Abstract
Adult neurogenesis has been implicated in learning and memory of complex spatial environments. However, new neurons also play a role in nonmnemonic behavior, including the stress response and attention shifting. Many commonly used spatial tasks are very simple, and unsuitable for detecting neurogenesis effects, or are aversively motivated, making it difficult to dissociate effects on spatial learning and memory from effects on stress. We have therefore created a novel complex spatial environment, the flex maze, to enable reward-mediated testing of spatial learning in a flexibly configurable labyrinth. Using a pharmacogenetic method to completely inhibit neurogenesis in adulthood, we found that rats lacking new neurons (TK rats) and wild type controls completed and remembered most mazes equally well. However, control rats were slower to complete peppermint-scented mazes than other mazes, while neurogenesis-deficient rats showed no effect of mint on maze behavior, completing these mazes significantly faster than control rats. Additional testing found that wild type and TK rats showed similar detection of, avoidance of, and glucocorticoid response to the mint odor. These results suggest that spatial learning and memory in a labyrinth task is unaffected by the loss of new neurons, but that these cells affect the ability of an aversive stimulus to distract rats from completing the maze.
Keywords: neurogenesis, neuronal plasticity, dentate gyrus, spatial learning, attention, rats
Introduction
Nearly 60 years ago, studies showed that rats with bilateral hippocampal lesions had deficits in learning of multiple-T and Hebb-Williams labyrinth mazes (Kaada et al., 1961; Kimble, 1963), suggesting that the hippocampus is involved in more than just short-term memory and emotionality, but in active forms of spatial navigation as well. Since that time, many studies have found that the hippocampus is important for learning or remembering spatial environments (Olton et al., 1978; Devan et al., 1996; Fox et al., 1998), an idea supported by the existence of hippocampal neurons that fire in specific parts of an environment, known as place cells (O’Keefe and Dostrovsky, 1971; Muller and Kubie, 1987; Quirk et al., 1990; Leutgeb et al., 2005). However, overrepresentation of rewarded locations in place fields, reward-driven changes in firing rates, and a critical role for granule cells in driving activity in rewarded places (Sasaki et al., 2018; Yuan and Leutgeb, 2020) suggest that the dentate gyrus may be more important for incorporating salient cues in spatial tasks than for navigation itself. And recent findings have challenged the primacy of the hippocampus for learning and memory in general (Basile et al., 2020).
One unique aspect of the dentate gyrus is the continual addition of neurons throughout life, which contributes to performance in spatial learning and memory tasks (Dupret et al., 2007, 2008; Lieberwirth et al., 2016). The Morris water maze (Morris, 1984) is the most common spatial task used to identify behavioral consequences of hippocampal manipulations, including ablation of adult neurogenesis, in rodents. Although a few studies have found impairments in adult neurogenesis-deficient animals in the water maze, several others find no requirement of new neurons for spatial water maze learning or memory (Snyder et al., 2005; Meshi et al., 2006; Saxe et al., 2006; Jessberger et al., 2009; Groves et al., 2013). The water maze utilizes aversive, stressful motivation (escape from forced swimming), which can complicate interpretations of spatial learning (Morris, 1984), in particular because the hippocampus and adult neurogenesis regulate responses to stress (Conrad, 2006; Snyder et al., 2011; Schoenfeld and Gould, 2012). And in fact, several studies find performance impairments in spatial water maze tasks that are not apparent in non-stressful, reward-mediated dry land spatial tasks (Whishaw and Tomie, 1996; Gonzalez et al., 2000; Whishaw and Pasztor, 2000; Gibson et al., 2001). Contextual fear conditioning, another task commonly used to test spatial functions of new neurons, and the hippocampus more generally, relies on footshock, another aversive, stressful motivation. Stressful aspects of common spatial learning tasks may mask the true effects of continued neurogenesis on spatial learning.
To test rodent behavior in complex spatial environments without the stress inherent in escape-motivated tasks, we created a new spatial apparatus, the flex maze, a labyrinth with interchangeable walls allowing for variable configurations within the same arena. We trained rats on multiple landscapes within the flex maze, each with distinctive olfactory and visual cues, and tested learning, allocentric navigation, and long-term memory following experience in multiple maze environments, which could produce memory interference. To test the functional importance of adult-born neurons on learning and navigation in these spatial environments, we inhibited adult neurogenesis using the GFAP-TK pharmacogenic rat model, in which an anti-viral drug that is orally administered to transgenic rats expressing a viral gene in neuronal precursor cells specifically inhibits generation of new neurons without affecting post-mitotic astrocytes (Snyder et al., 2016). Odor cues were introduced to provide context for each maze configuration, because although adult neurogenesis is inhibited in the olfactory bulb as well as the dentate gyrus, loss of new neurons does not impair odor detection (Imayoshi et al., 2008; Sakamoto et al., 2011; Alonso et al., 2019). Unexpectedly, one of the odors, peppermint, was found to be aversive to rats of both genotypes, providing an opportunity to investigate the interaction between adult neurogenesis, aversive cues, and spatial navigation in a complex dry land maze.
Our findings indicate that TK rats learn to navigate the mazes as well as wild type (WT) rats, use similar allocentric navigation strategies, and remember the paths for at least 3 weeks like WT rats. However, the presence of mint odor altered the maze performance of WT rats but not TK rats. The differential effect on performance was not due to olfactory deficits in TK rats, as they showed equal ability to WT rats to detect the mint odor and found it similarly aversive. The findings instead point to an effect of new neurons on the ability of the aversive odor to shift attention from the rewarded task toward the aversive odor.
Materials & Methods
GFAP-TK Rats and Experimental Design.
Male transgenic rats expressing herpes simplex virus thymidine kinase (HSV-TK) under the control of the human glial fibrillary acidic protein (GFAP) promoter on a Long Evans background (Snyder et al., 2016) were used for all experiments. Rats were bred in-house and housed 3–6/cage on a 12-hour reversed light-dark cycle (lights off at 9am). All rats were meal fed (15–16g chow/rat/day) from the time of weaning and given ad libitum access to water. The anti-viral drug valganciclovir (VGCV) was mixed into peanut butter and powdered chow (4mg VGCV/rat) and fed to both wild type (WT) and transgenic (TK) rats twice weekly beginning at 8 weeks of age; interaction of VGCV with the TK transgene prevents cell division in GFAP+ radial cells that normally give rise to neurons. WT and TK rats were given VGCV for 8 weeks before experiments commenced and maintained on the drug throughout the duration of the experiment. The 8-week time point eliminates a substantial population of relatively mature and immature adult-born neurons (Snyder et al., 2009; Snyder and Cameron, 2012; Cole et al., 2020), increasing the chances of seeing potential behavioral changes due to loss of adult neurogenesis relative to shorter time points. Behavioral effects on attention, sucrose-preference, motivation, novelty-suppressed feeding, and novel object-location preference have been observed following 8 weeks of VGCV (Snyder et al., 2016; Cameron and Schoenfeld, 2018; Karlsson et al., 2018; Weeden et al., 2019). All procedures followed the Institute of Laboratory Animal Research guidelines and were approved by the NIMH Animal Care and Use Committee.
Flex Maze Apparatus.
