Abstract
Biomineralization of enamel, dentin, and bone involves the deposition of apatite mineral crystals within an organic matrix. Bone and teeth are classic examples of biomaterials with unique biomechanical properties that are crucial to their function. The collagen-based apatite mineralization and the important function of noncollagenous proteins are similar in dentin and bone; however, enamel is formed in a unique amelogenin-containing protein matrix. While the structure and organic composition of enamel are different from those of dentin and bone, the principal molecular mechanisms of protein–protein interactions, protein self-assembly, and control of crystallization events by the organic matrix are common among these apatite-containing tissues. This review briefly summarizes enamel and dentin matrix components and their interactions with other extracellular matrix components and calcium ions in mediating the mineralization process. We highlight the crystallization events that are controlled by the protein matrix and their interactions in the extracellular matrix during enamel and dentin biomineralization. Strategies for peptide-inspired biomimetic growth of tooth enamel and bioinspired mineralization of collagen to stimulate repair of demineralized dentin and bone tissue engineering are also addressed.
Keywords: extracellular matrix (ECM), hydroxyapatite, amelogenin, collagen(s), noncollagenous proteins, mineralization
Introduction
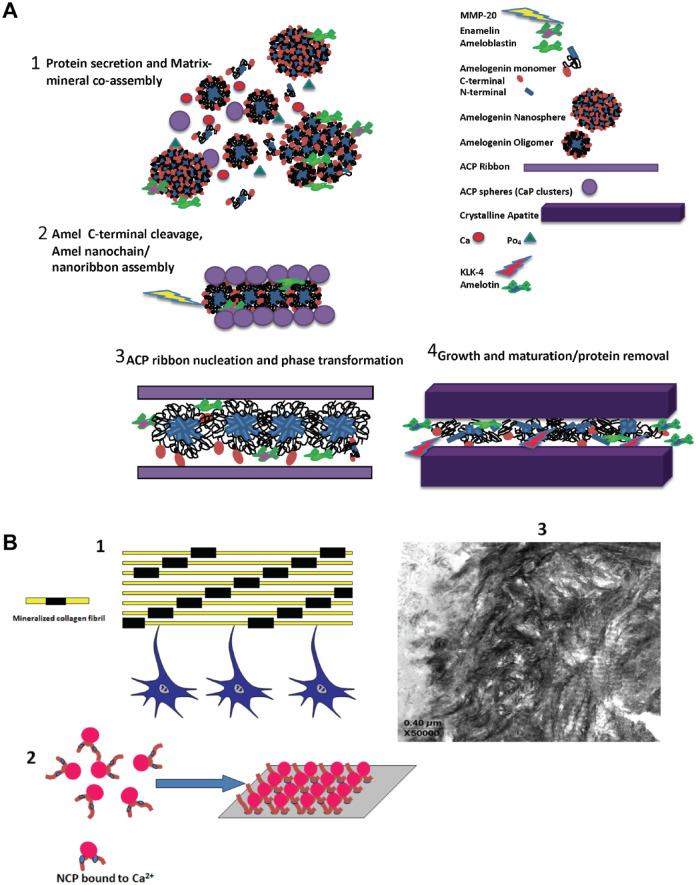
Biomineralization of enamel and dentin includes a complex cascade of events regulated by cells expressing matrix proteins that act as crystallization promoters or inhibitors (George and Veis 2008; Moradian-Oldak 2012). The plethora of matrix proteins synthesized by ameloblasts and odontoblasts depicts the complexity involved during the formation of these tissues. The formation of tooth enamel takes place in a confined extracellular environment between dentin and moving ameloblast cells. The amelogenin-rich extracellular matrix is being continuously secreted and assembled with nonamelogenins and the mineral to result in a “forming mineralized matrix” rather than the “preformed matrix” that has been defined for other mineralizing tissues such as dentin (Fig. 1A). In the matrix-mediated scheme for dentin formation, the self-assembled collagen fibril provides the template for the specific localization of the noncollagenous proteins at the gap zones, where the nucleating crystal is initially deposited, and guides further crystal growth (Fig. 1B). Thus, the collagen template provides the space for both the noncollagenous proteins and the mineral. Enamel and dentin have different mechanical properties fulfilling complementary functions in maintaining the mechanical stability of the tooth. These 2 biomineralizing systems are structurally different, and their tissue composition and extracellular matrix (ECM) macromolecular components are distinct. However, the principal molecular mechanisms of supramolecular organization, protein assembly, protein–protein interaction, and control of mineral nucleation, growth, and organization by the components of the ECM are common among enamel and dentin.
Figure 1.
Protein–mineral assembly in enamel and dentin. (A) A proposed conceptual model for protein-mediated enamel biomineralization in a continuously forming amelogenin-based matrix. (1) Amelogenin molecules are secreted in monomeric or oligomeric forms (dimers, trimers, hexamers) and spontaneously assemble into transient nanospheres (Moradian-Oldak 2012; Shaw et al. 2020). Amelogenin-mineral coassembly stabilizes amorphous calcium phosphate (ACP) particles (Wiedemann-Bidlack et al. 2011). Nanospheres may contain enamelin and ameloblastin molecules (Fan et al. 2009; Mazumder et al. 2014; Bapat et al. 2020). (2) Amelogenin C-terminus is cleaved by MMP-20 (Lu et al. 2008). Oligomers spontaneously assemble into elongated nanochains, promoting oriented nucleation of calcium phosphate clusters (Moradian-Oldak 2012). A model for nanoribbon-like structures has been also proposed and might be formed at this stage (Engelberth et al. 2018). (3) Calcium phosphate clusters are fused to form elongated ACP ribbons and later transform to crystalline apatite. ACP–apatite transformation is facilitated following the C-terminal cleavage of amelogenin. Amelotin, which is expressed during maturation stage, facilitates crystal growth. (4) With the cleavage of the amelogenin N-terminus by MMP-20, the disassembly of the nanospheres is promoted, and further degradation of amelogenin and other matrix proteins takes place in the maturation stage by the serine proteinase (KLK-4), allowing further growth of apatite mineral in thickness. (B) Dentin and bone tissues are formed by matrix-mediated mineralization: (1) Cartoon showing dentin and bone formation is a cellular event. Components secreted by osteoblasts/odontoblasts are responsible for calcified tissue formation. Yellow bars represent collagen fibrils; black bars represent calcium phosphate (CaP) mineral in the gap region. (2) Cartoon showing that noncollagenous proteins (NCPs) have the ability to bind Ca2+. These nanoclusters presumably localize on self-assembled templates such as collagen and provide the structural surface for stereospecific mineral nucleation. (3) Representative unstained TEM image of an unerupted bovine molar showing mineralized collagen fibrils.
Enamel Biomineralization
Enamel formation involves a series of programmed cellular, biochemical, and chemical events that control mineral nucleation, growth, and organization in a confined biological microenvironment (Fig. 2A). The key to achieving the organized architecture of enamel lies not only in the orchestrated movement and precise secretory activities of ameloblast cells but also in the way ECM components such as proteins and enzymes interact with each other as well as with mineralizing ions, cells, and the forming hydroxyapatite mineral (Smith 1998; Lu et al. 2008; Moradian-Oldak 2012).
Figure 2.
