Abstract
Study Objective
To determine the incidence of perioperative coronavirus disease (COVID-19) in women undergoing benign gynecologic surgery and to evaluate perioperative complication rates in patients with active, previous, or no previous severe acute respiratory syndrome coronavirus 2 infection.
Design
A multicenter prospective cohort study.
Setting
Ten institutions in the United States.
Patients
Patients aged >18 years who underwent benign gynecologic surgery from July 1, 2020, to December 31, 2020, were included. All patients were followed up from the time of surgery to 10 weeks postoperatively. Those with intrauterine pregnancy or known gynecologic malignancy were excluded.
Interventions
Benign gynecologic surgery.
Measurements and Main Results
The primary outcome was the incidence of perioperative COVID-19 infections, which was stratified as (1) previous COVID-19 infection, (2) preoperative COVID-19 infection, and (3) postoperative COVID-19 infection. Secondary outcomes included adverse events and mortality after surgery and predictors for postoperative COVID-19 infection. If surgery was delayed because of the COVID-19 pandemic, the reason for postponement and any subsequent adverse event was recorded. Of 3423 patients included for final analysis, 189 (5.5%) postponed their gynecologic surgery during the pandemic. Forty-three patients (1.3% of total cases) had a history of COVID-19. The majority (182, 96.3%) had no sequelae attributed to surgical postponement. After hospital discharge to 10 weeks postoperatively, 39 patients (1.1%) became infected with severe acute respiratory syndrome coronavirus 2. The mean duration of time between hospital discharge and the follow-up positive COVID-19 test was 22.1 ± 12.3 days (range, 4–50 days). Eleven (31.4% of postoperative COVID-19 infections, 0.3% of total cases) of the newly diagnosed COVID-19 infections occurred within 14 days of hospital discharge. On multivariable logistic regression, living in the Southwest (adjusted odds ratio, 6.8) and single-unit increase in age-adjusted Charlson comorbidity index (adjusted odds ratio, 1.2) increased the odds of postoperative COVID-19 infection. Perioperative complications were not significantly higher in patients with a history of positive COVID-19 than those without a history of COVID-19, although the mean duration of time between previous COVID-19 diagnosis and surgery was 97 days (14 weeks).
Conclusion
In this large multicenter prospective cohort study of benign gynecologic surgeries, only 1.1% of patients developed a postoperative COVID-19 infection, with 0.3% of infection in the immediate 14 days after surgery. The incidence of postoperative complications was not different in those with and without previous COVID-19 infections.
Keywords: COVID-19, SARS-CoV-2, Gynecologic surgery, Surgical outcomes, Adverse events, Nosocomial infections
In January of 2020, the Centers for Disease Control and Prevention confirmed the first US case of coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Washington state [1]. By March of 2020, the rapid spread of COVID-19 led to the halt of nonemergent healthcare services across the Unites States to preserve healthcare resources for patients with COVID-19.
Elective and nonurgent surgeries resumed in mid-2020 once personal protective equipment and COVID-19 testing became more readily available. To continue caring for patients with nonurgent conditions, we witnessed a rapid scale-up of telemedicine [2]. When surgical cases resumed, it was estimated that 41% of US adults delayed or deferred care during the pandemic [3]. This delay may reflect adherence to stay-at-home orders and pandemic circumstances making access to medical care difficult or fear of contracting or spreading COVID-19 despite the implementation of universal COVID-19 testing preoperatively across major institutions [4]. For patients who did subsequently proceed with surgery during the pandemic, there are limited data on surgical outcomes during the COVID-19 pandemic including outcomes in patients who are actively or previously infected with SARS-CoV-2.
This study was designed to understand perioperative COVID-19 infection rates and how COVID-19 affects surgical outcomes in women undergoing benign gynecologic surgery. The primary objective of our study is to determine the incidence of perioperative COVID-19 in women undergoing benign gynecologic surgeries. Our secondary aims were to determine the incidence of surgical morbidity and mortality in gynecologic patients undergoing surgery with an active SARS-CoV-2 infection, previous infection, or no infection and to identify predictors for postoperative COVID-19 infection.
Materials and Methods
This multicenter prospective cohort study was conducted at 10 institutions across the United States to evaluate the surgical outcomes of patients undergoing benign gynecologic surgeries during the COVID-19 pandemic (SOCOVID study). Ten institutions were selected to attempt to capture a wide geographic cohort and variety of benign gynecologic procedures. An institutional review board (IRB) approval was obtained at each site.
All patients aged >18 years who underwent benign gynecologic surgery from July 1, 2020, to December 31, 2020, were included. All patients were prospectively followed up from the time of surgery to 10 weeks postoperatively to capture wide practice variations across 10 sites. Those with intrauterine pregnancy or known gynecologic malignancy were excluded. Perioperative variables of interest were extracted from the medical record, including retrospective data for demographics and variables related to COVID-19 infection (Supplemental Table 1). The Charlson comorbidity index, a tool that has been studied to predict mortality based on scoring of medical comorbidities, was calculated for all patients with age and nonage adjustments [5].