A 1m × 1m open field (with 50cm-high outer walls) was constructed with a removable floor containing a 9 × 9 grid of 24cm-high cylindrical pins. Removable walls (8cm-wide × 24cm-high) were custom made to fit in between the pins and placed to create multiple maze configurations within the same open field. One week before flex maze training, all rats were given Froot Loops in their home cage to facilitate reward acquisition. During the first week of all flex maze experiments, all rats were habituated to the maze environment in 20 trials (4 trials/day) in which a Froot Loop reward was placed on a pedestal in the same corner of the maze and rats were started progressively farther from the pedestal. Rats eventually had to navigate all areas within the maze, but no choices were made; rats simply had to follow the corridor until they reached the end to receive the reward.
Initial Flex Maze Training and Testing Experiment.
Following the week of habituation, maze-trained WT and TK rats were trained on three different mazes, each with at least 9 directional choice points. Each maze configuration contained a different odor (cinnamon, peppermint, or banana; LorAnn Oils) at the start corner, and different high-contrast visual cues high on the walls of the testing room. Both odor and visual stimuli were intended as context cues that the rats could use to distinguish different configurations; the visual stimuli could also be used for spatial orientation in the maze, while the odor cue at the start point is not expected to aid in navigation. Rats were trained on each maze in 14 trials over 4 days: 4 trials per day on days 1–3, and 2 training trials on day 4. The maze was wiped with 70% ethanol and allowed to dry between each trial. Rats were run in random order blind to genotype and all rats completed one trial before any rats ran the next trial. The intertrial interval for each rat was between 1–2 hours, depending on the number of rats in a cohort and their completion times. On the fourth day, the 3rd and 4th trials were probe tests, in which rats started in each of the corners other than the start and end corners for that maze, to test whether WT and TK rats differed in their use of spatial knowledge of the environment or an idiothetic (turn by turn) strategy. Mazes were run sequentially over 3 consecutive weeks. One week following the last session in the third maze, rats were given one trial in each of the three maze configurations in random order to test long-term memory for the maze environments.
Maze Behaviors Analyzed.
The latency to reach and eat the reward was measured for all training and testing trials. In the initial experiment, the number of intersections crossed in the maze and number of vicarious trial-and-error (VTE) responses were also measured. Vicarious trial-and-error (VTE) is a rodent behavior commonly exhibited at decision points in spatial and non-spatial tasks (Redish, 2016), which involves looking or moving back and forth between two options before making an action choice and which is altered in TK rats performing a social preference task (Opendak et al., 2016).
Mint in Different Maze Configurations.
To better understand the effects of peppermint odor on maze performance, new cohorts of WT and TK rats were run as above but with mint in the final maze rather than the second maze or without mint in any of the three mazes. A third cohort of WT and TK rats was then divided into three groups to test the effects of peppermint in training versus testing. One group had peppermint present during training trials but not probe trials (mint train). A second group had no odor during training, but peppermint odor during the probe trials (mint probe). The third group had peppermint odor during training and probe trials both (mint both). Following this training, all rats learned two additional mazes, scented with vanilla and cinnamon, followed by one more trial in the original peppermint maze, scented with peppermint for all groups, to test long-term memory.
Mint Odor Testing.
An olfactory detection sensitivity test, the two-bottle olfactory discrimination test (Cheng et al., 2013) was performed to determine whether peppermint odor is differentially detected by, or aversive to, WT and TK rats. This test normally involves a procedure (e.g., pairing with LiCl) to make an odor aversive to the animal, but because we suspected that mint odor was naturally aversive we skipped this step. Single housed rats were habituated to 2 water bottles, one containing water and one containing water flavored with either peppermint or cinnamon (Bickford Flavors, 10−4 dilution during habituation) for 48 hours. After habituation, peppermint and cinnamon preferences were measured at different concentrations with 48-hour tests, swapping placement of water bottles after 24-hours (Cheng et al., 2013). Water bottles were weighed before and after tests and peppermint- and cinnamon-flavored water consumption were compared to that of unflavored water.
To test whether peppermint odor differentially activates the HPA axis, corticosterone was measured in blood collected from rats following peppermint and cinnamon odor exposure. All rats were tested during the inactive part of the light cycle (6–7am) to minimize baseline corticosterone levels, and each rat was used for only one measurement. Rats were single housed, and a scent (peppermint or cinnamon oil; LorAnn Oils) applied to a cotton ball was placed in the home cage for 5 minutes. Blood was collected from the tail vein 15 or 30 minutes following odor exposure or from control rats. Control rats were handled in a different procedure room to minimize any effects of residual odorant. Serum was extracted and analyzed using a corticosterone ELISA kit (Enzo Life Sciences) and 1420 multilabel counter (PerkinElmer).
To test the behavioral reactions of WT and TK rats to peppermint odor in an open field, rats were placed for 10 minutes into a 1m × 1m open field containing an undiluted peppermint oil-scented cotton ball (LorAnn Oils) in one corner. Locomotion through the corners (20cm × 20cm) and center (60cm × 60cm) of the open field was measured using TopScan (CleverSys Inc) to investigate peppermint avoidance and anxiety-like behavior.
Immunohistochemistry.
To verify the presence of new neurons in WT rats and absence in TK rats, a few sections through the hippocampus from each rat were immunostained for doublecortin (DCX). Free-floating sections were blocked with 3% normal donkey serum and 0.5% Tween-20 for 20 minutes then incubated in polyclonal goat anti-DCX (1:200 in blocking solution; Santa Cruz Biotechnology Cat# sc-8066, RRID:AB_2088494) for at least 5 days. Sections were then incubated in donkey anti-goat Alexa 488 fluorescent secondary (1:500; ThermoFisher Scientific Cat# A-11055, RRID:AB_2534102) and counterstained with Hoescht 33258 (1:1000), mounted, and coverslipped with Immu-Mount (ThermoFisher Scientific Cat# 9990402). The presence or absence of DCX+ cells in the dentate gyrus was qualitatively assessed using a BX-51 Olympus epifluorescent microscope with 40x oil-objective to confirm genotype and treatment efficacy. A separate group of WT and TK rats was treated for 8 weeks before analyzing DCX+ cells as above, to verify that these cells were absent at the time point when flex maze training began.
Statistical Analysis.
In the initial maze experiments, latency, intersections crossed, and VTE-like behavior across training were analyzed using 2×14 mixed-factorial ANOVAs (genotype × trial, with trial as a repeated measure). Independent-sample two-tailed t-tests were run for the probe (both probe tests were averaged together for each rat) and retrieval tests. In the mint testing maze experiments, latency analysis was performed as above for the training trials, but 2×3 (genotype × mint group) between-groups ANOVAs were used to analyze probe and retrieval tests. For mint preference testing, 2×2 (genotype × water) mixed-factorial ANOVAs were performed to compare the amounts of liquid consumed across groups. Corticosterone measurements were analyzed using 2×3 (genotype × odor) between-subjects ANOVAs. For mint open field experiments, independent samples, two-tailed t-tests were performed for locomotion and center time, and 2×2 (genotype × corner) mixed-factorial ANOVAs were performed for corner analysis. For all ANOVAs, Holm-Sidak post hoc tests were performed if necessary. All graphs represent means + sem.