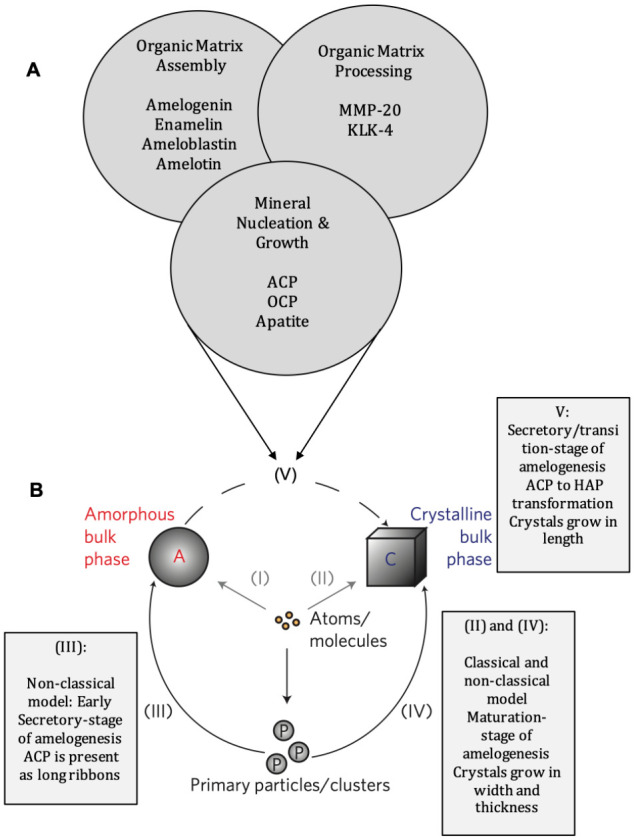
Extracellular events in enamel biomineralization. (A) Chemical and biochemical events that take place in the enamel extracellular matrix simultaneously, highlighting the main structural proteins and proteinases (MMP-20 and KLK-4) and the calcium phosphate precursor phases. (B) Nucleation pathways from ions and clusters of ions to the bulk crystal that can occur in all stages of enamel biomineralization. Both clusters and free ions can nucleate the crystalline bulk phase either directly or through an amorphous precursor phase. The model is a schematic representation of crystallization pathways proposed based on experimental data for magnetite crystals grown in solution (reproduced with permission from De Yoreo 2013). These pathways are common among other biomineralizing systems containing calcium carbonate or calcium phosphate (DeYoreo et al. 2015). (I) Atoms (molecules) join to form an amorphous bulk phase. (II) The crystal phase is formed directly from atoms (molecules). (III) Attachment of primary amorphous or crystalline particles (clusters) leads to the amorphous phase. (IV) Attachment of primary amorphous or crystalline particles (clusters) leads to the crystalline phase. (V) Transformation of an amorphous precursor phase to the bulk crystalline phase. Rectangles represent crystallization pathways that can occur at different stages of enamel formation.
In the secretory stage, ameloblasts play a central role in the secretion of scaffold proteins, in the transport of essential ions for mineralization, and in establishing the rod–interrod boundary via the Tomes’s processes (Simmer et al. 2010; Lacruz et al. 2017). Matrix proteins are continuously secreted, immediately assembled, and processed by MMP-20 while promoting mineralization (Lu et al. 2008) (Fig. 1A). The mineral is nucleated at the mineralization front, either on the already formed dentin crystallites or within the enamel matrix, and the crystals mainly grow in length (Simmer et al. 2010). Major gain in mineral content of enamel takes place during the maturation stage, when crystals grow in thickness and width (Robinson et al. 1988). The protein matrix is then rapidly degraded by KLK-4 and is eventually removed from the extracellular space to allow completion of mineralization (Fig. 1A). Although reduced by about 25% in number, ameloblast cells continue their critical role during the maturation stage by facilitating massive calcium and phosphate ion transport, regulating pH, and removing unwanted organic debris. Once all the ingredients for continuing apatite crystal growth are supplied to the extracellular matrix, control of crystal growth and modulation of crystal morphology may well be governed by interactions between the growing crystals and the environment, including other ions, proteolytic products, and polypeptides.
Experimental evidence has demonstrated that crystallization mechanisms are common among biomineralizing systems and most likely governed by a process called “particle attachment” (Fig. 2B, III and IV) (De Yoreo 2013; De Yoreo et al. 2015). This means that the crystals form by the addition and attachment of particles that range from multi-ion complexes to fully formed nanoparticles. Crystallization by particle attachment (CPA) involves nonclassical and classical models of crystallization, both of which can be influenced by the presence of proteins. The nonclassical pathway is achieved via the assembly of a variety of primary solid particles (P in Fig. 2B), in contrast to the classical pathway, which involves stepwise addition of ions or molecules to the growing crystal surfaces (II in Fig. 2B).
The nonclassical model of crystallization is supported by the presence of ribbon-like amorphous calcium phosphate (ACP) at the mineralization front (Beniash et al. 2009). This in vivo observation has been supported by in vitro crystallization studies that demonstrated the ability of enamel proteins such as amelogenin and enamelin to stabilize the ACP mineral phase and inhibit its transition to crystalline apatite (Friddle et al. 2011; Wiedemann-Bidlack et al. 2011; Tao et al. 2018). Evidence for nucleation and growth by particle attachment was recently supported in a mouse molar model (Jokisaari et al. 2019).
The rapid growth of the enamel mineral in thickness and width during the maturation stage and increase of mineral content from 30% to >95% may well be due to a combination of both classical and nonclassical crystallization. In vitro evidence for the latter comes from experiments that demonstrated the ability of enamel proteins amelogenin and enamelin to affect the morphology of calcium phosphate crystals (Iijima and Moradian-Oldak 2005; Iijima et al. 2010). The in vitro finding that domains within amelogenin have higher binding energy to certain crystal planes of apatite also support the classical theory of crystal formation and can explain the rapid growth of apatite crystals in thickness after the proteins are removed from the matrix (Friddle et al. 2011).
It is therefore possible that both classical and nonclassical pathways to crystallization dominate during different stages of enamel formation. In both, proteins and/or their fragments will interact with ions, lowering the barrier to nucleation by reducing interfacial energy (heterogeneous nucleators), modulating the kinetics of nucleation (stabilizing prenucleation clusters and inhibiting phase transformation), and finally modulating crystal growth (inhibiting or promoting growth of certain crystal faces).
The matrix composition of the developing enamel matrix is diverse, and the patterns of protein expression, secretion, processing, and assembly are complex and dynamic. It is now well known that the major structural proteins such as amelogenin, enamelin, ameloblastin, and amelotin, as well as proteinases, are essential for the formation of enamel with normal structure and are all directly or indirectly involved in controlling mineral assembly (Lacruz et al. 2017). However, the detailed molecular mechanisms underlying matrix–mineral assembly, control over the stability of calcium phosphate precursors and early nucleation events, and control over enamel apatite morphology and organization remain to be further elucidated.