As previously published, all patients received preoperative COVID-19 testing up to 5 days before their scheduled surgery per institutional protocols [4]. Our primary outcome was the incidence of perioperative COVID-19 infections defined as a documented positive SARS-CoV-2 polymerase chain reaction test from nasopharyngeal swabs or lower respiratory tract samples. We stratified COVID-19 infections by the timing of the infection relative to surgery as (1) previous COVID-19 infection, defined as a history of COVID-19 infection at any point independent of surgery; (2) preoperative COVID-19 infection, defined as COVID-19 infection detected preoperatively within 5 days of scheduled surgery; and (3) postoperative COVID-19 infection, defined as COVID-19 infection detected after surgery until the 10-week postoperative period. We defined COVID-19–related adverse events to include pneumonia, abnormal chest radiography, acute respiratory distress syndrome, intensive care unit admission, use of mechanical ventilation or extracorporeal membrane oxygenation, multisystem inflammatory syndrome, and involvement of other organ systems including gastrointestinal, neurologic, hematologic, dermatologic, and cardiac complications and death.
The reason for the postponement of surgery and any adverse events resulting from delayed surgery were recorded. Our secondary outcomes included adverse events and mortality after benign gynecologic surgery. Adverse events included blood transfusion intraoperatively or within 72 hours of surgery, venous thromboembolic disease, pneumonia, reintubation, renal insufficiency or failure, sepsis or septic shock, wound dehiscence, myocardial infarction or cardiac arrest, cerebral vascular accident, urinary tract infection, and deep or organ space infection. Additional events such as readmission, reoperation, emergency department utilization, and death after hospital discharge were recorded.
All study data were collected and managed using REDCap (Research Electronic Data Capture) [6] electronic data capture tools hosted at Indiana University School of Medicine. Study population means, medians, and proportions were calculated for all parametric and nonparametric continuous and categorical variables. Continuous variables were compared for patients with previous and preoperative COVID-19 infection and postoperative COVID-19 infection to all others using Student's t test and Mann-Whitney U test. Categorical variables were compared for patients who had previous and preoperative COVID-19 infection and postoperative COVID-19 infection with all others using chi-square test for association. Data reduction techniques were used to minimize the number of response categories for categorical data when small cell frequencies were observed for 2-by-2 cross tabulations. Unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for all 2-by-2 categorical comparisons. One parsimonious hierarchical multivariable logistic regression model was constructed to identify independent surgical predictors of postoperative COVID-19 infection after controlling for sociodemographic and clinical variables. Variables associated with postoperative COVID-19 infection at a significance level of .05 during bivariate analysis were included in the regression model. Adjusted ORs with 95% CIs were calculated to identify independent surgical predictors of postoperative COVID-19 infection after controlling for categorical sociodemographic, clinical, and surgical predictors of postoperative COVID-19 infection. p-values of <.05 were considered significant. IBM SPSS Statistics for Windows version 25 (IBM Corp., Armonk, NY) was used for all computational analyses.
Results
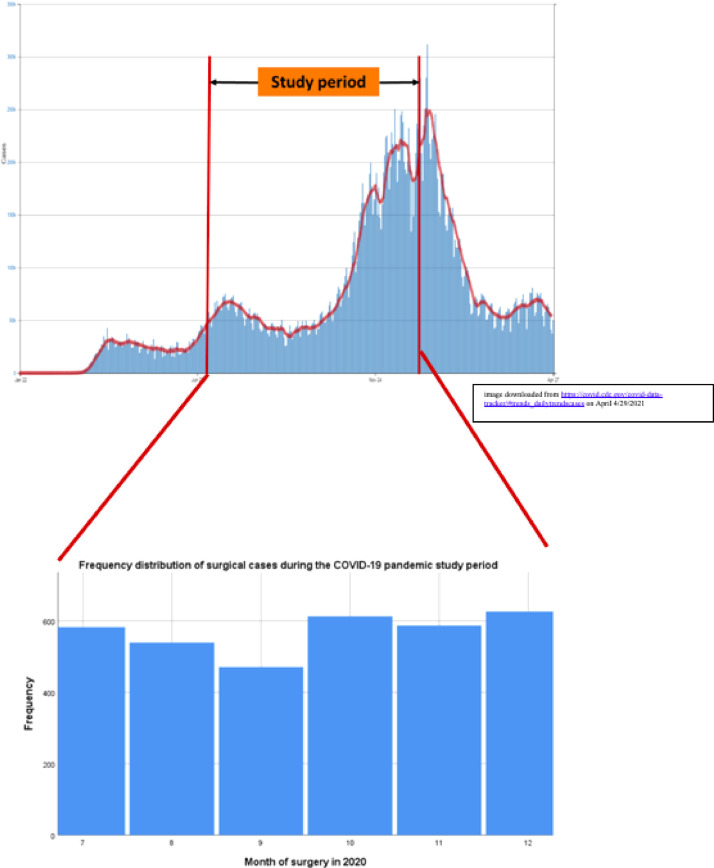
A total of 3541 patients were included in the prospective cohort from 10 surgical sites from July 1, 2020, to December 31, 2020. After removing entries for missing or inconsistent data, a total of 3423 entries (96.7%) were included for final analysis. Our study period occurred during the second and third peak of COVID-19 infections in the United States. The number of surgeries performed monthly in our cohort in relation to COVID-19 infections in the Unites States is presented in Fig. 1 . The sociodemographic and clinical variables of the patient cohort are presented in Table 1 . Surgical variables including the most commonly performed surgeries are presented in Table 2 . The most commonly performed surgery was hysteroscopy (17.5%), followed by laparoscopic adnexal surgery (13.6%) and then total laparoscopic hysterectomy (12.5%). Most surgeries were elective (96.5%).