Results
Adult neurogenesis is completely inhibited in TK rats.
DCX, a marker of immature granule neurons (Brown et al., 2003; Snyder et al., 2009), was used to qualitatively assess the presence of ongoing neurogenesis in WT and TK rats. As expected, based on previous work using this transgenic rat model (Opendak et al., 2016; Cahill et al., 2018; Galinato et al., 2018; Karlsson et al., 2018; Schoenfeld et al., 2019), treatment with valganciclovir completely eliminated new neurons in TK rats but not WT rats (Fig. 1). All wild type rats had numerous DCX+ cells, and all TK rats had ≤1 DCX+ cells per section, so no rats were excluded for problems with genotyping or drug treatment.
Figure 1: New neurons are absent in treated TK rats.

A) After treatment with valganciclovir for 8 weeks, many immature neurons labeled with antibodies to doublecortin (DCX, red) are visible in the granule cell layer (Hoechst counterstain, blue) of the dentate gyrus of wild type (WT) control rats. B) In rats expressing herpes virus thymidine kinase (TK) under the GFAP promoter, the same anti-viral drug treatment eliminates immature neurons.
Neurogenesis is not necessary for flex maze learning.
To test whether ongoing adult neurogenesis is critical for flexible maze learning, we trained WT and TK rats to navigate three unique mazes (Fig. 2A), tested sequentially. Odor cues of cinnamon, peppermint, and banana were used to contextualize each maze for long-term memory testing. In the first maze, scented with cinnamon (Fig. 2B), both WT and TK rats learned the maze rapidly over the course of one week (Fig. 2C), with no effect of genotype on performance (main effect of genotype: F1,12 = 1.48, p = 0.25; main effect of trials: F13,156 = 22.81, p < 0.0001, n = 7). When tested from probe corners (those other than the normal start and end corners, Fig. 2D), WT and TK rats reached the reward equally quickly (t12 = 0.05, P = .96, n = 7), suggesting that rats of both genotypes used the same strategy to guide behavior, likely allocentric navigation using extramaze cues. When tested 3 weeks later on the same maze (Fig. 2E), both WT and TK rats showed similar rapid performance (t12 = 0.80, P = .44, n = 7), suggesting intact long-term memory. When switched to the second maze, scented with peppermint (Fig. 2F), TK rats completed the new maze more quickly than WT rats during both training and probe trials (Fig. 2G–H; training: main effect of genotype: F1,12 = 9.21, P = 0.01; main effect of trials: F13,156 = 15.49, P < 0.0001; probe trials: t12 = 2.66, P = .02, n = 7). When tested 2 weeks later on the same maze (Fig. 2I), TK rats again reached the goal more quickly than WT rats (t12 = 3.32, P = .006, n = 7). In the third maze, scented with banana (Fig. 2J), both WT and TK rats had similar times on all trials during training (Fig. 2K), probe tests (Fig. 2L), and the 1-week retrieval test (Fig. 2M; training: main effect of genotype: F1,12 = 0.21, P = 0.66; main effect of trials: F13,156 = 8.36, P < 0.0001; probe trials: t12 = 0.56, P = .59; retrieval test: t12 = 0.09, P = .93, n = 7).
Figure 2: New neurons do not impair learning or navigation in the flex maze.
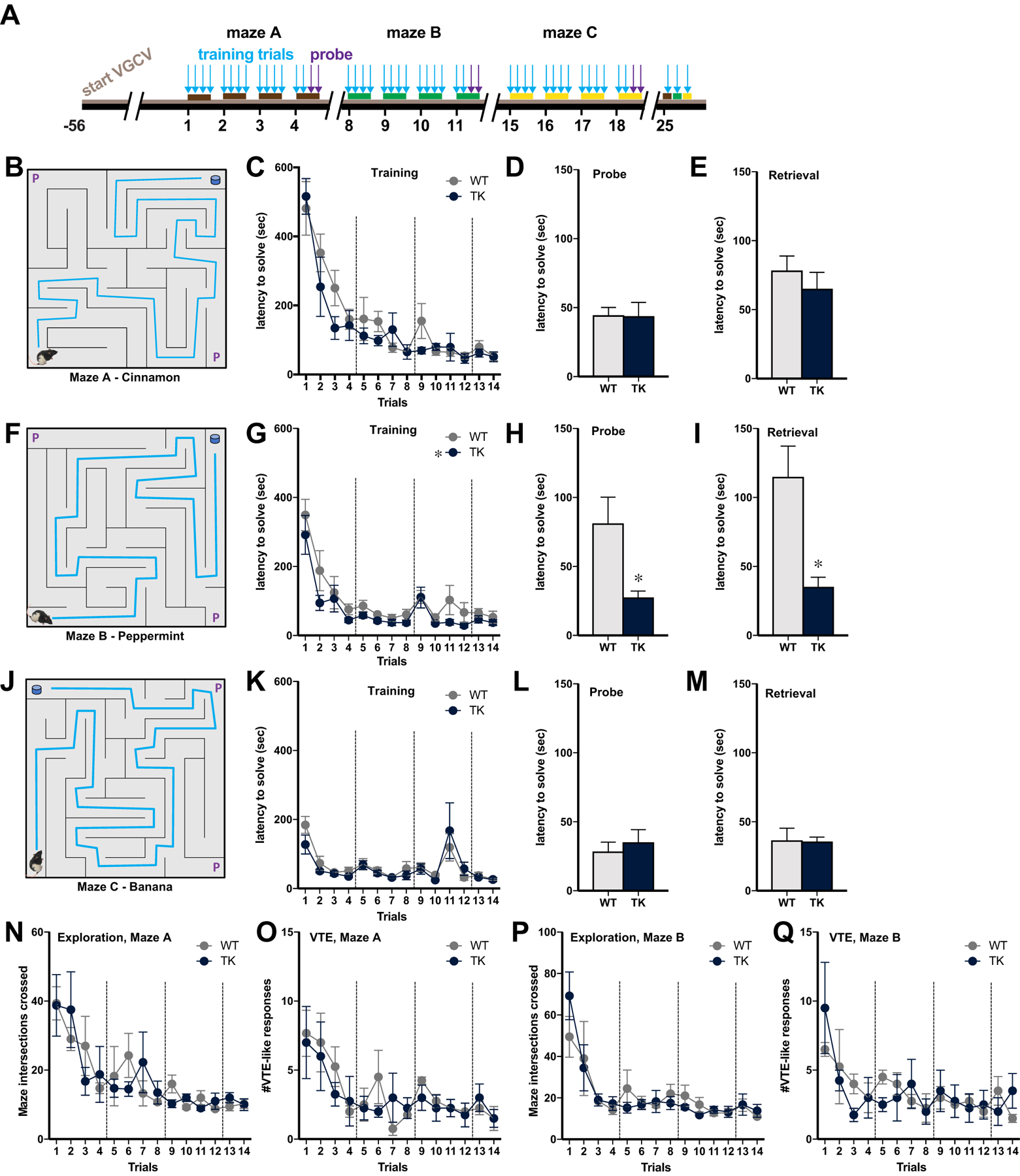
A) Wildtype rats (WT) and transgenic rats lacking adult neurogenesis (TK) were trained on three mazes, each with a different contextual odor cue, tested in probe trials with different starting points (marked P in drawings), and later tested for memory retrieval. B-E) In the cinnamon-scented maze (B), WT and TK rats performed similarly during learning (C), demonstrated similar spatial knowledge when started from probe corners (D), and showed similar long-term memory for the route (E). F-I) In the peppermint-scented maze (F), WT rats were slower than TK rats during acquisition (G), took longer to reach the goal from different start positions (H), and showed poorer performance after a delay (I). J-M) In the third maze, scented with banana (J), WT and TK rats again showed similar learning (K), probe performance (L), and long-term memory (M). N-Q) During learning in the cinnamon (N-O) and peppermint-scented mazes (P-Q), WT and TK rats explored the mazes to the same extent and showed similar numbers of vicarious trial and error (VTE)-like behaviors. * p < .05 compared to WT rats. All graphs reflect means + SEM.