Dentin Biomineralization
Like enamel, dentin is a hierarchically organized nanostructured biological composite formed by matrix-mediated biomineralization, as the cellular components control mineral deposition (George and Veis 2008). The consensus is that both matrix macromolecules and tissue architecture are of vital importance in dictating specific sites for mineral nucleation. Specifically, the organism controls the nature, crystal growth, crystal orientation, crystal morphology, and size regulation of the mineral by creating closed collagen compartments or defined channels in which the mineral crystal forms (Veis and Dorvee 2013). Thus, minerals of biogenic origin are created at near-ambient temperatures and pressures, with the matrix dictating the formation of unique patterns. Odontoblasts and osteoblasts, which are the principal cells for dentin and bone formation, are responsible for the synthesis and secretion of both the scaffolding proteins and the specialized proteins that are involved in crystal nucleation and growth (Fig. 1B) (Goldberg et al. 2008). Classical ion-mediated crystal nucleation theory was initially put forth for collagen intrafibrillar mineralization in bone and dentin. Recent investigation of the mechanism of particle-mediated crystallization suggests that prenucleation clusters are formed by the sequestration of calcium and phosphate ions by noncollagenous protein (NCPs) or their analogues. Aggregated clusters infiltrate into the collagen fibrils and undergo self-assembly and crystallographic alignment within the gap zone of the assembled collagen molecules (Ma et al. 2021).
Role of Intrinsically Disordered Proteins in Enamel and Dentin Biomineralization
Several of the proteins involved in biomineralization of enamel (i.e., amelogenin, ameloblastin) and dentin (i.e., small integrin-binding ligand, N-linked glycoproteins [SIBLINGs]) have been classified as intrinsically disordered proteins (IDPs) or have regions that do not fold into a 3-dimensional structure, which are referred to as intrinsically disordered protein regions (IDPRs) (Fisher and Fedarko 2003; Delak et al. 2009; Estroff and Cohen 2011; Veis and Dorvee 2013; Boskey and Villarreal-Ramirez 2016; Tavafoghi and Cerruti 2016; Wald et al. 2017).
IDPs are characterized by conformational flexibility as their structures are variable and do not fold into the conventional secondary structures seen in structural proteins. Such structural plasticity enables them to interact with different targets and engage in a wide array of biological functions that cannot be performed by structured proteins (Kalmar et al. 2012). For example, the conformational flexibility of dentin matrix protein 1 (DMP1) acts as a driving force in promoting binding to Ca2+ and initiating the process of mineral nucleation and subsequent formation of amorphous calcium phosphate mineral (He et al. 2003). Protein–protein interactions involving IDPs are important for biomineralization. The ability of amelogenin and ameloblastin to self-assemble and interact with each other, with cell membrane, and with the mineral is associated with their IDP character and structural flexibility (Fan et al. 2009; Delak et al. 2009; Bapat et al 2020). Moreover, intrinsic disorder is a unique structural feature that enables these proteins to participate in many signaling events.
Interactions of Matrix Proteins in Enamel
Amelogenin Is Essential for Enamel Biomineralization but Does Not Function Alone
Among all the structural proteins identified to date, amelogenin is the most abundant (>90%) and has been the most investigated (Shaw et al. 2020). The N- and C-terminal domains of amelogenin contain residues that are highly conserved, and the most critical motifs for amelogenin assembly and mineral binding have been identified within these domains. Amelogenin is not glycosylated but phosphorylated at a single Ser position. Phosphorylation plays important roles in regulation of calcium phosphate crystal formation (Wiedemann-Bidlack et al. 2011). In humans, mutations in the AMELX gene can affect amelogenin protein assembly, disturb interactions with ions or mineral, inhibit proteolytic digestion, and eventually lead to hypoplasia and/or hypomaturation phenotypes known as amelogenesis imperfecta (AI) (Wright et al. 2003). Enamel without amelogenin is thin with disorganized prisms, shorter crystals, and unwanted mineral phases such as octacalcium phosphate (Hu et al. 2016).
The self-assembly of amelogenin into oligomers, nanospheres, and nanoribbons has been extensively studied and reviewed but is not the subject of this review. Amelogenin self-assembly into hierarchical structures is highly dependent upon solution pH; protein, calcium, and phosphate ion concentration; and ionic strength (Engelberth et al. 2018). For more details on amelogenin structure and assembly, please refer to the recent review by Shaw et al. (2020).
In vitro experimental data collectively show that amelogenin has the potential to bind to an apatite surface (Friddle et al. 2011; Shaw et al. 2020), promote calcium phosphate nucleation, stabilize ACP in solution and inhibit its transformation to apatite (Wiedemann-Bidlack et al. 2011), promote elongated and organized growth of apatite crystals in solution (Moradian-Oldak 2012), and affect the morphology of calcium phosphate crystals by interacting with certain crystal surfaces (Iijima and Moradian-Oldak 2005).
Amelx null mice develop enamel with a disorganized microstructure, but the fact that enamel still forms and ribbon-like calcium phosphate crystals are still present is an indication that other enamel proteins such as enamelin and ameloblastin are intimately involved in many aspects of mineral formation, including nucleation, phase transformation, and organized growth. Our recent in vitro and in vivo studies have demonstrated that amelogenin interacts directly with enamelin and with ameloblastin (Fan et al. 2009; Gallon et al. 2013; Bapat et al. 2020). These studies support the notion of cooperative mechanisms between enamel matrix proteins in controlling processes of crystal nucleation and growth.
Ameloblastin–Amelogenin Colocalization and Coassembly
Ameloblastin, the second most abundant proline-rich enamel matrix glycoprotein, is intrinsically disordered. It is secreted together with amelogenin from secretory vesicles and is rapidly processed by MMP-20 (Zalzal and Nanci 1998) (Fig. 3A). The hydrophobic N-terminal cleavage products accumulate in the “sheath” space throughout the enamel layer while the calcium-binding C-terminal cleavage products accumulate on the enamel rods (Uchida et al. 1997). Ameloblastin is considered critical for proper enamel formation because a severely hypoplastic enamel layer appears on the teeth of Ambn mutant mice (Fukumoto et al. 2004). Its high calcium binding affinity is an indication that ameloblastin might be directly involved in controlling crystal formation (Vetyskova et al. 2020).
Figure 3.
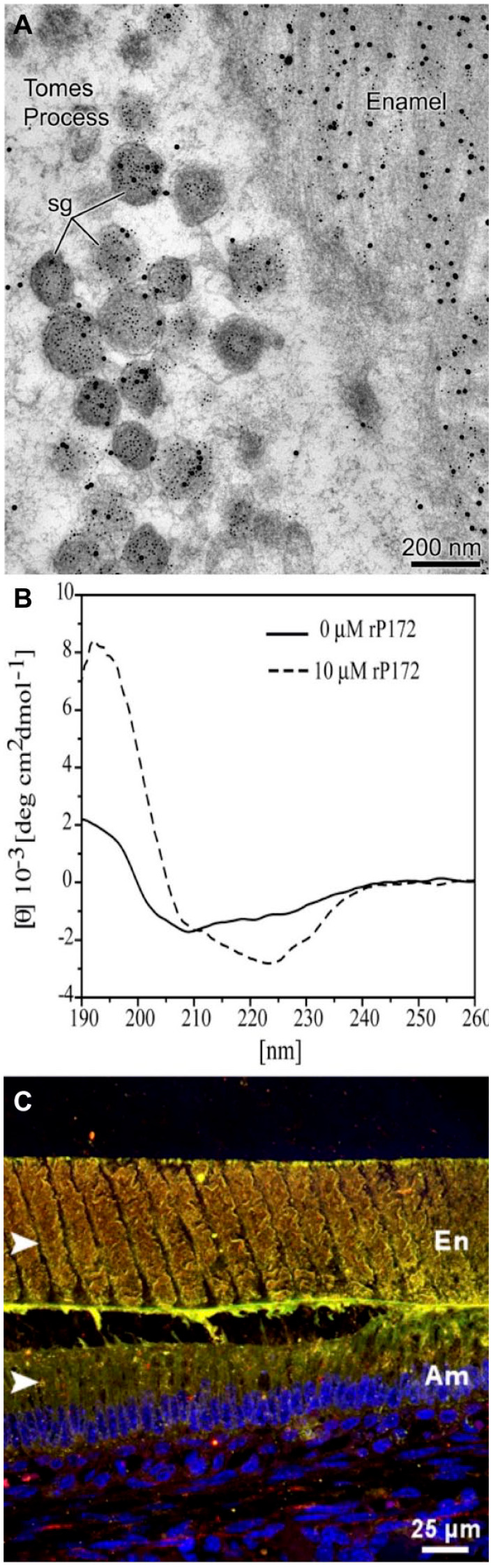
Amelogenin–ameloblastin common secretory pathway, interaction, and colocalization. (A) Transmission electron micrograph of rat incisors’ secretory-stage enamel showing that the number of amelogenin- and ameloblastin-containing secretory granules (sg) in Tomes’s processes increases dramatically. While most of them contain both ameloblastin (small gold particles) and amelogenin (large gold particles), some granules label only ameloblastin (reproduced with permission from Zalzal et al. 2008). (B) Circular dichroism of recombinant amelogenin rP172 showing secondary structural change following addition of ameloblastin (Mazumder et al 2014). (C) Immuno-colocalization patterns (yellow) of ameloblastin (red) and amelogenin (green) within the forming enamel layer of mouse mandibular molar sections at day P5 representing transition stage. Am, ameloblasts; En, enamel; TP, Tomes’s processes (Bapat et al. 2020).
