Abstract
Ovarian cancer is the deadliest gynecological malignancy due to its symptomless early stage, metastasis, and high recurrence rate. The tumor microenvironment contributes to the ovarian cancer progression, metastasis, and chemoresistance. Adipose-derived stem cell in the tumor microenvironment of ovarian cancer, as a key player, interacts with ovarian cancer cells to form the cancer-associated fibroblasts and cancer-associated adipocytes, and secretes soluble factors to activate tumor cell signaling, which can promote ovarian cancer metastasis and chemoresistance. We summarize in this review the recent progress in the studies of interactions between adipose-derived stem cell and ovarian cancer, thus, to provide some insight for ovarian cancer therapy through targeting adipose-derived stem cell.
Keywords: Ovarian cancer, adipose-derived stem cell, tumor microenvironment, metastasis, chemoresistance, cancer progression
Impact statement
The tumor microenvironment (TME) plays a critical role in ovarian cancer progression. As a key component of TME, adipose-derived stem cells (ADSCs) attract increasing attention and are considered as a treatment target for ovarian cancer. The present review pulls together and discusses the published evidence that supports the role of ADSCs in the TME of ovarian cancer and the pathways they activate to promote ovarian cancer progression, metastasis, and chemoresistance.
Introduction
Ovarian cancer (OC) is the most lethal disease among all gynecological malignancies. The incidence of OC in the United States and Europe is 11.7–12.1 per 100,000 individuals, and a little lower in Middle East and Asia. 1 More than 90% of malignant ovarian tumors originate from the epithelium of ovary and/or fallopian tubes. There are six major entities of OC classified by the World Health Organization (WHO), such as endometroid, mucinous, serous, clear cell, squamous, and transitional cell cancer. 2 OC has bad prognosis with a less than 40% five-year survival rate due to its early dissemination in the abdomen, late detection, and high recurrence rate. With modest symptoms in the early stage, most patients are diagnosed when the tumor already has extensive spreading in the peritoneal cavity, which is considered an advanced stage. OC is typically highly sensitive to chemotherapy. However, most patients suffer from a recurrence of the disease after a combination of debulking surgery and first-line chemotherapy. Early metastasis and high relapse rate contribute to the disastrous prognosis and significant mortality of OC.
In the progression of malignant tumor, cancer cells interact with adjacent cell populations and extracellular matrix in the tumor microenvironment (TME). Increasing studies has demonstrated the contribution of TME in regulating the malignant phenotypes of OC. 3 As an important component of TME of OC, adipose-derived stem cells (ADSCs) exert a key function in OC metastasis and chemoresistance. Herein, we review recent development of the studies about ADSCs in OC TME, discuss the interaction between ADSCs and OC cells, and the possible molecular mechanisms.
Tumor microenvironment and cancer progression
The TME is a complex network consisting of extracellular matrix (ECM), mesenchymal stem cells (MSCs), fibroblast, adipocytes, and immune cells. Current studies demonstrated the importance of TME as a player in the progression of cancer. Interaction with TME causes genetic and functional changes of cancer cells and regulates cancer cell proliferation, metastasis, and chemoresistance, making TME a potential therapeutic target.4–6
As an important component of TME, MSCs play a critical role in regulating tumor progression.7,8 MSCs are adult multipotent cells that have the capabilities of self-renewal and differentiation into multiple cell lineages including adipocytes, chondrocytes, osteoblasts, and fibroblasts. Based on the organ or tissue origin of MSCs these cells are named, for example, adipose-derived mesenchymal stem cells (ADSCs) and bone marrow-derived mesenchymal stem cells (BMSCs). The multilineage differentiation property of MSC suggests that MSC may be the precursor of multiple cell components in the TME. Based on recent evidence, the influences of MSCs on cancer cells are controversial. It has been demonstrated that MSCs in the TME induce the aggressive malignant phenotypes of different malignant tumors, such as breast cancer and colon cancer.9,10 However, MSCs also showed inhibitory effects on lung cancer and liver cancer.11,12 Different sources of MSCs, types of cancer, and methods of study can be ascribed to this dichotomous function of MSCs.
