ABSTRACT
Background: To evaluate the persistence of antibody for 10 years, and investigate the effect of one or two booster doses with Kanghua human diploid cells rabies vaccine (HDCV) in China.
Methods: Participants were re-recruited at year 10 post the primary phase 3 clinical study. Some of them in Kanghua HDCV group who had been boosted one dose at year 8, received one more dose at this boosted study. Participants who never boosted were randomly assigned to boost 1 or 2 doses of Kanghua HDCV. Blood samples were collected at day 0, 1, 3, 7, and 14. Safety was evaluated from day 0–14.
Results: At year 10 after primary vaccination, the seroconversion rates of neutralizing antibody were 98.28–100% in Kanghua and Pasteur groups.
After booster, the seroconversion rate in each group reached to 100% from day 7 to day 14. GMCs were similar in the groups with the same booster doses, and two doses of booster induced higher levels of antibody. The reported rates of solicited local and systemic adverse reaction were low, and no serious adverse events were found through the boosted study.
Conclusion: 5 doses of Kanghua HDCV maintained long-term immunity at least 10 years. One or two doses of booster, rapidly triggered 100% protection against rabies virus.
Trial registration: ClinicalTrials.gov: NCT03774628
KEYWORDS: Human diploid cells rabies vaccine, immunogenicity, safety, booster, clinical trial
Introduction
Rabies is a zoonotic disease with an estimated 60,000 human deaths worldwide each year. Most cases occurred in Africa and Asia regions, and 80% were in rural areas.1,2 The main transmission of rabies virus is by animal bites, and transmission to humans by dogs is responsible for 99% of cases. The incubation period of rabies varies from 1 week to 1 year, typically 2–3 months.2 The earliest specific symptom may be neuropathic pain at the bite site that is a cellular immune response caused by viral replication and inflammation in the corresponding dorsal root ganglion. Then, the rabies virus enters peripheral nerve, spreads to the spinal cord through the peripheral nervous system, and rises to the brain.3 Rabies is virtually fatal after clinical disease develops and rarely survives.4 Fortunately, rabies is a vaccine-preventable disease in both humans and animals. The main rabies vaccines used in many countries are cellular vaccines, including purified Vero cell rabies vaccine (PVRV), human diploid cell rabies vaccine (HDCV) and purified chicken embryo cell rabies vaccine (PCECV), which have shown good immune response and tolerance.5–7 Because of no carcinogenicity and any foreign animal impurity or neurotoxicity factor, HDCV which has excellent clinical performance, was regarded as the “gold standard” rabies vaccine in the WHO drug information in 2002.8 HDCV rabies vaccine had been extensively used in Europe and the United States since the 1970s and 1980s.9,10 However, the first domestic HDCV (Chengdu Kanghua Biological products Co., Ltd) was not approved until 2015 in China, which had shown good immunogenicity and safety profile.11 There is little information to date on long-term immunogenicity by the persistence of antibody and the responses to booster doses in participants immunized with this vaccine. This study compared the persistence of antibody after primary vaccination with two kinds of different vaccines 10 years ago, and the effect of different boosted doses and intervals all by Kanghua rabies vaccine.
Methods
Participants and study design
This randomized, parallel-controlled, partial-blind boosted study was conducted from August 2018 to March 2019, in Lianshui County of Jiangsu Province in China. Protocol was designed by the Jiangsu Provincial Center for Disease Control and Prevention (JSCDC) and Kanghua Biological products Co., Ltd (the study sponsor and manufacture of the boosted vaccine). It was approved by the institutional ethics committee of JSCDC.
Participants were re-recruited from an initial phase III clinical study which was completed in 2008. In initial phase III study, participants received 5 doses of Kanghua HDCV or Pasteur PVRV, according to the conventional Essen (1-1-1-1-1) regimen. A stratified randomization based on the type of vaccine received in the phase III clinical trial was performed according to a blocked randomization list (block = 8). Subjects from Kanghua HDCV group or Pasteur PVRV group, were divided into 2 sub-groups at a ratio of 1:1, respectively. Subjects who had a booster in the year 8 did not participate in randomization, and received one more dose of booster immunization.
