Since the beginning of the COVID-19 pandemic, divergent variants of concern (VoCs) of SARS-CoV-2 have evolved and become the most prevalent SARS-CoV-2 variants in distinct locations at different times. Currently, the Delta variant (B.1.617.2) dominates infection events in large parts of the world. Immunization campaigns, however, still use SARS-CoV-2 vaccines based on the spike (S) protein of the original Wuhan virus.
The S protein of the Delta variant of SARS-CoV-2 harbors mutations that support replication and transmission but also weaken the binding of neutralizing antibodies. The Delta variant has been reported to evade control by antibodies induced upon infection and, arguably more relevant, after BNT162b2 (BNT) vaccination [1, 2]. Likewise, the ChAdOx1 nCoV-19 (ChAd) vaccine appeared less effective than the BNT vaccine in preventing SARS-CoV-2 infection with the Delta variant [2, 3].
In addition to homologous prime-boost protocols, millions of ChAd-primed vaccinees received heterologous boost immunization with mRNA-based SARS-CoV-2 vaccines, as vaccination with ChAd was halted due to an increased risk of thrombotic events.
The results from randomized [4, 5] and observational studies [6–8] demonstrated that heterologous prime-boost protocols also induce robust humoral and cellular responses accompanied by acceptable reactogenicity. These reports prompted others to suggest that mixing vaccines might be a suitable strategy to combat emerging SARS-CoV-2 variants [9]. However, information is limited regarding the neutralization capacities against the Delta variant of various immunization regimens.
We assessed plasma from 85 individuals at a mean of 68 days (range 45–91 days) after ChAd priming and a mean of 17 days (range 13–23 days) after either homologous ChAd (n = 31, 20 females) or heterologous BNT (n = 54, 40 females) prime-boost protocols [6] for their capacity to neutralize the Delta variant by applying surrogate virus neutralization tests (sVNTs). For comparison, we also tested plasma from 30 individuals (21 females) at a mean of 21 days (range 18–27 days) after BNT priming and at a mean of 30 days (range 15–65 days) after homologous BNT prime-boost protocols.
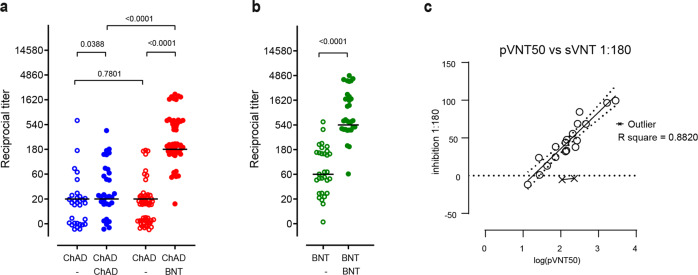
While homologous ChAd boosting only slightly increased neutralization of the Delta variant, heterologous ChAd/BNT vaccination led to a ninefold increase in neutralizing titers (Fig. 1a), resulting in detectable neutralizing sVNT titers in all individuals receiving this vaccination schedule. Similarly, homologous BNT prime-boost protocols also led to a ninefold increase but resulted in overall higher titers of neutralizing antibodies than heterologous immunization (Fig. 1b). As reported before for the Alpha, Beta, and Gamma VoCs [6], the results obtained with the Delta receptor binding domain-based sVNT were also closely correlated with data obtained with a vesicular stomatitis virus-based pseudotyped virus neutralization assay [10], which is based on particles harboring the spike protein of the Delta variant (Fig. 1c). Interestingly, although heterologous BNT boost after ChAd prime consistently resulted in higher neutralizing titers against the Alpha, Beta, and Gamma variants compared to homologous BNT vaccination [6], homologous BNT prime-boost appears to more efficiently induce neutralizing antibodies against the Delta variant.
Fig. 1.
Stronger humoral immune responses against the Delta SARS-CoV-2 variant following heterologous ChAdOx1 nCoV-19 (ChAd)/BNT162b2 (BNT) than homologous ChAd/ChAd vaccination. a Reciprocal titers of neutralizing antibodies against the Delta SARS-CoV-2-S variant measured using a surrogate virus neutralization test (sVNT). Data are from n = 31 biologically independent samples from the ChAd/ChAd group and n = 54 biologically independent samples from the ChAd/BNT group. b Reciprocal titers of neutralizing antibodies against the Delta SARS-CoV-2 variant measured using a surrogate virus neutralization test (sVNT). Data are from n = 30 biologically independent samples from BNT/BNT. c Efficacies of antibody neutralization against the SARS-CoV-2 Delta variant measured by surrogate virus neutralization tests (sVNT) show a strong positive correlation with those of pseudotyped virus neutralization tests (pVNT). Correlation (solid line) and 95% confidence intervals (dotted lines) between sVNT1:180 and antibody titers resulting in a 50% reduction in luciferase activity in pVNT, indicated as pVNT50. Open circles, values from individual donors, and outliers are marked with X and were defined as values with absolute residual value >2 SD of all residual values in each group of samples. Correlation was calculated using simple linear regression. a, b Chi-square test for trend; dots represent individual vaccinees, lines represent group median. Open symbols: preboost sera, filled symbols: postboost sera. For better visualization of identical titer values, data were randomly and proportionally adjusted closely around the precise titer result
The overall robust inhibition of the Delta variant further supports heterologous boosting with BNT of vaccinees initially primed with ChAd. However, in contrast to Alpha, Beta, and Gamma variants, homologous BNT prime-boost vaccination might be even more efficient in neutralizing the Delta variant.
Our data emphasize a high level of complexity in antibody responses that is affected not only by the vaccine or the vaccinee’s immune system but also by the VoC to be combatted. Moreover, our data indicate the urgent need for detailed studies to evaluate which vaccine schedules are best suited to combat COVID-19, once humoral immunity starts diminishing and the risk for infection and disease rises again in the vaccinated population.
Supplementary information
Acknowledgements
The study was approved by the Internal Review Board of Hannover Medical School (institutional review board no. 8973_BO-K_2020, amendment Dec 2020). All participants gave written informed consent. Supported by the German Center for Infection Research TTU 01.938 (Grant No. 80018019238 to GMNB and RF), by the German Center for Lung Research (Grant 82DZL002B1) by Deutsche Forschungsgemeinschaft, (DFG, German Research Foundation) Excellence Strategy EXC 2155 ‘RESIST’ (Project ID39087428 to RF), by funds of the State of Lower Saxony (14-76103-184 CORONA-11/20 to RF; 14-76103-184, MWK HZI COVID-19 to SP), by funds of the BMBF (NaFoUniMedCovid19 FKZ: 01KX2021; Projects B-FAST to RF, Projects 01KI2006D, 01KI20328A, 01KI20396, 01KX2021 to SP) and Deutsche Forschungsgemeinschaft, SFB 900/3 (Projects B1, 158989968 to RF; Projects PO 716/11-1, PO 716/14-1 to SP).
Author contributions
SIH analyzed data; SIH, BB, AD-J, GMNB and RF conceptualized the study; SIH, GB, MF, IR, MH and SP performed experiments; RF wrote manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Georg M. N. Behrens, Reinhold Förster.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00755-z.
References
- 1.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–80. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 2.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–94. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–6. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–30. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021. 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed]
- 7.Normark J, Vikström L, Gwon YD, Persson IL, Edin A, Björsell T, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021. 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed]
- 8.Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021. 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed]
- 9.Duarte-Salles T, Prieto-Alhambra D. Heterologous vaccine regimens against COVID-19. Lancet. 2021;398:94–95. doi: 10.1016/S0140-6736(21)01442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184:2384–93.e12. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.