Abstract
Purpose
To evaluate the potential opportunities and possible competitiveness of Avatera robotic system (ARS) (Avateramedical, Germany), and perform predictive cost-analysis for its implementation and dissemination.
Material and methods
Our study employed a projective quantitative research design. SWOT (strengths, weaknesses, opportunities, threats) analysis was used to map ARS internal competencies towards external contexts, and potential opportunities and risks in the robotic market. The ARS purchase and procedural costs were evaluated in two different scenarios.
Results
In the first scenario, setting the purchase cost of the Avatera at around $1.3–1.5 million, a total $400 procedural cost reduction compared to the RAS performed with the da Vinci Xi can be calculated. In the second scenario, with a purchase cos of the ARS of $700.000–800.000 and considering a 5-year period with an annual ARS volume of 500 procedures, only an additional $300 will be attributed to the robot itself. Our projections revealed that for an effective competition the purchase cost of ARS should range between $700.000 and $800.000 during the initial phase of market entry. The marketing strategy of the ARS should be oriented towards countries without any robotic system in operational use, followed by countries where the competition intensity in the marketplace is low.
Conclusion
The introduction of new robotic systems will greatly affect and reshape the market of robotic surgery. The ARS has all the technical capacity ensuring the performance of high-quality surgical procedures. A fast spread and implementation of the ARS could be expected should the purchase and maintenance costs be kept low.
Keywords: Robot-assisted surgery, Avatera robotic system, Da Vinci, Cost analysis, Business modeling, SWOT analysis
Introduction
During the last 2 decades, several robotic systems (RS) have been introduced in the medical market space with the aim to improve the quality of surgical services. The pioneer RS gaining wide popularity, the da Vinci robotic system (dVRS) (Intuitive Medical, Sunnyvale, CA, USA), was first introduced in 1999. With the huge increase in installations of Da Vinci robots > 5800 units worldwide and with the annual number of robotic surgeries > 8.5 million cases, urology, gynecology and visceral surgery represent the main fields utilizing those systems [1].
Among the proposed benefits of robotic surgery over the conventional laparoscopy are the avoidance of hand tremor, instrument tip movement with 7 degrees of freedom (df), three-dimensional vision, improved ergonomics and full control over 3 working instruments and camera by the operating surgeon, and potential reduction of the learning curve [2, 3]. Nevertheless, the current dVRSs are associated with several limitations, the main remaining its higher acquisition and maintenance costs, resulting in higher procedural costs compared to the open and laparoscopic counterparts [4].
The introduction of new cheaper RSs will potentially decrease the cost of robotic surgery and open the possibility for a more competitive landscape. In 2019, with the expiry of the key patents period of Intuitive Surgical, new RSs had the possibility to enter the market. The robotic alternatives to the dVRS include the Senhance system (Transenterix, Morrisville, NC, USA), Revo-I (Meere Company, Hwasong, Korea), Hugo RS (Medtronic, Dublin, Ireland), Versius (CMR Surgical, Cambridge, GB), Flex Surgical System (Medrobotics, Raynham, USA) and Avatera (Avateramedical, Jena, Germany) [5, 6]. The main features, advantages and disadvantages of newly developed RSs are summarized in Table 1. Some of these devices have been already tested clinically in Europe and Asia, while the others will appear in the market in near future. However, there is scarce literature evaluating the existing market gap and, to our knowledge, no study analyzing business opportunities of new emerging RSs. With the current paper, we aim to evaluate the potential opportunities and possible competitiveness of Avatera robotic system (ARS), and perform predictive cost-analysis for its implementation and dissemination.
Table 1.
