Abstract
There are a growing number of globally approved products and clinical trials utilizing autologous and allogeneic therapeutic cells for applications in regenerative medicine and immunotherapies. However, there is a need to develop rapid and cost-effective methods for manufacturing therapeutically effective cells. Furthermore, the resulting manufactured cells may exhibit heterogeneities that result in mixed therapeutic outcomes. Engineering approaches that can provide distinct microenvironmental cues to these cells may be able to enhance the growth and characterization of these cell products. This mini-review describes strategies to potentially enhance the expansion of therapeutic cells with biomaterials and bioreactors, as well as to characterize the cell products with microphysiological systems. These systems can provide distinct cues to maintain the quality attributes of the cells and evaluate their function in physiologically relevant conditions.
Keywords: Cell and gene therapies, cell manufacturing, biomaterials, bioreactors, microphysiological systems
Impact statement
This review discusses recent advancements in bioengineering approaches, including biomaterials, bioreactors, and microphysiological systems, that can help manufacture cells for therapies that are currently in clinical trials. Biomaterials and bioreactors can help grow cells more efficiently and maintain their function over longer periods of time. Microphysiological systems can test the cells in environments that are similar to conditions found in patients prior to their delivery. These approaches may help make these therapies more cost-effective and consistent in their therapeutic outcomes.
Introduction
Therapeutic cells are increasingly used in formulating cellular (e.g. human somatic cells) and gene therapy products (e.g. ex vivo genetically modified cells 1 ) to treat a variety of diseases and restore tissue function. Cellular and gene therapy products are biological products regulated by the FDA’s Center for Biologics Evaluation and Research (CBER). Clinical studies in humans require the submission of an investigational new drug application (IND) prior to initiating clinical studies in the United States. Marketing a cell or gene therapy product requires submission and approval of a biologics license application (BLA). 2 Growing interest and development in these therapeutic products have resulted in over 1700 active clinical trials 3 and 17 different FDA-approved products (Table 1) as of 27 March 2021. 4 More than half of these products are utilized for regenerative medicine applications; these include cord blood hematopoietic stem and progenitor cells (HSPCs) for the treatment of blood and immunodeficiency disorders, as well as tissue-specific cells (with or without scaffolds) for applications in oral soft tissue repair, skin cosmetics, or cartilage regeneration. The remaining products are for cancer immunotherapies, consisting of CAR T-cells for the treatment of B cell malignancies and dendritic cells (DC) for the treatment of prostate cancer. Current active clinical trials are utilizing a variety of cells, including mesenchymal stromal cells (MSCs), neural stem cells, natural killer (NK) cells, and blood mononuclear cells, for the treatment of a wide range of diseases, such as cancer, neurological, cardiovascular, respiratory, and inflammatory disorders, as well as for regenerative medicine applications.3,5,6
Table 1.
List of licensed cellular and patient-derived cellular gene therapy products from the Office of Tissues and Advanced Therapies at the U.S. Food and Drug Administration (current as of 27 March 2021)
| Trade name(proper name) | Manufacturer | Indications and usage |
|---|---|---|
| ALLOCORD(HPC Cord Blood) | SSM Cardinal Glennon Children's Medical Center | Allogeneic cord blood hematopoietic progenitor cell therapy indicated for use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| CLEVECORD(HPC, Cord Blood) | Cleveland Cord Blood Center | |
| DUCORD(HPC, Cord Blood) | Duke University School of Medicine | |
| HEMACORD(HPC, Cord Blood) | New York Blood Center, Inc. | |
| [No Trade Name](HPC, Cord Blood) | Clinimmune Labs, University of Colorado Cord Blood Bank | |
| [No Trade Name](HPC, Cord Blood) | MD Anderson Cord Blood Bank | |
| [No Trade Name] (HPC, Cord blood) | LifeSouth Community Blood Centers, Inc. | |
| [No Trade Name](HPC, Cord blood) | Bloodworks | |
| ABECMA (idecabtagene vicleucel) | Celegene Corporation, a Bristol-Myer Squibb Company | A B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. |
| BREYANI(lisocabtagene maraleucel) | Juno Therapeutics, Inc., a Bristol-Myers Squibb Company | A CD19-directed genetically modified autologous T-cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B. |
| KYMRIAH(tisagenlecleucel) | Novartis Pharmaceuticals Corporation | A CD19-directed genetically modified autologous T-cell immunotherapy indicated for the treatment of:• Patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse.• Adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. |
| TECARTUS(brexucabtagene autoleucel) | Kite Pharma, Inc. | A CD19-directed genetically modified autologous T-cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL). |
| YESCARTA (axicabtagene ciloleucel) | Kite Pharma, Incorporated | A CD19-directed genetically modified autologous T-cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and to DLBCL arising from follicular lymphoma. |
| GINTUIT(allogenic cultured keratinocytes and fibroblasts in bovine collagen) | Organogenesis Incorporated | An allogeneic cellularized scaffold product indicated for topical (non-submerged) application to a surgically created vascular wound bed in the treatment of mucogingival conditions in adults. |
| LAVIV (Azficel-T) | Fibrocell Tehcnologies, Inc. | An autologous cellular product indicated for improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults. |
| MACI(autologous cultured chondrocytes on a porcine collagen membrane) | Vericel Corporation | An autologous cellularized scaffold product indicated for the repair of symptomatic, single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults. |
| PROVENGE(Sipuleucel-T) | Dendreon Corporation | An autologous cellular immunotherapy indicated for the treatment of asymptomatic or minimally symptomatic metastatic castrate resistant (hormone refractory) prostate cancer. |
Despite the large number of clinical trials and significant number of approved products, there are many challenges in establishing reproducible, cost-effective manufacturing procedures for cell and gene therapy products. The procedures for manufacturing therapeutic cells typically consist of cell isolation from the patient (autologous cell therapy) or healthy donor (allogenic cell therapy), purification, genetic modification (for cellular gene therapy products), and large-scale expansion to approximately 106–10 9 therapeutic cells per clinical dose for adults prior to cell delivery to a patient or cryopreservation. Increasing the efficiency and rate of expansion, while simultaneously maintaining the function and quality attributes of the cells, is a critical challenge for ensuring the success of these therapies, as well as preventing patient attrition. Furthermore, improved expansion efficiencies and cost-effective approaches may help reduce the high price of these cell therapies, which can be prohibitively expensive; a single dose of YESCARTA and KYMRIAH CAR T-cell therapies, for example, currently cost US$373,000 and US$475,000, respectively. 7
Another challenge in the reproducible manufacturing of cell therapies is the functional heterogeneity of the cells and the variations in their resulting quality attributes. This includes intrinsic heterogeneity in cells due to donor-to-donor variations, intra-population heterogeneity, and variations in the treated patients, who may respond to the treatments in different ways and contain heterogenous disease microenvironments.8,9 Furthermore, heterogeneity is introduced during the manufacturing process, including variations in the isolation of the cells, cell culture expansion conditions, genetic modifications, and cryopreservation processes. In addition to improving manufacturing strategies, approaches to better characterize the cell products during the manufacturing process and predict their function in a patient-specific manner may help reduce heterogeneity in the therapeutic outcomes of these cell therapies.