Latency to complete a maze may be misleading as a measure of learning, because it is altered by the speed of locomotion as well as accuracy. Therefore, we used additional analyses to investigate the way in which adult neurogenesis affect maze performance. The total number of maze intersections crossed was analyzed as an indicator of divergence from the optimal path, and vicarious trial-and-error (VTE) behavior was assessed as an indication of intentional analysis of potential paths during maze completion. (Fig. 2N–Q). In both the cinnamon and mint mazes, WT and TK rats crossed fewer intersections and displayed fewer VTE-like behaviors across training, both indicative of learning, with no genotype difference in either behavior (cinnamon VTE: main effect of genotype: F1,6 = 0.15, P = 0.71; main effect of trials: F13,78 = 5.60, P < 0.0001; cinnamon intersections: main effect of genotype: F1,6 = 0.03, P = 0.88; main effect of trials: F13,78 = 7.82, P < 0.0001; mint VTE: main effect of genotype: F1,6 = 0.008, P = 0.93; main effect of trials: F13,78 = 3.37, P = 0.0004; mint intersections: main effect of genotype: F1,6 < 0.001, P = 0.99; main effect of trials: F13,78 = 9.13, P < 0.0001; n = 4). The lack of genotype effects in these measures in the mint maze suggests that the longer time to complete the maze by WT rats reflects slower movement and/or more frequent pausing, rather than a change in the path followed. In other words, these findings suggest that theeffect of new neurons is on performance rather than learning.
Peppermint odor impairs flex maze performance in WT rats.
To determine whether WT rats completed the second maze more slowly because of peppermint odor or because this was the first time rats experienced a change in maze conformation (somewhat akin to a reversal), we trained a new cohort of WT and TK rats in the flex maze, substituting a new odor (rosemary) for the second maze in place of peppermint (Fig. 3A–C). This time, WT and TK rats showed no difference in completion time in acquisition or probe tests (training: main effect of genotype: F1,182 = 1.02, P = 0.33; main effect of trials: F13,182 = 27.79, P < 0.0001; probe trials: t14 = 0.34, P = .74; n = 8). In a separate cohort of rats, the third maze, which is typically the fastest to learn of the three, was scented with peppermint (Fig. 3D–F), and WT rats again reached the goal more slowly than TK rats during training and probe tests (training: main effect of genotype: F1,208 = 5.76, P = 0.03; main effect of trials: F13,208 = 5.82, P < 0.0001; probe trials: t16 = 2.53, P = .02; n = 8 WTs, 10 TKs). These findings point to the presence of mint odor as the key maze feature determining whether new neurons affect performance, rather than maze configuration or maze testing order.
Figure 3: Peppermint odor affects real-time performance in the flex maze only in neurogenesis-intact rats.

A) WT and TK rats were tested on three mazes without mint odor. B-C) When rosemary was used in place of peppermint, WT and TK rats showed similar learning (B) and probe trial performance (C). D) WT and TK rats were tested on three mazes, with peppermint in the final, most rapidly learned maze. E-F) When peppermint was used in the third maze, WT rats were slower than TK rats during learning (E) and probe trials (F). G) Rats were tested with peppermint present at different phases of the task. H-K) When navigating maze configuration A in the absence of peppermint odor (H), WT and TK rats learned similarly. However, when peppermint odor was used in the same maze, WT rats were slower than TKs during learning (I). During probe trials in this maze (J), WT rats only showed poorer performance when peppermint odor was used during both acquisition and probe trials (Mint Both), and not when peppermint was absent during the probe trials (Mint Train) or first introduced in the probe trials (Mint Probe). During long-term retrieval trials in this maze (K), WT rats were slower than TK rats when peppermint odor was present during acquisition and retrieval testing (Mint Train and Mint Both) but performed similarly when peppermint was used only during probe trials and the retrieval trial (Mint Probe). * p < .05 compared to WT rats. All graphs reflect means + SEM.
To test whether the presence of peppermint affects real-time behavior or has lasting effects on maze performance, we tested three groups of WT and TK rats with peppermint present during various training and/or testing trials (Fig. 3G–K). During training, when mint was absent (mint probe group, Fig. 2H), WT and TK rats learned the maze similarly (main effect of genotype: F1,182 = 0.17, P = 0.68; main effect of trials: F13,182 = 8.30, P < 0.0001; n = 7 for WT, n = 9 for TK). When mint was present (mint train and mint both groups combined, Fig. 3I), WT rats were slower to complete the maze (main effect of genotype: F1,403 = 4.44, P = 0.04; main effect of trials: F13,403 = 5.71, P < 0.0001; n = 14 for WT, n = 19 for TK). With this result, the effect of mint on task performance was seen in all three maze configurations and maze orders.
In probe trials (Fig. 3J), a significant mint group × genotype interaction indicated that WT rats were slower than TK rats only when mint was present during both training and probe testing (genotype × mint group interaction: F2,41 = 5.71, P = .007; Holm-Sidak post hoc test WT vs TK: Mint Both: t41 = 3.73, P = .002; Mint Train: t41 = 0.67, P = .75; Mint Probe: t41 = 0.15, P = .88, n = 6 for WT Both group and n = 7 for both WT Mint Train and Mint Probe groups, n = 10 for TK Mint Both and Probe groups, n = 9 for TK Mint Train group). When tested three weeks later on a retrieval test, with peppermint odor present for all groups (Fig. 3K), a genotype × mint group interaction showed that TK rats were faster to complete the peppermint maze in the Mint Train and Mint Both groups but not the Mint Probe group (main effect of genotype: F1,43 = 9.59, P = 0.003; genotype × mint group interaction: F2,43 = 4.50, P = .017; Holm-Sidak post hoc test WT vs TK: Mint Both: t43 = 3.29, P = .006; Mint Train: t43 = 2.74, P = .018; Mint Both: t43 = 0.64, P = .52, n = 7 for all WT groups, n = 10 for TK Mint Both and Probe groups, n = 9 for TK Mint Train group). As all three groups were tested in the presence of mint odor, this finding indicates that peppermint must be present during training sessions in order to affect the performance of WT rats during retrieval. This finding is consistent with what was observed in the probe trials and suggests that mint first introduced after the maze is well-learned has no effect.
Peppermint odor is aversive and provokes a corticosterone response in both WT and TK rats.