| Name | Features | Use | Approval | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Open console robotic systems | |||||
| Senhance system (Transenterix, Morrisville, NC, USA) |
A 3D vision with glasses An infrared eye-tracking feature to control camera movement Up to 4 robotic arms on independent carts Feature of haptic feedback 5 and 3 mm mini-laparoscopic instruments |
Abd. surgery, gynecology, urology | FDA and CE approval in 2017 |
Haptic feedback 3 mm mini-laparoscopic instruments Independent carts Easier intraoperative communication No need for system-specific trocars |
Camera control with head movements (limits the surgeons comfort) 3D vision with glasses Handles similar to laparoscopic instruments 4 df range of movements similar to laparoscopy |
| Versius (CMR Surgical, Cambridge, GB) |
A 3D vision with glasses Up to 5 robotic arms on independent carts 7 df range of movements 5 mini-laparoscopic instruments |
Abd. surgery, gynecology, urology, thoracic surgery, ENT | Only CE approval in 2019 |
Independent carts Up to 5 arms Easier intraoperative communication No need for system-specific trocars |
Camera control with head movements (limits the surgeons comfort) 3D vision with glasses Instruments handling is ergonomic compared to “clutch” function |
| Hugo RAS (Medtronic, Dublin, Ireland) |
A 3D vision with glasses Up to 4 robotic arms on independent carts 7 df range of movements 5 mini-laparoscopic instruments |
Not applicable | CE approval projected in 2021 |
Independent carts Easier intraoperative communication |
Camera control with head movements (limits the surgeons comfort) 3D vision with glasses Instruments handling is ergonomic compared to “clutch” function |
| MiroSurge (German AeroSpace Center (DLR), Institute of robotics and mechatronics, Wessling, Germany) |
Feature of haptic feedback A 3D vision with glasses 3 robotic arms on independent carts 7 df range of movements 5 mini-laparoscopic instruments |
Not applicable | Not applicable |
Independent carts Easier intraoperative communication Haptic feedback Instrument collision detection feature |
Camera control with head movements (limits the surgeons comfort) 3D vision with glasses Instruments handling is ergonomic compared to “clutch” function |
| Flex surgical system (Medrobotics, Raynham, USA) |
Flexible design of camera system operated with a simplified joystick Accompanied by 3 mm flexible instruments with articulating wrists |
Colorectal, ENT | FDA and CE approval in 2019 |
Flexible camera Flexible 3 mm instruments |
Designed for ENT and colorectal surgeries |
| Close console robotic systems | |||||
| Revo-I (Meere Company, Hwasong, Korea) |
Closed-console control unit 3D HD vision Robotic cart with 4 robotic arms 7 df range of movements 5 mm instruments Clutch function Training module |
Abd. surgery, gynecology, urology | Korean FDA approval |
Closed-console control unit “Clutch” function Training module |
Obscured intraoperative communication Instrument clashing |
| Avatera (Avateramedical, Jena, Germany) |
Closed-console control unit 3D HD vision Slender eyepiece design (surgeon’s mouth and ears remain uncovered) Robotic cart with 4 robotic arms 7 df range of movements 5 mm single-use instruments Use of only bipolar energy “Clutch” function |
Abd. surgery, gynecology, urology | CE approval projected in 2021 |
Closed-console control unit Slender eyepiece design (surgeon’s mouth and ears remain uncovered) Single-use instruments Use of only bipolar energy Clutch function |
Instrument clashing |
Material and methods
The Avatera robotic system
The Avatera (Avateramedical, Jena, Germany) was founded in 2011 and represents the first German RS. Similar to the dVRS, it is a 2-component robotic system consisting of a separate closed-console control unit for an operating surgeon and a robotic cart with mounted 4 robotic arms. As such, it can be easily incorporated in most of the surgical theatres. It possesses a full HD resolution camera and is accompanied by 5 mm fully articulated working instruments with a 7 df range of movements. Likewise, dVRS, the movement of the instrument branches is accomplished with the clutch mechanism. The company offers a wide variety of instruments suitable for most visceral surgical procedures [7].
In addition to the main requirements, the ARS possesses several unique characteristics differentiating it from its competitors. The main such feature is the utilization of fully disposable instruments working exclusively with bipolar energy. Since the instruments are disposable, there is no risk cross-contamination. Moreover, the sterilization and cleaning of the instruments is time and cost demanding, and could cause their inadvertent damage [7]. An additional improved feature is the particular design of the eyepiece, leaving the surgeon’s ear and mouth uncovered, thus facilitating unobscured cooperation of the surgeon with his operating team during surgical procedures [7].
Study design and study tool
Our study implemented a projective quantitative research. Due to the absence of clinical data, a predictive analysis was conducted based on the performance data of dVRS. To identify the competitiveness of ARS and to assess the risk and potential opportunities of the ARS, the SWOT (strengths, weaknesses, opportunities, threats) analytical tool was implemented. The key features were included in 4 different quadrants and discussed separately for balanced decision-making. Due to space constraints, a detailed description of all key features for ARS is presented in Table 2.
Table 2.