The mechanisms involved in the protumor roles of MSCs include MSCs differentiating into tumor supporting cancer-associated fibroblasts (CAFs), promotion of epithelial-mesenchymal transition (EMT) and metastasis, activation of angiogenesis and cancer cell survival, and stimulation of cancer stem cells (CSCs). CAFs constitute a big portion of the local stroma of tumor and contribute substantially to cancer progression. 13 MSCs have been shown to differentiate into CAFs through transforming growth factor β (TGFβ) signaling in prostate carcinoma and breast cancer.14,15 MSCs also secrete soluble factors capable of promoting angiogenesis, cancer cell proliferation, and survival. 16 MSCs promote angiogenesis through induction of the extracellular signal-regulated kinase1/2 (ERK1/2) pathway, which enhances the expression of vascular endothelial growth factor (VEFG) and C-X-C chemokine receptor4 (CXCR4) in cancer cells. 16 Another study showed that MSCs promote the viability and proliferation of glioblastoma cells significantly. 17 EMT is a process during which epithelial cells gain mesenchymal properties through multiple changes. The mesenchymal properties include mobility and migration from the primary site, which is a key step for cancer cell metastasis. MSCs can activate EMT through promoting the secretion of matrix metalloproteinase (MMPs) in breast cancer cells. 18 It has also been demonstrated that MSCs enrich CSC population in various cancer types, such as breast cancer and gastric cancer.19,20
Although most studies demonstrate the protumor role of MSCs in TME, other studies showed evidence of the antitumor effects of MSCs. In a rat model of colon cancer, MSCs inhibited tumor growth by regulating immune response which results in increased monocytes and granulocytes infiltration in the TME. 21 MSCs showed an anti-tumor effect on mouse insulinoma, hepatoma, and lymphoma cells through the induction of apoptosis of tumor cells. 22
Interaction between ADSCs and OC
The TME has been gaining gradual recognition as a player in OC metastasis and chemoresistance and can contain potential biomarkers and targets for diagnosis and therapy, respectively. Unlike other solid malignant tumors that usually metastasize through the vasculature, OC predominantly metastasizes via dissemination directly from the primary tumor to organs in the abdomen and beyond the pelvic cavity. 23 The TME of OC establishes a communication network that enhances cancer cell invasion and metastasis via interactive signaling, which contributes significantly to OC progression. ADSCs in the TME, by secreting soluble factors, enhance the EMT and angiogenesis signaling, therefore promoting the progression of OC. Meanwhile, OC cells induce proliferation, migration, and differentiation of ADSCs into CAFs and other cells, which in turn create a favorable microenvironment for tumor progression.
Role of ADSCs in OC progression, metastasis, and chemoresistance
ADSCs are MSCs that reside in the adipose tissue and they share most features of MSCs. ADSC is the progenitor of adipocyte and has the potential to differentiate into osteoblast, chondrocyte, myocyte, etc. The protumor effects of ADSCs have been demonstrated in in vivo studies. Muehlberg et al. showed that intravenous injected ADSCs promote breast cancer cell growth and metastasis in a mouse model. 24 These effects of ADSCs on malignant tumor cells have been observed in murine models of pancreatic cancer, prostate tumor, endometrial tumor, and lung cancer.25–29 Soluble factors or chemokines secreted by ADSCs, such as platelet-derived growth factor (PDGF) and VEGF promote endothelial cell proliferation and angiogenesis, thus helping tumor growth.30,31
