ABSTRACT
This study examined whether future COVID-19 vaccine acceptance differed based on an experimental manipulation of the vaccine safety and effectiveness profile. Data come from the Detroit Metro Area Community Study, a population-based study conducted July 15–20, 2020. Participants were asked whether they would get a new COVID-19 vaccine after being randomly assigned information about the vaccine’s effectiveness (50% or 95%) and chance of fever (5% or 20%). Among 1,117 Detroiters, 51.3% would accept a COVID-19 vaccine that is 50% effective and 77.1% would accept a vaccine that is 95% effective. Women and adults ≥65 were more accepting of a vaccine; Black Detroiters were less accepting. Believing vaccines to be important, effective, and safe was associated with higher acceptance. Uptake of a COVID-19 may be limited, depending on perceived vaccine effectiveness and general attitudes toward vaccines. Public health approaches to modifying these attitudes will be especially important in the Black community.
KEYWORDS: COVID-19 vaccine, pandemics, Michigan, adult, race/ethnicity
Introduction
The novel coronavirus disease (COVID-19) is an ongoing pandemic which has caused huge disruptions to life in the United States and many countries globally. As Michigan’s most populous city, with more than 86% of the population Black or Hispanic, and more than one – third (36%) living in poverty,1 Detroit was particularly affected by COVID-19 early in the pandemic. Detroit remains one of the top 50 cities by number of cases. Moreover, Detroit’s case fatality rate, at 10.4%, is over double that of the state average of 4.9%.2,3 Due to the virulence and novelty of COVID-19, no current treatments can protect the population, thus a large government and industry focus in is on developing tests for disease/antibody response, therapeutic treatments, and a preventative vaccine.
The seasonal flu vaccine is the closest analog to what an optional COVID-19 vaccine program could look like. The average adult uptake of the seasonal flu vaccine throughout the U.S. during 2019–2020 was 48.4%, with Michigan’s uptake close behind at 48.3%.4 Coverage is lower among Black, Hispanic, Asian, and AI/AN adults and adults of other or multiple races than among whites.5
National surveys have shown sub-optimal support for a proposed COVID-19 vaccine. In the United States, a survey from August 2020 found 65% would support a vaccine for themselves,6 and another survey from September 2020 found 51% of Americans would take a COVID-19 vaccine.7 Globally, one survey found that there are wide variations across countries in vaccine acceptance, with the United States somewhere in the middle with an acceptance rate of 75.4%. 8 The variations in acceptance across studies could be due to policy and cultural contrasts, patterns of vaccine hesitancy (both COVID-19 specifically as well as more generally), 9 different samples, timing, and wording of the surveys. Acceptance of a proposed vaccine could change with fluctuations in perceptions tied to changes in the epidemiology of disease itself over time.10
Perceived effectiveness and safety of the vaccine could also influence proposed uptake. At the time of the study, results from phase III clinical trials were not available, but we assumed the effectiveness of the vaccine could vary theoretically from around 50%, as with the seasonal influenza vaccine11 to 95%, for the measles vaccine.12 Although results from the phase III clinical trials of the mRNA COVID-19 vaccine have shown efficacy >90%,13 other vaccines are in the pipeline, which may have lower efficacy and which may be made available to the public in the future.14
In this study, we surveyed adults in Detroit, Michigan, as part of the longitudinal Detroit Metro Area Community Study (DMACS). Participants were systematically offered information about the side effects and effectiveness of a hypothetical COVID-19 vaccine. The aims of this study were to determine how potential effectiveness and safety profiles could affect intent to obtain a vaccine, and if this acceptance was modified by socioeconomic status, threat perceptions or attitudes toward vaccines in general.
Methods
Study setting and population
The study survey took place between July 15 and 30, 2020. The questionnaire and toplines are available online.15
The Detroit Metro Area Communities Study (DMACS) is a panel survey of Detroit residents that began in 2016 to inform evidence-based decisions about investments and policies shaping Detroit communities; it has now completed 11 waves of data collection. DMACS is a truly representative sample of all adults in Detroit, built on a multistage probability sample. Shortly after the CDC declared the COVID-19 pandemic a national emergency, and with Detroit emerging as a “hot spot,” the Detroit Metro Area Communities Study (DMACS) launched rapid response surveys (each fielded over a two week period) about Detroiters’ experiences with COVID-19, inviting 1,802 existing panelists to take the survey (online or by phone). The first of these DMACS COVID surveys launched on March 31 2020, roughly 2.5 weeks after CDC declared a national emergency for COVID-19, with a 55.3% response rate, and this was followed by four more surveys launched on April 28 (61.8% response rate), May 28 (66.1% response rate), and July 15 (64.6% response rate), which was the first wave with vaccine questions asked, and which is analyzed in this paper.