Strengths, weaknesses, opportunities and costs of the Avatera robotic system
| Strengths | Weaknesses |
|---|---|
|
1. Unique device characteristics Single-use instruments Use of only bipolar energy Slender eye-piece design 2. Similar main features as the standard da Vinci RS 3. Easy-to-learn handling and modern training concept 4. Timely introduction (only one player in the market) 5. Reduced costs for robotic service |
1. No clinical studies on the Avatera platform 2. Well-established operational and functional features of da Vinci robot 3. Difficulties in changing surgeons’ attitudes and beliefs |
| Opportunities | Threats |
|---|---|
|
1. Further technological improvements Robotic sealing instruments (simultaneous cutting and hemostasis) 3D vision for all bedside surgical team Separate carts for each arm 2. Covid-19 Surgeries requiring minimal surgical personal involvement (true for all RS) Increasing need for one-day MAS surgeries |
1. Technical defects of the robot 2. Covid-19 associated issues Supply difficulties Training difficulties |
Calculation of ARS purchase cost
For calculation of purchase costs of the ARS, the current prices of the da Vinci Xi robot were considered [8]. Several technical components including purchase and maintenance costs of the robotic device itself, instrument sterilization and potential damage contribute to cost formation. In addition, to evaluate the cost-effectiveness of ARS procedures, perioperative clinical outcomes such as surgery duration and length of postoperative hospital stay, development of perioperative complications and readmission rates should be additionally encountered. To make our calculations simpler, we would assume that the alternative RS including the Avatera robot will possess a similar clinical safety profile for patients as that of the da Vinci robot.
To have a better understanding of the effect of the purchase and maintenance cost on the final procedural cost, two different scenarios with different purchase costs for ARS will be discussed.
For our projections, we have consulted experts, along with an analysis of secondary data and considered a hypothetical average cost of around €5.000 (≈ $6.000) for a RARP at a standard private hospital in Greece (considered a high-income country). Extracting administrative costs, medication, hospitalization, and surgery including charges for robotic instruments and maintenance (cost per-use = $1.500), a 20% (€1.000 ≈ $1.200) grossly estimated profit can be expected. With a purchase price for the da Vinci Xi robot of approximately $2 million, adding the annual maintenance costs, a mean annual number of 500 robot-assisted surgeries (RAS), will be required from the hospital to compensate for its purchase costs in 5 years.
From the alternative systems, the recently introduced Senhance surgical robot device is available with a purchase price of $1–1.2 million. Unlike the dVRS, the Senhance robotic system is associated with significantly reduced per-use costs of $200–500 for each procedure [8]. The official pricing of another CE-approved Versius robotic system is not available yet. However, the approximate possible purchase price can be around $1.5 million and with the approximate per-use costs of $1.500.
Results
Pricing of the ARS
In the first scenario, setting the purchase cost of the Avatera at around $1.3–1.5 million, in the same range as the Senhance and the Versius, a total $400 reduction of the procedural cost compared to the RAS performed with the da Vinci Xi can be calculated. Further, cost reduction can be expected with the utilization of cheaper single-use instruments (< $1.000) and elimination of their sterilization costs. As such, the price of the RARP at a standard private hospital in Greece can be expected to decrease by as much as €800–900 reaching a level of 4.100€ from the estimated 5.000€.
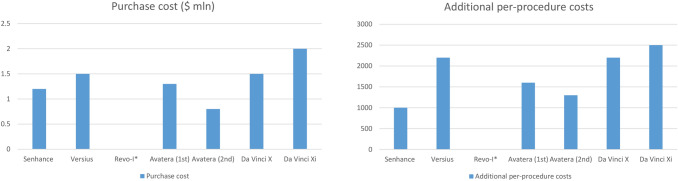
For the second scenario, the initial purchase cost of the Avatera robot will be set at $700.000–800.000. Considering a 5-year period with an annual ARS volume of 500 procedures, only an additional $300 will be attributed to the robot itself. Adding the per-use costs (≈ $1.000), an overall procedural costs of $1.300 can be expected, which will be significantly lower compared to the da Vinci Xi (procedure cost—$2.500) and a little higher compared to Senhance (procedure cost—$1.000). The comparisons of the per procedure costs of different RSs are portrayed in Fig. 1.
Fig. 1.
Additional per-procedural costs attributed to the utilization of the robotic system. Per-procedural costs calculated by adding per-use costs to the cost of each RS assuming an annual number of 500 RAS and 5-year period. *Not available
Discussion
In the current study, we performed a SWOT analysis and outlined present and future opportunities and threats of a new ARS. Based on our analysis and comparative data of the dVRS and Senhance RS, we performed a projected calculation of procedural cases assuming 2 scenarios with different purchase costs of ARS. We, then, performed a projective business analysis of the ARS for the next 10 years.
According to our SWOT, the differentiating characteristics of the ARS were the presence of single-use instruments, use of bipolar energy and slender eyepiece design. To achieve lower procedural costs, the company has implemented several key concepts including the incorporation of basic features together with the most used set of instruments limits and the elimination of instrument sterilization and damage costs due to their single-use design [7].
