospital mortality | 1,275 | 49,059 | 0.65 [0.54; 0.77] | 7828 | 0.63 [0.51; 0.78] |
| Subgroup with AEC | |||||
| Hospitalization | 6,547 | 27,983 | 0.80 [0.74; 0.85] | 6465 | 0.79 [0.73; 0.86] |
| ICU admission | 1,550 | 27,983 | 0.75 [0.65; 0.85] | 6465 | 0.71 [0.60; 0.84] |
| Hospital mortality | 1,096 | 27,983 | 0.68 [0.57; 0.79] | 6465 | 0.67 [0.55; 0.82] |
| Excluding patients with AR | |||||
| Hospitalization | 9,422 | 47,650 | 0.82 [0.77; 0.88] | 6580 | 0.71 [0.65; 0.77] |
| ICU admission | 2,367 | 47,650 | 0.77 [0.67; 0.87] | 6580 | 0.75 [0.63; 0.88] |
| Hospital mortality | 1,416 | 47,650 | 0.76 [0.64; 0.90] | 6580 | 0.73 [0.59; 0.90] |
AEC, Angiotensin-converting enzyme; AR, allergic rhinitis; CI, confidence interval; ICU, Intensive care unit; OR, odds ratio.
Online Repository. Appendix E1. Description of the Registry
The Cleveland Clinic COVID-19 Research Registry (CCCRR)
This study uses the CCCRR, which includes all patients tested for SARS-CoV-2 at the Cleveland Clinic Healthcare System (CCHS). Data on patients’ demographics, medications, comorbidities, history of SARS-CoV-2 exposure, national and international travel, disease manifestation on presentation, socioeconomic status, COVID-19-related therapy, disposition, and outcomes were extracted from electronic health records (EHR).E1 In addition, data related to hospitalization, critical care needs, and outcome were extracted for patients requiring hospitalization. Registry characterization and data collection reflect the clinical characteristics previously published on COVID-19.E2, E3, E4, E5, E6 Uniform clinical templates were implemented across the CCHS using EHR to standardize the care of patients tested for SARS-CoV-2 and to facilitate data extraction. Data extraction from EHR (Epic; Epic Systems Corporation, Wisc) at the CCHS was performed manually by a trained research team and electronically using predefined processes that have been previously published.E7 This study and the CCCRR were both approved by the Cleveland Clinic Institutional Review Board (IRB #20-283 and 20-391).
COVID-19 testing protocols
Specimens were collected using standardized protocols by trained medical personnel. Testing was performed by means of the CDC reverse transcription polymerase chain reaction (RT-PCR) SARS-CoV-2 assay, which uses Roche Magnapure extraction and ABI 7500 DX PCR instruments. All instruments and testing used are Food and Drug Administration approved under an Emergency Use Authorization.E1
References
- 1.Ng W.H., Tipih T., Makoah N.A., Vermeulen J.G., Goedhals D., Sempa J.B., et al. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. mBio. 2021;12:e03647-20. doi: 10.1128/mBio.03647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipworth B., Chan R., RuiWen Kuo C. COVID-19: start with the nose. J Allergy Clin Immunol. 2020;146:1214. doi: 10.1016/j.jaci.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., 3rd, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G., et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clinical Immunol Pract. 2021;9:1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.L., Montgomery M.T., et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Resp Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasela S., Ortega V.E., Martorella M., Garudadri S., Nguyen J., Ampleford E., et al. Genetic and non-genetic factors affecting the expression of COVID-19-relevant genes in the large airway epithelium. Genome Med. 2021;13:66. doi: 10.1186/s13073-021-00866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finney L.J., Glanville N., Farne H., Aniscenko J., Fenwick P., Kemp S.V., et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021;147:510–519.e5. doi: 10.1016/j.jaci.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishnan S., Nicolau D.V., Jr., Langford B., Mahdi M., Jeffers H., Mwasuku C., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milinovich A., Kattan M.W. Extracting and utilizing electronic health data from Epic for research. Ann Transl Med. 2018;6:42. doi: 10.21037/atm.2018.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zein J.G., Udeh B.L., Teague W.G., Koroukian S.M., Schlitz N.K., Bleecker E.R., et al. Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011-2012. PloS One. 2016;11:e0157301. doi: 10.1371/journal.pone.0157301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zein J., Whelan G., Erzurum S. Safety of influenza vaccine during COVID-19. J Clin Trans Sci. 2021;5:E49. [Google Scholar]
- 18.Rosenbaum P. Springer; New York: 2002. Observational Studies. [Google Scholar]
- 19.Rosenbaum P.R., Rubin D.B. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 20.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle Region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality. Appendix I: Immunocompromised state diagnosis and procedure codes 2016. Accessed May 2, 2021. https://www.qualityindicators.ahrq.gov/Downloads/Modules/PSI/V60-ICD10/TechSpecs/PSI_Appendix_I.pdf
- 25.Horwitz L.I., Jones S.A., Cerfolio R.J., Francois F., Greco J., Rudy B., et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16:90–92. doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
- 26.Dennis J.M., McGovern A.P., Vollmer S.J., Mateen B.A. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49:209–214. doi: 10.1097/CCM.0000000000004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Buuren S., Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 28.Rubin D.B. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 29.Izquierdo J.L., Almonacid C., Gonzalez Y., Del Rio-Bermudez C., Ancochea J., Cardenas R., et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57:2003142. doi: 10.1183/13993003.03142-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inselman J.W., Rank M.A., Zawada S.K., Jeffery M.M. Which people with asthma are most likely to be hospitalized with COVID-19 in the United States? J Allergy Clinical Immunol Pract. 2021;9:2080–2082. doi: 10.1016/j.jaip.2021.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keswani A., Dhana K., Rosenthal J.A., Moore D., Mahdavinia M. Atopy is predictive of a decreased need for hospitalization for coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125:479–481. doi: 10.1016/j.anai.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camiolo M., Gauthier M., Kaminski N., Ray A., Wenzel S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clinical Immunol. 2020;146:315–324.e7. doi: 10.1016/j.jaci.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowdhury A.Z., Jomo K.S. Responding to the COVID-19 pandemic in developing countries: lessons from selected countries of the Global South. Development (Rome) 2020;63:162–171. doi: 10.1057/s41301-020-00256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95:e01648-20. doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fajnzylber J., Regan J., Coxen K., Corry H., Wong C., Rosenthal A., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle Region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Milinovich A., Kattan M.W. Extracting and utilizing electronic health data from Epic for research. Ann Transl Med. 2018;6:42. doi: 10.21037/atm.2018.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]