Fig. 1.
Frequency distribution of US COVID-19 infections by month (top of figure) and frequency distribution of surgical cases by month during the study period (bottom of figure). COVID-19 = coronavirus disease.
Table 1.
Sociodemographic and clinical variables for study cohort (N = 3423)
| Variables | Value |
|---|---|
| Sociodemographic variables | |
| Age (yrs) | 46.6 ± 14.4 |
| Race and ethnicity, n (%) | |
| Non-Hispanic White | 1624 (47.7) |
| Non-Hispanic Black | 837 (24.6) |
| Hispanic or Latino | 654 (19.7) |
| Asian | 128 (3.7) |
| American Indian and Alaska Native | 11 (0.3) |
| Native Hawaiian and Other Pacific Islander | 6 (0.2) |
| Region of country | |
| Northwest | 785 (22.9) |
| Midwest | 1323 (38.7) |
| South | 447 (13.1) |
| Southwest | 866 (25.3) |
| Healthcare worker | |
| Yes | 386 (11.3) |
| No | 2293 (67.6) |
| Unknown | 713 (21) |
| Clinical variables | |
| Body mass index (kg/m2) | 30.3 ± 7.9 |
| Charlson comorbidity index (not age adjusted) | 0.45 ± 1.0 |
| Charlson comorbidity index (age adjusted) | 1.7 ± 1.8 |
| Previous surgery, n (%) | |
| None | 1412 (41.3) |
| Previous non-gynecologic abdominopelvic surgery | 1011 (29.5) |
| Previous non-gynecologic abdominopelvic surgery | 1493 (43.6) |
| Smoking history, n (%) | |
| None | 2633 (77) |
| Previous smoker, not currently | 495 (14.5) |
| Currently smoking | 259 (7.6) |
| Unknown | 32 (0.9) |
All data are presented as number (percentage) and mean ± standard deviation unless otherwise specified.
Table 2.
Surgical variables for study cohort (N = 3423)
| Variable | Value |
|---|---|
| Surgical variables | |
| Ten most common surgeries, n (%) | |
| Hysteroscopy/endometrial ablation | 597 (17.5) |
| Laparoscopic adnexal surgery | 463 (13.6) |
| Total laparoscopic hysterectomy with or without BSO | 428 (12.5) |
| Midurethral sling | 157 (4.6) |
| Laparoscopic colpopexy | 130 (3.8) |
| Laparoscopic myomectomy | 127 (3.7) |
| Vaginal hysterectomy with or without BSO | 99 (2.9) |
| Anterior and posterior colporrhaphy | 98 (2.9) |
| Laparoscopic supracervical hysterectomy with or without BSO | 98 (2.9) |
| Laparoscopic-assisted vaginal hysterectomy with or without BSO | 90 (2.6) |
| Other | 1297 (38) |
| Region of country, n (%) | |
| Northeast | 785 (22.9) |
| Midwest | 1323 (38.7) |
| South | 447 (13.1) |
| Southwest | 866 (25.3) |
| Need for surgery, n (%) | |
| Emergent | 118 (3.5) |
| Elective | 3301 (96.5) |
| Surgical route, n (%) | |
| Laparotomy | 237 (6.9) |
| Robotic/laparoscopic | 1525 (44.6) |
| Vaginal | 1544 (45.1) |
| Other | 115 (3.4) |
| Surgical subspecialty, n (%) | |
| General obstetrics and gynecology | 1249 (36.5) |
| MIGS | 925 (27) |
| Urogynecology | 1075 (31.4) |
| REI | 106 (3.1) |
| Other | 68 (2.0) |
| ASA class, n (%) | |
| ASA I | 512 (15.1) |
| ASA II | 1973 (59) |
| ASA III | 893 (26.3) |
| ASA IV | 19 (0.6) |
| ASA V | 2 (0.1) |
| Length of surgery (min) | 110.6 ± 90.4 (range = 1–620) |
| Length of hospital stay (h) | 18.8 ± 49.7 (range = 0.17–1620) |
ASA = American Society of Anesthesiologists; BSO = bilateral salpingo-oophorectomy; MIGS = minimally invasive gynecologic surgery; REI = reproductive endocrinology and infertility.
All data are presented as number (percentage) and mean ± standard deviation unless otherwise specified.