Prior to the boosting, participants were re-provided the written informed consents, and were excluded according to the exclusion criteria. Key exclusion criteria included that female subject who is breast-feeding, pregnant, or planning to be pregnant during the trial, subjects with severe allergies or allergic to any ingredient of vaccine, subjects with acute disease/infection or fever (axillary temperature >37.0°C on the day of vaccination), immunodeficiency conditions, history of asthma, diabetes (type I or II) and thyroidectomy, bleeding disorders or history of known thrombocytopenia, malignant tumor or cancer, anti-TB treatment or prevention is under way, asplenia or history of splenectomy, receipt of immunosuppressor, cytotoxic therapy or inhaled corticosteroids in past 6 months. Eligible participants from the initial Kanghua HDCV group, except those ones boosted at year 8, were randomly divided into two sub-groups (Sub-groups A and B, See Figure 1), to boost one or two doses (3 days interval) of Kanghua HDCV. Others in sub-groups D and E from the initial Pasteur PVRV group were boosted of Kanghua HDCV with the same two schedules. Subjects in sub-groups C from initial Kanghua HDCV group who had boosted one dose at year 8, received one more dose in this year 10 boosted study. Blood samples were collected pre-booster, and at day 1, 3, 7, and 14 post the first boosted vaccination. Safety data were collected from day 0 to day 14 post-the first dose and serious adverse event was observed during the 14 days.
Figure 1.
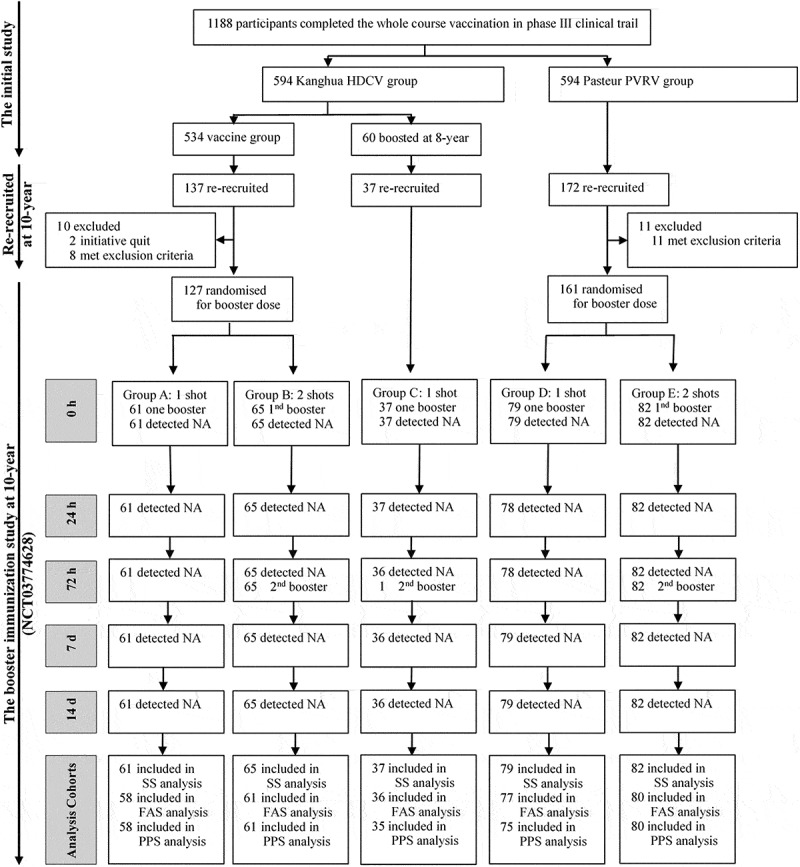
Participants flow chart
The full-analysis-set (FAS) population was defined as participants who had correctly received the priming 5-dose vaccinations in the previous phase 3 trial, received at least 1 booster dose, and had result of serologic tests at day 0, and at least 1 later time point. The per-protocol-set (PPS) population was defined as participants who had correctly received the priming 5-dose vaccinations in the previous phase 3 trial, received the booster dose in this complementary clinical trial 10 year later, and for whom antibody titers in serum both before and after booster injection were available. The safety-set (SS) Population was defined as participants who had received at least one booster dose and have at least one safety information in this complementary clinical trial at 10 year later. * for details, see Supplementary Table S1. #, ﹩, ☆, Δ, †, ‡, £, § for details, see Supplementary Table S2. Abbreviations: 1nd, the first; 2nd, the second; NA, neutralizing antibody.
Vaccines
The boosted vaccine was a lyophilized HDCV vaccine and developed by Chengdu Kanghua Biological products Co., Ltd. The vaccine currently available on the market in China (approval number: 20120007), prepared by infecting the Wi-38 human diploid cell strain with Pitman-Moore (PM) strain of rabies virus, which has also been approved by the China National Medical Products Administration(NMPA). After inoculating human diploid cells, the virus was cultured, harvested, purified, inactivated, and stabilized by freeze-drying. Finally, the potency of vaccine was no less than 2.5 IU/mL (specification:1 ml/dose). The effective ingredient is inactivated fixed rabies virus, the excipients are maltose and human serum albumin, and the vaccine diluent is sterilized PBS.