Evidence that hints at an interaction between amelogenin and ameloblastin begins at the secretory stage of enamel formation when these proteins are cosecreted through the same vesicles (Zalzal et al. 2008) (Fig. 3A) and continues into maturation stage when their N-terminal fragments colocalize around molar enamel rods (Mazumder et al. 2014). We have demonstrated direct interactions between recombinant amelogenin and ameloblastin in vitro by conformational changes in amelogenin’s secondary structure as the result of adding ameloblastin (Fig. 3B). We have provided in vivo evidence of amelogenin–ameloblastin colocalization using immunohistochemical methods (Fig. 3C). Using coimmunoprecipitation, we showed that ameloblastin binds to amelogenin via its previously identified, highly conserved Y/F-x-x-Y/L/F-x-Y/F self-assembly motif at the N-terminus of the region encoded by exon 5 (Wald et al. 2017; Bapat et al. 2020). Amelogenin and ameloblastin self-assembly motifs are therefore involved in their coassembly and can form heteromolecular assemblies in enamel extracellular space (Fig. 1A, step1).
Enamelin–Amelogenin Interactions Promote Crystal Nucleation and Growth
Enamelin is a large phosphorylated glycoprotein (186 kDa) that constitutes less than 5% of the ECM. Enamelin is also processed by proteinases immediately upon secretion, yielding the major and most stable cleavage product, the 32 kDa enamelin. Mutations to the ENAM gene have drastic consequences for human enamel formation (Hu and Yamakoshi 2003). The abnormally thin and disorganized enamel in AI patients suggests that enamelin is required to drive crystal formation, achieve structural organization of the apatite prisms, and even develop optimal enamel thickness. No true enamel or apatite mineral ribbons are formed in Enam-null mice (Hu et al. 2008).
The interaction between amelogenin and enamelin has an impact on amelogenin self-assembly and on the secondary structures of both proteins (Fan et al. 2009). Colocalization between amelogenin and enamelin has been demonstrated at the early stage of enamel formation (Fig. 4A) (Gallon et al. 2013). Addition of the 32 kDa enamelin to amelogenin affects ACP–apatite phase transformation and enhances calcium phosphate nucleation rates in a dose-dependent manner (Tao et al. 2018) (Fig. 4B). Synergistic effects between the 2 proteins on modulating octacalcium phosphate (OCP) crystal morphology have been also reported (Fig. 4C). The concept of enamelin dose-dependency has been demonstrated in transgenic mouse models, in which controlling the expression levels of the enamelin gene revealed that only an optimal quantity of enamelin will lead to normal enamel formation (Hu et al. 2008). Our recent findings that these proteins interact and colocalize during the early stage of enamel formation supports the notion that the stability of the ACP mineral phase in the early stage of enamel formation is not controlled merely by amelogenin but rather through cooperative function between amelogenin and enamelin (see Fig. 1A).
Figure 4.
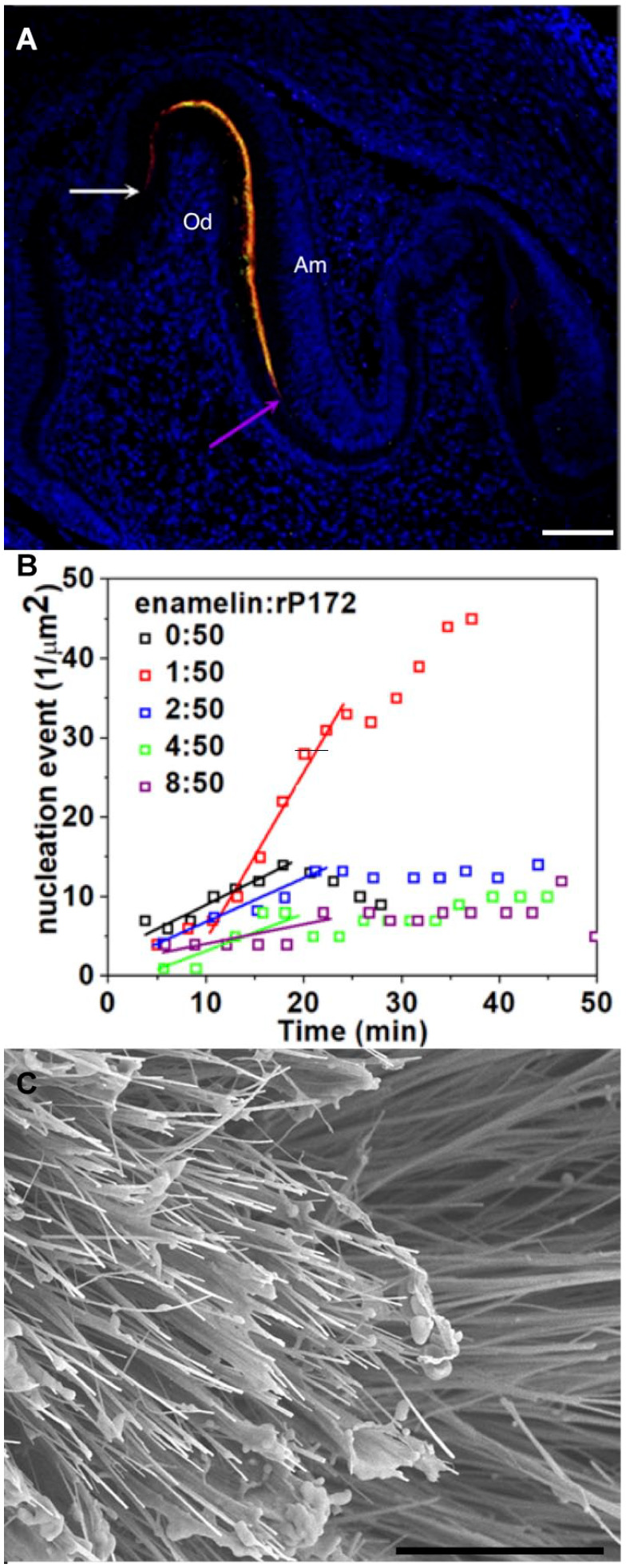
Amelogenin–enamelin interaction, colocalization, and control of calcium phosphate nucleation and growth. (A) Confocal image of mouse mandibular first molar showing immunofluorescence of amelogenin (green) and enamelin (red) at postnatal day 2. Colocalization of amelogenin and enamelin is revealed by overlapping signals resulting in yellow staining. Nuclei are stained with DAPI (blue) (Gallon et al 2013). White and purple arrows show the molar cusp and molar fissure between 2 cusps, respectively. Am, ameloblasts; Od, odontoblasts. Scale: 10 μm. (B) Dependence of the number of calcium phosphate nuclei on time (t) at 5 different enamelin/amelogenin (rP172) ratios obtained and detected from the atomic force microscopy. Note that the highest rate of nucleation was achieved at the optimal ratio of 1:50 enamelin/amelogenin (Tao et al. 2018). (C) An scanning electron microscopy image of octacalcium phosphate (OCP) crystals with high aspect ratios grown in 10% recombinant amelogenin rP148 with 40 μg/mL 32 kDa enamelin. Addition of enamelin to amelogenin enhanced the potential of amelogenin to stabilize the amorphous calcium phosphate (ACP) transient phase. The ratio of enamelin and amelogenin was crucial for stabilization of ACP and the growth of OCP crystals with larger aspect ratio. Scale: 10 μm (Iijima et al. 2010).