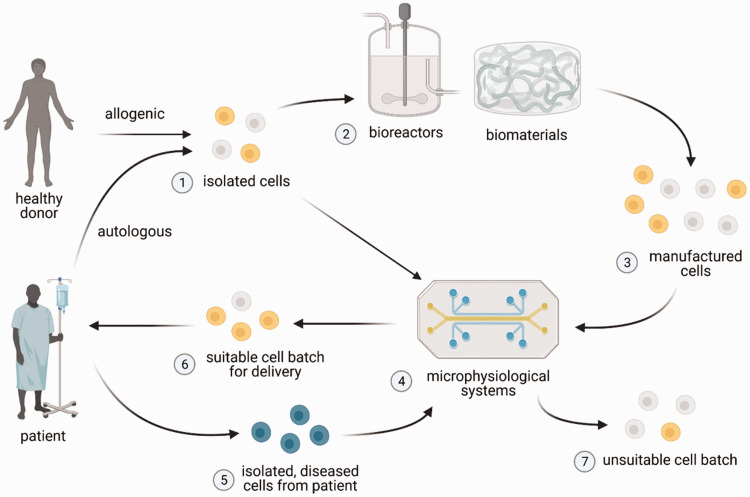
Bioengineering approaches, including bioreactors, biomaterials, and microphysiological systems, may offer ways to improve both the manufacturing and characterizations of various cellular therapy products (Figure 1). Bioreactors and biomaterials may provide precise control over the chemical and physical microenvironment of these cells by providing distinct cues, such as fluid flows, adhesion signals, mechanical cues, and/or microarchitecture, to enhance the expansion of the cell product and maintain its functional properties. Microphysiological systems, which can consist of microfluidic devices, organoids, and/or 3D cell culture systems per FDA definition, 10 may then provide tissue microenvironments to characterize the cell product in a physiologically relevant manner and identify therapeutically effective cell batches. This mini-review will first provide a brief overview of therapies utilizing T-cells, DCs, NK cells, hematopoietic stem cells, and MSCs, the five most prevalent cell types utilized in therapies in clinical trials that are delivered as a single cell suspension, as identified by Wang et al. 3 It will then review recent advancements, in the context of these prevalent cell therapies, on (1) strategies for the expansion of cells with biomaterials and/or bioreactors and (2) microphysiological systems that can be used to characterize the cell products.
Figure 1.
Potential utility of bioreactors, biomaterials, and microphysiological systems for cell manufacturing. Cells (1) are first isolated and purified from autologous (patient-derived) or allogenic (healthy donor) source, after which they can be grown and expanded into therapeutic cell numbers in bioreactors and/or biomaterials (2) that provide distinct microenvironmental cues. The expanded cells (3), however, may be heterogenous in their quality attributes. Microphysiological systems (4), which may incorporate patient-specific diseased cells (5) (i.e. cancer cells), can be used to characterize isolated cells (1) (in cases where expansion is not necessary) or manufactured cells (3), in order to identify batches of cell product that have suitable (6) or unsuitable (7) quality attributes for use in patients. Figure created with BioRender.com. (A color version of this figure is available in the online journal.)
Prevalent cell therapies in clinical trials
Leukocyte cell immunotherapies, which include T-cells, NK cells, and DCs, make up over 1000 of the active current clinical trials according to ClinicalTrials.gov (767 T-cell trials, 116 NK cell trials, and 136 DC trials). 3 The majority of clinical trials utilizing T-cells, DCs, and NK cells have applications in treating solid and liquid cancers, with the minority of trials used to treat a variety of other immunological disorders, including autoimmune, degenerative, infectious, or graft versus host diseases. 3 T-cells, which provide cell-mediated adaptive immunity, are utilized in clinical trial therapies with and without genetic-modification. The two most predominant genetically modified T-cell therapies, CAR T-cells and TCR T-cells, differ mainly in that CAR T-cells target surface antigens and are not restricted by MHC class, whereas TCR T-cells, which are in general less effective, can target both surface and intracellular targets 11 in a MHC-dependent manner. NK cells, which like T-cells provide cell-mediated immunity, are believed to induce fewer side effects compared to T-cells. 12 Therapies using NK cells are diversified by both the source of the cells (i.e. from peripheral blood mononuclear cells, stem cells, umbilical cord blood, or cell lines), as well as the combination of the cells with other therapies (i.e. chemotherapy, cytokines, immunoregulatory drugs, antibodies, CAR and cytokine genetic engineering). 13 Therapies that utilize DCs, which present antigen to and activate T-cells, can be categorized by the variety of methods to mature and load the cells with antigen (i.e. mRNA transfection, tumor lysate, viral vectors), as well as their route of administration (i.e. intra-dermal, subcutaneous, intra-nodal), 14 which can influence their efficacy. 15
Hematopoietic stem cells (HSC) and mesenchymal stromal (stem) cells (MSCs) are utilized in over 550 active clinical trials (274 HSC trials and 283 MSC trials) for applications in regenerative medicine and immunomodulation. 3 HSCs are stem cells that give rise to and differentiate into all other progenitor and mature blood cells and can regenerate the blood system after transplantation. These cells are used therapeutically by reconstituting the immune and blood system of patients who undergo myeloablative treatments (via chemotherapy or radiation therapy) to remove diseased or dysfunctional blood cells; for this reason, HSC transplantation is used to treat a variety of blood disorders, autoimmune diseases, transplant-related disorders, and leukemias. 16 MSCs are multipotent progenitor cells derived from various tissues, including bone marrow, fat, placenta, and umbilical cords, that are characterized by the surface marker expressions proposed by the International Society of Cellular Therapy (ISCT) (i.e. positive expression of CD105, CD73, CD90 and negative expression of CD45, CD34, cD14/CD11b, CD79/CD19, HLA-DR) and their ability to differentiate into bone, cartilage, and fat tissue in vitro. 17 Because of their ability to differentiate into various mesenchymal tissues, to secrete regenerative paracrine factors, and to modulate the immune system, MSCs are used to treat a wide range of diseases and disorders, including hematological, neurological, cardiovascular, kidney, lung, liver, and graft versus host diseases. 18
Advanced manufacturing strategies for cellular products
T-cells
The expansion of T-cells requires three signals that mimic antigen-presenting cells, which include (1) T-cell receptor engagement, (2) co-stimulation, and (3) pro-survival cytokines. Common methods of T-cell expansion include utilizing monocyte-derived DCs to present antigen to the T-cells or commercially available Dynabeads modified with anti-CD3 and anti-CD28 antibodies. However, the use of monocyte-derived DCs is costly, due to their extensive isolation and expansion procedures. Furthermore, anti-CD3/CD28 Dynabeads fail to provide these stimulatory cues in a physiologically relevant manner.
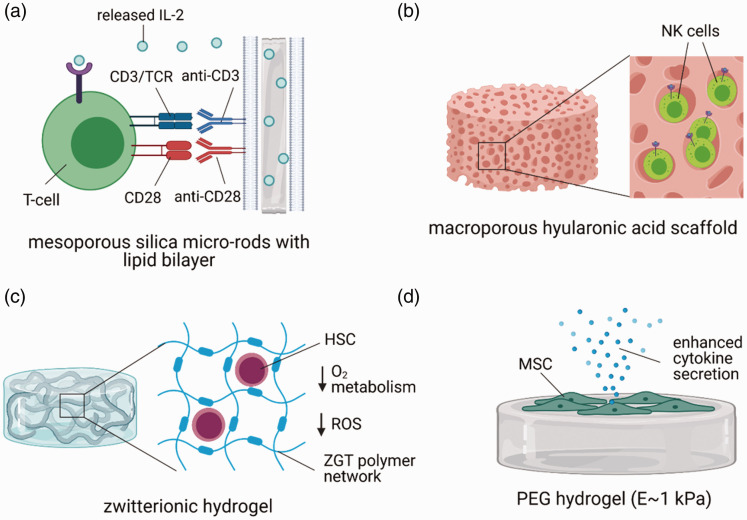
Biomaterial systems that are modified with stimulatory, co-stimulatory, and pro-survival cues with distinct structural cues and physical properties may provide more efficient ways to expand T-cells. Anti-CD3/CD28-coated polydimethylsiloxane beads induced greater expansion of T-cells than traditional Dynabeads, which provide different mechanical cues. 19 The role of physical cues on T-cell expansion was also demonstrated with hyaluronic acid hydrogels modified with anti-CD3/CD28, where softer hydrogels expanded mouse CD8+ T-cells more efficiently than stiffer hydrogels. 20 3D printed scaffolds fabricated from polycaprolactone and modified with anti-CD3/CD28 also provided a unique structural microenvironment that effectively induced the expansion of varying populations of primary human T-cells. 21 Notably, degradable, mesoporous silica micro-rods coated with interleukin (IL)-2 and a fluid lipid bilayer modified with anti-CD3/CD28 enhanced the expansion of both primary mouse and human T-cells 2 to 10-fold greater than traditional Dynabeads with soluble IL-222,23 (Figure 2(a)). Modification of these scaffolds with antigen peptides also induced antigen-specific expansion of rare T-cells to a greater extent than monocyte-derived DCs. 22 Decreasing the amount of anti-CD3/CD28 on these scaffolds both increased the proliferation of the T-cells and decreased their exhaustion markers. 22
Figure 2.