Immunogenicity
Rabies virus-neutralizing antibodies (RVNA) in the serum was determinated, using the rapid fluorescent focus inhibition test (RFFIT).12 The World Health Organization (WHO) accepted threshold level of seroconversion was 0.5 IU/ml based on the criteria.13,14 Immunogenicity was evaluated by the geometric mean concentration (GMC), seroconversion rate, and geometric mean fold increase (GMFI) of neutralizing antibodies.
Safety
Safety data were collected post each dose, including solicited inject-site and systemic adverse reaction (AR) and unsolicited adverse events (AEs). Solicited ARs classified as inject-site AEs (induration, redness, pain, swelling, itch) and systemic AEs (fever, diarrhea, cough, nausea/vomiting, fatigue, muscle pain, headache) were recorded within 7 days after each vaccination. Adverse reactions were graded according to the guidelines of vaccine clinical trials classification standard in China.15 Serious adverse events (SAEs) were collected from day 0 to 14.
Sample size and statistical analysis
Each of Kanghua HDCV and Pasteur PVRV group should contain more than 150 subjects re-recruited from 1128 subjects who had complete the initial phase III clinical trial. Subjects should be recruited as much as possible.
Participants, who completed at least one dose in each group were included in the analysis set of safety. The ones who competed full-vaccination and blood samples per protocol in each group, were included in the analysis set of immunogenicity.
Statistical comparisons were made using 2-sided tests with an α value of 0.05. GMC and GMFI were summarized with 95% confidence intervals (CIs) and compared by the Student’s t test or t’ test, respectively. Comparisons of seroconversion rate and adverse reactions/events rate were conducted by Chi–square test or Fisher’s exact test. The statistical analyses were performed by an independent statistician using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
This study began in August 2018 and was completed in August 2019. A total of 346 participants were re-recruited, including 137 subjects from initial Kanghua HDCV group who had never been boosted, 37 subjects were boosted in a 8-year immunity persistence study, and 172 subjects from initial Pasteur PVCV group. After screening, 127 participants from initial Kanghua HDCV group who had never been boosted were enrolled and randomly assigned into sub-groups A and B and 161 participants from initial Pasteur PVCV group were in sub-groups D and E. 37 participants from initial Kanghua HDCV group and boosted at year 8 were enrolled in sub-group C (Figure 1). All the enrolled participants were involved in the safety analysis set, while 12 participants were excluded for immunological analysis because of the history of receiving other rabies vaccination post primary vaccination (Figure 1, Tables S2). Demographic characteristics of all participants at enrollment are listed in Table 1, showing comparable age, sex, and BMI across groups.
Table 1.
Baseline demographic characteristics of participants
| Characteristic | Kanghua vaccine group |
Pasteur vaccine group |
P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | P Value | Group D | Group E | P Value | ||
| Participants, no. | 58 | 61 | 36 | 77 | 80 | |||
| Male sex, no. (%) | 22(37.93) | 24(39.34) | 16(44.44) | .8144 | 34(44.16) | 29(36.25) | .3123 | .8386* |
| Age, years | 54.30 ± 12.63 | 54.57 ± 10.83 | 55.10 ± 10.43 | .9470 | 51.58 ± 10.44 | 51.95 ± 12.89 | .8415 | .3139† |
| Height, m | 1.59 ± 0.07 | 1.61 ± 0.08 | 1.62 ± 0.09 | .1016 | 1.61 ± 0.07 | 1.59 ± 0.07 | .0917 | .1015† |
| Weight, kg | 64.20 ± 9.09 | 65.82 ± 11.69 | 66.01 ± 11.19 | .6308 | 66.26 ± 9.97 | 64.01 ± 10.96 | .1822 | .6061† |
| BMI | 25.37 ± 3.01 | 25.22 ± 3.63 | 24.96 ± 2.94 | .8330 | 25.47 ± 3.48 | 25.21 ± 3.87 | .6591 | .9594† |
The full-analysis-set (FAS) population was defined as participants who had correctly received the priming 5-dose vaccinations in the previous phase 3 trial, received at least one booster dose, and had result of serologic tests at day 0, and at least 1 later time point.
Abbreviations: Group A, Kanghua vaccine group with one dose of booster; Group B, Kanghua vaccine group with two doses of booster; Group C, Kanghua vaccine group with one dose of booster who had received one booster immunization after eight years of primary vaccination; Group D, Pasteur vaccine group with one dose of booster; Group E, Pasteur vaccine group with two doses of booster.
Abbreviations: BMI, body mass index.