Matrix Proteins in Dentin
Type I Collagen Forms a Dynamic and Interactive Template for Mineral Nucleation
Collagen fibril formation is an entropy-driven protein self-assembly process. Mineralization of self-assembled type I collagen is central to the function of bone and dentin. Several recent studies have shown that type I collagen forms a dynamic and 3-dimensional interactive scaffold for ordered mineral deposition (Nudelman et al. 2010; Wang et al. 2012; Yao et al. 2019). During fibrillogenesis, the ~300-nm rod-like collagen molecules self-assemble in a staggered manner, leaving gaps between the molecular ends. The gaps are registered in packed fibril-producing channels that run transverse to the fibril axis, and their reduced density produces the characteristic 67-nm periodicity in the fibrils. Due to the specific binding of the noncollagenous proteins in the gap regions of the collagen fibrils, the mineral crystals begin to grow initially in the gap spaces (Fig. 1B). Subsequently, the initial precipitated amorphous nanoclusters of Ca and P transform to needle-like crystals of hydroxyapatite, which eventually coalesce to form plate-like crystals and fill the gap space. The mineral crystals also form an interconnected mineral network with a helical morphology shaped by the collagen matrix. The unique feature of the deposited mineral crystals is that their c-axes align with the long axis of the collagen fibrils (Fig. 5). Thus, the alignment of the collagen fibrils is a requirement to promote long-range order and promote intrafibrillar mineralization (Yao et al. 2019). Besides the direct role of collagen in mineralization, it also directly interacts with many growth factors in the ECM, which in turn dictate cellular responses.
Figure 5.
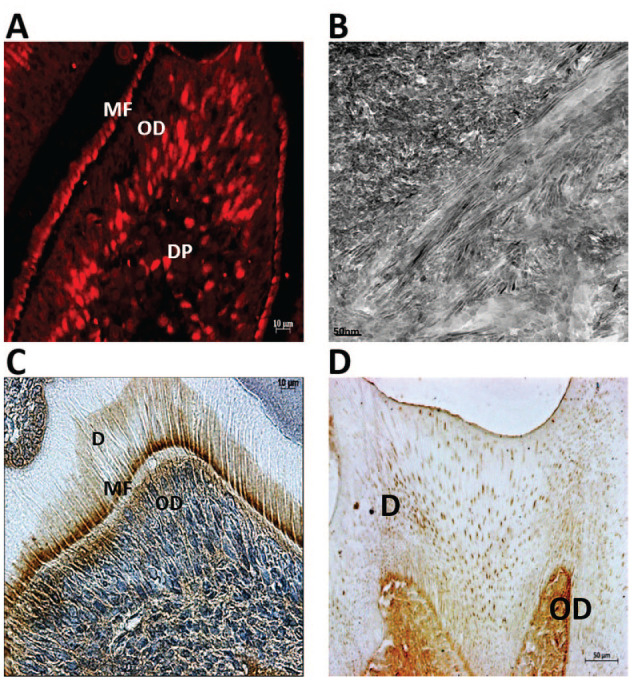
Role of noncollagenous proteins in mineral deposition. (A) Immunohistochemical staining with anti-DPP antibody shows specific localization of dentin phosphophoryn (DPP), a small integrin-binding ligand, N-linked glycoprotein (SIBLING) protein in the odontoblasts (ODs) and at the mineralizing front (MF) where the first mineral crystals are deposited during dentin formation in the molar of a 5-d-old mouse. (B) Demineralized dentin slices were trypsin-treated to remove noncollagenous proteins (NCPs) and incubated with dentin matrix protein 1 (DMP1) and subjected to in vitro nucleation assay. Representative unstained transmission electron microscopy (TEM) image shows that DMP1, a SIBLING protein in bone and dentin matrices, facilitates the deposition of oriented, needle-shaped calcium phosphate crystal. Alignment and orientation of the crystals were also dictated by the intact underlying collagen matrix. (C) Immunohistochemical staining with anti–type II TGF-β receptor interacting protein 1 (TRIP1) antibody shows specific localization of TRIP1, a newly identified NCP in dentin matrix. Note the presence of TRIP1 in the ODs, MF, and the newly formed dentin matrix in the molar of a 7-d-old mouse. (D) Immunohistochemical staining with anti–glucose regulatory protein 78 (GRP78) antibody shows specific localization of GRP78, a newly identified NCP in dentin matrix. Note the presence of GRP78 in the ODs and throughout the dentin matrix (D) in a 2-mo-old mouse molar.
NCPs
NCPs synthesized by osteoblasts and odontoblasts are responsible for mineral nucleation, crystal growth, hydroxyapatite crystallinity, inhibition of nonspecific mineral deposition, self-assembly of collagen fibrils, and coordination of cell–matrix interactions (Fig. 1B) (Boskey 1989; Kim et al. 2004).
The characteristic feature of the NCPs involved in dentin and bone mineralization is that they are acidic and negatively charged. The negative charge is contributed by amino acid residues, such as aspartic acid and glutamic acid, and by the posttranslational phosphorylation modification of amino acids, such as serine, threonine and tyrosine. Such negatively charged proteins/domains facilitate avid binding to Ca2+ and PO43− ions, which are supersaturated in the ECM milieu. Binding of Ca2+ and PO43− by collagen-immobilized NCPs is necessary to form the initial mineral nidus. NCPs can shape crystal morphology and promote the transformation of amorphous calcium phosphate to crystalline hydroxyapatite. Some of the proteins synthesized by odontoblasts and osteoblasts function as crystal inhibitors and prevent growth by binding to the surface of the nascent mineral nuclei (Giachelli 2005).