Postponement of Original Surgery
A total of 189 patients (5.5%) had their gynecologic surgery postponed from their original surgical date. Of these postponed cases, 43 (1.3% of all cases, 22.9% of all postponements) were because of a COVID-19 diagnosis, 11 (5.8%) were because of symptoms of COVID-19 or a COVID-19 exposure without a COVID-19 test result (either not yet tested or a test was pending at the time of postponement), 125 (66.5%) were delayed because of a moratorium on elective cases, and 9 (4.8%) were because of other causes such as surgeon quarantining, patient's fear of infection, and unknown causes. The mean duration of postponement time between the original date and their actual gynecologic surgery was 104.3 ± 60 days. Among the 189 patients who had their surgery postponed, 182 (96.3%) had no sequelae because of surgical postponement, 2 (1.1%) required a blood transfusion for bleeding, 3 (1.6%) required an emergency department visit, 1 (0.55%) had an office procedure, and 1 (0.55%) had a delayed diagnosis of endometrial intraepithelial neoplasia.
Previous COVID-19 Infection Independent of Surgery
A total of 114 patients (3.3%) had a positive COVID-19 test result more than 5 days before their gynecologic surgery. The mean duration of time between their previous COVID-19 diagnosis and their gynecologic surgery was 97.3 ± 64 days. The sociodemographic and clinical differences in the 128 study subjects who had a uniquely positive COVID-19 test result at any time before their gynecologic surgery (114 previous COVID-19 infection, 14 preoperative COVID-19 infection) compared with all others are shown in Table 3 . On bivariate analysis, there were significant differences in age and body mass index, race and ethnicity, region of country, and occupation in healthcare between groups.
Table 3.
Bivariate analysis of preoperative COVID-19 status at any time before gynecologic surgery on sociodemographic and clinical variables
| Positive preoperative COVID-19 test* | Negative preoperative COVID-19 test† | p value | |
|---|---|---|---|
| Age (n = 3403) | 42.2 ± 12.1 | 46.7 ± 14.5 | <.001 |
| BMI | 32.4 ± 8.1 | 30.3 ± 7.9 | .003 |
| Race and ethnicity | <.001 | ||
| Non-Hispanic White | 35/123 (28.5%) | 1572/3106 (50.6%) | |
| Non-Hispanic Black | 30/123 (24.4%) | 803/3106 (25.9%) | |
| Hispanic or Latino | 55/123 (44.7%) | 591/3106 (19.0%) | |
| Asian | 3/123 (2.4%) | 125/3106 (4.0%) | |
| American Indian and Alaskan Native | 0/123 (0%) | 10/3106 (0.3%) | |
| Native Hawaiian and Other Pacific Islanders | 0/123 (0%) | 5/3106 (0.2%) | |
| Region of country | <.001 | ||
| Northeast | 34/128 (26.6%) | 728/3254 (22.4%) | |
| Midwest | 36/128 (28.1%) | 1279/3254 (39.3%) | |
| South | 6/128 (4.7%) | 434/3254 (13.3%) | |
| Southwest | 52/128 (40.6%) | 813/3254 (25%) | |
| Healthcare worker | .003 | ||
| Yes | 21/125 (16.8%) | 365/3229 (11.3%) | |
| No | 67/125 (53.6%) | 2196/3229 (68%) | |
| Unknown | 37/128 (29.6%) | 668/3229 (20.7%) | |
COVID-19 = coronavirus disease; BMI = body mass index.
Only statistically significant bivariate associations are shown. All data are presented as number (percentage) and mean ± standard deviation unless otherwise specified.
Positive preoperative COVID-19 tests included those who had a positive test result remote from surgery or those who had a positive test result preoperatively per institutional testing protocols.
Negative preoperative COVID-19 tests include patients who had a negative test result or had pending or inconclusive tests.
Preoperative COVID-19 Infection
A total of 27 patients (0.8%) had a positive requisite preoperative COVID-19 test result. Among these 27 patients, 5 (18.5%) had a previous COVID-19 infection and retesting was not warranted per institutional protocol, 8 (29.6%) patients had a previous COVID-19 infection and had a positive test result preoperatively, and 14 (51.9%) had a positive preoperative COVID-19 test result obtained within 5 days of surgery. Of the 118 emergent surgeries, 10 (8.5%) were performed despite a positive COVID-19 test result. The indications for emergent surgeries included dilation and curettage, diagnostic and operative laparoscopy for adnexal pathology, and marsupialization of Bartholin's cyst abscess. There were no adverse sequelae for these 10 cases postoperatively. The mean duration of time between the requisite preoperative COVID-19 test and their actual gynecologic surgery was 2.9 ± 4.8 days.