GFAP-TK-mediated ablation leaves olfactory sensory neuron and some periglomerular neuron production intact but inhibits the birth of granule cells in the olfactory bulb as well as in the hippocampus (Cummings et al., 2014; Snyder et al., 2016). To determine whether TK rats fail to detect or show aversion to peppermint odor due to olfactory system changes, we tested olfactory detection sensitivity in a 2-bottle preference test (Cheng et al., 2013) (Fig. 4A–E). Both WT and TK rats drank more plain than flavored water at 10−3 and 10−4 dilutions of peppermint flavor and showed no preference at a 10−5 dilution (10−3: main effect of peppermint: F1,16 = 5.66, P = 0.03; main effect of genotype: F1,16 = 0.02, P = 0.92; 10−4: main effect of peppermint: F1,16 = 6.01, P = 0.026; main effect of genotype: F1,16 = 0.16, P = 0.70; 10−5: main effect of peppermint: F1,16 = 0.17, P = 0.68; main effect of genotype: F1,16 = 0.07, P = 0.80; n = 8). These findings suggesting that the presence of new neurons has no effect on either the innate aversion to peppermint or ability to detect it. Unexpectedly, WT rats showed a preference for cinnamon-flavored water at a 10−4 but not 10−5 dilution, while TK rats showed no preference or aversion to cinnamon-flavored water at either concentration (10−4: genotype × cinnamon interaction: F1,18 = 3.99, P = 0.06; Holm-Sidak post hoc test: no odor vs cinnamon: WT: t18 = 2.59, P = .036; TK: t18 = 0.23, P = .82; 10−5: main effect of cinnamon: F1,18 = 0.006, P = 0.94; main effect of genotype: F1,18 = 6.88, P = 0.02; n = 10).
Figure 4: Peppermint odor is similarly aversive to both WT and TK rats.

A-C) Both WT and TK rats preferred unflavored water to peppermint-flavored water at 1:1,000 (A) and 1:10,000 dilutions (B), but showed no discrimination at 1:100,000 dilutions (C). D-E) WT, but not TK rats, preferred cinnamon-flavored water at a 1:10,000 dilution (D), but no rats displayed discrimination at 1:100,000 (E). F-G) Both WT and TK rats showed transient increases in corticosterone 15-minutes after peppermint odor exposure (F) but not after cinnamon odor exposure (G). H-J) In an open field with peppermint oil placed in one corner, WT and TK rats both avoided the peppermint-containing corner relative to the other 3 corners (H) and displayed similar overall locomotion (I) and anxiety-like behavior (J). * p < .05 odor or time point group (collapsed across genotype) different from treatment groups. † p < .05 compared to WT water group. All graphs reflect means + SEM.
To determine whether peppermint odor activates the HPA axis, we presented rats with the odor in their home cage for 5 minutes, collected blood 15 and 30 minutes later, and measured serum corticosterone (Fig. 4F–G). Both WT and TK rats showed a transient increase in corticosterone 15 minutes after peppermint odor presentation, which returned to baseline within 30 minutes of odor exposure (main effect of peppermint: F1,32 = 31.57, P < 0.0001; Holm-Sidak post hoc tests: 15 min vs control: t32 = 7.61, P < .0001; 15 min vs 30 min: t32 = 6.27, P < .0001; 30 min vs control: t32 = 0.24, P = .81; main effect of genotype: F1,32 = 0.44, P = 0.51; n = 8 for WT control, n = 10 for TK control, n = 6 for TK peppermint 15, n = 5 for TK peppermint 30 and for WT peppermint 15, n = 4 for WT peppermint 30). To compare the stress hormone response to peppermint odor and cinnamon odor, we collected blood from WT and TK rats 15 minutes after exposure to either odor. WT and TK rats both had transient corticosterone increase to peppermint odor but not cinnamon odor (main effect of odor: F1,22 = 13.58, P = 0.0001; Holm-Sidak post hocs: peppermint vs control: t22 = 4.66, P = .0004; peppermint vs cinnamon: t22 = 4.25, P = .0007; cinnamon vs control: t22 = 0.65, P = .52; main effect of genotype: F1,22 = 3.13, P = 0.09; n = 4 for WT and TK control, n = 5 for WT and TK for both peppermint and cinnamon odor), suggesting that only peppermint odor provokes a hypothalamic-pituitary-adrenal axis response indicative of stress, threat, or arousal.
To test whether peppermint odor differentially affects locomotion and avoidance behavior during exploration of a novel arena, we analyzed behavior of WT and TK rats for 10 minutes in an open field with a peppermint oil-laced cotton ball in one corner of the field (Fig 4H–J). Both WT and TK rats avoided the mint corner, spending more time in non-peppermint corners (main effect of corner: F1,15 = 43.40, P < 0.0001; main effect of genotype: F1,15 = 0.24, P = 0.63; n = 8 for WT, n = 9 for TK), suggesting that peppermint odor was aversive and altered exploration in this environment similarly for both genotypes. WT and TK rats did not differ in their general locomotion or the amount of time spent in the center of the open field, a measure of anxiety-like behavior (total distance: t15 = 0.03, P = .98; time in center: t15 = 0.15, P = .88), suggesting that WT and TK rats also showed similar exploration of a novel space in the presence of peppermint odor.
Discussion
We characterized the role of adult neurogenesis in a reward-motivated spatial maze task in the presence or absence of an aversive odor cue. These experiments showed that ongoing adult neurogenesis had no effect on learning or remembering complex and changing spatial environments or on navigation strategy in the flex maze. However, new neurons did alter maze performance in the presence of an aversive olfactory stimulus, peppermint odor. Rats lacking neurogenesis performed better than normal rats, reaching the goal more quickly, in the presence of mint, which appeared to result from a slowing effect of mint selectively in the WT rats. Peppermint odor altered performance in WT rats only when it was present and had been present during introduction to the maze. Further investigation found that control and neurogenesis-deficient rats detected, avoided, and activated the HPA axis to the same degree in response to peppermint, suggesting that the effect of new neurons on maze behavior was not related to changes in olfaction or odor aversiveness. Rats without ongoing adult neurogenesis did not differ from controls in the number of errors they made while completing the mazes, regardless of the presence of peppermint odor, indicating that the observed differences in maze behavior reflect altered performance, rather than learning, in the presence of an aversive threat cue.
Peppermint is aversive
We serendipitously found an effect of new neurons on maze behavior only in the presence of mint odor and then investigated further, observing that the mint odor was aversive to the rats. Two decades-old studies have suggested that peppermint is mildly aversive in adult and juvenile rats (Barnett and Spencer, 1953; Galef and Kaner, 1980), and popular internet wisdom touts mint odor as a rodent pest repellant. However, peppermint has been used as a neutral stimulus for odor discrimination learning and as a context cue in many studies (Sullivan and Leon, 1986; Eichenbaum et al., 1989; Hess et al., 1995; Schafe et al., 1999; Yuan et al., 2003; Amano et al., 2010; Cao et al., 2012), without noting its innate aversiveness to rodents. In the current study, peppermint was avoided by adult male rats, both in drinking water and during exploration of an open field, indicating that it has unconditioned aversive properties. Although we directly tested the aversiveness of only peppermint and cinnamon, none of the other five odorants used in our study (banana and rosemary – with lavender, apple, and clove used in unpublished experiments) produced a genotype effect on flex maze performance, or noticeably slowed maze performance, suggesting that peppermint is the only one of our odor cues that is aversive for adult rats. Given its aversiveness, the use of peppermint odor should be carefully considered when interpreting the results of sensitive behavioral tasks.