Vaccine profile experiment
Participants were randomized into one of four groups. Each group of participants was given different information about the safety and effectiveness of a hypothetical COVID-19 vaccine. The vaccine profile differed by safety (5% chance of fever vs. 20% chance of fever) and effectiveness (95% effective vs. 50% effective). The prompt for one profile is shown below:
“A vaccine is currently not available for the coronavirus. For this next question, imagine that a new coronavirus vaccine has just been developed and approved, and it is available for free. Would you get a coronavirus vaccine that is 95% effective, with a 5% chance of a side effect like fever? 95% effective means that there is a 95% reduction in disease among those vaccinated compared to those unvaccinated.”
Perceived threat and vaccine hesitancy
Threat perceptions were assessed with two questions. Participants responded to a question about their likelihood of contracting SARS-CoV-2 in the next three months, using a response scale of 0% to 100% (chance of contracting) with 10% intervals. Perceived severity was assessed with the question, “How serious a problem would you say the COVID-19 pandemic is right now” with four response options: very serious, somewhat serious, not too serious, and not at all serious. The latter two responses were collapsed together in the analysis.
Vaccine hesitancy was quantified using three questions from the Vaccine Confidence Project:16 “Vaccines are important”, “Vaccines are effective,” and “Vaccines are safe.” All three were assessed on a 5-point Likert scale.
Statistical analysis
We analyzed the effect of the vaccine safety and effectiveness profile on acceptance using Poisson regression models with robust standard errors that output risk ratios (RR) and 95% confidence intervals (CI).17 The first model only included the safety and effectiveness vaccine profile as independent variables.
In a subsequent analysis, we adjusted for the factors that were posited to modify the relationship between the vaccine profile and vaccine acceptance. These factors include socioeconomic status (sex, education, income, age, and race/ethnicity), perceived risk of contracting SARS-CoV-2, perceived severity of COVID-19, and general vaccine hesitancy. Each of these factors was entered separately, but all models were adjusted by socioeconomic status. In each model, we entered an interaction term between the factor of interest and the vaccine effectiveness attribute, and then estimated the marginal mean vaccine acceptance at 50% and 95% vaccine effectiveness. We did not conduct an analysis by vaccine safety levels because vaccine safety was not significant in the first model. All data analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC). Results are weighted to be representative of the population of Detroit. The code used to analyze the data, along with results from regression models, is available at: https://doi.org/10.6084/m9.figshare.14166491.
IRB approval
The protocol was approved by the University of Michigan Institutional Review Board (#HUM00112364). Participants read over an informed consent form and agreed to it electronically prior to any data collection.
Results
DMACS is a longitudinal study, and in July, 1,772 existing panelists were invited to participate in this wave of data collection. In total 1,138 (64.2%) responded, and 1,117 completed the experimental questions regarding the vaccine profile. Demographic characteristics of the respondents are shown in Table 1. The majority of respondents were Black (76.5%) and had an educational attainment of high school or less (51.8%). There was wide variation in perceived risk of contracting COVID-19 over the next three months, with 27.4% reporting that they had a 0% chance, and 27.4% saying they had a at least a 50% chance. Most (75.0%) agreed that COVID-19 was very serious. Attitudes toward vaccines also varied, but 44.7% strongly agreed vaccines were important, 31.7% strongly agreed that vaccines were effective, and 29.8% strongly agreed that vaccines were safe.
Table 1.