Matrix Proteins That Regulate Dentin Mineralization
SIBLING Proteins
The SIBLING family of NCPs consists of DMP1, osteopontin (OPN), bone sialoprotein (BSP), matrix extracellular phosphoglycoprotein (MEPE), and dentin sialophosphoprotein (DSPP). DSPP is a compound protein consisting of dentin sialoprotein (DSP) and dentin phosphophoryn (DPP) (Fig. 5A, B). Although the SIBLINGs have poor homologies at the amino acid level, they all contain conserved regions such as the integrin-binding RGD motif, the NXS/T motif for N-linked oligosaccharides, and multiple casein kinase II–type phosphorylation sites (Fisher and Fedarko 2003). Posttranslational modifications such as phosphorylation increase their negative charge and aid in biomineralization by localizing to the collagen fibril microdomains and interacting with Ca2+ to stabilize mineral–protein complexes. Several of these proteins also promote the self-assembly of the collagen matrix; in particular, OPN plays a major role during the formation and remodeling of the organic matrix prior to mineralization. Numerous human disorders have been attributed to dysfunctional SIBLING proteins, emphasizing their importance to bone and dentin formation. For the detailed role of SIBLING proteins and their role in mineralization, readers are referred to published reviews (George and Veis 2008; Staines et al. 2012).
Novel NCPs Associated with the Mineral In Bone and Dentin Matrix
Type II TGF-β receptor interacting protein 1 (TRIP1) is a member of a family of structurally conserved proteins, the WD-40 repeat proteins. The WD-40 proteins contain 4 or more copies of a conserved Trp-Asp motif, the so-called WD-40 repeat, which forms a scaffold for binding other proteins. A typical WD-40 domain consists of 6 to 8 structurally conserved WD-40 repeats, each of which contains a 4-stranded anti–β-sheet, which then folds into a β-propeller structure often comprising 7 blades. These structures are stabilized by hydrophobic interactions. TRIP1 has been identified as a functional component of eukaryotic translation initiator factor 3 (eiF3) multiprotein complexes (Asano et al. 1997).
TRIP1, a Collagen- and Mineral-Binding Ncp inthe Mineralized Matrices of Bone and Dentin
The expression of TRIP1 in the dentin matrix is interesting. At day 3, the odontoblasts are fully polarized and secrete type I collagen and several NCPs necessary for matrix mineralization. During this process, TRIP1 is localized at the mineralization front, where the first crystals of calcium phosphate are deposited, and its expression is observed at all stages of tooth development (Ramachandran et al. 2012) (Fig. 5C).
A role for TRIP1 in dentin biomineralization was demonstrated by an in vitro nucleation assay. Demineralized and deproteinized dentin wafers that contain intact collagenous matrix were used to demonstrate its ability to nucleate calcium phosphate. Sparse mineral deposits were observed at 7 d within the collagen gap and overlap zones of the dentin wafer adsorbed with TRIP1, while increased deposits were observed at the end of 14 d (Ramachandran et al. 2018). Interestingly, at low concentrations of TRIP1, self-assembly of the protein into fibrillar structures was clearly evident. With increasing TRIP1 concentrations, nanosized calcium phosphate deposits were embedded within the protein meshwork. These particles coalesced to form agglomerates of hydroxyapatite (HAP) with higher concentrations of TRIP1. Transmission electron microscopy (TEM) imaging showed the characteristic diffraction rings corresponding to the crystallographic planes of HAP (Ramachandran et al. 2018). It is possible that the fibrillary supramolecular structure of TRIP1 may function as a nucleating template and that higher amounts of TRIP1 would bind more calcium phosphate nanoclusters, lower the interfacial energy for nucleation, and promote HAP formation.
The possibility of TRIP1 to nucleate calcium phosphate suggested that TRIP1 could bind to type I collagen. Surface plasmon resonance analysis suggested that TRIP1 bound to type I collagen in a dose-dependent manner with a KD, a measure of the affinity between the 2 molecules, of 48.5 µM and with fast association and dissociation rates (Ramachandran et al. 2016). Immunogold labeling also suggested that TRIP1 could bind to monomeric collagen aggregates. These observations suggest that TRIP1 in the ECM could bind both collagen and calcium ions to initiate matrix mineralization (Chen and George 2018).
GRP78, a Newly Identified NCP, Binds to Collagen and Mineral in Bone and Dentin ECM
Glucose regulated protein 78 (GRP78/BiP) is a member of the heat shock protein 70 family. Its primary function is to act as a molecular chaperone in the endoplasmic reticulum (ER) and facilitate the proper folding and assembly of membrane and secretory proteins. Although GRP78 is known for its function within the ER lumen, the protein has functions outside of the ER, namely, at the cell surface. Under conditions of stress within the cell, such as handling large amounts of intracellular Ca2+, GRP78 has the ability to relocate to the cell surface. Cell surface GRP78 was shown to function as a receptor and transport extracellular ligands such as DMP1 to the nucleus and promote cellular differentiation.
During the process of biomineralization, transport of calcium ions is of utmost importance, and their regulation by calcium-sequestering proteins in the ECM plays a central role in the nucleation of calcium phosphate. Immobilized GRP78 has the potential to bind Ca2+ and nucleate calcium phosphate crystals in vitro. Based on our observations, it is tempting to speculate that GRP78 might function in maintaining a local reservoir of calcium ions in the ECM during mineralized matrix formation. This speculation is further strengthened by the developmental expression pattern of GRP78 that shows increased expression in cells responsible for mineralized matrix formation, such as hypertrophic chondrocytes, osteoblasts, osteocytes, and odontoblasts, and also in the bone and dentin extracellular matrix (Ravindran et al. 2012) (Fig. 5D).
Binding of GRP78 to type I collagen was demonstrated by solid-phase binding assay. The dissociation constant was estimated to be 34 nM, which indicates fairly strong binding. In addition, the secreted pool of proteins from MC3T3-E1 cells was able to bind to type I collagen-coated plates, and the secretome from cell cultures under differentiation conditions contained a higher concentration of GRP78 when compared with control cultures in normal growth conditions (Ravindran et al. 2011).
Identification of unconventional intracellular proteins such as GRP78 and TRIP1 in bone and dentin matrix demonstrates the complexity that organisms use in biomineralization. Controlled mineral nucleation and growth mediated by various proteins permits the formation of exquisite mineral structures to perform various structural and signaling functions.
Biomimetic and Bioinspired Approaches to Dentin and Enamel Repair
Matrix-mediated biomineralization of enamel and dentin offers a wealth of scientific principles that can be used by scientists in the fields of regenerative medicine and dentistry. In regenerative medicine, nature’s biomineralization strategy for bone and dentin formation can be envisaged to induce biomimetic intra- and extrafibrillar mineralization of collagen fibrils using NCPs, NCP-derived peptides, synthetic IDPs, polymer analogues, and small molecules to generate collagen-based materials (Padovano et al. 2015; Zhu et al. 2020; Yu and Wei 2021; Zhao and Tang 2021). Fine-tuning of the additives used can promote the self-assembly of the collagen fibrils and also facilitate formation of ACP precursors with subsequent transformation into nanocrystalline hydroxyapatite tightly bound to the collagen matrix. Collagen scaffolds reinforced with mineral have better osteoconductive properties than unmineralized collagen. Thus, bioinspired mineralization of collagen is a promising strategy for repair of demineralized dentin and bone tissue engineering. The strategies of biomineralization can also be used to generate cell-instructive biomimetic scaffolds with intrinsic ability to recruit stem cells with regenerative potential (Reznikov et al. 2016).