Examples of biomaterials for enhancing the expansion of therapeutic cells. (a) Mesoporous silica micro-rods releasing IL-2 with a lipid bilayer that presents anti-CD3 and anti-CD28 enhance the proliferation and reduce the exhaustion of primary T-cells. (b) A macroporous hyaluronic acid scaffold provides a 3D microenvironment to natural killer cells to enhance their proliferation. (c) Zwitterionic poly(carboxybetamine)-based hydrogels (ZGT) enhance the expansion of long-term HSCs by providing a 3D microenvironment, reducing oxygen-related metabolism, and decreasing reaction oxygen species (ROS) production. (d) Culture of MSCs on poly(ethylene glycol) (PEG) hydrogels with ∼1 kPa elastic modulus enhanced MSC secretion of cytokines over later passages. Figure created with BioRender.com. (A color version of this figure is available in the online journal.)
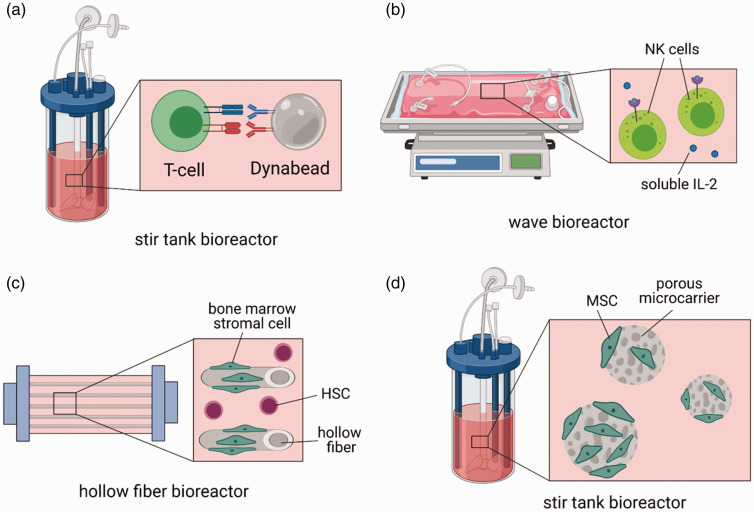
Bioreactors, which provide dynamic culture environments and automated expansion procedures, have also been utilized for expanding primary T-cells. Notably, culture of CAR T-cells with anti-CD3/CD28 Dynabeads in a stir-tank bioreactor demonstrated greater expansion of the cells than growth in static tissue culture flasks growth was enhanced to a greater extent with increasing agitation speeds 24 (Figure 3(a)). A commercially available bioreactor from Miltenyi Biotec, the CliniMACS Prodigy, offers an automated, closed system for the isolation, viral transduction, and expansion of CAR T-cells; the feasibility of this system was demonstrated in a study evaluating the clinical grade production of CAR T-cells for 28 CAR T-cell products for a phase I clinical trial for CD19+ B-cell malignancies. 25 The Cocoon platform, offered by Lonza, is another commercially available, closed bioreactor system that has multiple all-in-one units that are amendable to isolation, viral transduction, and expansion of CAR T-cells. 26
Figure 3.
Example of bioreactors for enhancing the expansion of therapeutic cells. (a) A stir tank bioreactor containing primary T-cells and anti-CD3/CD28 Dynabeads enhances the proliferation of the T-cells with increasing agitation speeds. (b) Dynamic culture of NK cells in a wave bioreactor enhances the cytotoxicity and purity of the manufactured cells. (c) Culture of HSCs in a hollow fiber bioreactor with adherent bone marrow stromal cells enhances the expansion of the HSCs. (d) MSC culture on porous poly-ε-caprolactone microcarriers in a stir tank bioreactor enables greater growth of the cells. Figure created with BioRender.com. (A color version of this figure is available in the online journal.)
Natural killer cells and dendritic cells
Due to their low frequency in peripheral blood, therapies involving human NK cells and DCs require special expansion or differentiation protocols. The proliferation of human NK cells is readily induced with soluble IL-2. Due to the fact that primary human DCs do not proliferate in culture, DCs are often derived from peripheral blood monocytes that are differentiated into immature DCs with IL-4 and GM-CSF. These immature DCs are subsequently matured and pulsed with antigen and maturing cytokines/adjuvants, such as TNF-α, PGE2, and/or LPS. The total number of mature DCs obtained is a fraction of the total number of monocytes initially isolated.
Both bioreactor and biomaterial strategies are emerging to enhance the expansion of NK cells. Comparison of NK production in tissue culture flasks, fluoropolymer cell culture bags, and the closed Wave Bioreactor system demonstrated that NK cells produced in the Wave Bioreactor displayed the highest cytotoxic capacity by expression of NKp44. 27 A similar study comparing the Wave Bioreactor to tissue culture flask expansion also demonstrated enhanced NK purity and functional cytotoxicity of NK cells cultured in the bioreactor 28 (Figure 3(b)). Furthermore, culture of NK cells in a 3D macroporous hyaluronic acid hydrogel resulted in greater cytokine production, proliferation, and intrinsic anti-tumor efficacy than NK cells cultured in 2D Petri dishes (Figure 2(b)); similar effects were observed when culturing the NK cells in porous collagen sponges, suggesting that the macroporous 3D architecture was integral to this observed enhancement. 29
Several semi-closed or closed bioreactor systems have been utilized for the culture and differentiation of monocyte-derived DCs. The CliniMACS closed bioreactor system offered by Miltenyi Biotec has been utilized in combination with various non-adherent, fluoropolymer culture bags to produce functional mature DCs from monocytes pulsed with tumor lysate.30,31 These cell culture bags offer decreased risk of contamination and are amenable to scale up, but in general do not induce a marked difference in the phenotype of manufactured mature DCs relative to tissue culture plastic culture. 32 Furthermore, culture of monocyte-derived DCs in a dynamic hollow-fiber bioreactor yielded similar quantities of functional mature DCs as a static culture bag system, with the advantage of processing a single apheresis at once. 33
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) derived from umbilical cord blood, which have a reduced risk of inducing graft versus host disease compared to HSCs from bone marrow, are limited therapeutically by their small cell numbers and require cell expansion methods to expand their clinical use. 34 Methods for expanding HSCs on 2D tissue culture polystyrene (TCPS) with soluble cytokines and small molecules are often hampered by the loss of HSC stemness, limited expansion ability, and diminished homing ability of the cells. These limitations are likely due to the lack of microenvironment cues that mimic the in vivo HSC niche.