Critically for the current study, WT and TK rats showed similar abilities to detect peppermint and similar inclinations to avoid it. This lack of effect on olfactory responsiveness may be surprising, given that olfactory bulb adult neurogenesis, like dentate gyrus neurogenesis, is inhibited in TK mice and rats treated with valganciclovir (Snyder et al., 2016). However, it is consistent with previous studies showing that olfactory sensitivity and discrimination are relatively spared following inhibition of adult neurogenesis; impairments have been observed only in olfactory tests that require discrimination of mixtures of very similar odors (Imayoshi et al., 2008; Alonso et al., 2019). The genotype effects on behavior seen here are therefore unlikely to be caused by differences in the ability to sense or respond to peppermint odor but instead are likely to reflect differential behavior or attention toward the aversive stimulus in the face of a competing goal.
The increase in corticosterone release following mint exposure and the known effects of new neurons on stress responses (Snyder et al., 2011; Opendak et al., 2016; Schoenfeld et al., 2019) suggest that mint odor may alter behavior by acting as a stressor. However, corticosterone release occurs in response to emotional arousal regardless of whether the provoking stimulus is a stressor/threat (Woodson et al., 2003; Koolhaas et al., 2011). Stressors are generally studied in the context of their effects on physiology and behavior that outlast the presence of the threat, and this ability to produce lasting effects may be considered as a defining feature of a stressor. Aversive odors have been studied in this context (McGregor et al., 2002; Blanchard et al., 2003; Fendt and Endres, 2008), with several studies demonstrating that exposure to either cat fur odor or cat saliva odors alter emotional behaviors at later time points. In contrast, several other noxious odors that provoke corticosterone release, controversially including predator odor components such as TMT, fail to produce emotional conditioning and are therefore not considered by many investigators to provoke true fear or to act as stressors. As an edible plant odor with trigeminal activating properties, it seems likely that mint odor is similarly noxious or irritating rather than a fear-inducing stressor (Galliot et al., 2012). Although the presence or absence of lasting effects of mint odor exposure was directly investigated in the current study, we did observe that rats exposed to mint in training trials did not show slowed performance when mint was removed for probe trials later in the same session (“mint train” group), consistent with an absence of lasting emotional effect of mint. Earlier work has also found no effect of mint odor exposure on adult neurogenesis, consistent with a lack of lasting stress effect (Tanapat et al., 2001).
Even noxious odors that do not produce lasting stress effects stimulate defensive behaviors including avoidance, investigation, and escape (McGregor et al., 2002; Blanchard et al., 2003; Fendt and Endres, 2008). Aversive cues of various types are frequently used to motivate particular behaviors in learning tasks. However, in the current study, the aversive cue was not required for learning and instead affected behavior through actions as a distractor and/or by altering physiology. Both the hypothalamic pituitary adrenal axis and the sympathetic adrenomedullary (SAM) axis are activated by arousing stimuli, but glucocorticoid release is likely to be too slow to affect behavior in flex maze trials, which generally lasted less than two minutes (Tanapat et al., 2001; Koolhaas et al., 2011). SAM activation, one the other hand, is very rapid, and noradrenaline plays a role in attentional control (Thiele and Bellgrove, 2018), suggesting that physiological effects of this system may drive attention away from the maze goal and toward the arousing mint odor. More generally, the physiological arousal that is the earliest part of the stress response may act in part to drive attention toward the salient stimulus, which may or may not end up being a true threat, and away from the previously predominant goal or behavior.
Maze learning and memory are normal in the absence of new neurons
Impairments in spatial learning observed in numerous hippocampal lesion studies, as well as the existence of hippocampal place cells, make spatial tasks clear targets for investigating the function of adult neurogenesis. However, several studies have failed to find any effect of new neuron ablation in various spatial learning and memory tasks (Deng et al., 2010; Cameron and Glover, 2015). This lack of a critical function for new neurons in many spatial tasks is supported by the current findings showing that rats without adult neurogenesis learned all of the flex maze configurations as well as wildtype controls.
An early T-maze study of rats with extensive granule cell loss, following developmental irradiation, suggested that a key feature of affected tasks was their difficulty, as operationalized by the number of trials required to perform above chance (Gazzara and Altman, 1981). This idea is consistent with the focus on a role for adult neurogenesis in “pattern separation” tasks (Clelland et al., 2009; Sahay et al., 2011), which are difficult versions of discrimination tasks (Santoro, 2013). Most of these pattern separation studies have used spatial tasks (either context cues or navigation), but impairments in eyeblink conditioning tasks point to a difficulty-dependent role for new neurons in non-spatial tasks as well (Beylin et al., 2001; Shors et al., 2001).
The flex maze, as utilized in the current study, is more spatially complex than the T-maze, plus maze, radial arm maze, Morris water maze, or Barnes maze, as it required rats to navigate nine or more correct choice points in each of three potentially interfering maze configurations. However, it was clearly not more difficult than many spatial tasks, as rats were able to learn efficient routes to reward within 4–12 trials, even after changing to new configurations. Many spatial tasks are made difficult, independent of spatial complexity, by utilizing complex task rules or stringent success criteria, long delays or performance sequences that tax memory, or cues that are difficult to identify or distinguish. For example, the postnatal or adult neurogenesis-dependent versions of tasks in earlier studies required well over a hundred trials (Gazzara and Altman, 1981; Beylin et al., 2001). The ability of TK rats to learn the flex mazes normally is not therefore inconsistent with a role for new neurons in difficult tasks, but a real test of this relationship would require a much larger and more difficult labyrinth.
New neurons affect performance in the presence of an aversive stimulus
In contrast to their normal behavior in most of the mazes, rats without ongoing neurogenesis differed from controls in performance of the maze in the presence of an aversive odor cue. Performance of WT rats was altered by mint odor, while rats lacking neurogenesis behaved similarly in all three maze configurations regardless of the odor stimuli – despite showing normal sensitivity and aversion to the odor (see above). Maze performance was unaffected in WT rats when mint odor was present throughout training but absent during testing, suggesting that the aversive odor affected performance but not learning. Intriguingly, mint odor introduced for the first time during testing also failed to affect maze performance. This pattern suggests that the aversive stimulus was ignored by rats of both genotypes when performing a highly-trained behavior in an environment that was otherwise familiar and previously non-threatening, consistent with predator odor response in a foraging task (Stryjek et al., 2018). Alternatively, mint may become noxious only after repeated exposure, a possibility that is supported by the similar behavior of WT and TK rats in the first two learning trials (Fig 3C,I).