Demographic characteristics of survey participants in the Detroit Metro Area Community Study (DMACS), July 2020, N = 1138
| Counta | Weighted % ± SE | ||
|---|---|---|---|
| Gender | Male | 336 | 46.4% ± 2.1% |
| Female | 799 | 53.6% ± 2.1% | |
| Education | High school or less | 307 | 51.8% ± 2.1% |
| Some college | 478 | 32.8% ± 1.9% | |
| Bachelor’s degree | 199 | 8.8% ± 0.9% | |
| Graduate degree | 147 | 6.6% ± 0.7% | |
| Income | <$10,000 | 234 | 18.9% ± 1.7% |
| $10,000-$29,999 | 270 | 27.3% ± 2.0% | |
| $30,000-$49,999 | 233 | 22.7% ± 1.8% | |
| ≥$50,000 | 312 | 31.0% ± 2.0% | |
| Age | 18–39 years | 388 | 41.5% ± 2.1% |
| 40–64 years | 548 | 40.2% ± 2.0% | |
| ≥65 years | 189 | 18.3% ± 1.6% | |
| Race/ethnicity | Non-Hispanic Black | 797 | 76.5% ± 1.7% |
| Non-Hispanic White | 147 | 10.8% ± 1.2% | |
| Hispanic | 67 | 7.8% ± 1.2% | |
| Other | 127 | 4.9% ± 0.8% | |
| Perceived risk of contracting SARS-CoV-2 | 0% | 246 | 27.4% ± 2.1% |
| 10% or 20% | 292 | 31.2% ± 2.1% | |
| 30% or 40% | 139 | 14.0% ± 1.6% | |
| ≥50% | 277 | 27.4% ± 2.0% | |
| Perceived seriousness of COVID-19 | Not serious | 66 | 7.0% ± 1.1% |
| Somewhat serious | 232 | 18.0% ± 1.6% | |
| Very serious | 829 | 75.0% ± 1.8% | |
| Vaccines are important | Strongly disagree | 136 | 11.5% ± 1.3% |
| Somewhat disagree | 89 | 10.0% ± 1.4% | |
| Neither agree nor disagree | 176 | 16.6% ± 1.6% | |
| Somewhat agree | 217 | 17.2% ± 1.6% | |
| Strongly agree | 502 | 44.7% ± 2.1% | |
| Vaccines are effective | Strongly disagree | 90 | 7.7% ± 1.1% |
| Somewhat disagree | 113 | 11.9% ± 1.5% | |
| Neither agree nor disagree | 215 | 19.0% ± 1.6% | |
| Somewhat agree | 341 | 29.6% ± 2.0% | |
| Strongly agree | 348 | 31.7% ± 2.0% | |
| Vaccines are safe | Strongly disagree | 122 | 9.5% ± 1.2% |
| Somewhat disagree | 137 | 13.7% ± 1.5% | |
| Neither agree nor disagree | 216 | 21.7% ± 1.8% | |
| Somewhat agree | 312 | 25.3% ± 1.8% | |
| Strongly agree | 328 | 29.8% ± 1.9% |
aThe number of participants with missing data was 3 for gender, 7 for education, 89 for income, 13 for age, 184 for perceived risk, 11 for perceived severity, 18 for vaccines are important, 31 for vaccines are effective, and 23 for vaccines are safe.
Table 2 shows the distribution of participants across four randomized groups. For example, the proportion who were Black varied between 73.9% and 78.7% across the four groups. This table also shows the proportion who would accept a vaccine, broken down by socioeconomic status and vaccine profile. The proportion of Black Detroiters who would accept a COVID-19 vaccine was 56.5% if the vaccine was 95% effective with a 5% risk of fever, and this proportion decreased as the vaccine decreased in effectiveness and had an increased risk of fever. Only 30.6% of Black Detroiters would accept a vaccine 50% effective with a 20% risk of fever.
Table 2.