Expansion of HSCs in biomaterial scaffolds that provide 3D microenvironments with distinct adhesive and mechanical cues can better maintain the stemness and enhance the proliferation of the cells relative to 2D culture. 3D culture of HSCs in zwitterionic hydrogels and polyethylene glycol (PEG) hydrogels demonstrated improved ability to preserve the primitive HSC population relative to 2D culture on the same hydrogels. 35 In particular, 3D materials that provide engagement with RGD integrins may provide distinct improvement in expansion, as HSCs were shown to proliferate to the greatest extent on fibrin porous scaffolds compared to collagen, poly(lactic-co-glycolic acid), or polycaprolactone scaffolds. 36 These findings were further supported by studies demonstrating improved HSC expansion on fibronectin-coated 3D PCL nano-scaffolds relative to 2D TCP. 37 In addition to adhesion cues, mechanical cues may also regulate HSC maintenance, as glycosaminoglycan(GAG)-based hydrogels that spatially confine the proliferation of HSCs with increased stiffness better maintained the stemness of these cells. 38
Methods that precisely control soluble factors that are inhibitory and/or conducive for HSC maintenance can also dramatically improve HSC expansion. Utilization of a fed-batch media dilution approach for expanding human HSCs was able to reduce the concentration of inhibitory factors secreted by differentiating HSCs and resulted in a 11-fold increase of HSCs. 39 Similarly, zwitterionic hydrogels were shown to reduce the production of inhibitory reactive oxygen species by HSCs by suppressing O2-related metabolism, resulting in a 73-fold increase in long term-HSC frequency 35 (Figure 2(c)). Also, the sequestration of conducive cytokines for HSC maintenance within GAG-based hydrogels modified with sulfated heparin enhanced the frequency of long-term culture initiating HSCs. 39
Biomaterial systems and bioreactors that enable the co-culture of HSCs with stromal cells can further enhance the expansion of HSCs. Efforts to mimic both the cellular and microporous architecture of the HSC niche with RGD-modified microporous poly(ethylene glycol) diacrylate hydrogels seeded with bone marrow-derived MSCs yielded a cell scaffold that better maintained the stemness of the HSCs than standard 2D cell culture methods. 40 Similarly, MSCs cultured on fibrin macroporous scaffolds were able to more greatly enhance the expansion of HSCs relative to other natural and synthetic scaffolds. 36 Furthermore, co-culture of HSCs with the H5-S bone marrow stromal cell line in a hollow fiber bioreactor supported greater progenitor expansion than a 2D TCPS control with both cell types as well 41 (Figure 3(c)).
Mesenchymal stromal cells
There is a critical need to expand MSCs for clinical trials of immunotherapy and wound healing, as there is a low frequency of these cells in available tissue and the required infusion doses are high (>1× 10 6 cells/kg patient). Expansion on static TCPS is most commonly used, although this method is limited by high costs for scale-up, labor intensive protocols, inability to precisely control culture parameters, and serial loss of MSC therapeutic potential with expansion. For example, it is well characterized that with increasing passage number on 2D TCPS, MSCs lose their immunomodulatory, osteogenic, and chondrogenic capacities.42–44
Numerous bioreactor strategies have been explored for the expansion of MSCs that can provide dynamic culture conditions to enhance expansion and potentially reduce manufacturing costs. Several microcarrier systems have been developed, including porous, biodegradable poly-ε-caprolactone microcarriers, which enable greater growth of MSCs in a stirred tank bioreactor than conventional 2D TCPS culture 45 (Figure 3(d)). Commercially available CultiSphere-S®, consisting of microporous gelatin-coated beads, have also been extensively evaluated in various bioreactor systems; 46 incorporation of these beads with a conventional spinner flask bioreactor can induce higher cell yields than incorporation into the Wave Bioreactor. 47 Combined analysis of cell yields and bioprocess economic modeling of microcarrier systems have also shown that microcarrier systems in a vertical wheel bioreactor can reduce the total manufacturing costs per dose by nearly a half relative to standard tissue culture plastic expansion. 48 The combined analysis of manufacturing costs and yield was shown to be particularly valuable in comparing the manufacturing of MSCs in a multi-layer vessel, stirred tank bioreactor with microcarriers, a hollow fiber bioreactor, and a packed bed bioreactor; while MSCs had the greatest fold expansion in the hollow fiber bioreactor, multi-layer vessels were shown to be the most cost-effective in terms of yield. 49
Biomaterial substrates of distinct mechanical properties may provide enhanced tools for maintaining the therapeutic capacity of MSCs during expansion. Serial expansion of MSCs on PEG hydrogels of approximately 1 kPa stiffness showed greater maintenance of surface marker expression and greater secretome production capacity than MSCs cultured on TCPS 50 (Figure 2(d)). Furthermore, priming of MSCs on physiologically soft PDMS substrates suppressed fibrogenesis relative to stiff substrates by inhibiting microRNA miR-21 accumulation, thus enhancing their capacity to promote tissue repair. 51 The notion that soft substrates may enhance the phenotype of MSCs was further supported by work showing that inflammatory priming of MSCs with TNF-α on soft alginate hydrogels more greatly upregulated inflammatory cytokines than priming on stiff hydrogels. 52
Microphysiological systems for characterizing manufactured cells
T-cells and natural killer cells
The majority of on-going clinical trials for T-cells are for cancer immunotherapy. Microphysiological systems of cancer have been used to evaluate the efficacy of CAR T-cells in solid tumors. This is particularly important since T-cell therapies have demonstrated reduced efficacy in solid tumors due to the immunosuppressive tumor microenvironment and reduced T-cell infiltration into solid tumors. 53 Tumor spheroids, which mimic the high cellular density of solid tumors, are increasingly used to evaluate the efficacy of CAR T-cells, as well as their migratory ability into solid tumors in vitro.54,55 The incorporation of spheroids or single tumor cells into extracellular matrices, such as collagen, provides a method of further evaluating the migration of the T-cells into tumor-associated extracellular matrix mimics; 56 these assays are also amendable to incorporation of inflammatory cytokines and hypoxic conditions. 56 Another model utilizing tumor cells seeded into a porcine decellularized scaffold with intact basement membrane was able to evaluate the migration and cytotoxicity of the CAR T-cells under dynamic media flow conditions. 57
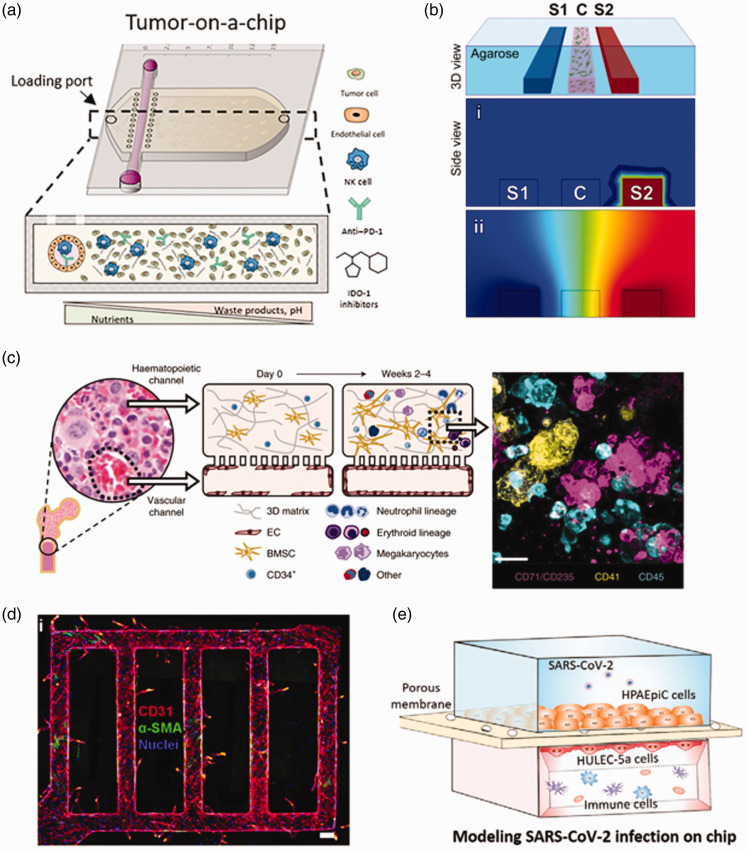
Similar 3D models and microphysiological systems of cancer have been used to evaluate NK cell function in targeting solid tumor cells. The migration of NK.92 NK cells in RGD-modified and MMP-degradable PEG gels was shown to be impaired in the presence of H1299 cancer lines that secreted anti-inflammatory TGF-β. 58 A more complex tumor on a chip model was developed that co-cultured NK.92 cells with MCF7 breast cancer cells in the presence of a endothelial cell-lined lumen; the cancer cells provided an immunosuppressive environment to the NK cells that diminished their cytotoxicity and resulted in their exhaustion, even after removal from the device 59 (Figure 4(a)).