Postoperative COVID-19 Infection
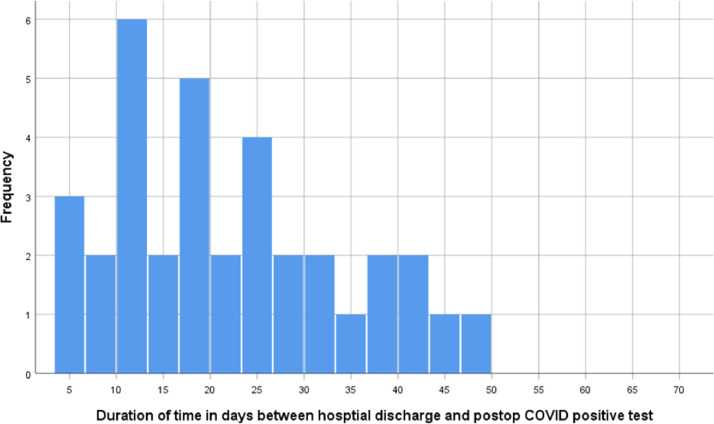
After hospital discharge to 10 weeks postoperatively, 39 patients (1.1%) developed a COVID-19 infection. The mean duration of time between hospital discharge after gynecologic surgery and the postoperative positive COVID-19 test was 22.1 ± 12.3 days (range, 4–50 days). Eleven (31.4% of postoperative COVID-19 infections, 0.3% of total cases) of the newly diagnosed COVID-19 infections occurred within 14 days of hospitalization for gynecologic surgery, with most of the diagnoses of COVID-19 infections occurring 15 to 20 days postoperatively (Fig. 2 ). The median length of hospital stay in hours was longer for patients who developed a postoperative COVID-19 infection than those who did not (median, 17.7 ± 24, vs median, 8.7 ± 18.1; p = .002). The length of hospital stay after gynecologic surgery was >24 hours for 18 (46.2%) of the 39 patients with postoperative COVID-19 infections. The remaining sociodemographic, clinical, and surgical differences in the 39 study subjects who had a newly diagnosed COVID-19 infection on follow-up compared with all others are presented in Table 4 .
Fig. 2.
The frequency distribution of time between hospital discharge and newly diagnosed postoperative COVID-19 infection. COVID-19 = coronavirus disease.
Table 4.
Bivariate analysis of patients with postoperative COVID-19 infections compared with those without COVID-19 infections
| Postoperative COVID-19 infections (n = 39) | No postoperative COVID-19 infection (n = 3384) | p value | |
|---|---|---|---|
| Charlson comorbidity index (not age adjusted) | 0.9 ± 1.2 | 0.4 ± 1.0 | .001 |
| Charlson comorbidity index (age adjusted) | 2.2± 1.7 | 1.7 ± 1.8 | .02 |
| Region of country | <.001 | ||
| Northeast | 3/39 (7.7%) | 782/3382 (23.1%) | |
| Midwest | 14/39 (35.9%) | 1309/3382 (38.7%) | |
| South | 1/39 (2.6%) | 446/3382 (13.2%) | |
| Southwest | 21/39 (53.8%) | 845/3382 (25%) | |
| Surgical specialty | .04 | ||
| General obstetrics and gynecology | 15/39 (38.5%) | 1234/3384 (36.5%) | |
| MIGS | 13/39 (33.3%) | 912/3384 (27%) | |
| Urogynecology | 8/39 (20.5%) | 1067/3384 (31.5%) | |
| REI | 0/39 (0%) | 106/3384 (3.1%) | |
| Other | 3/39 (7.7%) | 65/3384 (1.9%) | |
| ASA class | .004 | ||
| Class I | 6/39 (15.4%) | 506/3360 (15.1%) | |
| Class II | 19/39 (48.7%) | 1954/3360 (58.2%) | |
| Class III | 12/39 (30.8%) | 881/3360 (26.2%) | |
| Class IV | 2/39 (5.1%) | 17/3360 (0.5%) | |
| Class V | 0/39 (0%) | 2/3360 (0.1%) | |
| Length of hospital stay (hours) | Median, 17.7; IQR, 24 | Median, 8.7; IQR, 18.1 | .002 |
ASA = American Society of Anesthesiologist; COVID-19 = coronavirus disease; IQR = interquartile range; MIGS = minimally invasive gynecologic surgery; REI = reproductive endocrinology and infertility.
Only statistically significant bivariate associations are shown. All data are presented as number (percentage) and mean ± standard deviation unless otherwise specified.
There were no adverse clinical outcomes as a result of postoperative COVID-19 infection in 27 cases (69.2%) whereas 12 patients had adverse clinical outcomes including 5 cases of pneumonia (12.8%), 3 cases of abnormal chest radiography (7.7%), 3 cases of hospital readmission (7.7%), 1 case of gastrointestinal symptoms (2.6%), 1 case of neurologic symptoms (2.6%), and 1 case of sinusitis symptoms (2.6%). None of the patients with a postoperative COVID-19 infection required an intensive care unit admission, mechanical ventilation, or extracorporeal membrane oxygenation and did not develop coagulopathy, cardiomyopathy, multisystem inflammatory syndrome, or death.
On the multivariable parsimonious hierarchical logistic regression model of postoperative COVID-19 infections, living in the Southwest region (adjusted OR, 6.8; 95% CI, 2.0–23.1) of the Unites States increased the odds of a postoperative COVID-19 infection after discharge compared with the Northeast region. A single-unit increase in age-adjusted Charlson comorbidity increased the odds of a postoperative COVID-19 infection by 20% (adjusted OR, 1.2; 95% CI, 1.002–1.35). There was a statistical trend showing that for each 1-hour increase in hospital stay, the odds of postoperative COVID-19 infections increased by 0.3% (adjusted OR, 1.003; 95% CI, 1.001–1.005).