When behavior was affected by adult neurogenesis, the animals lacking new neurons were not impaired but were instead faster to reach the goal than the controls. Improved behavioral performance has previously been observed in a radial arm maze task in mice following irradiation to prevent adult neurogenesis (Saxe et al., 2007) and in Y maze and shuttle box tasks following hippocampal disruption (Isaacson et al., 1961; Gaffan et al., 2001). In one of these studies (Gaffan et al., 2001), enhanced attention to the key stimuli by animals with lesions appeared to be responsible for the improvement – an explanation that is consistent with decreased distractibility in runway and operant tasks observed following hippocampal lesions (Raphelson et al., 1965; Gustafson and Koenig, 1979) and decreased distractibility in TK rats in an orienting task (Weeden et al., 2019). A similar decrease in distraction by the mint odor stimulus likely explains the better maze performance observed in TK rats in the current study. Whether increased attention to particular stimuli improves behavior depends on the task design. Improved performance should occur in tasks like the current one, in which the odor stimulus is not critical for performance and can be considered as a potential distractor. The same increase in attention to stimuli, however, should have the opposite effect if the task required a change in behavior in response to a cue. The specific measures used to assess behavior are important as well: ablation of neurogenesis affected task performance as measured by the latency to reach the reward, but it had no effect on the number of errors made along the route.
Previous studies have shown that hippocampal lesions in macaques decrease distraction by aversive stimuli in a task requiring the monkeys to reach over neutral or potentially threatening stimuli to retrieve a reward. Normal monkeys had much slower responses in the presence of fearful versus neutral stimuli, whereas monkeys with hippocampal damage showed little change in the presence of the potential threat (Chudasama et al., 2008, 2009). These results mirror the current findings, in which the aversive mint odor was distracting to the intact, but not the neurogenesis-deficient, rats. In real-world situations, attention to novel neutral cues may or may not be beneficial, depending on whether the cues later prove valuable for predicting important outcomes or events. Attention to innately aversive stimuli is more important, as these cues are likely to signal potential danger. Shifting behavior away from foraging and exploration and towards self-preservation is adaptive in the face of a potential threat, but this shift decreases reward attainment and would be maladaptive if the novel neutral stimuli are unimportant. Although rats lacking adult neurogenesis performed better in our task by ignoring threat cues, they should be more likely to experience harm in natural situations if they ignore threat cues in novel environments. Taken together, these findings suggest that new neurons in the hippocampus are important for monitoring and adaptively incorporating information from highly salient cues into ongoing goal-directed behavior.
Acknowledgements:
This work was supported by the Intramural Program of the NIH, National Institute of Mental Health, ZIAMH002784 (H.A.C).
Data sharing statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alonso SB, Reinert JK, Marichal N, Massalini S, Berninger B, Kuner T, Calegari F. 2019. An increase in neural stem cells and olfactory bulb adult neurogenesis improves discrimination of highly similar odorants. Embo J 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Paré D. 2010. Synaptic correlates of fear extinction in the amygdala. Nature Neuroscience 13:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA, Spencer MM. 1953. Responses of wild rats to offensive smells and tastes. British Journal of Animal Behaviour. [Google Scholar]
- Basile BM, Templer VL, Gazes RP, Hampton RR. 2020. Preserved visual memory and relational cognition performance in monkeys with selective hippocampal lesions. Sci Adv 6:eaaz0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. 2001. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiology of learning and memory 76:447–461. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. 2003. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuro-psychopharmacology Biological Psychiatry 27:1177–1185. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. 2003. Transient expression of doublecortin during adult neurogenesis. The Journal of Comparative Neurology 467:1–10. [DOI] [PubMed] [Google Scholar]
- Cahill SP, Cole JD, Yu RQ, Clemans-Gibbon J, Snyder JS. 2018. Differential Effects of Extended Exercise and Memantine Treatment on Adult Neurogenesis in Male and Female Rats. Neuroscience 390:241–255. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Glover LR. 2015. Adult neurogenesis: beyond learning and memory. Annual review of psychology 66:53–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Schoenfeld TJ. 2018. Behavioral and structural adaptations to stress. Frontiers in neuroendocrinology 49:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao A, Yu L, Wang Y, Wang J, Yang L, Lei G-F. 2012. Effects of methylphenidate on attentional set-shifting in a genetic model of attention-deficit/hyperactivity disorder. Behavioral and brain functions : BBF 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Bai L, Steuer E, Belluscio L. 2013. Olfactory functions scale with circuit restoration in a rapidly reversible Alzheimer’s disease model. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:12208–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, Murray EA. 2009. Distinct contributions of the amygdala and hippocampus to fear expression. The European journal of neuroscience 30:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. 2008. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological psychiatry 63:1084–1091. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JD, Espinueva D, Seib DR, Ash AM, Cooke MB, Cahill SP, O’Leary T, Kwan SS, Snyder JS. 2020. Adult-born hippocampal neurons undergo extended development and are morphologically distinct from neonatally-born neurons Prolonged development of adult-born neurons. J Neurosci 40:5740–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. 2006. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behavioral and cognitive neuroscience reviews 5:41–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Snyder JS, Brewer M, Cameron HA, Belluscio L. 2014. Adult neurogenesis is necessary to refine and maintain circuit specificity. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:13801–13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. 2010. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature reviews Neuroscience 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL. 1996. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiology of learning and memory [Internet] 66:305–323. Available from: http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WNM-45MGVK3-7&_user=10843&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000000150&_version=1&_urlVersion=0&_userid=10843&md5=a084745d0cad9813c31f1710b1da1e8c [DOI] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, Lamarque S, Lemaire V, Oliet SHR, Piazza P-V, Abrous DN. 2007. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS biology 5:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest J-M, Koehl M, Ichas F, Giorgi FD, Costet P, Abrous DN, Piazza PV. 2008. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 3:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, MATHEWS P, Cohen NJ. 1989. Further-Studies of Hippocampal Representation During Odor Discrimination-Learning. Behavioral Neuroscience 103:1207–1216. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T. 2008. 2,3,5-Trimethyl-3-thiazoline (TMT), a component of fox odor - just repugnant or really fear-inducing? Neurosci Biobehav R 32:1259–66. [DOI] [PubMed] [Google Scholar]
- Fox GB, Fan L, LeVasseur RA, Faden AI. 1998. Effect of traumatic brain injury on mouse spatial and nonspatial learning in the Barnes circular maze. Journal of neurotrauma 15:1037–1046. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Bannerman DM, Warburton EC, Aggleton JP. 2001. Rats’ processing of visual scenes: effects of lesions to fornix, anterior thalamus, mamillary nuclei or the retrohippocampal region. Behavioural brain research 121:103–117. [DOI] [PubMed] [Google Scholar]
- Galef BG, Kaner HC. 1980. Establishment and maintenance of preference for natural and artificial olfactory stimuli in juvenile rats. Journal of comparative and physiological psychology 94:588–595. [DOI] [PubMed] [Google Scholar]
- Galinato MH, Takashima Y, Fannon MJ, Quach LW, Silva RJM, Mysore KK, Terranova MJ, Dutta RR, Ostrom RW, Somkuwar SS, Mandyam CD. 2018. Neurogenesis during Abstinence Is Necessary for Context-Driven Methamphetamine-Related Memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 38:2029–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliot E, Laurent L, Hacquemand R, Pourié G, Millot J-L. 2012. Fear-like behavioral responses in mice in different odorant environments: Trigeminal versus olfactory mediation under low doses. Behav Process 90:161–6. [DOI] [PubMed] [Google Scholar]
- Gazzara RA, Altman J. 1981. Early postnatal x-irradiation of the hippocampus and discrimination learning in adult rats. J Comp Physiol Psychol 95:484–495. [DOI] [PubMed] [Google Scholar]