An important point is the timely introduction of the ARS along with the other recently developed alternative robotic platforms. Although some of these RSs already received approvals, the overall number of studies is limited and the pace of their spread is very slow [6]. With a clearly defined marketing strategy, Avatera has all the potentials to become the main competitor to the dVRS. In contrast to the ARS, there is a huge piece of evidence accumulated during the last 20 years from almost all surgical disciplines. The dVRS is accepted as the standard robotic platform associated with high-quality clinical outcomes [9]. In addition, there is a developed positive attitude among surgeons and patients towards the dVRS due to substantial media marketing [10]. Changing these beliefs and attitudes can be somehow challenging and may require some time and effort. Given this, a significantly reduced purchase cost for ARS might be needed to make the system more appealing for hospitals and decision-makers. Thus, a purchase cost between $700.000 and 800.000 seems reasonable during the initial phases of market entrance.
Even with the latter purchase cost of the ARS a lower procedural cost can be achieved with the Senhance system. Nevertheless, the latter system should be viewed more as an alternative/competitor to conventional laparoscopy. The system consists of separate a surgeon-console and 3 robotic arms, has some features of other RSs and is claimed to improve the ergonomics for the surgeons and. However, it still lacks one of the main and distinctive functions, “Endowrist” function with 7 df of movements, responsible for the reported better clinical outcomes [5, 6]. When compared to RSs which have the latter feature, the ARS will be the least expensive and more appealing. Further specific marketing strategies and identifying primary target groups can facilitate the faster spread of the ARS.
The first and the biggest target group for Avatera will be the hospitals that are not equipped with any RS yet. This category can include public and private hospitals in countries with already existing dVRS and most important in countries, where dVRS is not affordable due to its higher purchase and maintenance costs. Up to now, most of the da Vinci systems have been installed in Northern America and Europe, with limited numbers in Asia, Africa and Southern America [11]. This opens a huge field for a new system to easily enter the market and become the leading player in those countries.
For the second target immediate and delayed possibilities for the installment of the ARS can be visualized. Currently, the RAS utilizing dVRS is reserved for more complex oncological surgeries with limited use for benign conditions [12]. Reducing the per-use costs can allow the hospitals to utilize the robotic platform for procedures deemed to be not cost-effective with da Vinci assistance.
It can be expected that there will be an increased need for RAS in the near future. The role of RSs has been more emphasized during last year due to the Covid-19 pandemic. Many of the planned surgical procedures were postponed due to the overload of hospitals and the caution of nosocomial transmission [13, 14]. Even with optimistic predictions, the Covid-19 situation will continue for a long time [15]. The given necessitates use of RSs together with the use of artificial intelligence to decrease the number of involved surgical personnel [16]. Although the clinical application of artificial intelligence in the operative room is very limited [17], its development integration in the medical systems can eliminate the need for bedside assistance.
On the other hand, the Covid-19 itself might introduce some logistic issues for the ARS as a result of closed borders and impaired inter-country communications. The supply and service for the system as well as on-site training might be complicated in some countries. Apparently, these are global issues not dependent on a single company.
Our research was associated with several limitations. A major criticism can be the absence of any clinical data for the Avatera system. Although its effectiveness has been proven in the cadaver studies, and interviews with experts have provided us with important insights, no data on human studies is available so far. Another drawback of the research is that the ARS has not been launched and the actual costs of the system are not known yet. To minimize the inaccuracies for calculations of procedural costs, two different scenarios with different purchase costs (based on experts’ evaluations) were presented and discussed. As such this research demonstrated what could be expected if the purchase prices of the Avatera system were in the range of $1.2–1.5 million and $700,000–800,000. For ease of comparison, the calculations were performed with the annual number of RAS of 500 cases.
Finally, it should be stated that in our study we did not evaluate the scalability of the pricing as well as the long-term costs of using disposable instruments. A further potential solution can be the introduction of recyclable single-use instruments to minimize the environmental pollution caused by them.
None withstanding the discussed limitations, the current study is the first to evaluate market opportunities and competitive potentials for the new Avatera robotic system using the SWOT analytical framework.
Conclusion
The introduction of new RSs will greatly affect and reshape the market of robotic surgery. The ARS has all the technical capacity ensuring the performance of high-quality surgical procedures. A fast spread and implementation of the ARS could be expected should the purchase and maintenance costs be kept low.
Acknowledgements
Not applicable
Authors contribution
EL: study concept and project development, data analysis and interpretation, manuscript editing; AT: data analysis and interpretation, manuscript drafting; IK: data interpretation, critical revision; PK: manuscript editing, critical revision; DM: manuscript drafting, critical revision; AM: project development, data analysis and manuscript editing.