Immunogenicity
At year 10 after primary vaccination, the seroconversion rates of neutralizing antibody were 98.28–100%, and GMCs were 2.18–2.44 IU/ml in Kanghua HDCV (sub-groups A and B) and Pasteur PVRV (sub-groups D and E) groups. There were no significant differences between the groups. But in the sub-group C, the seroconversion rate of neutralizing antibody was 100%, and GMC was 8.43 IU/ml which showed significantly higher than those of other four sub-groups (P < .0001) (Table 2).
Table 2.
Immune response to a booster dose of rabies vaccine in the per-protocol-set (PPS) population
| Variable | Kanghua vaccine group (primary vaccination) |
Pasteur vaccine group (primary vaccination) |
P Value | |||
|---|---|---|---|---|---|---|
| Group A (n = 58) | Group B (n = 61) | Group C (n = 35) | Group D (n = 75) | Group E (n = 80) | ||
| Pre-booster | ||||||
| GMC(95% CI) | 2.27(1.79–2.87) | 2.41(1.91–3.06) | 8.43(5.97–11.90) | 2.44(1.98–3.01) | 2.18(1.83–2.60) | <.0001 |
| GMC-SNK test | B | B | A | B | B | |
| Titer ≥0.5IU/ml | ||||||
| Participants, No. | 57 | 60 | 35 | 75 | 79 | |
| Participants, %(95% CI) | 98.28(90.76–99.96) | 98.36(91.20–99.96) | 100.00(90.00–100.00) | 100.00(95.20–100.00) | 98.75(93.23–99.97) | .8534 |
| Post-booster at day 1 | ||||||
| GMC(95% CI) | 2.42(1.92–3.05) | 2.54(2.03–3.18) | 8.22(5.86–11.54) | 2.46(1.99–3.02) | 2.24(1.86–2.69) | <.0001 |
| GMC-SNK-test | B | B | A | B | B | |
| GMFI (95% CI) | 1.06(1.01–1.12) | 1.05(0.98–1.13) | 0.98(0.93–1.03) | 1.01(0.95–1.06) | 1.03(0.55–2.34) | .2801 |
| Titer ≥0.5IU/ml | ||||||
| Participants, No. | 57 | 61 | 35 | 75 | 80 | |
| Participants, %(95% CI) | 98.28(90.76–99.96) | 100(94.13–100.00) | 100(90.00–100) | 100(95.20–100.00) | 100(95.49–100.00) | .3010 |
| Post-booster at the day 3 | ||||||
| GMC(95% CI) | 2.55(2.01–3.24) | 2.65(2.12–3.31) | 8.31(5.91–11.69) | 2.47(2.00–3.03) | 2.39(1.97–2.92) | <.0001 |
| GMC-SNK-test | B | B | A | B | B | |
| GMFI (95% CI) | 1.12(1.03–1.23) | 1.10(1.01–1.20) | 0.99(0.93–1.04) | 1.01(0.95–1.07) | 1.10(1.00–1.21) | .1777 |
| Titer ≥0.5IU/ml | ||||||
| Participants, No. | 57 | 61 | 35 | 74 | 80 | |
| Participants, %(95% CI) | 98.28(90.76–99.96) | 100(94.13–100.00) | 100(90.00–100) | 98.67(92.79–99.97) | 100(95.49–100.00) | .5777 |
| Post-booster at the day 7 | ||||||
| GMC(95% CI) | 11.61(8.83–15.28) | 13.08(9.94–17.22) | 17.02(12.96–22.35) | 13.47(10.60–17.12) | 11.60(9.31–14.47) | .3711 |
| GMFI (95% CI) | 5.12(4.01–6.52) | 5.42(4.26–6.89) | 2.02(1.68–2.43) | 5.51(4.44–6.85) | 5.33(4.36–6.51) | <.0001 |
| GMFI-SNK-test | A | A | B | A | A | |
| Titer ≥0.5IU/ml | ||||||
| Participants, No. | 58 | 61 | 35 | 75 | 80 | |
| Participants, %(95% CI) | 100(93.84–100.00) | 100(94.13–100.00) | 100(90.00–100) | 100(95.20–100.00) | 100(95.49–100.00) | - |
| Post-booster at day 14 | ||||||
| GMC(95% CI) | 27.18(20.40–36.20) | 46.51(36.21–59.74) | 24.50(19.13–31.39) | 35.89(28.24–45.62) | 42.23(34.74–51.35) | .0024 |
| SNK-test | B | A | B | B*A | A | |
| GMFI (95% CI) | 11.97(8.88–16.14) | 19.27(14.34–25.89) | 2.91(2.29–3.68) | 14.70(11.09–19.48) | 19.39(15.33–24.53) | <.0001 |
| GMFI-SNK-test | A | A | B | A | A | |
| Titer ≥0.5IU/ml | ||||||
| Participants, No. | 58 | 61 | 35 | 75 | 80 | |
| Participants, %(95% CI) | 100.00(93.84–100.00) | 100.00(94.13–100.00) | 100.00(90.00–100.00) | 100.00(95.20–100.00) | 100.00(95.49–100.00) | - |
The per-protocol-set (PPS) population was defined as participants who had correctly received the priming 5-dose vaccinations in the previous phase 3 trial, boosted in this complementary clinical trial at 10 year later, and for whom antibody titers in serum both before and after booster injection were available.