Figure 4.
Examples of microphysiological systems that may be used to evaluate cell quality attributes. (a) A tumor-on-a chip with an engineered blood vessel and encapsulated tumor cells and NK cells can be used to evaluate NK cell exhaustion in a tumor microenvironment. Reprinted from [Ref. 59]. © The Authors, some rights reserved; exclusive licensee AAAS. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) https://creativecommons.org/licenses/by-nc/4.0/. (b) A 3D microfluidic device consisting of agarose and DCs embedded in collagen and matrigel can create stable gradients of CCL19 and CCL21 to evaluate DC migration. 60 (c) A bone marrow on a chip model consisting of a vascular channel lined with endothelial cells and a fibrin-collagen hydrogel containing bone marrow-derived MSCs and HSCs can recapitulate the bone marrow microenvironment. 61 Reprinted by permission from Nature Publishing Group. (d) Endothelialized microfluidic vessels in a collagen matrix with pericytes can recapitulate endothelial cell sprouting, an early stage of angiogenesis. 62 (e) A lung on a chip co-culturing lung epithelial cells, endothelial cells, circulating immune cells, and SARS-COV-2 can be used to recapitulate key immune responses of SARS-COV-2 infection in vitro. 63 (A color version of this figure is available in the online journal.)
Advancements in the incorporation of other cellular components of the tumor microenvironment into these models may provide more physiologically relevant platforms to test the efficacy of these cells in solid tumors. Microfluidic platforms have been used to induce blood vessel growth into 3D tumor spheroids in the presence of fibroblasts and ECM mimicking matrices, in order to integrate both key stromal cell types and to model solid tumors with high cell density.64,65 Furthermore, macrophages can be integrated into these systems to evaluate the role of anti-inflammatory tumor-associated macrophages. 66 Cancer-associated fibroblasts, which play an important role in immunosuppression, have also been integrated into these devices to evaluate their role on cancer cell migration and invasion.67,68
Dendritic cells
Monocyte-derived DCs in clinical trials are typically administered intradermally, but may also be injected intravenously, subcutaneously, and intranodally. 3 For DCs injected intradermally and subcutaneously, mature DCs, with upregulated CCR7, follow gradients of CCL21 secreted by lymphatic endothelial cells into lymphatic vessels, where they eventually migrate into the subcapsular sinus of the lymph node. Here, they then follow gradients of CCL19 and CCL21 to the lymph node paracortex, where they present antigen to and activate T-cells on top of a network of fibroblastic reticular cells.
Microphysiological systems and 3D cell culture can be used to evaluate the migration and antigen presentation of DCs in physiologically relevant microenvironments. 3D collagen and matrigel hydrogels, for example, have been used to evaluate the migration of mature and immature monocyte-derived DCs. 69 Furthermore, 3D microfluidic devices have been used to evaluate how DCs migrate under varying gradients of CCL19 and CCL21 to mimic migration into the lymph node paracortex 60 (Figure 4(b)). Co-culture of OVA-pulsed DCs with T-cells in a 3D collagen scaffold can evaluate DC antigen presentation to T-cells in physiologically relevant extracellular matricies. 70 Furthermore, 3D models of the lymph node paracortex, which contain CCL21 secreting fibroblastic reticular cells cultured on 3 D scaffolds, may provide microenvironments to test the migration and antigen presentation of DCs. 71
Hematopoietic stem cells
The success of HSCs therapies for the treatment of blood disorders relies not only on the self-renewal capacity of the cells but also their ability to engraft into the host bone marrow. Engraftment of the HSCs into the HSC niche requires a complex series of recruitment steps that include: (1) “homing” of circulating HSCs to bone marrow vasculature and transendothelial migration, (2) “transmarrow migration” into the endosteal region of the marrow, and (3) “lodgment” into the HSC niche. 72 Surface markers expressed by HSCs that are critical to the ability of HSCs to engraft into the host bone marrow microenvironment include CXCR4 (which responds to gradients of SDF-1 expressed by bone marrow cells), VLA-4/VLA-5 (which bind to VCAM-1 on bone marrow endothelial cells), ICAM-1 (which facilitates transendothelial migration), and CD49e (which binds to fibronectin).72,73
Perfusable biomaterials that recapitulate key features of the bone marrow microenvironment and express the HSC recruitment factor SDF-1 may provide useful tools to evaluate the engraft of manufactured HSCs. A bone marrow on a chip, formed initially in vivo in a mouse in a PDMS device, consisted of new bone with a trabecular network containing long-term hematopoietic stem and progenitor cells that did not need exogenous cytokines to maintain their phenotype for one week in vitro. 74 Notably, the bone marrow chip consisted of SDF-1-expressing stromal cells associated with the surface of the bone and blood vessels. 74 Another study partially recapitulated the structural and functional properties of the human bone marrow with a hydroxyapatite scaffold seeded with osteoblastic-differentiated MSCs and perfused with umbilical cord HSCs. Furthermore, the niche was customizable by incorporating SDF-1 over expressing MSCs into the system, which enhanced the quiescence of the HSCs. 74 Both of these systems, however, are limited by the lack of vasculature compartment, which diminishes the in vivo relevancy of HSC recruitment.
Compartmentalized microfluidic systems enable modeling of multiple bone marrow niches, including the vascular niche. A recent bone marrow chip, designed with two parallel PDMS channels containing (1) a fibrin-collagen gel seeded with CD34+ HSCs and MSCs and (2) lined vascular endothelial cells, was demonstrated to support both differentiation and maturation of multiple blood lineages and HSC maintenance. 61 The utility of these systems for studying HSC recruitment was shown in a similar system consisting of endothelial cell-lined vascular networks in a collagen gel with co-cultured marrow fibroblasts; monocyte-fibroblast crosstalk was critical to enhancing the adhesion of the HSCs to the endothelial network. 75 Another recent compartmentalized chip was utilized to incorporate three parts of the bone marrow niche, including a endosteal compartment, consisting of a layer of MSCs induced to osteogenic differentiation, and a central marrow and perivascular compartment, which consists of human endothelial cells, HSCs, and MSCs seeded in a fibrin-collagen hydrogel; the addition of the endosteal compartment reduced the proliferation but enhanced the maintenance of the HSCs. 76
Mesenchymal stromal cells
One globally approved product (Cartistem®, approved by Ministry of Food and Drug Safety, Korea (MFDS)) and 30 ongoing clinical trials are utilizing MSCs for the treatment of osteoarthritis. 3 Several microphysiological systems may be useful to model this disease and/or evaluate MSC-induced chonodrogenesis. Previous work has evaluated donor-to-donor variations in the ability of MSCs to form chondrogenic spheroids, which offers a physiologically relevant method for evaluating their chondrogenesis. 44 Furthermore, MSC chondrogenesis in spheroids was shown to be impaired by application of cytokines that recapitulate the inflammatory microenvironment of osteoarthritis. 77 MPS systems that also mimic hyperphysiological compression in cartilage during osteoarthritis, which was shown to induce catabolism, inflammation, and hypertrophy in the in vitro tissue, 78 may serve as a more physiologically relevant test platform for MSC chondrogenic capacity in diseased cartilage.