Intraoperative Complications
There were 104 intraoperative complications (3%) associated with gynecologic surgery. There were 22 cases of urinary tract injuries (0.6% of total surgeries), 19 cases of required additional/unplanned surgery (0.6%), 18 cases of hemorrhage complications (0.5%), 8 cases of gastrointestinal tract injuries (0.2%), 6 cases of injuries to organs distant from the primary surgical site (0.1%), 5 cases of pulmonary complications (0.1%), 3 cases of anesthetic complications (0.08%), and 2 cases of major vessel injuries (0.06%). Twenty patients (0.5%) had other complications ranging from unanticipated surgical complexity (n = 11), uterine perforation (n = 5), transient cardiac arrhythmias (n = 2), transient ureteral obstruction (n = 1), and use of cell saver (n = 1).
Postoperative Complications during Hospitalization
In the cohort, 277 patients (8.1%) developed immediate postoperative complications. The most common were pain, nausea/vomiting, and blood product transfusion. Details are available in Supplemental Table 2. There were no cases of newly diagnosed COVID-19 infection in the immediate postoperative hospitalization period. There was 1 death postoperatively during hospitalization, which was not because of a COVID-19 diagnosis or a surgical complication.
Postoperative Complications after Hospital Discharge
Postoperatively, 2479 patients (72.4%) presented for in-person follow-up, whereas 447 patients (13.1%) followed up virtually by telephone or video virtual visit. There were 1066 postoperative complications (31.1%) after hospital discharge with the most common being pain (n = 299, 8.7% of all patients), return to emergency department unrelated to COVID-19 infection (n = 216, 6.3% of all patients), urinary tract infection (n = 119 cases, 3.5% of total surgeries), hospital readmission unrelated to COVID-19 infection (n = 81, 2.4% of all patients), nausea and vomiting (n = 70, 2% of all patients), and surgical site infection (n = 62, 1.8% of all patients). There were 2 deaths postoperatively after hospital discharge because of cancer-related complications, which were not because of a COVID-19 diagnosis or a surgical complication.
Any intraoperative, immediate postoperative, or postdischarge follow-up complications were not significantly higher in patients who had a positive COVID-19 test result at any time before gynecologic surgery compared with all others (Table 5 ).
Table 5.
Perioperative complications in patients with and without previous COVID-19 tests
| Positive preoperative COVID-19 test* | Negative preoperative COVID-19 test† | p value | |
|---|---|---|---|
| Intraoperative complications | 5/128 (3.9%) | 99/3275 (3.0%) | .57 |
| Postoperative complications (before discharge) | 5/128 (3.9%) | 201/3275 (6.1%) | .30 |
| Postoperative complications (after discharge) | 30/128 (23.4%) | 1031/3275 (31.5%) | .05 |
COVID-19 = coronavirus disease.
Positive preoperative COVID-19 tests included those who had a positive test result remote from surgery or those who had a positive test result preoperatively per institutional testing protocols.
Negative preoperative COVID-19 tests include patients who had a negative test result or had pending or inconclusive tests.
Discussion
To date, the SOCOVID study is the largest known national prospective cohort study in the Unites States that evaluates benign gynecologic surgery outcomes during the SARS-CoV-2 pandemic. We found a low incidence (1.1%) of postoperative COVID-19 infections in the 10 weeks after surgery. Those living in the Southwest had higher odds of developing postoperative COVID-19 infection. There were no differences in intraoperative or postoperative complications between those with previous COVID-19 infections and those without.
Our study showed that only 0.3% of the study population had a new positive test result for COVID-19 within 14 days of hospitalization for gynecologic surgery. Given that the Centers for Disease Control and Prevention reports a 14-day incubation period for COVID-19 [7], with another study reporting that the most onset of symptoms is within 11.5 days of infection [8], we conclude that the risk of nosocomial COVID-19 infection during hospitalization for gynecologic surgery was very low. Furthermore, this 0.3% may overestimate nosocomial transmissions because some patients may have acquired COVID-19 in the community postoperatively. The low conversion rate may be valuable for counseling patients who are concerned about COVID-19 exposure in the hospital setting; however, because universal postoperative COVID-19 testing was not conducted as part of our study, the reported incidence may be an underestimation of postoperative infections.
In this study of mostly minimally invasive gynecologic surgery, only 1.3% of benign surgical cases from July 1 to December 31, 2020, were postponed because of a preoperative positive COVID-19 test result. Fortunately, most of these patients did not experience any sequelae as a result of surgical postponement although this likely is because most patients in our cohort were undergoing elective surgeries. Most cases for benign gynecologic conditions such as uterine myomas, abnormal uterine bleeding, or pelvic organ prolapse were categorized as tier 1a (defined as low acuity and non–life-threatening illness) with the previously published “Elective Surgery Acuity Scale” [9]. This may affirm that the tiered ranking systems developed to reintroduce elective cases appropriately triaged cases. Furthermore, in the event that elective procedures are to be postponed or delayed in the future, our data show that postponement of these types of elective surgery did not lead to significant morbidity or mortality.