Abbreviations: CI, confidence interval; GMFI, geometric mean fold increase; GMC, geometric mean concentration, SNK, Student-Newman-Keuls.
After the first boosted dose, the seroconversion rates and GMCs were barely changed from day 0 to day 3 in sub-groups A, B, C, and D, with the GMFIs of 1.01–1.12. The variables showed no significant difference between the four sub-groups. The levels of antibody started to increase during day 3–7, seroconversion rates up to 100% from day 7, and GMCs were sharply evoked up to 27.18–46.51 IU/ml at day 14, with significantly higher level difference in sub-groups B and E (P = .0024). This variation tendency can also be shown in terms of GMFIs. On the day 7, GMFIs were up to over 5 in sub-groups A, B, D and E with a relatively stable low value nearly 1 from day 0 to 3. To the day 14, GMFIs reached to about 11.97 and 14.70 in sub-groups A and D, and 19.27and 19.39 in sub-groups B and E. In the sub-group C, seroconversion rate maintained 100% at all the visit time, GMC was hardly changed with a value over 8 IU/ml, and significantly higher than others sub-groups from day 0 to 3 (all P < .0001). GMC in sub-group C also began to increase during day 3–7, but not significantly higher than other sub-groups from day 7. To the day 14, the GMC was similar with those in sub-groups A and D, and lower than those in sub-groups B and E (See Table 2).
Reactogenicity and safety
Within 7 days after vaccination, overall number of solicited adverse reactions of injection-site were 4(6.56%), 2(3.08%), 3(8.11%), 8(10.13%), 11(13.41%), respectively, in each sub-groups, which were comparable with no significant difference (Table 3). The main reported local symptom was pain, the reported rates were 3.08–13.41%, and similar between each sub-groups. Total number of solicited systematic adverse reactions were 0(0.00%), 1(1.54%), 0(0.00%), 3(3.80%), 7(8.54%), respectively. With a little higher reported rates in sub-group E (P = .0329). The most common systemic adverse reaction was fever, especially in sub-group E. the reported rates of other local symptoms (induration and itch) at the injection site and systemic symptoms (muscle pain and fatigue) were below 2.7%. All the adverse reactions were mild, and none of grade 3 adverse events was reported. No serious adverse events were reported through the booster study.
Table 3.
Frequency of adverse reactions within 14 days after the first booster dose of rabies vaccine in the safety-set (SS) population
| Adverse Reaciona | Kanghua vaccine group |
Pasteur vaccine group |
P Value | |||
|---|---|---|---|---|---|---|
| Group A (n = 61) | Group B (n = 65) | Group C (n = 37) | Group D (n = 79) | Group E (n = 82) | ||
| Injection-site reaction | ||||||
| Pain | ||||||
| Participants, No. | 4 | 2 | 2 | 7 | 11 | |
| Participants, %(95% CI) | 6.56(1.82–15.95) | 3.08(0.37–10.68) | 5.41(0.66–18.19) | 8.86(3.64–17.41) | 13.41(6.89–22.74) | .2006 |
| Induration | ||||||
| Participants, No. | 0 | 0 | 0 | 1 | 0 | |
| Participants, %(95% CI) | 0(0.00–5.87) | 0(0.00–5.52) | 0(0.00–9.49) | 1.27(0.03–6.85) | 0(0.00–4.40) | .7469 |
| Itch | ||||||
| Participants, No. | 0 | 0 | 1 | 0 | 0 | |
| Participants, %(95% CI) | 0(0.00–5.87) | 0(0.00–5.52) | 2.70(0.07–14.16) | 0(0.00–4.56) | 0(0.00–4.40) | .1142 |
| Overall | ||||||
| Participants, No. | 4 | 2 | 3 | 8 | 11 | |
| Participants, %(95% CI) | 6.56(1.82–15.95) | 3.08(0.37–10.68) | 8.11(1.70–21.91) | 10.13(4.47–18.98) | 13.41(6.89–22.74) | .2410 |
| Systemic reaction | ||||||
| Feverb | ||||||
| Participants, No. | 0 | 1 | 0 | 2 | 7 | |
| Participants, %(95% CI) | 0(0.00–5.87) | 1.54(0.04–8.28) | 0(0.00–9.49) | 2.53(0.31–8.85) | 8.54(3.50–16.80) | .0329 |
| Muscle pain | ||||||
| Participants, No. | 0 | 0 | 0 | 1 | 0 | |
| Participants, %(95% CI) | 0(0.00–5.87) | 0(0.00–5.52) | 0(0.00–9.49) | 1.27(0.03–6.85) | 0(0.00–4.40) | .7469 |
| Fatigue | ||||||
| Participants, No. | 0 | 0 | 0 | 0 | 1 | |
| Participants, %(95% CI) | 0(0.00–5.87) | 0(0.00–5.52) | 0(0.00–9.49) | 0(0.00–4.56) | 1.22(0.03–6.61) | 1.0000 |
| Overall | ||||||
| Participants, No. | 0 | 1 | 0 | 3 | 7 | |
| Participants, %(95% CI) | 0(0.00–5.87) | 1.54(0.04–8.28) | 0(0.00–9.49) | 3.80(0.79–10.70) | 8.54(3.50–16.80) | .0406 |
aSeverity grades were defined as follows, unless otherwise indicated: grade 1, mild (ie, no interference with activity); grade 2, moderate (ie, some interference with activity); grade 3, severe (ie, prevented activity).
bGrade 1, axillary temperature of 37.1°C–37.5°C; grade 2, axillary temperature of 37.6°C–39.0°C; grade 3: axillary temperature of ≥39.1°C.
Abbreviation: CI, confidence interval.
The safety-set (SS) Population was defined as participants who had received at least one booster dose and have at least one safety information in this complementary clinical trial at 10 year later.