Not only enhancing collagen mineralization but also promoting bioinspired regrowth of enamel have important implications in preventive and restorative dentistry. A recent review (Pandya and Diekwisch 2019) summarizes different physicochemical and biological strategies implemented by investigators toward enamel tissue engineering. Among them, protein matrix-guided enamel crystal growth and enamel surface remineralization have been the subject of numerous basic and clinical investigations (Moradian-Oldak and Fan 2010). Biomimetic remineralization of enamel that relies on the function of biomineralizing polypeptides could be developed for fabrication of future generations of enamel restorative materials. For example, amelogenin-inspired bioactive peptides with retained functional domains demonstrated an effective enamel remineralization potential in situ by forming organized aprismatic enamel while imparting significant mechanical properties (Mukherjee et al. 2018). They were also effective in remineralization of dentin by promoting collagen mineralization and enhancing mechanical strength of demineralized dentin (Mukherjee et al. 2020). This peptide-based biomimetic translational approach still needs to be optimized for the development of clinically viable complex biomaterials.
Author Contributions
J. Moradian-Oldak, A. George, contributed to conception and design, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the National Institute of Dental & Craniofacial Research (NIDCR)–National Institutes of Health (NIH). A. George has been supported by NIDCR-NIH grants DE011657 and DE028531 and the Brodie Endowment Fund. J. Moradian-Oldak has been supported by NIDCR-NIH grants DE027529, DE013414, and DE027632.
References
- Asano K, Kinzy TG, Merrick WC, Hershey JW. 1997. Conservation and diversity of eukaryotic translation initiation factor eIF3. J Biol Chem. 272(2):1101–1109. [DOI] [PubMed] [Google Scholar]
- Bapat RA, Su J, Moradian-Oldak J. 2020. Co-immunoprecipitation reveals interactions between amelogenin and ameloblastin via their self-assembly domains. Front Physiol. 11:622086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniash E, Metzler RA, Lam RSK, Gilbert P. 2009. Transient amorphous calcium phosphate in forming enamel. J Struct Biol. 166(2):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL. 1989. Noncollagenous matrix proteins and their role in mineralization. Bone Miner. 6(2):111–123. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Villarreal-Ramirez E. 2016. Intrinsically disordered proteins and biomineralization. Matrix Biol. 52–54:43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, George A. 2018. TRIP-1 promotes the assembly of an ECM that contains extracellular vesicles and factors that modulate angiogenesis. Front Physiol. 9:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delak K, Harcup C, Lakshminarayanan R, Sun Z, Fan Y, Moradian-Oldak J, Evans JS. 2009. The tooth enamel protein, porcine amelogenin, is an intrinsically disordered protein with an extended molecular configuration in the monomeric form. Biochemistry. 48(10):2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Yoreo J. 2013. Crystal nucleation: more than one pathway. Nat Mater. 12(4):284–285. [DOI] [PubMed] [Google Scholar]
- De Yoreo JJ, Gilbert PU, Sommerdijk NA, Penn RL, Whitelam S, Joester D, Zhang H, Rimer JD, Navrotsky A, Banfield JF, et al. 2015. Crystal growth: crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science. 349(6247):aaa6760. [DOI] [PubMed] [Google Scholar]
- Engelberth SA, Bacino MS, Sandhu S, Li W, Bonde J, Habelitz S. 2018. Progression of self-assembly of amelogenin protein supramolecular structures in simulated enamel fluid. Biomacromolecules. 19(10):3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estroff LA, Cohen I. 2011. Biomineralization: micelles in a crystal. Nat Mater. 10(11):810–811. [DOI] [PubMed] [Google Scholar]
- Fan D, Du C, Sun Z, Lakshminarayanan R, Moradian-Oldak J. 2009. In vitro study on the interaction between the 32 kDa enamelin and amelogenin. J Struct Biol. 166(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS. 2003. Six genes expressed in bones and teeth encode the current members of the sibling family of proteins. Connect Tissue Res. 44(Suppl 1):33–40. [PubMed] [Google Scholar]
- Friddle RW, Battle K, Trubetskoy V, Tao J, Salter EA, Moradian-Oldak J, De Yoreo JJ, Wierzbicki A. 2011. Single-molecule determination of the face-specific adsorption of amelogenin’s C-terminus on hydroxyapatite. Angew Chem Int Ed Engl. 50(33):7541–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB, Yamada Y. 2004. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 167(5):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallon V, Chen L, Yang X, Moradian-Oldak J. 2013. Localization and quantitative co-localization of enamelin with amelogenin. J Struct Biol. 183(2):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A, Veis A. 2008. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 108(11):4670–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli CM. 2005. Inducers and inhibitors of biomineralization: lessons from pathological calcification. Orthod Craniofac Res. 8(4):229–231. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Lacerda-Pinheiro S, Priam F, Jegat N, Six N, Bonnefoix M, Septier D, Chaussain-Miller C, Veis A, Denbesten P, et al. 2008. Matricellular molecules and odontoblast progenitors as tools for dentin repair and regeneration. Clin Oral Investig. 12(2):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. 2003. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2(8):552–558. [DOI] [PubMed] [Google Scholar]
- Hu JC, Hu Y, Smith CE, McKee MD, Wright JT, Yamakoshi Y, Papagerakis P, Hunter GK, Feng JQ, Yamakoshi F, et al. 2008. Enamel defects and ameloblast-specific expression in Enam knock-out/lacZ knock-in mice. J Biol Chem. 283(16):10858–10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Yamakoshi Y. 2003. Enamelin and autosomal-dominant amelogenesis imperfecta. Crit Rev Oral Biol Med. 14(6):387–398. [DOI] [PubMed] [Google Scholar]
- Hu Y, Smith CE, Cai Z, Donnelly LA, Yang J, Hu JC, Simmer JP. 2016. Enamel ribbons, surface nodules, and octacalcium phosphate in C57BL/6 Amelx(−/−) mice and Amelx(+/−) lyonization. Mol Genet Genomic Med. 4(6):641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Fan D, Bromley KM, Sun Z, Moradian-Oldak J. 2010. Tooth enamel proteins enamelin and amelogenin cooperate to regulate the growth morphology of octacalcium phosphate crystals. Cryst Growth Des. 10(11):4815–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Moradian-Oldak J. 2005. Control of apatite crystal growth in a fluoride containing amelogenin-rich matrix. Biomaterials. 26(13):1595–1603. [DOI] [PubMed] [Google Scholar]
- Jokisaari JR, Wang C, Qiao Q, Hu X, Reed DA, Bleher R, Luan X, Klie RF, Diekwisch TGH. 2019. Particle-attachment-mediated and matrix/lattice-guided enamel apatite crystal growth. ACS Nano. 13(3):3151–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar L, Homola D, Varga G, Tompa P. 2012. Structural disorder in proteins brings order to crystal growth in biomineralization. Bone. 51(3):528–534. [DOI] [PubMed] [Google Scholar]