Vaccine acceptance by socioeconomic status and randomized vaccine profile in the Detroit Metro Area Community Study (DMACS), July 2020, N = 1138
| Vaccine 95% effective, 5% fever risk |
Vaccine 95% effective, 20% fever risk |
Vaccine 50% effective, 5% fever risk |
Vaccine 95% effective, 20% fever risk |
|||||
|---|---|---|---|---|---|---|---|---|
| Count (col. %) | Vaccine acceptance (row %) | Count (col. %) | Vaccine acceptance (row %) | Count (col. %) | Vaccine acceptance (row %) | Count (col. %) | Vaccine acceptance (row %) | |
| Gender | ||||||||
| Male | 90 (47.7%) | 58 (61.4%) | 68 (46.2%) | 45 (61.3%) | 75 (41.8%) | 42 (57.2%) | 102 (48.8%) | 45 (45.5%) |
| Female | 199 (52.3%) | 118 (63.2%) | 202 (53.8%) | 114 (52.4%) | 216 (58.2%) | 69 (27.6%) | 182 (51.2%) | 60 (34.1%) |
| Education | ||||||||
| High school or less | 79 (49.8%) | 47 (56.9%) | 68 (54.7%) | 32 (55.9%) | 82 (53.7%) | 27 (36.9%) | 77 (49.1%) | 28 (39.7%) |
| Some college | 132 (37.1%) | 74 (65.2%) | 113 (30.1%) | 67 (56.0%) | 116 (30.6%) | 37 (37.4%) | 117 (32.8%) | 33 (32.0%) |
| Bachelor’s degree | 40 (5.9%) | 27 (78.0%) | 51 (9.8%) | 34 (57.5%) | 58 (10.3%) | 33 (64.3%) | 50 (9.8%) | 20 (47.5%) |
| Graduate degree | 36 (7.2%) | 27 (69.5%) | 37 (5.5%) | 26 (70.6%) | 35 (5.4%) | 14 (39.7%) | 39 (8.4%) | 24 (62.1%) |
| Income | ||||||||
| <$10,000 | 53 (16.2%) | 27 (39.3%) | 56 (18.8%) | 31 (59.6%) | 60 (22.7%) | 17 (33.5%) | 65 (18.6%) | 20 (29.6%) |
| $10,000-$29,999 | 68 (25.9%) | 39 (58.3%) | 61 (24.4%) | 37 (54.3%) | 77 (31.7%) | 25 (27.8%) | 64 (27.6%) | 16 (30.4%) |
| $30,000-$49,999 | 70 (27.1%) | 44 (69.2%) | 50 (22.8%) | 29 (47.8%) | 51 (15.2%) | 25 (60.9%) | 62 (25.5%) | 28 (39.3%) |
| ≥$50,000 | 77 (30.9%) | 54 (67.6%) | 77 (34.0%) | 51 (67.7%) | 85 (30.4%) | 40 (52.6%) | 72 (28.4%) | 37 (53.4%) |
| Age | ||||||||
| 18–39 years | 95 (37.7%) | 58 (65.2%) | 88 (41.1%) | 49 (46.5%) | 105 (44.3%) | 38 (39.4%) | 99 (42.8%) | 45 (41.3%) |
| 40–64 years | 137 (41.4%) | 82 (60.0%) | 140 (44.1%) | 77 (61.3%) | 143 (39.3%) | 48 (32.2%) | 128 (36.6%) | 34 (36.4%) |
| ≥65 years | 56 (20.9%) | 36 (65.3%) | 38 (14.8%) | 31 (73.6%) | 40 (16.4%) | 24 (59.8%) | 55 (20.6%) | 26 (45.5%) |
| Race/ethnicity | ||||||||
| Non-Hispanic Black | 208 (77.6%) | 116 (56.5%) | 191 (78.7%) | 99 (48.0%) | 204 (76.6%) | 62 (35.3%) | 194 (73.9%) | 59 (30.6%) |
| Non-Hispanic White | 37 (11.3%) | 33 (89.8%) | 40 (8.6%) | 37 (94.9%) | 33 (11.0%) | 23 (72.7%) | 37 (12.0%) | 21 (65.6%) |
| Hispanic | 15 (7.0%) | 9 (72.4%) | 16 (9.6%) | 12 (89.5%) | 20 (7.7%) | 8 (49.8%) | 16 (7.3%) | 11 (81.4%) |
| Other | 31 (4.1%) | 20 (95.0%) | 23 (3.0%) | 11 (61.8%) | 34 (4.7%) | 18 (24.3%) | 38 (6.8%) | 15 (50.0%) |
All percentages are weighted.
col., column.
From a Poisson regression model which only included the vaccine profile as a predictor, we estimated that there were large and significant differences in vaccine acceptance associated with the effectiveness of a hypothetical vaccine – 77.1% of those who presented with a hypothetical vaccine that was 95% effective reported they would accept the vaccine compared to only 51.3% of those who were presented with a vaccine that was only 50% effective (RR = 0.67, P < .0001). No significant difference in vaccine acceptance by vaccine safety (RR 1.07, P = .4317) was uncovered.
There were significant differences in acceptance across the different vaccine effectiveness profiles by age, race/ethnicity, and measures of vaccine hesitancy (Figure 1). Overall, older age groups were more accepting of a vaccine, and Black Detroiters were less accepting than their white counterparts. Having more positive beliefs about vaccines (believing them to be important, effective, and safe) was also associated with higher rates of acceptance. Notably, there were large differences in acceptance if the vaccine was 50% vs 95% effective, except among those with the least positive views about vaccines. Among these groups, i.e., those strongly disagreeing that vaccines were important, effective, or safe, the vaccine would not be accepted regardless of its level of effectiveness.