ADSCs from human omentum enhance OC growth and metastasis. Nowicka et al. isolated omentum ADSCs from OC patients and co-culture these ADSCs with OC cells; they found that omentum ADSCs increase OC cell proliferation, migration, and chemoresistance. 32 In another study with interactions between omentum ADSCs and OC cells, co-culture of ADSCs and OC cells revealed that ADSCs promote significant proliferation and invasion of OC cells through stimulation of MMPs secretion from OC cells. 33 As reported, the surface marker of ADSCs, CD44, interacts with some MMPs, which influence the ECM remodeling and is associated with cancer cell infiltration. 34 Selective blocking of MMP2 and MMP9 by SB-3CT partially attenuated the proliferation and invasion effects of ADSCs on OC cells. This phenomenon has also been observed in a mouse xenograft model of OC. 33 Another study performed a proteomics analysis in OC cells to evaluate their protein expression when treated with ADSCs conditioned medium. In this study, the authors identified thymosin beta 4 X-linked (TMSB4X) as one of the factors contributing to the protumor effects of ADSCs. TMSB4X plays a part in regulating actin polymerization and is also involved in cell differentiation, migration, and proliferation. TMSB4X levels in OC cells increased significantly when cultured in ADSC-conditioned medium. Inhibition of TMSB4X attenuated the protumor effects of ADSCs both in vivo and in vitro. 35 The same group reported that human omentum ADSCs promote autophagy through activating the STAT3 signaling pathway. Knockdown of STAT3 with siRNA decreased hypoxia-induced autophagy and reduced the proliferation and metastasis of OC. 36
ADSCs in TME can promote cancer progression by differentiating into CAFs or cancer-associate adipocytes (CAAs).37,38 CAFs have been proved to contribute to OC progression in multiple ways. CAFs regulate collagen deposition and other ECM component to facilitate cancer cell proliferation, invasion, and migration.6,39 CAFs also secrete soluble factors, such as VEGF, TGF-β, IL-6, urokinase-type plasminogen activator (uPA), COX-2, and CXCL-1 to support growth and metastasis of OC cells.40,41 CAAs interfere with both lipid storage and signaling regulation. CAAs produce a variety of growth factors and cytokines that aid in ECM remodeling, invasion, resistance to treatment, and EMT. 8 Nieman et al. reported that co-cultures of OC cells and CAAs promoted OC cell migration and invasion by increasing the production of IL-8/fatty acid binding protein-4; CAAs provide energy for rapid growth and metastasis of OC cells. 42
ADSCs promote OC progression by increasing the number of CSC. Studies by Coffman et al. showed that ADSCs, compared to other MSCs, expressed high levels of bone morphogenetic proteins (BMPs) and promoted OC growth by rising numbers of CSCs. They found a positive feedback loop when OC cell-secreted Hedgehog (HH) induced the expression of BMP4 in ADSCs, next when ADSCs secreted BMP4 they observed an increase in the expression of OC cell HH. Interruption of this loop prevented enrichment of CSCs and reversed resistance to chemotherapy. 43
ADSCs promote resistance of OC cells to chemotherapy. The first-line chemotherapy for OC patient consists of the combination of a platinum and a taxane, such as carboplatin and paclitaxel, to kill cancer cells via inhibiting DNA synthesis and cell dividing. Omental ADSCs-derived conditioned medium from control adult females inhibited cisplatin apoptotic effects via decreasing cleaved caspase-3 and platinum accumulation in epithelial OC cells. 44 ADSCs conditioned medium from metastatic omentum in OC patient induced levels of cytokines and growth factors such as IL-6, IL-8, VEGF, and TNFα when compared with non-metastatic omentum ADSCs, and promoted chemoresistance against cisplatin and paclitaxel. This chemoresistance could be partially reversed by epigenetic drugs. 45
Although most studies report the protumor effects of ADSCs, few studies showed the inhibitory effects of ADSCs on OC. An in vitro study by Khalil et al. used MSCs from different origin, including BMSCs, ADSCs, and MSCs from umbilical cord. Co-culture of these MSCs and OC cell lines decreased the invasion and aggressiveness of OC cell lines, along with an increase in cellular apoptosis. 46 In another study, OC cell lines were treated with ADSCs-derived conditioned medium and this blocked cell cycle and activated apoptosis. 47 The discrepancy of the effects of ADSCs on OC cells might be attributed to different OC cell lines, different culture conditions, or different sources of ADSCs. As a baseline to understand the function of ADSCs in OC, we first would need to differentiate and classify what cytokines, growth factors, and pathways within ADSCs may contribute to the inhibitory or stimulatory effects seen in OC.