Discussion
Most vaccine manufacturers recommend 1 booster dose after 1 year of primary vaccination, and vaccination was recommended every 5 years or, ideally, as required for regular anti-rabies antibody tests to ensure protection in persons at continued risk. No further Pre-exposure prophylaxis (PrEP) booster doses following a primary series are required for people living in, or traveling to high-risk areas.8 In the other hand, studies with the HDCV have shown different persistence of antibody post-vaccination.
The study of Thraenhart et al showed that 18 vaccinees who received pre- or post-vaccination had 100% seroconversion of neutralizing antibody after 2–14 years, with the concentrations was 1.3–5.3 IU/ml at year 9–14.16 However, Kuwert et al. administered HDCV to 17 subjects with 3 doses on day 0, 7, and 14, two of three participants had antibody levels of 1.0 IU/ml at the end of one year, but after two years none had detectable antibody.17 The study of E Rosanoff and H Tint in 1979, showed that participants received pre-vaccination of 1–50 IU/ml HDVC, had GMCs from 8.8 to 34.5 IU/ml at day 35. but to 9–12 months later, the detectable antibody levels were 0.5–3.0 IU/ml. A single dose of booster induced extremely high levels of antibody up to 100 IU/ml.18 In the initial study, the full course injection of HDVC or purified vero cells rabies vaccine (PVRV) resulted in the development of high levels of neutralizing antibody. The GMCs were up to over 37 IU/ml at day 14 post full course of vaccination (data was not published). To the year 10, our study showed that the primary vaccination could induce long-term persistence of antibody, the GMCs were maintained at a level approximately 2.5 IU/ml, and the seroconversion rates were over 98%. The initial HDCV group had the similar results with the PVRV group. Although it was difficult to compare the results from different studies with the diversity of doses and schedules, studies found that no significant differences in vaccine-induced neutralizing antibody (VNA) were observed in association with number of doses of vaccine received or the length of time after primary vaccination.19
The anamnestic responses can be elicited by booster doses whether the subjects had a detectable antibody concentration prior to the booster or not. However, the completely anamnestic responses occurred at least since the day 5–7.20,21The anamnestic responses to the booster of Kanghua HDCV was after day 3 and showed increased GMCs and 100% seroconversion of vaccine-induced neutralizing antibody of since day 7. Study suggested that the circulating neutralizing antibody will have begun to appear after day 7 following the first rabies vaccine dose.3,11 Rabies immunoglobulin (RIG) was recommended and administered as soon as possible after the initiation of post-expoure prophylaxis (PEP), to provides passive immunization before the immune system can respond to the vaccine by producing VNAs.3 During the period before day 7 post first dose of booster, subjects with antibody <0.5IU/ml, who is subsequently exposed to rabies, RIG should be required. In this study, the boosted vaccination induced peak levels, for the same primary vaccination groups, 2 doses boosters evoked higher levels of antibody compared to that of 1 dosed group with the 100% seroconversion in each group, respectively. The results from Strady et al confirmed that if the subjects had an antibody concentration of 30 IU/ml or more after the booster, their antibody level would remain ≥0.5 IU/ml for the next 10 years.22 After 2 doses of booster, the GMCs was increased from approximate 2.41 and 2.18 up to 46.51 and 42.23 in sub-groups B and E. The results indicated that two booster doses of Kanghua HDCV might induce long-term immunogenicity. However, participants who received 2 doses of booster with a 2-year interval, had lower GMC of antibody about 24.50 IU/ml than those in sub-groups B and E with 2 booster dose, had the similar level with the one booster groups. According to the algorithm from the previous study, 2 doses of boots with a short interval might be a better interval for subsequent boosters, even if there were shown well immunogenicity in each sub-group.22,23
There was no recommendation for the timing of subsequent booster vaccinations for the Pesteur PVRV. In the same schedule sub-groups, participants who received Pesteur PVRV boosted with Kanghua HDCV at the 10-year after primary vaccination, had similar level of VNAs with those with Kanghua vaccine through the primary and boosted study. The results suggested that subjects received Pesteur PVRV of primary vaccination, could boost with Kanghua HDCV, and the course of vaccination also conducted satisfactory immunogenicity.