Figure 1.
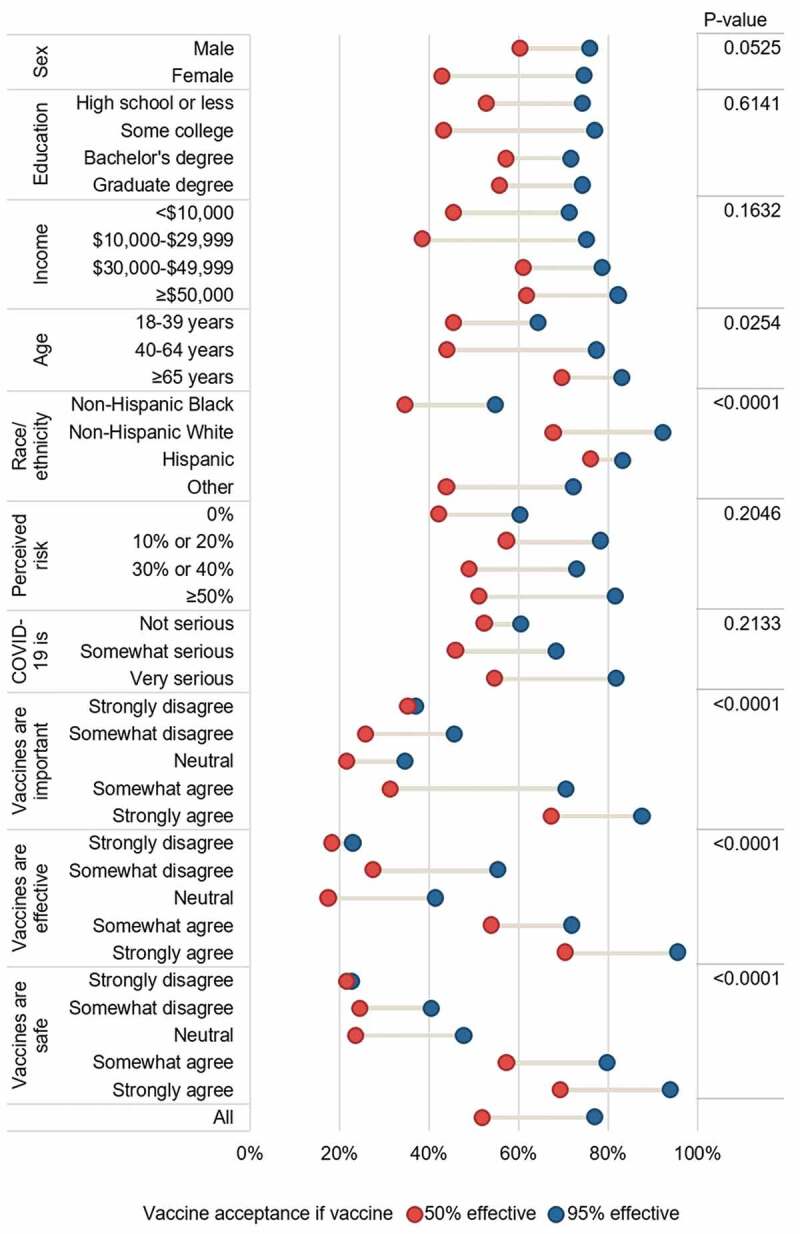
Dumbbell plot of vaccine acceptance if vaccine was 50% versus 95% effective, adjusted for gender, education, income, age, and race/ethnicity. P-value is for main effect of variable
Discussion
Insuring an equitable and efficient distribution of the COVID-19 vaccine needs to be a key component of any successful strategy to control and prevent the spread of COVID-19 now and in the future. Despite that, polls have repeatedly shown that a large proportion of Americans may not accept a COVID-19 vaccine, which could seriously delay or prevent positive control efforts. In this probability based sample of Detroiters, a large proportion would not accept a vaccine, particularly if its effectiveness was low or if they held generally negative views of vaccines in terms of not being important, effective, or safe.