Changes in ADSCs induced by OC cells
The influences of cancer cells on ADSCs have also been studied in several tumors. Pinilla et al. reported that under the influence of breast cancer cells, human ADSCs produced CCL5 which promoted the invasion of breast cancer cells in vitro. 48 Weigand et al. selectively isolated ADSCs from normal breast and breast cancers and found that ADSCs from breast cancers showed enhanced adipogenic differentiation. 49
Evidence has shown that OC cells or OC TME can facilitate ADSCs to the phenotypes that provide a favorable environment for tumor progression. In the TME of OC, the presence of exosomes from OC cells can increase the expression of SDF-1, α-SMA, and TGF-β in ADSCs, indicating the differentiation of ADSCs into CAFs. Moreover, exosomes from different cells hold the ability to activate different signaling pathways related to either phosphorylated-SMAD2 (SMAD-dependent pathway) or phosphorylated-AKT (SMAD-independent pathway). 37 SMADs conform a family of transducers for receptors of the TGF-β family. Mclean et al. identified the existence of cancer-associated MSCs (CA-MSCs) in the tissue of human OC. These CA-MSCs had a normal morphology and karyotype and were not capable of forming tumors. However, CA-MSCs promoted tumor growth more effectively than normal ADSCs by increasing CSCs and expressed higher BMPs. 50 RNA-seq analysis of omental ADSCs and ovarian CA-MSCs found that the latter one occur from OC-mediated reprograming of ADSCs from local normal tissue. This process only happened in the in vivo ovarian TME. 51
Conclusions and perspectives
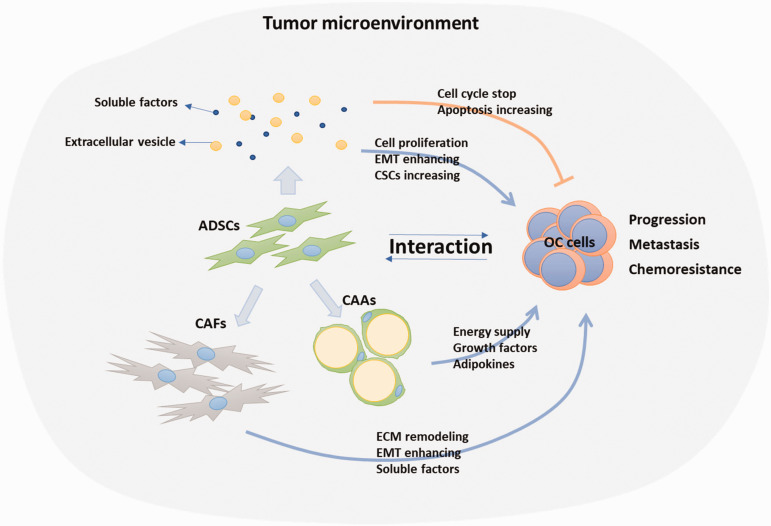
OC is the deadliest gynecological malignancy due to an insidious onset, early metastasis, and high rate of disease relapse after standard care. With more and more attention on the influences of TME on tumor progression, understanding the roles of ADSCs in OC progression becomes more important. The interaction between ADSCs and OC cells via direct or indirect reciprocal communications provides a leading force for OC cell proliferation, invasion, metastasis, and even contribute to chemoresistance, which make ADSCs in TME become an anti-tumor target (Figure 1). ADSCs in tumor regions show increased expression of BMP2, BMP4, and BMP6. Therefore, inhibition of BMP may be a good therapeutic option for OC. 50 Furthermore, with the nature of ADSCs being in stroma and activated by cancer cells and homing to cancer site, ADSC has the potential to be a drug delivery tool. Bioengineered ADSCs have been demonstrated to actively target both tumor necrotic regions and stroma of OC. This may also be feasible for the therapy of other cancers, such as liver, kidney, and GI tract. Moreover, ADSCs loaded with superparamagnetic iron oxide nanoparticles can be used by surgeons to pre-mark the areas with metastasis by magnetic resonance imaging. 52
Figure 1.