Another globally approved therapy (Cellgram™, approved by MFDS) and 10% of on-going MSC clinical trials are used for the treatment of cardiovascular diseases. Existing microphysiological systems for heart and blood vessel function may be utilized to explore the mechanisms by which MSCs may treat these diseases. One of the main mechanisms by which MSCs improve cardiovascular disease is through induction of angiogenesis, of which several 3D MPS systems have recapitulated.62,79–81 Co-culture of MSCs and endothelial cells in microfluidic devices have been used to demonstrate the ability of MSCs to stabilize newly formed vessels. 82 Many of these trials deal with the treatment of heart diseases, such as myocardial infarction. There are several MPS systems that model adult heart contractile function and structure, as well as various heart pathologies.83,84
There are also several MSC clinical trials for the treatment of neural diseases, such as Alzheimer’s disease and amyotrophic lateral sclerosis (ALS). There have been several recent advancements in microphysiological systems that can recapitulate these diseases, although none of these have yet been used to evaluate MSCs. An MPS model of ALS, consisting of 3D skeletal muscle bundles co-cultured with motor neuron spheroids derived from ALS patients, recapitulated key degenerative phenotypes of the disease and responded to ALS drugs. 85 Furthermore, advancements in 3D culture models have been able to recapitulate key pathological hallmarks of Alzheimer’s disease, including beta-amyloid aggregation, phosphorylated tau acclamation, and neuroinflammatory activity, with a triculture system with neurons, astrocytes, and microglia. 86 Furthermore, microfluidic models have been used to model the blood–brain barrier (BBB) in Alzheimer’s disease, with co-cultures of brain endothelial cells and neural cells that recapitulate key BBB dysfunction in AD. 87
Nearly 50% of ongoing clinical trials with MSCs are for inflammatory diseases. Many of these diseases, including chronic obstructive pulmonary disease (COPD) and COVID-19, can be modeled with lung microphysiological systems or organoids. A lung on a chip system, which involves co-culture of microvascular endothelial cells with lung epithelial cells from individuals with COPD, was able to recapitulate key features of the disease, including cytokine secretion and neutrophil recruitment. 88 These chips are amendable to studies with infection by influenza, as well as with pseudotyped SARS-COV-2 viruses. 89 A similar model with co-cultured alveolar epithelium, microvascular endothelium, and circulating immune cells demonstrated key features of viral infection and exacerbated inflammation when infected with SARS-COV-2. 63 Furthermore, several studies have explored the infection of lung organoids derived from human pluripotent stem cells with SARS-COV-2 and demonstrated upregulation of cytokines representative of what was observed in patients.90,91
Conclusion
There are a wide range of bioreactors, biomaterials, and microphysiological systems developed that can provide distinct microenvironments to cells to enhance their efficiency of expansion, to maintain their quality attributes during expansion, and to characterize the cells in a physiologically relevant setting. A greater understanding of the cues that regulate the proliferation and maintenance of therapeutic cells, as well the development of biomaterials that provide greater spatial and temporal control over cellular microenvironments are necessary to further improve the expansion of these cells. While not addressed in this review, bioreactors can also be used to further the development and maturation of cells grown on scaffolds, in order to enhance ex vivo tissue formation. Furthermore, while microphysiological systems currently may not perfectly recapitulate in vivo microenvironments and replace the use of animal models for preclinical toxicology and efficacy studies of cells, they may help significantly reduce and refine animal usage. The integration and combination of advanced methods to model human physiology, including 3D printing, organoids, and fluidically linked microphysiological systems, may also provide more physiologically relevant models for modeling human disease and creating microenvironments to control cell phenotype and growth.
ACKNOWLEDGMENTS
We acknowledge Pankaj Mandal, Haritha Vallabhaneni, Carolyn Laurencot, and Raj Puri for their review for this manuscript.
AUTHORS’ CONTRIBUTIONS: All authors participated in the design, interpretation of the studies, writing, and review of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by B.J.K.’s appointment to the Research Participation Program at CBER administered by the Oak Ridge Institute for Science and Education through the US Department of Education and US Food and Drug Administration. This work was also partially supported by research funds from the Division of Cellular and Gene Therapies.
ORCID iD: Brian J Kwee https://orcid.org/0000-0002-4426-1363
References
- 1.What is Gene Therapy?, https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy (accessed 27 March 2021)
- 2.Sung KE, Arcidiacono J, Fink DW, Jr., Gray A, Lam J, Tang W, Wu I, Puri RK. The regulatory process from concept to market. In: Lanza R, Langer R, Joesph V, Atala A (eds) Principles of tissue engineering. 5th ed. Cambridge, MA: Academic Press, 2020, pp.1553–72 [Google Scholar]
- 3.Wang LLW, Janes ME, Kumbhojkar N, Kapate N, Clegg JR, Prakash S, Heavey MK, Zhao Z, Anselmo AC, Mitragotri S. Cell therapies in the clinic. Bioeng Transl Med 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Approved Cellular and Gene Therapy Products, https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed 27 March 2021)
- 5.Roh K-H, Nerem RM, Roy K. Biomanufacturing of therapeutic cells: state of the art, current challenges, and future perspectives. Annu Rev Chem Biomol Eng 2016; 7:455–78 [DOI] [PubMed] [Google Scholar]
- 6.Golchin A, Farahany TZ. Biological products: cellular therapy and FDA approved products. Stem Cell Rev Rep 2019; 15:166–75 [DOI] [PubMed] [Google Scholar]
- 7.Fiorenza S, Ritchie DS, Ramsey SD, Turtle CJ, Roth JA. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant 2020; 55:1706–15 [DOI] [PubMed] [Google Scholar]
- 8.Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem 2012; 113:2806–12 [DOI] [PubMed] [Google Scholar]
- 9.Ho A, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy 2008; 10:320–30 [DOI] [PubMed] [Google Scholar]
- 10.Advancing Alternative Methods at FDA, https://www.fda.gov/science-research/about-science-research-fda/advancing-alternative-methods-fda (accessed 27 March 2021)
- 11.Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol 2019; 10:2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine 2020; 59:102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suen WC-W, Lee WY-W, Leung K-T, Pan X-H, Li G. Natural killer cell-based cancer immunotherapy: a review on 10 years completed clinical trials. Cancer Invest 2018; 36:431–57 [DOI] [PubMed] [Google Scholar]
- 14.Constantino J, Gomes C, Falcão A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res 2017; 65:798–810 [DOI] [PubMed] [Google Scholar]
- 15.Lesterhuis WJ, de Vries IJM, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, Scharenborg NM, van de Rakt MW, de Boer AJ, Croockewit S. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res 2011; 17:5725–35 [DOI] [PubMed] [Google Scholar]
- 16.Chabannon C, Kuball J, Bondanza A, Dazzi F, Pedrazzoli P, Toubert A, Ruggeri A, Fleischhauer K, Bonini C. Hematopoietic stem cell transplantation in its 60s: a platform for cellular therapies. Sci Transl Med 2018; 10: eaap9630. [DOI] [PubMed] [Google Scholar]
- 17.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006; 8:315–7 [DOI] [PubMed] [Google Scholar]