An important finding was that acceptance was lower in Black Detroiters than other groups. This accords with current influenza vaccine programs – influenza vaccination coverage is lower among Black compared to white Americans (39.4% vs 48.7% in 2018–19).18 Lower vaccination coverage in the Black population could be driven by diverse issues, such as convenient access to vaccination providers and mistrust in health care workers given their experiences in the medical setting. For some groups, including Black Americans, the root concern is not hesitancy toward vaccines, but mistrust in the medical establishment.19 Nevertheless, Black Americans have engaged in behaviors to limit the spread of SARS-CoV-2, like social distancing, in similar proportions to white Americans.20 Given the substantial burden of COVID-19 in Black Americans and other minority groups,21 it will be important to adopt public health measures to promote vaccination within groups, like Black Americans, who have been historically and currently neglected by the medical and public health establishment.
A previous survey found that the speed of development and concern about side effects were among the most frequently cited reasons for mistrust in the COVID-19 vaccine,22 and so these concerns could be addressed in vaccine promotions. Addressing concerns about the effectiveness of the COVID-19 vaccine could increase uptake. Unfortunately, the COVID-19 vaccine development process has become politicized,23 even more so since this study was conducted, and so people’s perceptions of how safe or effective the vaccine is, may be influenced by politics rather than public health policy or scientific evidence. It is also important to note that these perceptions of vaccination are in addition to baseline political differences in perceived risk of COVID-19 infection.20
We examined the relationship between perceived threat of COVID-19 and vaccine acceptance, with perceived threat decomposed into risk and severity perceptions. We did not find a relationship between perceived severity of disease and vaccine acceptance. However, previous experimental evidence from a hypothetical pandemic found that participants were more likely to want to get a vaccine if they were told the disease posed severe consequences. Importantly, if they were told that some cases were more severe and others less severe, the participants were less likely to want to get the vaccine.24 This indicates that differing information about the threat of COVID-19 could overwhelm risk calculations in an individual and negate any impact on vaccine acceptance. In the current pandemic, a wide assortment of stories from the media and from personal acquaintances about COVID-19 cases of varying severity could overwhelm individual perceptions of the seriousness of the pandemic, and limit acceptance of a vaccine. Another study, from Hong Kong, found substantively similar results, although they found that perceived severity, but not perceived risk, was significantly related to vaccine intent.25 The differences between these two studies could be tied to baseline epidemiological differences between Detroit and Hong Kong, or by how the survey questions were worded. Nevertheless, these two studies show a limited ability to predict intent to vaccinate based on threat perceptions.
Strengths and limitations
A limitation of the current analysis was the assessment of risk perceptions at one point in time, prior to the licensure of the vaccine. These perceptions may change over time, and with the implementation of an actual vaccine, so influence eventual acceptance of a COVID-19 vaccine. Additionally, the standards we used for effectiveness and safety differ slightly from empirical evidence from currently available vaccines. For example, although the Moderna and Pfizer vaccines are >90% effective, the Johnson & Johnson vaccine is 66% effective in preventing moderate to severe COVID-19.26 We also acknowledge that other factors between these vaccines could influence uptake, including number of doses required.27 Other factors, including knowledge of COVID-19 and history of receipt of other vaccines, like influenza vaccine, were not assessed in this study, but could be important predictors of COVID-19 vaccination intent, and could vary across race.18 A strength of this study was its use of a probability-based sample from a city hard hit by COVID-19 early on in the pandemic.
Conclusions
Acceptance of a COVID-19 vaccine could be strongly affected by how effective the vaccine is perceived to be, even if actual effectiveness is well-described. In a study in Detroit, uptake of a COVID-19 could be lower than 50%, especially if the vaccine is believed to have low effectiveness, among Black Detroiters, and among those with vaccine hesitant attitudes and beliefs.
Acknowledgments
We acknowledge the role of the data collection team in enrolling participants prior and during the outbreak of the COVID-19 pandemic.
Funding Statement
ALW’s salary was supported by an award from the National Science Foundation, Division of Social and Economic Sciences [2027836].
Disclosure of potential conflicts of interest
All authors report no conflict of interest. The funder of this study had no role in study design, analysis, interpretations, or writing of the manuscript.