Interaction between ADSCs and OC cells. ADSCs affect OC cells by secreting soluble factors or extracellular vesicles. OC cells influence ADSCs to differentiate into CAFs or CAAs which in turn promote OC progression, metastasis, and chemoresistance through multiple mechanisms. ADSC, adipose-derived stem cell; CAF, cancer-associate fibroblast; CAA, cancer-associate adipocyte; OC, ovarian cancer; EMT, epithelial-mesenchymal transition; ECM, extracellular matrix. (A color version of this figure is available in the online journal.)
On the other hand, ADSC has become a popular source material for regenerative medicine research because of its abundant amount, multilineage differentiation potential, easily isolating and expanding procedures, and immunomodulatory properties. The roles of ADSCs in OC or other cancers should be considered in the application of ADSCs in regenerative medicine. Further understanding of the network crosstalk among ADSCs and OC cells will expand our knowledge of the numerous functions of ADSCs in OC progression and therapy response, thereby providing development of more effective and safer ADSC-related therapeutic strategies in both cancer treatment and regenerative medicine.
ACKNOWLEDGMENTS
The authors thank Christopher A. Walker for language revision of the manuscript.
AUTHORS’ CONTRIBUTIONS: WZ designed the review, WZ and CTR wrote the manuscript, JY and BZ revised the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by UTHSC/WCHSU CORNET Award.
ORCID iDs: Wenjing Zhang https://orcid.org/0000-0002-6694-6072
Carolina Torres-Rojas https://orcid.org/0000-0001-6373-5467
References
- 1.Nash Z, Menon U. Ovarian cancer screening: current status and future directions. Best Pract Res Clin Obstet Gynaecol 2020; 65:32–45 [DOI] [PubMed] [Google Scholar]
- 2.Colombo N.Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, Sessa C, Castiglione M. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21:v23–30 [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Wang C, Zhou S. Targeting tumor microenvironment in ovarian cancer: premise and promise. Biochim Biophys Acta Rev Cancer 2020; 1873:188361. [DOI] [PubMed] [Google Scholar]
- 4.Ansell SM, Vonderheide RH. Cellular composition of the tumor microenvironment. Am Soc Clin Oncol Educ Book 2013;DOI: 10.1200/EdBook_AM.2013.33.e91 [DOI] [PubMed] [Google Scholar]
- 5.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19:1423–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Z.Wang Q, Lau WB, Lau B, Xu L, Zhao L, Yang H, Feng M, Xuan Y, Yang Y, Lei L, Wang C, Yi T, Zhao X, Wei Y, Zhou S. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett 2016; 377:174–82 [DOI] [PubMed] [Google Scholar]
- 7.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol 2018; 35:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atiya H, Frisbie L, Pressimone C, Coffman L. Mesenchymal stem cells in the tumor microenvironment. Adv Exp Med Biol 2020; 1234:31–42 [DOI] [PubMed] [Google Scholar]
- 9.Albarenque SM, Zwacka RM, Mohr A. Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Res 2011; 7:163–71 [DOI] [PubMed] [Google Scholar]
- 10.Shinagawa K.Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W, Chayama K. Mesenchymal stem cells enhance growth and metastasis of Colon cancer. Int J Cancer 2010; 127:2323–33 [DOI] [PubMed] [Google Scholar]
- 11.Liu T.Zhu K, Ke C, Yang S, Yang F, Li Z, Zhang Z. Mesenchymal stem cells inhibited development of lung cancer induced by chemical carcinogens in a rat model. Am J Transl Res 2017; 9:2891–900 [PMC free article] [PubMed] [Google Scholar]
- 12.Yulyana Y.Ho IA, Sia KC, Newman JP, Toh XY, Endaya BB, Chan JK, Gnecchi M, Huynh H, Chung AY, Lim KH, Leong HS, Iyer NG, Hui KM, Lam PY. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/akt signaling. Mol Ther 2015; 23:746–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med 2014; 211:1503–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barcellos-de-Souza P.Comito G, Pons-Segura C, Taddei ML, Gori V, Becherucci V, Bambi F, Margheri F, Laurenzana A, Del Rosso M, Chiarugi P. Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-beta1. Stem Cells 2016; 34:2536–47 [DOI] [PubMed] [Google Scholar]