- 18.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 2016; 25:829–48 [DOI] [PubMed] [Google Scholar]
- 19.Lambert LH, Goebrecht GK, De Leo SE, O’Connor RS, Nunez-Cruz S, Li T-D, Yuan J, Milone MC, Kam LC. Improving T cell expansion with a soft touch. Nano Lett 2017; 17:821–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey JW, Dong Y, Chung JW, Salathe SF, Pruitt HC, Li X, Chang C, Fraser AK, Bessell CA, Ewald AJ. Engineering an artificial T‐cell stimulating matrix for immunotherapy. Adv Mater 2019; 31:1807359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delalat B, Harding F, Gundsambuu B, De-Juan-Pardo EM, Wunner FM, Wille M-L, Jasieniak M, Malatesta KA, Griesser HJ, Simula A. 3D printed lattices as an activation and expansion platform for T cell therapy. Biomaterials 2017; 140:58–68 [DOI] [PubMed] [Google Scholar]
- 22.Cheung AS, Zhang DK, Koshy ST, Mooney DJ. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat Biotechnol 2018; 36:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang DK, Cheung AS, Mooney DJ. Activation and expansion of human T cells using artificial antigen-presenting cell scaffolds. Nat Protoc 2020; 15:773–98 [DOI] [PubMed] [Google Scholar]
- 24.Costariol E, Rotondi MC, Amini A, Hewitt CJ, Nienow AW, Heathman TR, Rafiq QA. Demonstrating the manufacture of human CAR‐T cells in an automated stirred‐tank bioreactor. Biotechnol J 2020; 15:2000177. [DOI] [PubMed] [Google Scholar]
- 25.Castella M, Caballero-Baños M, Ortiz-Maldonado V, González-Navarro EA, Suñé G, Antoñana-Vidósola A, Boronat A, Marzal B, Millán L, Martín-Antonio B. Point-Of-Care CAR T-cell production (ARI-0001) using a closed semi-automatic bioreactor: experience from an academic phase I clinical trial. Front Immunol 2020; 11:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai X, Mei Y, Cai D, Han W. Standardizing CAR-T therapy: getting it scaled up. Biotechnol Adv 2019; 37:239–45 [DOI] [PubMed] [Google Scholar]
- 27.Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, Alici E. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy 2010; 12:1044–55 [DOI] [PubMed] [Google Scholar]
- 28.Meng Y, Sun J, Hu T, Ma Y, Du T, Kong C, Zhang G, Yu T, Piao H. Rapid expansion in the WAVE bioreactor of clinical scale cells for tumor immunotherapy. Hum Vaccin Immunother 2018; 14:2516–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn YH, Ren L, Kim SM, Seo S-H, Jung C-R, Noh J-Y, Lee SY, Lee H, Cho MY, Jung H. A three-dimensional hyaluronic acid-based niche enhances the therapeutic efficacy of human natural killer cell-based cancer immunotherapy. Biomaterials 2020; 247:119960. [DOI] [PubMed] [Google Scholar]
- 30.Eyrich M, Schreiber SC, Rachor J, Krauss J, Pauwels F, Hain J, Wölfl M, Lutz MB, de Vleeschouwer S, Schlegel PG. Development and validation of a fully GMP-compliant production process of autologous, tumor-lysate-pulsed dendritic cells. Cytotherapy 2014; 16:946–64 [DOI] [PubMed] [Google Scholar]
- 31.Nava S, Dossena M, Pogliani S, Pellegatta S, Antozzi C, Baggi F, Gellera C, Pollo B, Parati EA, Finocchiaro G. An optimized method for manufacturing a clinical scale dendritic cell-based vaccine for the treatment of glioblastoma. PLoS One 2012; 7:e52301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fekete N, Béland AV, Campbell K, Clark SL, Hoesli CA. Bags versus flasks: a comparison of cell culture systems for the production of dendritic cell-based immunotherapies. Transfusion 2018; 58:1800–13 [DOI] [PubMed] [Google Scholar]
- 33.Uslu U, Erdmann M, Wiesinger M, Schuler G, Schuler-Thurner B. Automated good manufacturing practice–compliant generation of human monocyte-derived dendritic cells from a complete apheresis product using a hollow-fiber bioreactor system overcomes a major hurdle in the manufacture of dendritic cells for cancer vaccines. Cytotherapy 2019; 21:1166–78 [DOI] [PubMed] [Google Scholar]
- 34.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013; 122:491–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai T, Li J, Sinclair A, Imren S, Merriam F, Sun F, O’Kelly MB, Nourigat C, Jain P, Delrow JJ. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat Med 2019; 25:1566–75 [DOI] [PubMed] [Google Scholar]
- 36.Ferreira MSV, Jahnen-Dechent W, Labude N, Bovi M, Hieronymus T, Zenke M, Schneider RK, Neurs S. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 2012; 33:6987–97 [DOI] [PubMed] [Google Scholar]
- 37.Mousavi SH, Abroun S, Soleimani M, Mowla SJ. 3-Dimensional nano-fibre scaffold for ex vivo expansion of cord blood haematopoietic stem cells. Artif Cells Nanomed Biotechnol 2018; 46:740–8 [DOI] [PubMed] [Google Scholar]
- 38.Gvaramia D, Müller E, Müller K, Atallah P, Tsurkan M, Freudenberg U, Bornhäuser M, Werner C. Combined influence of biophysical and biochemical cues on maintenance and proliferation of hematopoietic stem cells. Biomaterials 2017; 138:108–17 [DOI] [PubMed] [Google Scholar]
- 39.Csaszar E, Kirouac DC, Yu M, Wang W, Qiao W, Cooke MP, Boitano AE, Ito C, Zandstra PW. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell 2012; 10:218–29 [DOI] [PubMed] [Google Scholar]
- 40.Raic A, Rödling L, Kalbacher H, Lee-Thedieck C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials 2014; 35:929–40 [DOI] [PubMed] [Google Scholar]
- 41.Xue C, Kwek KY, Chan JK, Chen Q, Lim M. The hollow fiber bioreactor as a stroma‐supported, serum‐free ex vivo expansion platform for human umbilical cord blood cells. Biotechnol J 2014; 9:980–9 [DOI] [PubMed] [Google Scholar]
- 42.Klinker MW, Marklein RA, Surdo JLL, Wei C-H, Bauer SR. Morphological features of IFN-γ–stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc Natl Acad Sci U S A 2017; 114:E2598–E607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marklein RA, Lo Surdo JL, Bellayr IH, Godil SA, Puri RK, Bauer SR. High content imaging of early morphological signatures predicts long term mineralization capacity of human mesenchymal stem cells upon osteogenic induction. Stem Cells 2016; 34:935–47 [DOI] [PubMed] [Google Scholar]
- 44.Lam J, Bellayr IH, Marklein RA, Bauer SR, Puri RK, Sung KE. Functional profiling of chondrogenically induced multipotent stromal cell aggregates reveals transcriptomic and emergent morphological phenotypes predictive of differentiation capacity. Stem Cells Transl Med 2018; 7:664–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam AT-L, Li J, Toh JP-W, Sim EJ-H, Chen AK-L, Chan JK-Y, Choolani M, Reuveny S, Birch WR, Oh SK-W. Biodegradable poly-ε-caprolactone microcarriers for efficient production of human mesenchymal stromal cells and secreted cytokines in batch and fed-batch bioreactors. Cytotherapy 2017; 19:419–32 [DOI] [PubMed] [Google Scholar]
- 46.Yuan Y, Kallos MS, Hunter C, Sen A. Improved expansion of human bone marrow‐derived mesenchymal stem cells in microcarrier‐based suspension culture. J Tissue Eng Regen Med 2014; 8:210–25 [DOI] [PubMed] [Google Scholar]
- 47.da Silva JS, Severino P, Wodewotzky TI, Covas DT, Swiech K, Marti LC, Suazo CAT. Mesenchymal stromal cells maintain the major quality attributes when expanded in different bioreactor systems. Biochem Eng J 2020; 161:107693 [Google Scholar]
- 48.de Sousa Pinto D, Bandeiras C, de Almeida Fuzeta M, Rodrigues CA, Jung S, Hashimura Y, Tseng RJ, Milligan W, Lee B, Ferreira FC. Scalable manufacturing of human mesenchymal stromal cells in the vertical‐wheel bioreactor system: an experimental and economic approach. Biotechnol J 2019; 14:1800716. [DOI] [PubMed] [Google Scholar]
- 49.Mizukami A, Chilima TDP, Orellana MD, Neto MA, Covas DT, Farid SS, Swiech K. Technologies for large-scale umbilical cord-derived MSC expansion: experimental performance and cost of goods analysis. Biochem Eng J 2018; 135:36–48 [Google Scholar]