The limitations of the study included: (1) Although the participants had recruited as possible as we can, the sample size in the Kanghua HDCV subgroup was lower, because of the reasons of losing follow-up, working outside. Significantly, this is the first report for the persistence and booster of Kanghua HDCV in China, which was essential. (2) The time points of observation of immunogenicity were fewer, there no observation during the period post-primary vaccination to the tenth year. We could conduct a long-time follow-up post booster, immunity durability will be researched in future studies. (3) Studies found that completely anamnestic responses occurred at least since the day 5–7,20,21 the variation of antibody from day 3 to 7 was unclear in this boosted study. It was better to add observation of immunogenicity between day 3 and 7.
In conclusion, this study confirmed that two schedules of booster induced well immune response with higher GMC in the two doses groups, especially in the short interval sub-groups. It will be more important to provide the data for persistence and alternative enhanced immunity of HDCV in China.
Supplementary Material
Acknowledgments
The authors would like to thank the volunteers who participated in this study, and also thank all the investigators of Jiangsu Provincial Center for Diseases Control and Prevention and Yancheng Center for Diseases Control and Prevention, who were responsible for collecting data and managing participants.
Funding Statement
This work was supported by Chengdu Kanghua Biological products Co., Ltd.
Abbreviations
| HDCV | human diploid cells rabies vaccine |
| PVRV | purified Vero cell rabies vaccine |
| GMC | Geometric mean concentration |
| GMFI | Geometric mean fold increase |
| SAE | serious adverse event |
| AR | adverse reaction |
| AE | adverse events |
| CI | confidence interval |
| NIFDC | National Institute for Food and Drug Control |
| SNK | Student-Newman-Keuls |
Authors’ contributions
F-C Zhu, F-Y Meng, W-L Hou, R Zhou, H Liu, X-H Gan contributed to conception and design of the study; F-Y Meng and W-L Hou contributed to collecting data and managing participants; M-W Wei, S-Y Wang analyzed the data; J-L Hu, M-W Wei, S-Y Wang wrote the paper; All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1906601.
References
- 1.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(4):e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO fact sheets on rabies 2020. [accessed 2020 Apr]. https://www.who.int/en/news-room/fact-sheets/detail/rabies.