- Kim HM, Himeno T, Kawashita M, Kokubo T, Nakamura T. 2004. The mechanism of biomineralization of bone-like apatite on synthetic hydroxyapatite: an in vitro assessment. J R Soc Interface. 1(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Habelitz S, Wright JT, Paine ML. 2017. Dental enamel formation and implications for oral health and disease. Physiol Rev. 97(3):939–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP. 2008. Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem. 389(6):695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YX, Hoff SE, Huang XQ, Liu J, Wan QQ, Song Q, Gu JT, Heinz H, Tay FR, Niu LN. 2021. Involvement of prenucleation clusters in calcium phosphate mineralization of collagen. Acta Biomater. 120:213–223. [DOI] [PubMed] [Google Scholar]
- Mazumder P, Prajapati S, Lokappa SB, Gallon V, Moradian-Oldak J. 2014. Analysis of co-assembly and co-localization of ameloblastin and amelogenin. Front Physiol. 5:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradian-Oldak J. 2012. Protein-mediated enamel mineralization. Front Biosci. 17:1996–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradian-Oldak J, Fan Y. 2010. Tooth-inspired nanocomposites. In: Kumar C. editor. Nanomaterials for the life sciences: biomimetic and bioinspired nanomaterials. Weinheim (Germany): Willey-VCH. [Google Scholar]
- Mukherjee K, Ruan Q, Nutt S, Tao J, De Yoreo JJ, Moradian-Oldak J. 2018. Peptide-based bioinspired approach to regrowing multilayered aprismatic enamel. ACS Omega. 3(3):2546–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Visakan G, Phark JH, Moradian-Oldak J. 2020. Enhancing collagen mineralization with amelogenin peptide: toward the restoration of dentin. ACS Biomater Sci Eng. 6(4):2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, Hilbers PA, de With G, Sommerdijk NA. 2010. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 9(12):1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovano JD, Ravindran S, Snee PT, Ramachandran A, Bedran-Russo AK, George A. 2015. DMP1-derived peptides promote remineralization of human dentin. J Dent Res. 94(4):608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Diekwisch TGH. 2019. Enamel biomimetics-fiction or future of dentistry. Int J Oral Sci. 11(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, He K, Huang CC, Shahbazian-Yassar R, Shokuhfar T, George A. 2018. TRIP-1 in the extracellular matrix promotes nucleation of calcium phosphate polymorphs. Connect Tissue Res. 59(Suppl 1):13–19. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Ravindran S, George A. 2012. Localization of transforming growth factor beta receptor II interacting protein-1 in bone and teeth: implications in matrix mineralization. J Histochem Cytochem. 60(4):323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Ravindran S, Huang CC, George A. 2016. TGF beta receptor II interacting protein-1, an intracellular protein has an extracellular role as a modulator of matrix mineralization. Sci Rep. 6:37885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Gao Q, Ramachandran A, Blond S, Predescu SA, George A. 2011. Stress chaperone GRP-78 functions in mineralized matrix formation. J Biol Chem. 286(11):8729–8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Gao Q, Ramachandran A, Sundivakkam P, Tiruppathi C, George A. 2012. Expression and distribution of GRP-78/BiP in mineralizing tissues and mesenchymal cells. Histochem Cell Biol. 138(1):113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov N, Steele JAM, Fratzl P, Stevens MM. 2016. A materials science vision of extracellular matrix mineralization. Nat Rev Mater. 1(8):16041. [Google Scholar]
- Robinson C, Kirkham J, Hallsworth AS. 1988. Volume distribution and concentration of protein, mineral and water in developing bovine enamel. Arch Oral Biol. 33(3):159–162. [DOI] [PubMed] [Google Scholar]
- Shaw WJ, Tarasevich BJ, Buchko GW, Arachchige RMJ, Burton SD. 2020. Controls of nature: secondary, tertiary, and quaternary structure of the enamel protein amelogenin in solution and on hydroxyapatite. J Struct Biol. 212(3):107630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, Hu JC. 2010. Regulation of dental enamel shape and hardness. J Dent Res. 89(10):1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. 1998. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 9(2):128–161. [DOI] [PubMed] [Google Scholar]
- Staines KA, MacRae VE, Farquharson C. 2012. The importance of the sibling family of proteins on skeletal mineralisation and bone remodelling. J Endocrinol. 214(3):241–255. [DOI] [PubMed] [Google Scholar]
- Tao J, Fijneman A, Wan J, Prajapati S, Mukherjee K, Fernandez-Martinez A, Moradian-Oldak J, De Yoreo JJ. 2018. Control of calcium phosphate nucleation and transformation through interactions of enamelin and amelogenin exhibits the “goldilocks effect.” Cryst Growth Des. 18(12):7391–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavafoghi M, Cerruti M. 2016. The role of amino acids in hydroxyapatite mineralization. J R Soc Interface. 13(123):20160462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Murakami C, Dohi N, Wakida K, Satoda T, Takahashi O. 1997. Synthesis, secretion, degradation, and fate of ameloblastin during the matrix formation stage of the rat incisor as shown by immunocytochemistry and immunochemistry using region-specific antibodies. J Histochem Cytochem. 45(10):1329–1340. [DOI] [PubMed] [Google Scholar]
- Veis A, Dorvee JR. 2013. Biomineralization mechanisms: a new paradigm for crystal nucleation in organic matrices. Calcif Tissue Int. 93(4):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetyskova V, Zouharova M, Bednarova L, Vanek O, Sazelova P, Kasicka V, Vymetal J, Srp J, Rumlova M, Charnavets T, et al. 2020. Characterization of AMBN I and II isoforms and study of their Ca(2+)-binding properties. Int J Mol Sci. 21(23):9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald T, Spoutil F, Osickova A, Prochazkova M, Benada O, Kasparek P, Bumba L, Klein OD, Sedlacek R, Sebo P, et al. 2017. Intrinsically disordered proteins drive enamel formation via an evolutionarily conserved self-assembly motif. Proc Natl Acad Sci U S A. 114(9):E1641–E1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Azaïs T, Robin M, Vallée A, Catania C, Legriel P, Pehau-Arnaudet G, Babonneau F, Giraud-Guille M-M, Nassif N. 2012. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat Mater. 11(8):724–733. [DOI] [PubMed] [Google Scholar]
- Wiedemann-Bidlack FB, Kwak SY, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011. Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro. J Struct Biol. 173(2):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JT, Hart PS, Aldred MJ, Seow K, Crawford PJ, Hong SP, Gibson CW, Hart TC. 2003. Relationship of phenotype and genotype in x-linked amelogenesis imperfecta. Connect Tissue Res. 44(Suppl 1):72–78. [PubMed] [Google Scholar]
- Yao S, Xu Y, Shao C, Nudelman F, Sommerdijk N, Tang R. 2019. A biomimetic model for mineralization of type-i collagen fibrils. Methods Mol Biol. 1944:39–54. [DOI] [PubMed] [Google Scholar]
- Yu L, Wei M. 2021. Biomineralization of collagen-based materials for hard tissue repair. Int J Mol Sci. 22(2):944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalzal SF, Smith CE, Nanci A. 2008. Ameloblastin and amelogenin share a common secretory pathway and are co-secreted during enamel formation. Matrix Biol. 27(4):352–359. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tang R. 2021. Improvement of organisms by biomimetic mineralization: a material incorporation strategy for biological modification. Acta Biomater. 120:57–80. [DOI] [PubMed] [Google Scholar]
- Zhu H, Gomez M, Xiao J, Perale G, Betge F, Lyngstadaas SP, Haugen HJ. 2020. Xenohybrid bone graft containing intrinsically disordered proteins shows enhanced in vitro bone formation. ACS Appl Bio Mater. 3(4):2263–2274. [DOI] [PubMed] [Google Scholar]