- Gibson BM, Shettleworth SJ, McDonald RJ. 2001. Finding a goal on dry land and in the water: differential effects of disorientation on spatial learning. Behavioural brain research [Internet] 123:103–111. Available from: http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6SYP-433NPH8-B&_user=10843&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000000150&_version=1&_urlVersion=0&_userid=10843&md5=6266737c48e4a9960c2c7e2e97629e3f [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Kolb B, Whishaw IQ. 2000. A cautionary note regarding drug and brain lesion studies that use swimming pool tasks: partial reinforcement impairs acquisition of place learning in a swimming pool but not on dry land. Behavioural brain research [Internet] 112:43–52. Available from: http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6SYP-40HV0NW-5&_user=10843&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000000150&_version=1&_urlVersion=0&_userid=10843&md5=9ec3805e04ddfc73d200f1299569afa8 [DOI] [PubMed] [Google Scholar]
- Groves JO, Leslie I, Huang G-J, Mchugh SB, Taylor A, Mott R, Munafo M, Bannerman DM, Flint J. 2013. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS genetics 9:e1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson JW, Koenig LJ. 1979. Hippocampal function in distractibility and generalization: a behavioral investigation. Physiology & behavior 22:297–303. [DOI] [PubMed] [Google Scholar]
- Hess US, Lynch G, Gall CM. 1995. Changes in c-fos mRNA expression in rat brain during odor discrimination learning: differential involvement of hippocampal subfields CA1 and CA3. The Journal of neuroscience : the official journal of the Society for Neuroscience 15:4786–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. 2008. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nature Neuroscience 11:1153–1161. [DOI] [PubMed] [Google Scholar]
- Isaacson RL, Douglas RJ, Moore RY. 1961. The effect of radical hippocampal ablation on acquisition of avoidance response. Journal of Comparative and Physiological Psychology, 54(6), 625–628. [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, Squire LR, Gage FH. 2009. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learning & memory (Cold Spring Harbor, NY) 16:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaada BR, Rasmussen EW, Kveim O. 1961. Effects of hippocampal lesions on maze learning and retention in rats. Exp Neurol 3:333–355. [DOI] [PubMed] [Google Scholar]
- Karlsson R-M, Wang AS, Sonti AN, Cameron HA. 2018. Adult neurogenesis affects motivation to obtain weak, but not strong, reward in operant tasks. Hippocampus 28:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble DP. 1963. The effects of bilateral hippocampal lesions in rats. Journal of comparative and physiological psychology 56:273–283. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, Boer SFD, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. 2011. Stress revisited: a critical evaluation of the stress concept. Neuroscience and biobehavioral reviews 35:1291–1301. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser M-B, Moser EI. 2005. Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol 15:738–746. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Pan Y, Liu Y, Zhang Z, Wang Z. 2016. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Research 1644:127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. 2002. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res 129:1–16. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. 2006. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nature Neuroscience 9:729–731. [DOI] [PubMed] [Google Scholar]
- Morris R 1984. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods 11:47–60. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. 1987. The Effects of Changes in the Environment on the Spatial Firing of Hippocampal Complex-Spike Cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 7:1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research 34:171–175. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Gage FH. 1978. Hippocampal Connections and Spatial Discrimination. Brain Research 139:295–308. [DOI] [PubMed] [Google Scholar]
- Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, Gould E. 2016. Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:7027–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. 1990. The Firing of Hippocampal Place Cells in the Dark Depends on the Rats Recent Experience. The Journal of neuroscience : the official journal of the Society for Neuroscience 10:2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphelson AC, Isaacson RL, Douglas RJ. 1965. The effect of distracting stimuli on the runway performance of limbic damaged rats. Psychonomic Science [Internet] 3:483–484. Available from: message:%3C3290ff98-8770-4774-99f1-e5ce7d4333ae@CESEDGE01.nih.gov%3E [Google Scholar]
- Redish AD. 2016. Vicarious trial and error. Nature reviews Neuroscience 17:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, Kageyama R. 2011. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proceedings of the National Academy of Sciences of the United States of America 108:8479–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A 2013. Reassessing pattern separation in the dentate gyrus. Frontiers in Behavioral Neuroscience 7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Piatti VC, Hwaun E, Ahmadi S, Lisman JE, Leutgeb S, Leutgeb JK. 2018. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nature Neuroscience 21:258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. 2006. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America 103:17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. 2007. Paradoxical influence of hippocampal neurogenesis on working memory. Proceedings of the National Academy of Sciences of the United States of America [Internet] 104:4642–4646. Available from: http://www.pnas.org/content/104/11/4642/suppl/DC1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, Ledoux JE. 1999. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learning & memory (Cold Spring Harbor, NY) 6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. 2012. Stress, stress hormones, and adult neurogenesis. Experimental neurology 233:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rhee D, Martin L, Smith JA, Sonti AN, Padmanaban V, Cameron HA. 2019. New neurons restore structural and behavioral abnormalities in a rat model of PTSD. Hippocampus 29:848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. 2001. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Cameron HA. 2012. Could adult hippocampal neurogenesis be relevant for human behavior? Behavioural brain research 227:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. 2009. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:14484–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Grigereit L, Russo A, Seib DR, Brewer M, Pickel J, Cameron HA. 2016. A Transgenic Rat for Specifically Inhibiting Adult Neurogenesis. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. 2005. A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. 2011. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryjek R, Mioduszewska B, Spaltabaka-Gędek E, Juszczak GR. 2018. Wild Norway Rats Do Not Avoid Predator Scents When Collecting Food in a Familiar Habitat: A Field Study. Sci Rep-uk 8:9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. 1986. Early Olfactory Learning Induces an Enhanced Olfactory-Bulb Response in Young-Rats. Brain research Developmental brain research 27:278–282. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. 2001. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. The Journal of Comparative Neurology 437:496–504. [DOI] [PubMed] [Google Scholar]
- Thiele A, Bellgrove MA. 2018. Neuromodulation of Attention. Neuron 97:769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden CSS, Mercurio JC, Cameron HA. 2019. A role for hippocampal adult neurogenesis in shifting attention toward novel stimuli. Behavioural brain research 376:112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Pasztor TJ. 2000. Rats alternate on a dry-land but not swimming-pool (Morris task) place task: implications for spatial processing. Behavioral Neuroscience 114:442–446. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. 1996. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiology & behavior [Internet] 60:1191–1197. Available from: http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T0P-3WBNMSW-1&_user=10843&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000000150&_version=1&_urlVersion=0&_userid=10843&md5=2772393102075d32e8b2407ece58fda8 [DOI] [PubMed] [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. 2003. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learning & memory (Cold Spring Harbor, NY) [Internet] 10:326–336. Available from: http://learnmem.cshlp.org/content/10/5/326.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Leutgeb S. 2020. Hippocampal firing rates count. Nat Neurosci 23:597–599. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Darby-King A, Neve RL, McLean JH. 2003. Early odor preference learning in the rat: bidirectional effects of cAMP response element-binding protein (CREB) and mutant CREB support a causal role for phosphorylated CREB. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:4760–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]