- 15.Shangguan L.Ti X, Krause U, Hai B, Zhao Y, Yang Z, Liu F. Inhibition of TGF-beta/smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells 2012; 30:2810–9 [DOI] [PubMed] [Google Scholar]
- 16.Zhu W.Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 2012; 315:28–37 [DOI] [PubMed] [Google Scholar]
- 17.Rodini CO, Goncalves da Silva PB, Assoni AF, Carvalho VM, Okamoto OK. Mesenchymal stem cells enhance tumorigenic properties of human glioblastoma through independent cell-cell communication mechanisms. Oncotarget 2018; 9:24766–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karnoub AE.Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449:557–63 [DOI] [PubMed] [Google Scholar]
- 19.Maffey A.Storini C, Diceglie C, Martelli C, Sironi L, Calzarossa C, Tonna N, Lovchik R, Delamarche E, Ottobrini L, Bianco F. Mesenchymal stem cells from tumor microenvironment favour breast cancer stem cell proliferation, cancerogenic and metastatic potential, via ionotropic purinergic signalling. Sci Rep 2017; 7:13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura K, Semba S, Aoyagi K, Sasaki H, Yokozaki H. Mesenchymal stem cells provide an advantageous tumor microenvironment for the restoration of cancer stem cells. Pathobiology 2012; 79:290–306 [DOI] [PubMed] [Google Scholar]
- 21.Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol 2003; 75:248–55 [DOI] [PubMed] [Google Scholar]
- 22.Lu YR.Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, Li SF, Long D, Tan WD, Mao YQ, Zhang J, Li YP, Cheng JQ. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther 2008; 7:245–51 [DOI] [PubMed] [Google Scholar]
- 23.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010; 177:1053–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muehlberg FL.Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E, Liu W, Arlinghaus RB, Alt EU. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis 2009; 30:589–97 [DOI] [PubMed] [Google Scholar]
- 25.Ji SQ.Cao J, Zhang QY, Li YY, Yan YQ, Yu FX. Adipose tissue-derived stem cells promote pancreatic cancer cell proliferation and invasion. Braz J Med Biol Res 2013; 46:758–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prantl L.Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, Alt EU. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate 2010; 70:1709–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klopp AH.Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, Debeb B, Woodward W, Schmandt R, Broaddus R, Lu K, Kolonin MG. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res 2012; 18:771–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y.Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res 2009; 69:5259–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heo SC.Heo SC, Lee KO, Shin SH, Kwon YW, Kim YM, Lee CH, Kim YD, Lee MK, Yoon MS, Kim JH. Periostin mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth in a xenograft lung adenocarcinoma model. Biochim Biophys Acta 2011; 1813:2061–70 [DOI] [PubMed] [Google Scholar]
- 30.Li W, Xu H, Qian C. c-Kit-positive adipose tissue-derived mesenchymal stem cells promote the growth and angiogenesis of breast cancer. Biomed Res Int 2017; 2017:7407168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salha S.Gehmert S, Brebant V, Anker A, Loibl M, Prantl L, Gehmert S. PDGF regulated migration of mesenchymal stem cells towards malignancy acts via the PI3K signaling pathway. Clin Hemorheol Microcirc 2018; 70:543–51 [DOI] [PubMed] [Google Scholar]
- 32.Nowicka A.Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, Broaddus R, Kolonin M, Mok SC, Thompson MS, Woodward WA, Lu K, Salimian B, Nagrath D, Klopp AH. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PloS One 2013; 8:e81859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu Y.Tang H, Guo Y, Guo J, Huang B, Fang F, Cai J, Wang Z. Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer. Exp Cell Res 2015; 337:16–27 [DOI] [PubMed] [Google Scholar]