- 50.Rao VV, Vu MK, Ma H, Killaars AR, Anseth KS. Rescuing mesenchymal stem cell regenerative properties on hydrogel substrates post serial expansion. Bioeng Transl Med 2019; 4:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, Hinz B. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 2017; 16:379–89 [DOI] [PubMed] [Google Scholar]
- 52.Wong SW, Lenzini S, Cooper MH, Mooney DJ, Shin J-W. Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Sci Adv 2020; 6:eaaw0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newick K, O'Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med 2017; 68:139–52 [DOI] [PubMed] [Google Scholar]
- 54.Szöőr Á, Tóth G, Zsebik B, Szabó V, Eshhar Z, Abken H, Vereb G. Trastuzumab derived HER2-specific CARs for the treatment of trastuzumab-resistant breast cancer: CAR T cells penetrate and eradicate tumors that are not accessible to antibodies. Cancer Lett 2020; 484:1–8 [DOI] [PubMed] [Google Scholar]
- 55.Dillard P, Köksal H, Inderberg E-M, Wälchli S. A spheroid killing assay by CAR T cells. J Vis Exp 2018; 10:58785. [DOI] [PubMed] [Google Scholar]
- 56.Pavesi A, Tan AT, Koh S, Chia A, Colombo M, Antonecchia E, Miccolis C, Ceccarello E, Adriani G, Raimondi MT. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight 2017; 2:e89762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallstabe L, Göttlich C, Nelke LC, Kühnemundt J, Schwarz T, Nerreter T, Einsele H, Walles H, Dandekar G, Nietzer SL. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight 2019; 4: e126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Temples MN, Adjei IM, Nimocks PM, Djeu J, Sharma B. Engineered three-dimensional tumor models to study natural killer cell suppression. ACS Biomater Sci Eng 2020; 6:4179–99 [DOI] [PubMed] [Google Scholar]
- 59.Ayuso JM, Rehman S, Virumbrales-Munoz M, McMinn PH, Geiger P, Fitzgerald C, Heaster T, Skala MC, Beebe DJ. Microfluidic Tumor-on-a-chip model to evaluate the role of tumor environmental stress on NK cell exhaustion. Sci Adv 2021; 7:eabc2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc Natl Acad Sci U S A 2011; 108:5614–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, MacDonald A, Bölükbaşı ÖV, Joyce CE, Teixeira LSM. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng 2020; 4:394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 2012; 109:9342–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M, Wang P, Luo R, Wang Y, Li Z, Guo Y, Yao Y, Li M, Tao T, Chen W. Biomimetic human disease model of SARS‐CoV‐2‐induced lung injury and immune responses on organ chip system. Adv Sci 2021; 8:2002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haase K, Offeddu GS, Gillrie MR, Kamm RD. Endothelial regulation of drug transport in a 3D vascularized tumor model. Adv Funct Mater 2020; 30:2002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nashimoto Y, Okada R, Hanada S, Arima Y, Nishiyama K, Miura T, Yokokawa R. Vascularized cancer on a chip: the effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020; 229:119547. [DOI] [PubMed] [Google Scholar]
- 66.Bi Y, Shirure VS, Liu R, Cunningham C, Ding L, Meacham JM, Goedegebuure SP, George SC, Fields RC. Tumor-on-a-chip platform to interrogate the role of macrophages in tumor progression. Integr Biol 2020; 12:221–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truong DD, Kratz A, Park JG, Barrientos ES, Saini H, Nguyen T, Pockaj B, Mouneimne G, LaBaer J, Nikkhah M. A human organotypic microfluidic tumor model permits investigation of the interplay between patient-derived fibroblasts and breast cancer cells. Cancer Res 2019; 79:3139–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sung KE, Yang N, Pehlke C, Keely PJ, Eliceiri KW, Friedl A, Beebe DJ. Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr Biol 2011; 3:439–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cougoule C, Lastrucci C, Guiet R, Mascarau R, Meunier E, Lugo-Villarino G, Neyrolles O, Poincloux R, Maridonneau-Parini I. Podosomes, but not the maturation status, determine the protease-dependent 3D migration in human dendritic cells. Front Immunol 2018; 9:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunzer M, Schäfer A, Borgmann S, Grabbe S, Zänker KS, Bröcker E-B, Kämpgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity 2000; 13:323–32 [DOI] [PubMed] [Google Scholar]
- 71.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol 2009; 183:4273–83 [DOI] [PubMed] [Google Scholar]
- 72.Nilsson SK, Simmons PJ, Bertoncello I. Hemopoietic stem cell engraftment. Exp Hematol 2006; 34:123–9 [DOI] [PubMed] [Google Scholar]
- 73.Zheng Y, Watanabe N, Nagamura-Inoue T, Igura K, Nagayama H, Tojo A, Tanosaki R, Takaue Y, Okamoto S, Takahashi TA. Ex vivo manipulation of umbilical cord blood-derived hematopoietic stem/progenitor cells with recombinant human stem cell factor can up-regulate levels of homing-essential molecules to increase their transmigratory potential. Exp Hematol 2003; 31:1237–46 [DOI] [PubMed] [Google Scholar]
- 74.Torisawa Y-S, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat Methods 2014; 11:663–9 [DOI] [PubMed] [Google Scholar]
- 75.Kotha SS, Hayes BJ, Phong KT, Redd MA, Bomsztyk K, Ramakrishnan A, Torok-Storb B, Zheng Y. Engineering a multicellular vascular niche to model hematopoietic cell trafficking. Stem Cell Res Ther 2018; 9:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson MR, Ghoshal D, Mejías JC, Rubio DF, Keith E, Roy K. A multi-niche microvascularized human bone marrow (hBM) on-a-chip elucidates key roles of the endosteal niche in hBM physiology. Biomaterials 2021; 270:120683. [DOI] [PubMed] [Google Scholar]
- 77.Mohanraj B, Huang AH, Yeger‐McKeever MJ, Schmidt MJ, Dodge GR, Mauck RL. Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro‐inflammatory cytokines. J Orthop Res 2018; 36:2901–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Occhetta P, Mainardi A, Votta E, Vallmajo-Martin Q, Ehrbar M, Martin I, Barbero A, Rasponi M. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat Biomed Eng 2019; 3:545–57 [DOI] [PubMed] [Google Scholar]
- 79.Nguyen D-HT, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A 2013; 110:6712–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bischel LL, Young EW, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 2013; 34:1471–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013; 13:1489–500 [DOI] [PubMed] [Google Scholar]
- 82.Trkov S, Eng G, Di Liddo R, Parnigotto PP, Vunjak‐Novakovic G. Micropatterned three‐dimensional hydrogel system to study human endothelial–mesenchymal stem cell interactions. J Tissue Eng Regen Med 2010; 4:205–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019; 176:913–27.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018; 556:239–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osaki T, Uzel SG, Kamm RD. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci Adv 2018; 4:eaat5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, Kim DY, Tanzi RE, Cho H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci 2018; 21:941–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD, Tanzi RE. Blood–brain barrier dysfunction in a 3D in vitro model of Alzheimer's disease. Adv Sci 2019; 6:1900962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee H-H, Alves SE, Salmon M, Ferrante TC, Weaver JC. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods 2016; 13:151–7 [DOI] [PubMed] [Google Scholar]
- 89.Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, Nurani A, Zhu DY, Goyal G, Gilpin SE. Human organs-on-chips as tools for repurposing approved drugs as potential influenza and COVID19 therapeutics in viral pandemics. bioRxiv 2020. 10.1101/2020.04.13.039917 [DOI] [Google Scholar]
- 90.Han Y, Duan X, Yang L, Nilsson-Payant BE, Wang P, Duan F, Tang X, Yaron TM, Zhang T, Uhl S. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 2021; 589:270–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 2020; 27:125–36. e7 [DOI] [PMC free article] [PubMed] [Google Scholar]