When looking at the 1.1% of patients who developed postoperative COVID-19 infection in the 10-week postoperative period, patients living in the Southwest had higher odds of developing a postoperative COVID-19 infection. This is likely because the timing of our study coincided with an increase of cases in the Southwest region, suggesting that the higher incidence of community spread may play a role in postoperative COVID-19 infections beyond nosocomial spread. Our study demonstrated a statistical trend that each 1-hour increase in hospital stay was associated with increased odds of postoperative COVID-19 infections by 0.3%; in other words, shorter hospital stays reduced the odds of developing COVID-19 postoperatively. Before the pandemic, same-day discharge was shown to be feasible [10] with no differences seen in readmission after hysterectomy [11] or pelvic organ prolapse surgery [12]. In light of the current pandemic, our study suggests the additional benefit of limiting the duration of postoperative hospital stay to also include reduced odds of acquiring nosocomial COVID-19 infection.
In patients with active COVID-19 infections undergoing surgery, there is a reported increase in surgical mortality and complications [13], specifically increased pulmonary complications [14] and venous thromboembolism [15,16]. However, current data remain mixed with some showing no change in the rate of postoperative morbidity and mortality [17,18]. In a large international multicenter cohort of 1128 patients undergoing surgery at 235 hospitals in 24 countries from January 1 to March 21, 2020, the COVID-19 infection rate was 26.1% preoperatively, with 51.2% experiencing postoperative pulmonary complications and overall 30-day mortality at 23.8% [14]. In another multicenter international prospective cohort study of 140 231 surgical patients from 116 countries, 2.2% had a preoperative positive COVID-19 test result and the adjusted OR for 30-day mortality was significantly higher in patients having surgery within 7 weeks of a COVID-19 infection [19]. In the SOCOVID study, we did not find a difference in complications between patients with and without a history of COVID-19 infection. Furthermore, the rate of complications from the SOCOVID cohort does not differ from known 30-day postoperative complications reported from large registries such as the American College of Surgeons National Surgical Quality Improvement Program. In a National Surgical Quality Improvement Program study of 27 167 hysterectomies, the overall 30-day complication rate was 8.1% [20]. The most likely difference for the relative low rate of complications in patients with a history of COVID-19 is related to the average delay from the time of COVID-19 diagnosis to surgery in our study, which was approximately 14 weeks. Although our study was not designed to determine the ideal time frame to delay surgery after a positive COVID-19 test result, it certainly challenges current institutional preoperative COVID-testing protocols [4] with a mean waiting time of 10 days (range, 10–30 days) after a COVID-19 infection. Perhaps, the ideal waiting time should be reconsidered particularly with recent data showing that patients who have surgery less than 7 weeks after COVID-19 infection experience greater postoperative mortality [19], although more large scale studies are needed to support this recommendation.
There are several strengths to this study. This prospective study design allowed us to follow up all patients during the study period to capture all perioperative COVID-19 infections and the associated complications. The multicenter design of this study allowed the inclusion of a diverse population of women undergoing a variety of mostly elective, minimally invasive, and outpatient gynecologic surgeries. All centers underwent training to ensure standardized data entry.
There are several limitations to this study. To coordinate IRB approval across 10 study sites, data collection did not begin until July 1, 2020, which was after the initial peak of cases in the Northeast. Furthermore, we do not have a complete geographic representation of the United States. We planned to have sites from the West coast; however, because of IRB-related issues, our West coast sites were unable to enroll subjects. Because the incidence of new COVID-19 cases in the Unites States varied from month to month in different regions of our country, this may have skewed the overall number of new cases in certain regions of the United States. Finally, our reported incidence of postoperative COVID-19 infection may be underestimated because patients did not undergo universal COVID-19 testing postoperatively.
Conclusion
In this large multicenter prospective cohort study of benign gynecologic surgeries, only 1.1% of patients developed a postoperative COVID-19 infection, with 0.3% of patients with an infection in the immediate 14 days after surgery. For those with a previous COVID-19 infection remote from surgery, the incidence of surgical complications was low and not statistically different from those without a previous COVID-19 infection.
Acknowledgments
The authors thank Nancy Frankel, Anjani Kapadia, Rebecca Zhou, Karen Schirm, Erryn Tappy, Madhuri Gottam, Neha Gaddam, Emily Sendukas, Deina Bossa, Juanita Bonilla, Surabhi Tewari, Miguel Luna Russo, Megan S. Orlando, Hannah Millimet, Garland Almquist, Amanda Wagner, Kerri Andre, Vyvian Borse, and Sylwia Clarke.
Footnotes
Dr. Ascher-Walsh owns Expert Alternatives and produces alternative treatments for myomas. Dr. Mueller is an expert witness for Ethicon and is a recipient of National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Research Grant. The other authors declare that they have no conflict of interest.
This paper was presented at the general session of the 47th Society of Gynecologic Surgeons 47th Annual Scientific Meeting in Palm Springs, California in June of 2021.
Institutional review board approval number: IRB# 20-702 Cleveland Clinic.
Karl Storz provided funding for data management and statistical analysis.
Supplementary Material
Supplemental Table 1.