References
- 1.US Census . QuickFacts Detroit city, Michigan [Internet]; 2019. [accessed 2020 Oct 30]. https://www.census.gov/quickfacts/detroitcitymichigan
- 2.Mack J.Why is Michigan’s Coronavirus death rate so high? [Internet]. MLive; 2020. [accessed 2020 Oct 28]. https://www.mlive.com/public-interest/2020/06/why-is-michigans-coronavirus-death-rate-so-high.html
- 3.Michigan.gov . Coronavirus: Michigan data [Internet]; 2020. [accessed 2020 Oct 28]. https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173—,00.html
- 4.Centers for Disease Control and Prevention (CDC) . 2010–11 through 2019–20 influenza seasons vaccination coverage trend report [Internet]; 2020. [accessed 2020 Oct 28]. https://www.cdc.gov/flu/fluvaxview/reportshtml/trends/index.html
- 5.CDC . Flu vaccination coverage, United States, 2018–19 influenza season [Internet]; 2019. [accessed 2020 Dec 19]. https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm
- 6.Mullen O’Keefe S. One in Three Americans would not get COVID-19 vaccine [Internet]. Gallup; 2020. [accessed 2020 Oct 28. https://news.gallup.com/poll/317018/one-three-americans-not-covid-vaccine.aspx
- 7.Tyson A, Johnson C, Funk C. Concerns about the safety and effectiveness of possible vaccine, pace of approval process [Internet]. Pew Res; 2020. [accessed 2020 Oct 28]. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
- 8.Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–59. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 10.Gidengil CA, Parker AM, Zikmund-Fisher BJ. Trends in risk perceptions and vaccination intentions: a longitudinal study of the first year of the H1N1 pandemic. Am J Public Health. 2012;102(4):672–79. doi: 10.2105/AJPH.2011.300407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention . Seasonal influenza vaccine effectiveness, 2004–2018 [Internet]. Influ; 2019. [accessed 2019 Mar 19]. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm
- 12.Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J Infect Dis. 2011; 204:S133–48. [DOI] [PubMed] [Google Scholar]
- 13.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325(13):1318. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 15.Detroit Metro Area Communities Study . 2020 Toplines [Internet]; 2020. [accessed 2020 Dec 19]. https://detroitsurvey.umich.edu/findings/2020-2/
- 16.de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396(10255):898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 18.Grohskopf LA, Liburd LC, Redfield RR. Addressing influenza vaccination disparities during the COVID-19 pandemic. JAMA. 2020;324(11):1029. doi: 10.1001/jama.2020.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson LD, Smith MA, Bigman CA. Does discrimination breed mistrust? Examining the role of mediated and non-mediated discrimination experiences in medical mistrust. J Health Commun. 2019;24(10):791–99. doi: 10.1080/10810730.2019.1669742. [DOI] [PubMed] [Google Scholar]
- 20.Masters NB, Shih S-F, Bukoff A, Akel KB, Kobayashi LC, Miller AL, Harapan H, Lu Y, Wagner AL. Social distancing in response to the novel coronavirus (COVID-19) in the United States. PLoS ONE. 2020;15:e0239025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke D, Fresques H. Early data shows African Americans have contracted and died of coronavirus at an alarming rate [Internet]. ProPublica; 2020. [accessed 2020 Apr 14]. https://www.propublica.org/article/early-data-shows-african-americans-have-contracted-and-died-of-coronavirus-at-an-alarming-rate
- 22.Latkin CA, Dayton L, Yi G, Konstantopoulos A, Boodram B. Trust in a COVID-19 vaccine in the U.S.: a social-ecological perspective. Soc Sci Med. 2005;162:113684. doi: 10.1016/j.socscimed.2021.113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020;579(7799):321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]
- 24.Zikmund-Fisher BJ, Scherer AM, Knaus M, Das E, Fagerlin A. Discussion of average versus extreme case severity in pandemic risk communications. Emerg Infect Dis. 2017;23(4):706–08. doi: 10.3201/eid2304.161600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong MCS, Wong ELY, Huang J, Cheung AWL, Law K, Chong MKC, Ng RWY, Lai CKC, Boon SS, Lau JTF, et al. Acceptance of the COVID-19 vaccine based on the health belief model: a population-based survey in Hong Kong. Vaccine. 2021;39(7):1148–56. doi: 10.1016/j.vaccine.2020.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livingston EH, Malani PN, Creech CB. The Johnson & Johnson vaccine for COVID-19. JAMA. 2021. doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Wagner AL, Lin M, Sun X, Zikmund-Fisher BJ, Boulton ML, Ren J, Prosser LA. Preferences for vaccination program attributes among parents of young infants in Shanghai, China. Hum Vaccin Immunother. 2020;16(8):1905–10. doi: 10.1080/21645515.2020.1712937. [DOI] [PMC free article] [PubMed] [Google Scholar]