- 34.Hass R, Otte A. Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun Signal 2012; 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu Y.You M, Zhang J, Gao G, Han R, Luo W, Liu T, Zuo J, Wang F. Adipose-derived mesenchymal stem cells enhance ovarian cancer growth and metastasis by increasing thymosin beta 4X-linked expression. Stem Cells Int 2019; 2019:9037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu Y.Wang Y, Li K, Liu M, Zhang Y, Li Y, Hu X, Liu C, Zhou H, Zuo J, Peng W. Human omental adipose-derived mesenchymal stem cells enhance autophagy in ovarian carcinoma cells through the STAT3 signalling pathway. Cell Signal 2020; 69:109549. [DOI] [PubMed] [Google Scholar]
- 37.Cho JA.Park H, Lim EH, Kim KH, Choi JS, Lee JH, Shin JW, Lee KW. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol 2011; 123:379–86 [DOI] [PubMed] [Google Scholar]
- 38.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol 2012; 40:130–8 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y.Tang H, Cai J, Zhang T, Guo J, Feng D, Wang Z. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett 2011; 303:47–55 [DOI] [PubMed] [Google Scholar]
- 40.Spaeth EL.Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PloS One 2009; 4:e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schauer IG, Sood AK, Mok S, Liu J. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia 2011; 13:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieman KM.Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011; 17:1498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coffman LG.Choi YJ, McLean K, Allen BL, di Magliano MP, Buckanovich RJ. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget 2016; 7:6916–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen Y, Guo Y, Huang Z, Cai J, Wang Z. Adipose‑derived mesenchymal stem cells attenuate cisplatin‑induced apoptosis in epithelial ovarian cancer cells. Mol Med Rep 2017; 16:9587–92 [DOI] [PubMed] [Google Scholar]
- 45.Sookram J.Zheng A, Linden KM, Morgan AB, Brown SA, Ostrovsky O. Epigenetic therapy can inhibit growth of ovarian cancer cells and reverse chemoresistant properties acquired from metastatic omentum. Int J Gynecol Obstet 2019; 145:225–32 [DOI] [PubMed] [Google Scholar]
- 46.Khalil C.Moussa M, Azar A, Tawk J, Habbouche J, Salameh R, Ibrahim A, Alaaeddine N. Anti-proliferative effects of mesenchymal stem cells (MSCs) derived from multiple sources on ovarian cancer cell lines: an in-vitro experimental study. J Ovarian Res 2019; 12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reza A, Choi YJ, Yasuda H, Kim JH. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep 2016; 6:38498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinilla S.Alt E, Abdul Khalek FJ, Jotzu C, Muehlberg F, Beckmann C, Song YH. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett 2009; 284:80–5 [DOI] [PubMed] [Google Scholar]
- 49.Weigand A.Boos AM, Tasbihi K, Beier JP, Dalton PD, Schrauder M, Horch RE, Beckmann MW, Strissel PL, Strick R. Selective isolation and characterization of primary cells from normal breast and tumors reveal plasticity of adipose derived stem cells. Breast Cancer Res 2016; 18:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLean K.Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, Buckanovich RJ. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest 2011; 121:3206–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coffman LG.Pearson AT, Frisbie LG, Freeman Z, Christie E, Bowtell DD, Buckanovich RJ. Ovarian carcinoma-associated mesenchymal stem cells arise from tissue-specific normal stroma. Stem Cells 2019; 37:257–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malekshah OM.Sarkar S, Nomani A, Patel N, Javidian P, Goedken M, Polunas M, Louro P, Hatefi A. Bioengineered adipose-derived stem cells for targeted enzyme-prodrug therapy of ovarian cancer intraperitoneal metastasis. J Control Release 2019; 311-312:273–87 [DOI] [PMC free article] [PubMed] [Google Scholar]