- 3.WHO . Rabies vaccines: WHO position paper, April 2018 - recommendations. Vaccine. 2018Sept5;36(37):5500–03. doi: 10.1016/j.vaccine.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Jackson AC.Human rabies: a 2016 update. Curr Infect Dis Rep. 2016;18(11):38. doi: 10.1007/s11908-016-0540-y. [DOI] [PubMed] [Google Scholar]
- 5.Ramezankhani R, Shirzadi MR, Ramezankhani A, Poor Mozafary J. A comparative study on the adverse reactions of purified chick embryo cell vaccine (PCECV) and purified vero cell rabies vaccine (PVRV). Arch Iran Med. 2016;19(7):502–07. [PubMed] [Google Scholar]
- 6.Shi N, Zhang Y, Zheng H, Zhu Z, Wang D, Li S, Li Y, Yang L, Zhang J, Bai Y, et al. Immunogenicity, safety and antibody persistence of a purified vero cell cultured rabies vaccine (Speeda) administered by the Zagreb regimen or Essen regimen in post-exposure subjects. Hum Vaccines Immunother. 2017;13(6):1–8. doi: 10.1080/21645515.2017.1279770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moro PL, Woo EJ, Paul W, Lewis P, Petersen BW, Cano M. Post-marketing surveillance of human rabies diploid cell vaccine (Imovax) in the vaccine adverse event reporting system (VAERS) in the United States, 1990–2015. PLoS Negl Trop Dis. 2016;10(7):e0004846. doi: 10.1371/journal.pntd.0004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. WHO position on rabies vaccine. WHO Drug Information. 2002:16(1). [Google Scholar]
- 9.Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection. Post-exposure treatment with the new human diploid cell rabies vaccine and antirabies serum. 1976. Wilderness Environ Med. 2000;11(1):42–46. doi: 10.1580/1080-6032(2000)011[0042:PTWTNH]2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Tsiang H. Rabies vaccines: a review of progress towards improved efficacy and safety. BioDrugs. 1998;10(4):317–28. doi: 10.2165/00063030-199810040-00006. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Wu X, Cai Y, Cao S, Zhu X, Lv Q, Chen H, Shi L, Li J, Wang X, et al. Pre-marketing immunogenicity and safety of a lyophilized purified human diploid cell rabies vaccine produced from microcarrier cultures: a randomized clinical trial. Hum Vaccines Immunother. 2019;15(4):828–33. doi: 10.1080/21645515.2018.1549450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalan E, Wilson C, Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand. 1979July;7(3):213–20. doi: 10.1016/S0092-1157(79)80024-4. [DOI] [PubMed] [Google Scholar]
- 13.Organization WH . WHO recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies. 1997.
- 14.Organization WH. WHO Expert Consultation on Rabies. Second report. World Health Organ Tech Rep Ser, 2013. [PubMed]
- 15.Chu K, Hu J, Meng F, Li J, Luo L, Xu J, Yuan Z, Li Z, Chen W, Jiao L, et al. Immunogenicity and safety of subunit plague vaccine: a randomized phase 2a clinical trial. Hum Vaccines Immunother. 2016;12(9):2334–40. doi: 10.1080/21645515.2016.1175261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thraenhart O, Kreuzfelder E, Hillebrandt M, Marcus I, Ramakrishnan K, Fu ZF, Dietzschold B. Long-term humoral and cellular immunity after vaccination with cell culture rabies vaccines in man. Clin Immunol Immunopathol. 1994;71(3):287–92. doi: 10.1006/clin.1994.1088. [DOI] [PubMed] [Google Scholar]
- 17.Kuwert EK, Marcus I, Hoher PG. Neutralizing and complement-fixing antibody responses in pre- and post-exposure vaccinees to a rabies vaccine produced in human diploid cells. J Biol Stand. 1976;4(4):249–62. doi: 10.1016/S0092-1157(76)80010-8. [DOI] [PubMed] [Google Scholar]
- 18.Rosanoff E, Tint H. Responses to human diploid cell rabies vaccine: neutralizing antibody responses of vaccinees receiving booster doses of human diploid cell rabies vaccine. Am J Epidemiol. 1979;110(3):322–27. doi: 10.1093/oxfordjournals.aje.a112817. [DOI] [PubMed] [Google Scholar]
- 19.Mclean HQ, World Health Organization . The immunological basis for immunization series: module 17: rabies. 2011.
- 20.Horman JT, Rullan JV, Myers RA, Bond JO, Israel E, Joseph JM. Antibody response after a two-year intradermal booster of rabies human diploid cell vaccine. J Am Vet Med Assoc. 1987;191:185–87. [PubMed] [Google Scholar]
- 21.Gherardin AW, Scrimgeour DJ, Lau SC, Phillips MA, Kass RB. Early rabies antibody response to intramuscular booster in previously intradermally immunized travelers using human diploid cell rabies vaccine. J Travel Med. 2001;8(3):122–26. doi: 10.2310/7060.2001.24445. [DOI] [PubMed] [Google Scholar]
- 22.Strady A, Lang J, Lienard M, Blondeau C, Jaussaud R, Plotkin S. Antibody persistence following preexposure regimens of cell-culture rabies vaccines: 10-year follow-up and proposal for a new booster policy. J Infect Dis. 1998;177(5):1290–95. doi: 10.1086/515267. [DOI] [PubMed] [Google Scholar]
- 23.Kessels JA, Recuenco S, Navarro-Vela AM, Deray R, Vigilato M, Ertl H, Durrheim D, Rees H, Nel LH, Abela-Ridder B, et al. Pre-exposure rabies prophylaxis: a systematic review. Bull World Health Organ. 2017;95(3):210–9C. doi: 10.2471/BLT.16.173039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