Variables collected from medical record
| Preoperative data | Perioperative data | Postoperative data |
|---|---|---|
| Date of birth, height, weight, race, ethnicity, occupational status as healthcare worker, past medical history (Charlson comorbidity index), history of gynecologic and/or nongynecologic abdominal-pelvic surgery, smoking history | Date of surgery, perioperative COVID-19 test date, type (NP, nasal, oral secretions, sputum, other) and result (positive, negative, pending at the time of surgery), date and time of hospital admission and discharge, need for surgery (elective vs emergent), lead surgical CPT code, ASA classification, Surgical approach (robotic/laparoscopic, open, vaginal, other), class of surgery (inpatient vs outpatient), location of surgery (ambulatory/outpatient center vs hospital), specialty of surgeon, level of trainee involvement (resident and/or fellow), anesthesia type, use of antibiotic and VTE prophylaxis, duration of surgery and anesthesia, EBL, need for blood product transfusion, intraoperative complications | Postoperative complications before discharge or until the time of clinic follow-up including new COVID-19 infection, emergency department visits or hospital readmissions unrelated to COVID-19, surgical site infection, VTE events, UTI, myocardial infarction, acute kidney injury, renal failure, congestive heart failure, cerebrovascular events, small bowel obstruction, sepsis, shock, Clostridium difficile colitis, neuropathy, arrhythmia, pulmonary edema, pneumonia, cardiac arrest, postoperative blood transfusion, ileus, nausea and vomiting, undiagnosed injury to urinary or gastrointestinal tracts, unplanned return to operating room, ICU admission, death |
ASA = American Society of Anesthesiology; CPT = Current Procedural Terminology; EBL = estimated blood loss; ICU = intensive care unit; NP = nasopharyngeal; UTI = urinary tract infection; VTE = venous thromboembolism.
Supplemental Table 2.
Postoperative complications (before discharge)
| Postoperative complications | All surgeries (N = 3423) |
|---|---|
| Pain | 84 (2.5) |
| Nausea/vomiting | 48 (1.4) |
| Blood product transfusion | 44 (1.3) |
| Other complications | 24 (0.7) |
All numbers are reported as number (percentage).
References
- 1.Centers for Disease Control and Prevention. First travel-related case of 2019 novel corona virus detected in United States. CDC Newsroom. Available at: https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html. Accessed March 20, 2021.
- 2.Grimes CL, Balk EM, Crisp CC, et al. A guide for urogynecologic patient care utilizing telemedicine during the COVID-19 pandemic: review of existing evidence. Int Urogynecol J. 2020;31:1063–1089. doi: 10.1007/s00192-020-04314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns – United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlando MS, Chang OH, Luna Russo MA, Kho RM. Institutional protocols for coronavirus disease 2019 testing in elective gynecologic surgery across sites for the Society of Gynecologic Surgeons’ Surgical Outcomes during the COVID-19 pandemic (SOCOVID) study. Am J Obstet Gynecol. 2021;224:540–542. doi: 10.1016/j.ajog.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed May 12, 2021.
- 8.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joint statement on re-introduction of hospital and office-based procedures for the practicing urogynecologist and gynecologist. J Minim Invasive Gynecol. 2020;27:1030–1032. doi: 10.1016/j.jmig.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsholm M, Mogensen O, Jeppesen MM, Lysdal VK, Traen K, Jensen PT. Systematic review of same-day discharge after minimally invasive hysterectomy. Int J Gynaecol Obstet. 2017;136:128–137. doi: 10.1002/ijgo.12023. [DOI] [PubMed] [Google Scholar]
- 11.Sheyn D, El-Nashar S, Billow M, Mahajan S, Duarte M, Pollard R. Readmission rates after same-day discharge compared with postoperative day 1 discharge after benign laparoscopic hysterectomy. J Minim Invasive Gynecol. 2018;25:484–490. doi: 10.1016/j.jmig.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Berger AA, Tan-Kim J, Menefee SA. Comparison of 30-day readmission after same-day compared with next-day discharge in minimally invasive pelvic organ prolapse surgery. Obstet Gynecol. 2020;135:1327–1337. doi: 10.1097/AOG.0000000000003871. [DOI] [PubMed] [Google Scholar]
- 13.Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155:691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collaborative COVIDSurg. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannis D, Barish MA, Goldin M, et al. Incidence of venous thromboembolism and mortality in patients with initial presentation of COVID-19. J Thromb Thrombolysis. 2021;51:897–901. doi: 10.1007/s11239-021-02413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith ACD, Miranda BH, Strong B, et al. St Andrew's COVID-19 surgery safety (StACS) study: the Burns Centre experience. Burns. 2021;47:1547–1555. doi: 10.1016/j.burns.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seretis C, Archer L, Lalou L, et al. Minimal impact of COVID-19 outbreak on the postoperative morbidity and mortality following emergency general surgery procedures: results from a 3-month observational period. Med Glas (Zenica) 2020;17:275–278. doi: 10.17392/1229-20. [DOI] [PubMed] [Google Scholar]
- 19.COVIDSurg Collaborative, GlobalSurg Collaborative Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76:748–758. doi: 10.1111/anae.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie M, Strassle PD, Moulder JK, Dizon AM, Schiff LD, Carey ET. Uterine weight and complications after abdominal, laparoscopic, and vaginal hysterectomy. Am J Obstet Gynecol. 2018;219:480.e1–480.e8. doi: 10.1016/j.ajog.2018.06.015. [DOI] [PubMed] [Google Scholar]