Funding
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
No ethical approval was required for this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rassweiler JJ, Autorino R, Klein J, Mottrie A, Goezen AS, Stolzenburg JU, Rha KH, Schurr M, Kaouk J, Patel V, Dasgupta P, Liatsikos E. Future of robotic surgery in urology. BJU Int. 2017;120(6):822–841. doi: 10.1111/bju.13851. [DOI] [PubMed] [Google Scholar]
- 2.Matanes E, Boulus S, Lowenstein L. The implementation of robotic surgery in Israel. Isr Med Assoc J. 2015;17(9):563–566. [PubMed] [Google Scholar]
- 3.Kaye DR, Mullins JK, Carter HB, Bivalacqua TJ. Robotic surgery in urological oncology: patient care or market share? Nat Rev Urol. 2015;12(1):55–60. doi: 10.1038/nrurol.2014.339. [DOI] [PubMed] [Google Scholar]
- 4.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. 2012;187(4):1392–1398. doi: 10.1016/j.juro.2011.11.089. [DOI] [PubMed] [Google Scholar]
- 5.Gozen A, Rassweiler J. Robotic surgery in urology: new kids on the block. Urologe A. 2020;59(9):1044–1050. doi: 10.1007/s00120-020-01293-8. [DOI] [PubMed] [Google Scholar]
- 6.Kinross JM, Mason SE, Mylonas G, Darzi A. Next-generation robotics in gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2020;17(7):430–440. doi: 10.1038/s41575-020-0290-z. [DOI] [PubMed] [Google Scholar]
- 7.Avatera (2020) Avatera system. https://www.avatera.eu/en/avatera-system. Accessed 26 Mar 2021
- 8.Rao PP. Robotic surgery: new robots and finally some real competition! World J Urol. 2018;36(4):537–541. doi: 10.1007/s00345-018-2213-y. [DOI] [PubMed] [Google Scholar]
- 9.Intuitive S (2020) Sustainability report 2020. https://www.intuitive.com/en-us/-/media/Project/Intuitive-surgical/files/pdf/2020-intuitive-sustainability-report.pdf. Accessed 27 Mar 2021
- 10.Wright JD, Raglan GB, Schulkin J, Fialkow MF. Attitudes and beliefs regarding the utility of robotically assisted gynecologic surgery among practicing gynecologists. J Healthc Qual. 2017;39(4):211–218. doi: 10.1097/JHQ.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 11.Azhar RA, Elkoushy MA, Aldousari S. Robot-assisted urological surgery in the Middle East: where are we and how far can we go? Arab J Urol. 2019;17(2):106–113. doi: 10.1080/2090598X.2019.1601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal RP, Koupparis AJ. Expanding the indications of robotic surgery in urology: a systematic review of the literature. Arab J Urol. 2018;16(3):270–284. doi: 10.1016/j.aju.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhat KRS, Moschovas MC, Rogers T, Onol FF, Corder C, Roof S, Sighinolfi C, Rocco B, Patel VR. COVID-19 model-based practice changes in managing a large prostate cancer practice: following the trends during a month-long ordeal. J Robot Surg. 2020 doi: 10.1007/s11701-020-01100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amparore D, Campi R, Checcucci E, Sessa F, Pecoraro A, Minervini A, Fiori C, Ficarra V, Novara G, Serni S, Porpiglia F. Forecasting the future of urology practice: a comprehensive review of the recommendations by International and European Associations on priority procedures during the COVID-19 pandemic. Eur Urol Focus. 2020;6(5):1032–1048. doi: 10.1016/j.euf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orskov S, Nielsen BF, Fons S, Sneppen K, Simonsen L. The COVID-19 pandemic: key considerations for the epidemic and its control. APMIS. 2021 doi: 10.1111/apm.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steward JE, Kitley WR, Schmidt CM, Sundaram CP. Urologic surgery and COVID-19: how the pandemic is changing the way we operate. J Endourol. 2020;34(5):541–549. doi: 10.1089/end.2020.0342. [DOI] [PubMed] [Google Scholar]
- 17.Checcucci E, Autorino R, Cacciamani GE, Amparore D, De Cillis S, Piana A, Piazzolla P, Vezzetti E, Fiori C, Veneziano D, Tewari A, Dasgupta P, Hung A, Gill I, Porpiglia F, Uro-technology and SoMe Working Group of the Young Academic Urologists Working Party of the European Association of Urology Artificial intelligence and neural networks in urology: current clinical applications. Minerva Urol Nefrol. 2020;72(1):49–57. doi: 10.23736/S0393-2249.19.03613-0. [DOI] [PubMed] [Google Scholar]